Fatty Acids and Fatty Acid Trophic Markers in Two Holothurian Species from the Central Mediterranean Sea
Abstract
1. Introduction
2. Materials and Methods
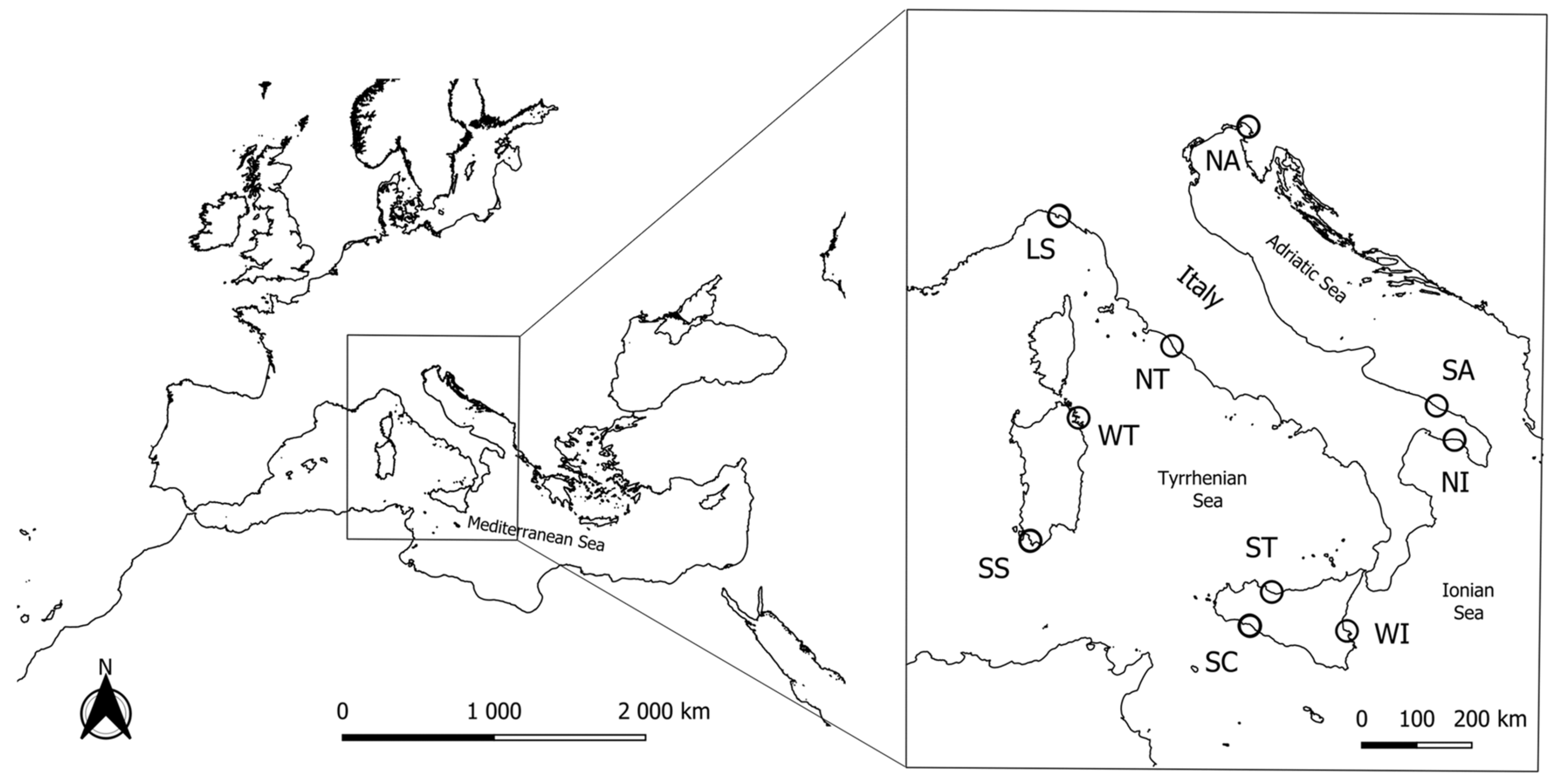
2.1. Sample Collection
2.2. Sample Preparation
2.3. Total Lipid Extraction and GC-FID Analysis
2.4. GC-MS Analysis
2.5. Fatty Acid Trophic Markers (FATM)
2.6. Data Analysis
3. Results
3.1. Fatty Acid Characterization
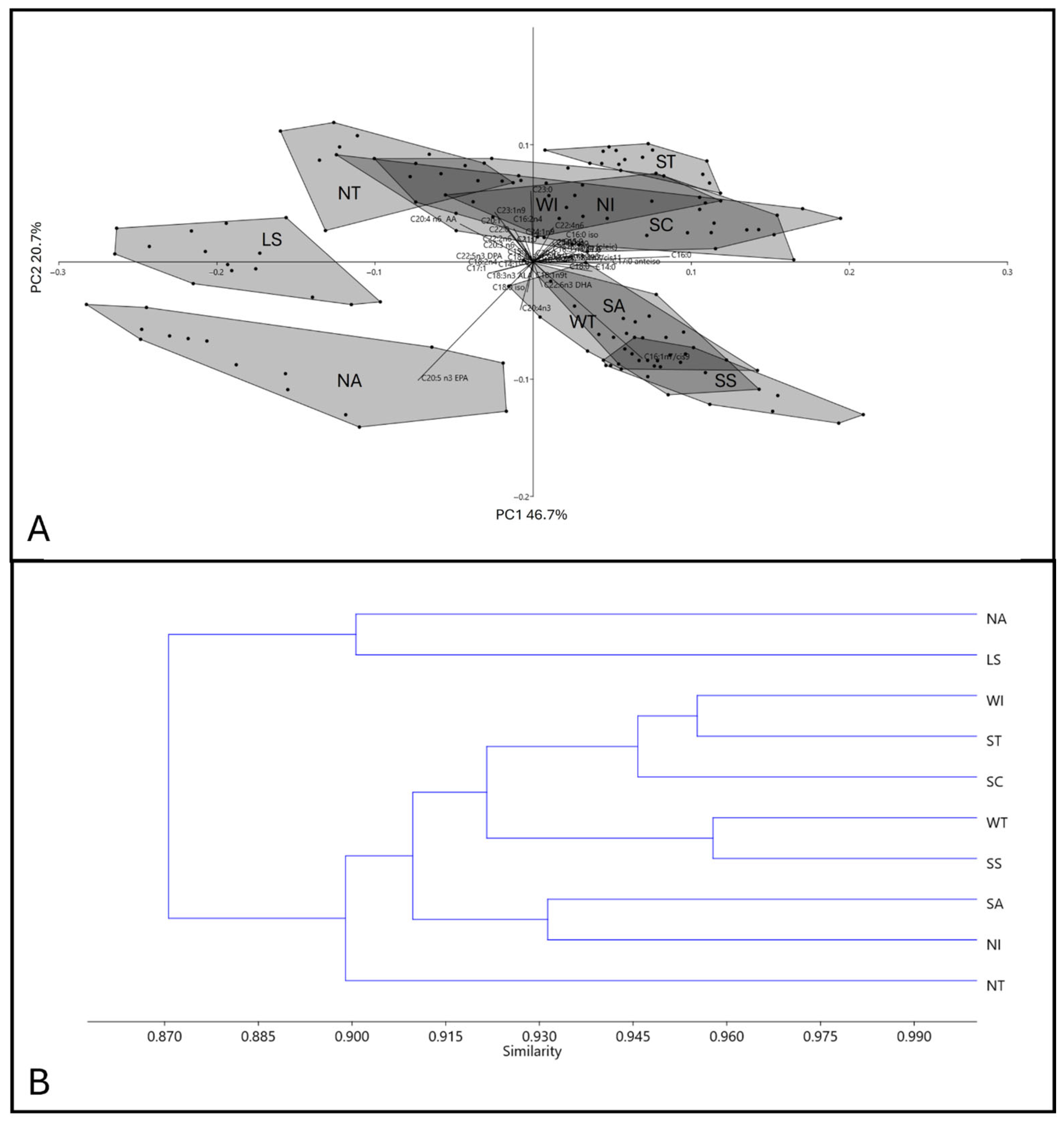
3.1.1. Holothuria polii
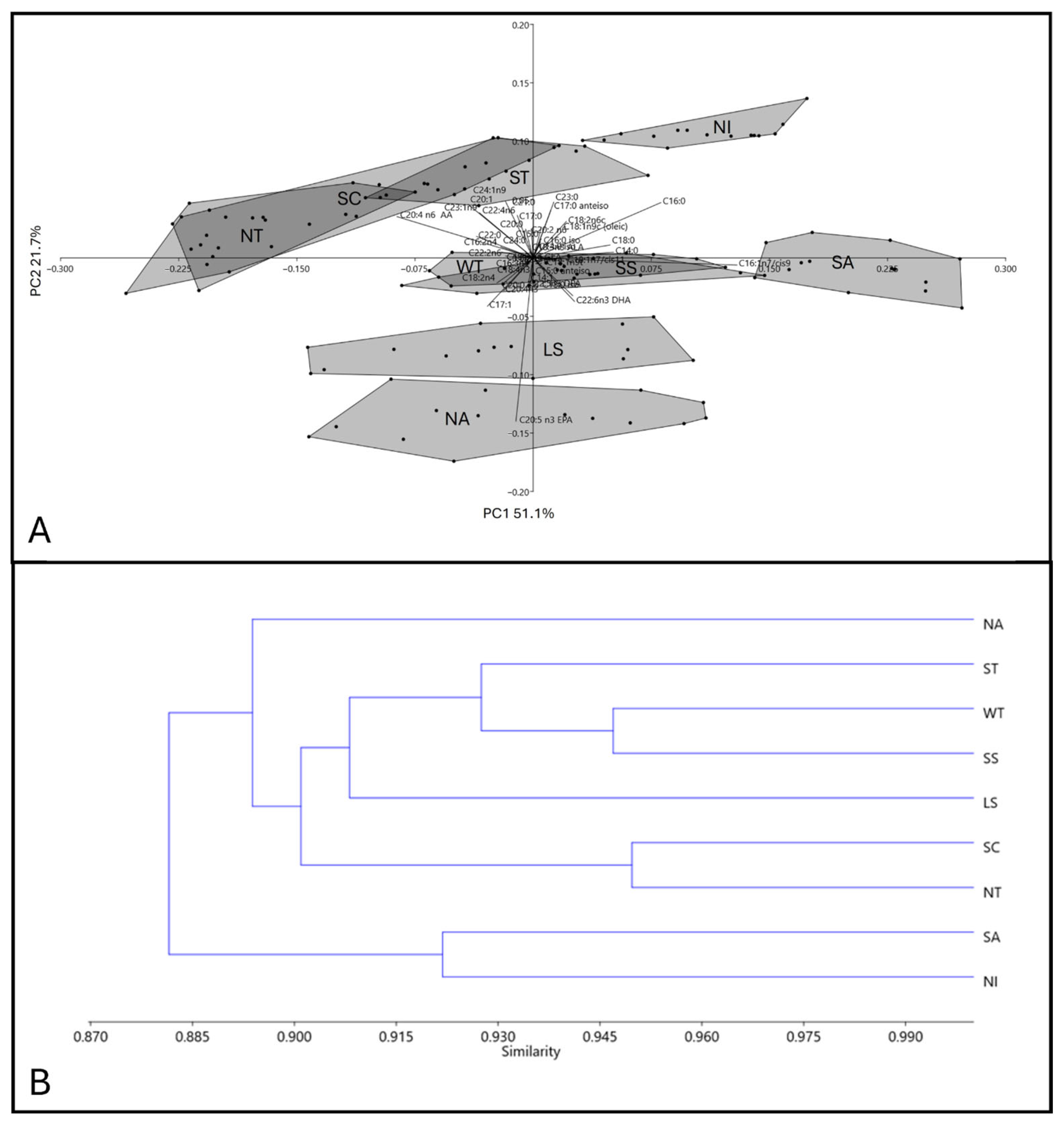
3.1.2. Holothuria tubulosa
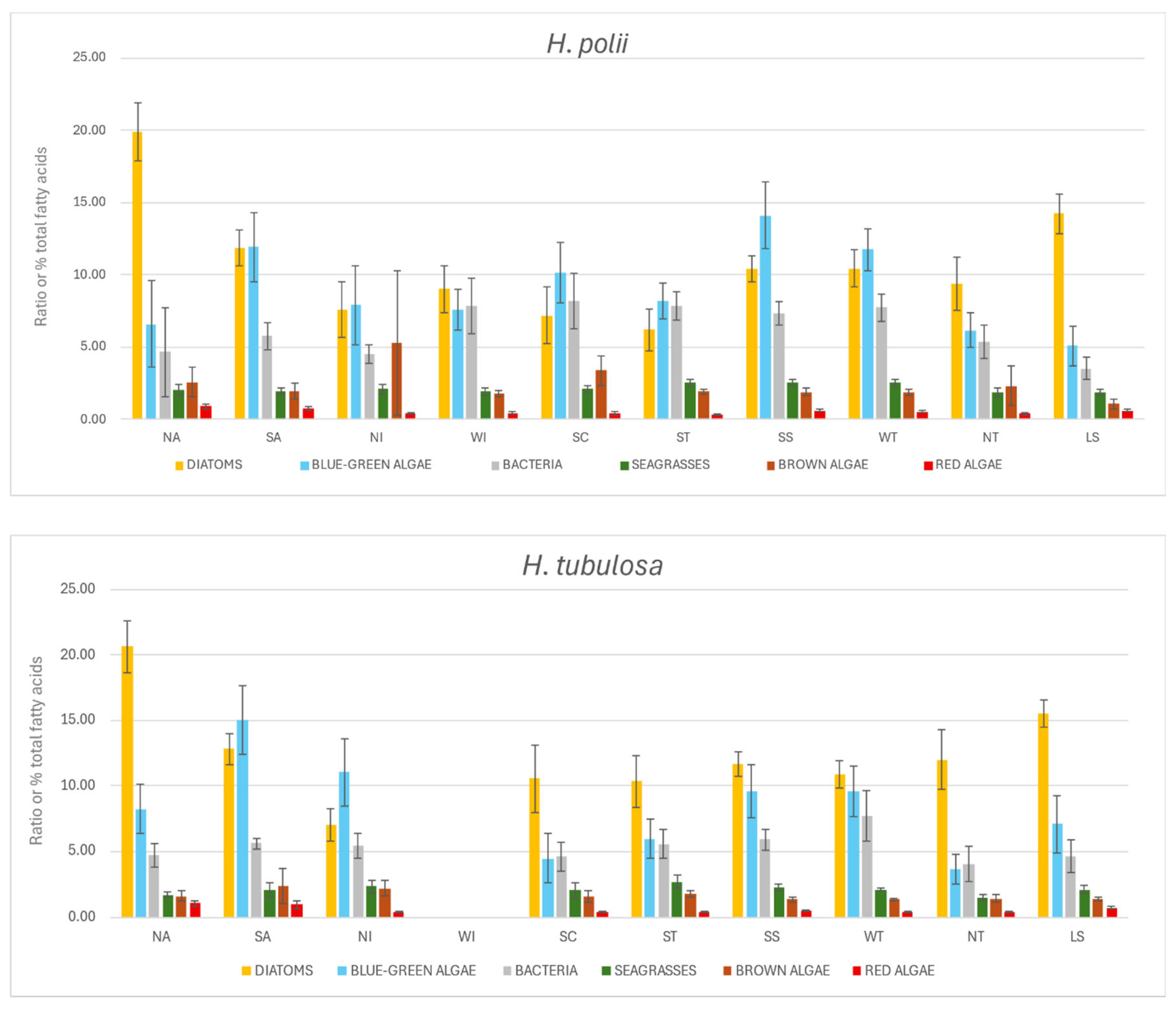
3.2. Trophic Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brusca, R.C.; Brusca, G.J. Invertebrates; Sinauer Associates: Sunderland, MA, USA, 2003; Volume 347. [Google Scholar]
- Drazen, J.C.; Phleger, C.F.; Guest, M.A.; Nichols, P.D. Lipid, sterols and fatty acid composition of abyssal holothurians and ophiuroids from the North-East Pacific Ocean: Food web implications. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2008, 151, 79–87. [Google Scholar] [CrossRef]
- Zhukova, N.V. Fatty Acids of Echinoderms: Diversity, Current Applications and Future Opportunities. Mar. Drugs 2023, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, V.; Giglioli, A.A.; Pusceddu, A.; Addis, P. Biology, ecology and management perspectives of overexploited deposit-feeders sea cucumbers, with focus on Holothuria tubulosa (Gmelin, 1788). Adv. Oceanogr. Limnol. 2021, 12. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Gkalogianni, E.Z.; Apostologamvrou, C.; Voulgaris, K.; Varkoulis, A.; Vafidis, D. Proximate Compositions and Fatty Acid Profiles of Raw and Processed Holothuria polii and Holothuria tubulosa from the Aegean Sea. Sustainability 2024, 16, 6048. [Google Scholar] [CrossRef]
- Costa, V.; Mazzola, A.; Vizzini, S. Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Biol. Ecol. 2014, 461, 226–232. [Google Scholar] [CrossRef]
- Boncagni, P.; Rakaj, A.; Fianchini, A.; Vizzini, S. Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and Holothuria tubulosa. Estuar. Coast. Shelf Sci. 2019, 231, 106464. [Google Scholar] [CrossRef]
- Uthicke, S.; Karez, R. Sediment patch selectivity in tropical sea cucumbers (Holothurioidea: Aspidochirotida) analysed with multiple choice experiments. J. Exp. Mar. Bio. Ecol. 1999, 236, 69–87. [Google Scholar] [CrossRef]
- Kinch, J. Importance of Sea cucumber fisheries and trade for small island communities: A case study in Papua New Guinea. In The World of Sea Cucumbers Challenges, Advances, and Innovations; Academic Press: Cambridge, MA, USA, 2024; pp. 133–148. [Google Scholar] [CrossRef]
- Kinch, J.; Purcell, S.W.; Uthicke, S.; Friedman, K. Population status, fisheries and trade of sea cucumbers in the Western Central Pacific. In Sea Cucumbers. A Global Review of Fisheries and Trade; FAO Fisheries and Aquaculture Technical Paper No. 516; FAO: Rome, Italy, 2008; pp. 7–55. [Google Scholar]
- Purcell, S.W.; Mercier, A.; Conand, C.; Hamel, J.F.; Toral-Granda, M.V.; Lovatelli, A.; Uthicke, S. Sea cucumber fisheries: Global analysis of stocks, management measures and drivers of overfishing. Fish Fish. 2013, 14, 34–59. [Google Scholar] [CrossRef]
- Toral-Granda, V. Population status, fisheries and trade of sea cucumbers in Latin America and the Caribbean. In Sea cucumbers. A Global Review of Fisheries and Trade; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2008; pp. 213–229. [Google Scholar]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Dakrory, A.I.; Fahmy, S.R.; Soliman, A.M.; Mohamed, A.S.; Amer, S.A.M. Protective and Curative Effects of the Sea Cucumber Holothuria atra Extract against DMBA-Induced Hepatorenal Diseases in Rats. BioMed Res. Int. 2015, 2015, 563652. [Google Scholar] [CrossRef]
- Anderson, S.C.; Flemming, J.M.; Watson, R.; Lotze, H.K. Serial exploitation of global sea cucumber fisheries. Fish Fish. 2011, 12, 317–339. [Google Scholar] [CrossRef]
- David, F.; Hubas, C.; Laguerre, H.; Badou, A.; Herault, G.; Bordelet, T.; Ameziane, N. Food sources, digestive efficiency and resource allocation in the sea cucumber Holothuria forskali (Echinodermata: Holothuroidea): Insights from pigments and fatty acids. Aquac. Nutr. 2020, 26, 1568–1583. [Google Scholar] [CrossRef]
- Sadoul, B.; Caprioli, J.P.; Barrier-Loiseau, C.; Cimiterra, N.; Laugier, T.; Lagarde, F.; Chary, K.; Callier, M.D.; Guillermard, M.O.; Roque d’Orbcastel, E. Is Holothuria tubulosa the golden goose of ecological aquaculture in the Mediterranean Sea? Aquaculture 2022, 554, 738149. [Google Scholar] [CrossRef]
- Azevedo and Silva, F.; Brito, A.C.; Pombo, A.; Simões, T.; Marques, T.A.; Rocha, C.; Madruga, A.S.; Sousa, J.; Venâncio, E.; Félix, P.M. Spatiotemporal Distribution Patterns of the Sea Cucumber Holothuria arguinensis on a Rocky-Reef Coast (Northeast Atlantic). Estuaries Coasts 2023, 46, 1035–1045. [Google Scholar] [CrossRef]
- González-Wangüemert, M.; Roggatz, C.C.; Rodrigues, M.J.; Barreira, L.; da Silva, M.M.; Custódio, L. A new insight into the influence of habitat on the biochemical properties of three commercial sea cucumber species. Int. Aquat. Res. 2018, 10, 361–373. [Google Scholar] [CrossRef]
- Sicuro, B.; Manuela, P.; Francesco, G.; Abete, M.C.; Antonio, D.; Franco, D.; Mioletti, S. Food quality and Safety of Mediterranean Sea Cucumbers Holothuria tubulosa and Holothuria polii in Southern Adriatic Sea. Asian J. Anim. Vet. Adv. 2012, 7, 851–859. [Google Scholar] [CrossRef]
- Meloni, D.; Esposito, G. Hygienic and commercial issues related to the illegal fishing and processing of sea cucumbers in the Mediterranean: A case study on over-exploitation in Italy between 2015 and 2017. Reg. Stud. Mar. Sci. 2018, 19, 43–46. [Google Scholar] [CrossRef]
- Rakaj, A.; Fianchini, A. Mediterranean sea cucumbers—Biology, ecology, and exploitation. In The World of Sea Cucumbers Challenges, Advances, and Innovations; Academic Press: Cambridge, MA, USA, 2024; pp. 753–773. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Thomas, F.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. Shelf Sci. 2006, 70, 271–286. [Google Scholar] [CrossRef]
- Hazel, J.R.; Williams, E.E.; Livermore, R.; Mozingo, N. Thermal adaptation in biological membranes: Functional significance of changes in phospholipid molecular species composition. Lipids 1991, 26, 277–282. [Google Scholar] [CrossRef]
- Parrish, C.C.; Abrajano, T.A.; Budge, S.M.; Helleur, R.J.; Hudson, E.D.; Pulchan, K.; Ramos, C. Lipid and Phenolic Biomarkers in Marine Ecosystems: Analysis and Applications. In Marine Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; pp. 193–223. [Google Scholar]
- Stowasser, G.; Pond, D.W.; Collins, M.A. Fatty acid trophic markers elucidate resource partitioning within the demersal fish community of South Georgia and Shag Rocks (Southern Ocean). Mar. Biol. 2012, 159, 2299–2310. [Google Scholar] [CrossRef]
- Hiltunen, M.; Strandberg, U.; Brett, M.T.; Winans, A.K.; Beauchamp, D.A.; Kotila, M.; Keister, J.E. Taxonomic, Temporal, and Spatial Variations in Zooplankton Fatty Acid Composition in Puget Sound, WA, USA. Estuaries Coasts 2022, 45, 567–581. [Google Scholar] [CrossRef]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Salant, C.D.; Shanks, A.L.; Schram, J.B.; Galloway, A.W.E. Trophic Biomarkers Indicate Coastal Surf Zone Hydrodynamics Affect Resource Assimilation by Mytilus californianus Mussels. Estuaries Coasts 2021, 44, 2212–2221. [Google Scholar] [CrossRef]
- Dalsgaard, J.; St John, M.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar] [CrossRef]
- Coelho, H.; Lopes da Silva, T.; Reis, A.; Queiroga, H.; Serôdio, J.; Calado, R. Fatty acid profiles indicate the habitat of mud snails Hydrobia ulvae within the same estuary: Mudflats vs. seagrass meadows. Estuar. Coast. Shelf Sci. 2011, 92, 181–187. [Google Scholar] [CrossRef]
- Iverson, S.J. Tracing aquatic food webs using fatty acids: From qualitative indicators to quantitative determination. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; pp. 281–308. [Google Scholar]
- Nerot, C.; Meziane, T.; Schaal, G.; Grall, J.; Lorrain, A.; Paulet, Y.M.; Kraffe, E. Spatial changes in fatty acids signatures of the great scallop Pecten maximus across the Bay of Biscay continental shelf. Cont. Shelf Res. 2015, 109, 1–9. [Google Scholar] [CrossRef]
- Penha-Lopes, G.; Torres, P.; Narciso, L.; Cannicci, S.; Paula, J. Comparison of fecundity, embryo loss and fatty acid composition of mangrove crab species in sewage contaminated and pristine mangrove habitats in Mozambique. J. Exp. Mar. Biol. Ecol. 2009, 381, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, Y.; Wang, H. The application of compound-specific isotope analysis of fatty acids for traceability of sea cucumber (Apostichopus japonicus) in the coastal areas of China. J. Sci. Food Agric. 2017, 97, 4912–4921. [Google Scholar] [CrossRef]
- Kharlamenko, V.I.; Zhukova, N.V.; Khotimchenko, S.V.; Svetashev, V.I.; Kamenev, G.M. Fatty acids as markers of food sources in a shallow-water hydrothermal ecosystem (Kraternaya Bight, Yankich Island, Kurile Islands). Mar. Ecol. Prog. Ser. 1995, 120, 231–242. [Google Scholar] [CrossRef]
- Rajendran, N.; Suwa, Y.; Urushigawa, Y. Distribution of phospholipid ester-linked fatty acid biomarkers for bacteria in the sediment of Ise Bay, Japan. Mar. Chem. 1993, 42, 39–56. [Google Scholar] [CrossRef]
- Napolitano, G.E. Fatty acids as chemical and trophic markers in freshwater ecosystems. In Lipids in Freshwater Ecosystems; Springer: New York, NY, USA, 1999; pp. 21–44. [Google Scholar]
- Falk-Petersen, S.; Dahl, T.M.; Scott, C.L.; Sargent, J.R.; Gulliksen, B.; Kwasniewski, S.; Hop, H.; Millar, R.M. Lipid biomarkers and trophic linkages between ctenophores and copepods in Svalbard waters. Mar. Ecol. Prog. Ser. 2002, 227, 187–194. [Google Scholar] [CrossRef]
- Johns, R.B.; Nichols, P.D.; Perry, G.J. Fatty acid composition of ten marine algae from Australian waters. Phytochemistry 1979, 18, 799–802. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E. Distribution of C 20 Polyenoic Fatty Acids in Red Macrophytic Algae. Bot. Mar. 1990, 33, 525–528. [Google Scholar] [CrossRef]
- Wannigama, G.P.; Volkman, J.K.; Gillan, F.T.; Nichols, P.D.; Johns, R.B. A comparison of lipid components of the fresh and dead leaves and pneumatophores of the mangrove Avicennia marina. Phytochemistry 1981, 20, 659–666. [Google Scholar] [CrossRef]
- Derrien, M.; Yang, L.; Hur, J. Lipid biomarkers and spectroscopic indices for identifying organic matter sources in aquatic environments: A review. Water Res. 2017, 112, 58–71. [Google Scholar] [CrossRef]
- Gopi, K.; Mazumder, D.; Sammut, J.; Saintilan, N. Determining the provenance and authenticity of seafood: A review of current methodologies. Trends Food Sci. Technol. 2019, 91, 294–304. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Stable carbon isotope fractionation of fatty acid in sea cucumber (Apostichopus japonicus): Insights from an experimental study. N. Z. J. Mar. Freshw. Res. 2022, 56, 234–246. [Google Scholar] [CrossRef]
- Iverson, S.J.; Field, C.; Don Bowen, W.; Blanchard, W. Quantitative fatty acid signature analysis: A new method of estimating predator diets. Ecol. Monogr. 2004, 74, 211–235. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Koopman, H.N. Studying Trophic Ecology in Marine Ecosystems Using Fatty Acids: A Primer on Analysis and Interpretation. Mar. Mammal. Sci. 2006, 22, 759–801. [Google Scholar] [CrossRef]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Owen, J.M.; Adron, J.W.; Sargent, J.R.; Cowey, C.B. Studies on the nutrition of marine flatfish. The effect of dietary fatty acids on the tissue fatty-acids of the plaice Pleuronectes platessa. Mar. Biol. 1972, 13, 160–166. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Venegas-Venegas, E.; Rincón-Cervera, M.Á.; Suárez, M.D. Fatty acid profiles of livers from selected marine fish species. J. Food Compos. Anal. 2011, 24, 217–222. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Bowen, W.D.; Ackman, R.G. Among- and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and Southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 2002, 59, 886–898. [Google Scholar] [CrossRef]
- Tortonese, E. Fauna d’Italia Vol. VI—Echinodermata; Calderini: Bologna, Italy, 1965. [Google Scholar]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef]
- IUPAC. Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed.; Blackwell Jevent: Oxford, UK, 1992. [Google Scholar]
- Ackman, R.G. Gas-liquid chromatography of fatty acids and esters. Methods Enzymol. 1969, 14, 329–381. [Google Scholar]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological Roles of Exploited Sea Cucumbers. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 173–191. [Google Scholar] [CrossRef]
- Capoccioni, F.; Contò, M.; Failla, S.; Cataudella, S.; Ciccotti, E. Fatty acid profiles of migrating female silver eel from Mediterranean coastal lagoons as integrative descriptors of spawners biological quality. Estuar. Coast. Shelf Sci. 2018, 210, 87–97. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Massin, C.; Jangoux, M. Les observations écologiques se rapportant aux Holothuries des mers d’ Europe et, plus particulièrement, aux Aspidochirotes sont fort rares. Les renseignements donnés par les ouvrages généraux (Ludwig, 1889–1891). Cah. Biol. Mar. 1976, 17, 45–59. [Google Scholar]
- Mezali, K.; Zupo, V.; Francour, P. Population Dynamics of Holothuria (Holothuria) tubulosa and Holothuria (Lessonothuria) polii of an Algerian Posidonia oceanica meadow. Biol. Mar. Medit. 2006, 13, 158–161. [Google Scholar]
- Hudson, I.; Pond, D.; Billett, D.; Tyler, P.; Lampitt, R.; Wolff, G. Temporal variations in fatty acid composition of deep-sea holothurians: Evidence of bentho-pelagic coupling. Mar. Ecol. Prog. Ser. 2004, 281, 109–120. [Google Scholar] [CrossRef]
- Belbachir, N.-E.; Mezali, K. Food preferences of four aspidochirotid holothurians species (Holothuroidea: Echinodermata) inhabiting the Posidonia oceanica meadow of Mostaganem area (Algeria). SPC Beche-De-Mer. Inf. Bull. 2018, 36, 55–59. [Google Scholar]
- Nichols, P.D.; Klumpp, D.W.; Johns, R.B. Lipid components of the epiphyte material, suspended particulate matter and cultured bacteria from a seagrass, Posidonia australis, community as indicators of carbon source. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 315–325. [Google Scholar] [CrossRef]
- Kanjer, L.; Mucko, M.; Car, A.; Bosak, S. Epiphytic diatoms on Posidonia oceanica (L.) Delile leaves from eastern Adriatic Sea. Nat. Croat. 2019, 28, 1–20. [Google Scholar]
- Mabrouk, L.; Ben Brahim, M.; Hamza, A.; Mahfoudhi, M.; Bradai, M.N. A comparison of abundance and diversity of epiphytic microalgal assemblages on the leaves of the seagrasses Posidonia oceanica (L.) and Cymodocea nodosa (Ucria) asch in Eastern Tunisia. J. Mar. Biol. 2014, 2014, 275305. [Google Scholar] [CrossRef]
- Cutajar, K.; Falconer, L.; Massa-Gallucci, A.; Cox, R.E.; Schenke, L.; Bardócz, T.; Andolina, C.; Signa, G.; Vizzini, S.; Sprague, M.; et al. Stable isotope and fatty acid analysis reveal the ability of sea cucumbers to use fish farm waste in integrated multi-trophic aquaculture. J. Environ. Manag. 2022, 318, 115511. [Google Scholar] [CrossRef]
- Mfilinge, P.L.; Tsuchiya, M. Changes in Sediment Fatty Acid Composition during Passage through the Gut of Deposit Feeding Holothurians: Holothuria atra (Jaeger, 1883) and Holothuria leucospilota (Brandt, 1835). J. Lipids 2016, 2016, 4579794. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Q.; Yang, H. Seasonal variations of food sources in Apostichopus japonicus indicated by fatty acid biomarkers analysis. J. Fish. China 2010, 34, 760–767. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, L.; Tang, X.; Xia, X.; Hu, W.; Zhou, P. Season and geography induced variation in sea cucumber (Stichopus japonicus) nutritional composition and gut microbiota. J. Food Compos. Anal. 2021, 101, 103838. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, Y.; Zhao, X. Identification of the geographical origins of sea cucumber Population status, fisheries and trade of sea cucumbers in Latin America and the Caribbean. Sea cucumbers. A global review of fisheries and trade (Apostichopus japonicus) in northern China. Food Chem. 2017, 218, 269–276. [Google Scholar] [CrossRef]
- Nemova, N.N.; Fokina, N.N.; Nefedova, Z.A.; Ruokolainen, T.R.; Bakhmet, I.N. Modifications of gill lipid composition in littoral and cultured blue mussels Mytilus edulis L. under the influence of ambient salinity. Polar Rec. 2013, 49, 272–277. [Google Scholar] [CrossRef]
- CMEMS. Marine Data Store (MDS). Mediterranean Sea Physics Reanalysis; E.U. Copernicus Marine Service Information, Marine Data Store (MDS): Lisbon, Portugal, 2024. [Google Scholar]
- Silina, A.V.; Zhukova, N.V. Growth variability and feeding of scallop Patinopecten yessoensis on different bottom sediments: Evidence from fatty acid analysis. J. Exp. Mar. Biol. Ecol. 2007, 348, 46–59. [Google Scholar] [CrossRef]
- Hill, W.R.; Rinchard, J.; Czesny, S. Light, nutrients and the fatty acid composition of stream periphyton. Freshw. Biol. 2011, 56, 1825–1836. [Google Scholar] [CrossRef]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef]
| Source | Biomarker |
|---|---|
| Diatoms 1 | C20:5n-3 |
| Bacteria 2 | ∑15:0 + ∑17:0 + C18:1n-7 |
| Seagrasses 3 | C18:2n-6 + C18:3n-3 |
| Brown algae 4 | C18:1n-9 |
| Red algae 5 | C20:5n-3/C20:4n-6 > 10 |
| Blue-green algae 6 | C16:1n-7 + C18:1n-7 |
| Fatty Acid | Northern Adriatic Sea (NA) | Southern Adriatic Sea (SA) | Northern Ionian Sea (NI) | Western Ionian Sea (WI) | Sicilian Channel (SC) | Southern Tyrrhenian Sea (ST) | Sea of Sardinia (SS) | Western Tyrrhenian Sea (WT) | Northern Tyrrhenian Sea (NT) | Ligurian Sea (LS) |
|---|---|---|---|---|---|---|---|---|---|---|
| iso-C14:0 | 0.3 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.4 ± 0.1 |
| C14:0 | 1.0 ± 0.6 | 2.8 ± 0.5 | 1.6 ± 0.5 | 2.0 ± 0.5 | 2.6 ± 0.5 | 2.2 ± 0.4 | 2.8 ± 0.4 | 2.5 ± 0.4 | 1.6 ± 0.5 | 1.1 ± 0.4 |
| anteiso-C15:0 | 0.8 ± 0.7 | 0.9 ± 0.3 | 0.9 ± 0.3 | 1.3 ± 0.5 | 1.3 ± 0.4 | 1.0 ± 0.1 | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.9 ± 0.3 | 0.8 ± 0.2 |
| C15:0 | 0.4 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.2 | 1.1 ± 0.4 | 1.5 ± 0.5 | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.4 ± 0.2 |
| iso-C16:0 | 0.4 ± 0.4 | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.5 ± 0.1 |
| C16:0 | 3.4 ± 1.6 | 8.4 ± 1.1 | 5.2 ± 1.2 | 7.6 ± 1.6 | 8.9 ± 1.7 | 9.4 ± 1.6 | 10.6 ± 1.8 | 8.7 ± 1.4 | 5.2 ± 1.2 | 3.6 ± 1.0 |
| anteiso-C17:0 | 0.2 ± 0.2 | 0.6 ± 0.1 | 0.0 ± 0.0 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| C17:0 | 0.6 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.2 | 0.2 ± 0.1 |
| iso-C18:0 | 0.3 ± 0.1 | 0.4 ± 0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.1 |
| C18:0 | 3.1 ± 0.8 | 6.4 ± 0.9 | 3.9 ± 0.4 | 4.4 ± 0.5 | 4.9 ± 0.5 | 5.0 ± 0.6 | 5.9 ± 0.6 | 5.6 ± 0.7 | 3.9 ± 0.4 | 4.1 ± 0.5 |
| iso-C20:0 | 0.4 ± 0.1 | 0.3 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.1 ± 0.2 |
| C20:0 | 2.3 ± 0.3 | 2.7 ± 0.2 | 2.4 ± 0.6 | 2.7 ± 0.2 | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.2 ± 0.3 | 2.3 ± 0.2 | 2.4 ± 0.6 | 2.4 ± 0.1 |
| C21:0 | 2.2 ± 0.3 | 2.9 ± 0.1 | 2.6 ± 0.2 | 2.6 ± 0.3 | 2.2 ± 0.4 | 1.8 ± 0.3 | 1.3 ± 0.2 | 1.5 ± 0.2 | 2.6 ± 0.2 | 2.2 ± 0.3 |
| C22:0 | 1.6 ± 0.3 | 1.2 ± 0.2 | 2.4 ± 0.4 | 2.1 ± 0.4 | 1.8 ± 0.3 | 1.8 ± 0.3 | 1.1 ± 0.2 | 1.6 ± 0.3 | 2.4 ± 0.4 | 2.3 ± 0.2 |
| C23:0 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.6 ± 0.3 |
| C24:0 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.3 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| ∑SFA | 16.9 ± 3.6 | 29.4 ± 1.7 | 31.8 ± 2.3 | 29.4 ± 3.9 | 31.1 ± 4.2 | 30.3 ± 2.6 | 29.7 ± 2.6 | 28.8 ± 2.6 | 23.0 ± 2.4 | 19.1 ± 1.9 |
| C14:1n-5 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.1 | 0.6 ± 0.1 |
| C15:1n-5 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| C16:1n-7 | 3.9 ± 1.6 | 9.2 ± 2.1 | 3.4 ± 0.8 | 4.0 ± 1.0 | 6.6 ± 1.7 | 4.2 ± 0.8 | 10.4 ± 2.2 | 8.0 ± 1.3 | 3.4 ± 0.8 | 3 ± 1.1 |
| C17:1n-7 | 1.0 ± 0.4 | 0.1 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.1 | 1.5 ± 0.6 |
| C18:1n-9t | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.2 | 0.4 ± 0.0 |
| C18:1n-9c | 2.6 ± 1.0 | 1.9 ± 0.6 | 2.3 ± 1.4 | 1.8 ± 0.2 | 3.4 ± 1.0 | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 2.3 ± 1.4 | 1.1 ± 0.3 |
| C18:1n-7 | 2.7 ± 1.8 | 2.8 ± 0.4 | 2.8 ± 0.6 | 3.7 ± 0.8 | 3.6 ± 0.7 | 4.0 ± 0.6 | 3.7 ± 0.3 | 3.8 ± 0.3 | 2.8 ± 0.6 | 2.1 ± 0.3 |
| C20:1n-9 | 7.8 ± 1.4 | 7.5 ± 0.9 | 9.7 ± 0.7 | 10.2 ± 1.5 | 7.6 ± 1.4 | 9.0 ± 1.1 | 7.9 ± 1.1 | 7.9 ± 1.0 | 9.7 ± 0.7 | 11.2 ± 0.8 |
| C23:1n-9 | 7.2 ± 0.7 | 7.0 ± 2.1 | 9.2 ± 0.9 | 5.6 ± 0.6 | 7.8 ± 1.8 | 6.9 ± 0.7 | 4.6 ± 0.4 | 4.5 ± 0.6 | 9.2 ± 0.9 | 7.8 ± 0.9 |
| C24:1n-9 | 4.9 ± 0.9 | 5.0 ± 0.8 | 5.6 ± 0.6 | 6.0 ± 0.7 | 5.3 ± 0.7 | 5.1 ± 0.5 | 4.8 ± 0.7 | 4.2 ± 0.9 | 5.6 ± 0.6 | 5.7 ± 0.3 |
| ∑MUFA | 30.5 ± 1.7 | 34.5 ± 1.8 | 33.6 ± 1.01 | 32.5 ± 1.0 | 35.8 ± 1.8 | 32.3 ± 1.0 | 34.5 ± 1.3 | 31.8 ± 1.0 | 34.0 ± 1.6 | 33.7 ± 1.1 |
| C16:2n-4 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| C16:3n-4 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| C18:2n-6c (LA) | 0.5 ± 0.3 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.6 ± 0.2 | 0.3 ± 0.1 |
| C18:2n-4 | 0.6 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0 | 0.1 ± 0.1 | 0.3 ± 0.4 |
| C18:3n-6 (GLA) | 0.2 ± 0.3 | 0.1 ± 0.1 | 0.6 ± 0.6 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.6 ± 0.6 | 0.1 ± 0.1 |
| C18:3n-3 (ALA) | 1.6 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.2 |
| C18:4n-3 | 0.8 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| C20:2n-6 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| C20:3n-6 | 1.8 ± 0.2 | 1.5 ± 0.3 | 1.8 ± 0.1 | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.8 ± 0.1 | 1.9 ± 0.1 |
| C20:4n-6 (ARA) | 23.0 ± 3.7 | 16.7 ± 1.6 | 24.7 ± 1.7 | 20.5 ± 2.5 | 17.3 ± 3.4 | 21.7 ± 1.8 | 17.1 ± 2.2 | 19.8 ± 2.1 | 24.7 ± 1.7 | 24.2 ± 2.5 |
| C20:4n-3 | 0.7 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0 | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.1 |
| C22:2n-6 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0 | 0.4 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 |
| C20:5n-3 (EPA) | 19.9 ± 2.0 | 11.9 ± 1.3 | 9.4 ± 1.9 | 9.0 ± 1.7 | 7.2 ± 2.0 | 6.2 ± 1.5 | 10.4 ± 0.9 | 10.4 ± 1.3 | 9.4 ± 1.9 | 14.2 ± 1.4 |
| C22:4n-6 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0 | 0.1 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 |
| C22:5n-3 (DPA) | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.7 ± 0.1 |
| C22:6n-3 (DHA) | 0.9 ± 0.3 | 1.2 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 1.0 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.6 ± 0.2 | 0.9 ± 0.2 |
| ∑PUFA | 52.5 ± 4.4 | 36.0 ± 2.1 | 34.7 ± 2.5 | 38.1 ± 3.5 | 33.0 ± 4.5 | 37.4 ± 2.8 | 35.77 ± 2.7 | 39.4 ± 2.4 | 43.0 ± 2.9 | 47.19 ± 2.1 |
| total n-6 | 27.2 ± 3.6 | 20.6 ± 1.8 | 18.0 ± 2.6 | 25.3 ± 2.6 | 21.8 ± 3.4 | 26.9 ± 1.8 | 21.0 ± 2.2 | 24.4 ± 2.3 | 29.9 ± 1.9 | 28.11 ± 2.6 |
| total n-3 | 24.3 ± 1.8 | 15.1 ± 1.5 | 16.2 ± 1.3 | 11.9 ± 1.7 | 10.6 ± 1.7 | 9.6 ± 1.2 | 14.3 ± 0.7 | 14.5 ± 1.2 | 12.2 ± 1.8 | 18.13 ± 1.6 |
| n3/n6 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.65 ± 0.1 |
| Fatty Acid | Northern Adriatic Sea (NA) | Southern Adriatic Sea (SA) | Northern Ionian Sea (NI) | Sicilian Channel (SC) | Southern Tyrrhenian Sea (ST) | Sea of Sardinia (SS) | Western Tyrrhenian Sea (WT) | Northern Tyrrhenian Sea (NT) | Ligurian Sea (LS) |
|---|---|---|---|---|---|---|---|---|---|
| iso-C14:0 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.4 | 0.4 ± 0.2 | 0.4 ± 0.1 |
| C14:0 | 2.2 ± 1.3 | 3.4 ± 0.4 | 2.7 ± 0.6 | 1.3 ± 0.7 | 1.8 ± 0.5 | 2.0 ± 0.5 | 2.3 ± 0.5 | 0.9 ± 0.4 | 1.8 ± 0.7 |
| anteiso-C15:0 | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.2 | 1.2 ± 0.4 | 0.6 ± 0.3 | 1.1 ± 0.3 |
| C15:0 | 0.4 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.2 | 1.0 ± 0.4 | 0.7 ± 0.2 | 1.1 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.2 |
| iso-C16:0 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.3 | 0.5 ± 0.2 | 0.5 ± 0.2 |
| C16:0 | 4.6 ± 1.6 | 10.2 ± 1.3 | 8.3 ± 1.6 | 5.1 ± 2.8 | 7.2 ± 1.6 | 7.2 ± 2.2 | 7.6 ± 1.6 | 2.9 ± 1.0 | 5.5 ± 1.9 |
| anteiso-C17:0 | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.1 | 0.0 ± 0.0 |
| C17:0 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.7 ± 0.1 | 0.3 ± 0.2 |
| iso-C18:0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.3 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.1 |
| C18:0 | 4.2 ± 1.0 | 7.4 ± 1.0 | 6.0 ± 0.7 | 3.8 ± 0.6 | 4.4 ± 0.4 | 5.0 ± 0.6 | 4.9 ± 0.7 | 3.4 ± 0.3 | 5.3 ± 0.8 |
| iso-C20:0 | 0.6 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.6 ± 0.2 | 0.5 ± 0 | 0.2 ± 0.1 |
| C20:0 | 2.3 ± 0.2 | 2.5 ± 0.3 | 3.1 ± 0.3 | 2.9 ± 0.2 | 3.0 ± 0.2 | 2.7 ± 0.4 | 2.3 ± 0.7 | 2.8 ± 0.1 | 2.7 ± 0.2 |
| C21:0 | 1.9 ± 0.2 | 2.7 ± 0.5 | 2.8 ± 0.3 | 2.6 ± 0.5 | 2.5 ± 0.5 | 2.0 ± 0.4 | 1.7 ± 0.3 | 2.7 ± 0.2 | 1.8 ± 0.3 |
| C22:0 | 1.4 ± 0.4 | 1.0 ± 0.2 | 1.5 ± 0.2 | 2.4 ± 0.6 | 2.1 ± 0.3 | 1.8 ± 0.4 | 1.8 ± 0.3 | 2.7 ± 0.2 | 2.0 ± 0.5 |
| C23:0 | 0.0 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.2 | 0.3 ± 0.2 |
| C24:0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| ∑SFA | 20.6 ± 4.2 | 31.8 ± 2.3 | 29.0 ± 2.8 | 22.7 ± 3.9 | 26.3 ± 2.7 | 25.3 ± 2.5 | 27.5 ± 3.6 | 19.32 ± 2.7 | 23.15 ± 3.4 |
| C14:1n-5 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.1 | 0.6 ± 0.2 |
| C15:1n-5 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 |
| C16:1n-7 | 5.5 ± 1.7 | 12.2 ± 2.6 | 8.2 ± 2.3 | 2.2 ± 1.5 | 3.6 ± 1.1 | 6.4 ± 1.8 | 5.7 ± 1.4 | 1.8 ± 0.7 | 4.5 ± 1.8 |
| C17:1n-7 | 0.8 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 1.5 ± 0.5 |
| C18:1n-9t | 0.4 ± 0.2 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.4 | 0.3 ± 0.1 |
| C18:1n-9c | 1.6 ± 0.4 | 2.4 ± 1.3 | 2.2 ± 0.6 | 1.6 ± 0.4 | 1.8 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.2 |
| C18:1n-7 | 2.7 ± 0.4 | 2.9 ± 0.2 | 2.9 ± 0.6 | 2.3 ± 0.5 | 2.4 ± 0.4 | 3.2 ± 0.4 | 3.9 ± 1.0 | 1.9 ± 0.5 | 2.6 ± 0.6 |
| C20:1n-9 | 6.7 ± 1.1 | 6.0 ± 1.0 | 8.7 ± 1.4 | 10.4 ± 1.5 | 9.7 ± 1.2 | 9.4 ± 1.1 | 8.1 ± 1.1 | 10.6 ± 1.1 | 9.1 ± 1.2 |
| C23:1n-9 | 7.6 ± 0.8 | 5.2 ± 1.1 | 8.7 ± 1.8 | 7.8 ± 1.2 | 6.5 ± 0.6 | 5.1 ± 0.4 | 4.7 ± 0.5 | 9.9 ± 1.0 | 6.0 ± 1.4 |
| C24:1n-9 | 3.6 ± 0.5 | 3.6 ± 0.5 | 5.6 ± 0.8 | 5.7 ± 1.1 | 4.9 ± 0.4 | 4.8 ± 1.0 | 3.4 ± 0.4 | 5.1 ± 0.5 | 4.3 ± 0.6 |
| ∑MUFA | 29.2 ± 1.4 | 33.6 ± 1.0 | 37.0 ± 1.2 | 31.5 ± 2.1 | 30.0 ± 1.0 | 31.6 ± 1.9 | 28.5 ± 1.1 | 31.8 ± 1.0 | 30.5 ± 1.2 |
| C16:2n-4 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| C16:3n-4 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| C18:2n-6c (LA) | 0.5 ± 0.1 | 1.0 ± 0.5 | 1.0 ± 0.4 | 0.6 ± 0.5 | 1.2 ± 0.4 | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.3 |
| C18:2n-4 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.6 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.2 |
| C18:3n-6 (GLA) | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0 | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.0 | 0.1 ± 0.1 |
| C18:3n-3 (ALA) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.4 ± 0.1 | 1.1 ± 0.2 | 1.6 ± 0.1 |
| C18:4n-3 | 1.0 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| C20:2n-6 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.2 |
| C20:3n-6 | 1.2 ± 0.1 | 1 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.1 |
| C20:4n-6 (ARA) | 20.2 ± 3.9 | 14.2 ± 2.8 | 18.8 ± 2.8 | 26.3 ± 2.2 | 24.6 ± 1.8 | 22.8 ± 2.8 | 24.5 ± 3.2 | 28.6 ± 2.5 | 21.9 ± 3.1 |
| C20:4n-3 | 0.5 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.0 |
| C22:2n-6 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 |
| C20:5n-3 (EPA) | 20.6 ± 2.0 | 12.8 ± 1.2 | 7.0 ± 1.3 | 10.6 ± 2.6 | 10.3 ± 2 | 11.6 ± 0.9 | 10.9 ± 1.0 | 12.0 ± 2.2 | 15.5 ± 1.1 |
| C22:4n-6 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.0 ± 0.0 |
| C22:5n-3 (DPA) | 0.6 ± 0.3 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.4 | 0.3 ± 0.2 |
| C22:6n-3 (DHA) | 1.8 ± 0.9 | 1.3 ± 0.1 | 1.1 ± 0.3 | 0.5 ± 0.2 | 0.7 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.5 ± 0.2 | 1.5 ± 0.4 |
| ∑PUFA | 50.2 ± 4.2 | 34.6 ± 2.5 | 33.9 ± 3.4 | 45.8 ± 4.0 | 43.7 ± 3.2 | 43.1 ± 2.9 | 44.0 ± 3.9 | 48.8 ± 3.5 | 46.4 ± 2.9 |
| total n-6 | 23.9 ± 4.1 | 18.0 ± 2.6 | 23.2 ± 2.7 | 31.2 ± 2.3 | 29.6 ± 1.9 | 26.6 ± 2.6 | 28.7 ± 3.4 | 33.1 ± 2.57 | 25.5 ± 2.8 |
| total n-3 | 25.8 ± 2.2 | 16.2 ± 1.3 | 10.4 ± 0.9 | 13.7 ± 2.4 | 13.5 ± 1.7 | 15.1 ± 1.0 | 14.8 ± 1.0 | 14.8 ± 2.0 | 19.7 ± 1.2 |
| n3/n6 | 1.1 ± 0.3 | 0.9 ± 0.2 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonachella, N.; Contò, M.; Martinoli, M.; Martini, A.; Fianchini, A.; Fontanesi, L.; Gallucci, F.; Paris, E.; Pulcini, D.; Rakaj, A.; et al. Fatty Acids and Fatty Acid Trophic Markers in Two Holothurian Species from the Central Mediterranean Sea. Diversity 2025, 17, 576. https://doi.org/10.3390/d17080576
Tonachella N, Contò M, Martinoli M, Martini A, Fianchini A, Fontanesi L, Gallucci F, Paris E, Pulcini D, Rakaj A, et al. Fatty Acids and Fatty Acid Trophic Markers in Two Holothurian Species from the Central Mediterranean Sea. Diversity. 2025; 17(8):576. https://doi.org/10.3390/d17080576
Chicago/Turabian StyleTonachella, Nicolò, Michela Contò, Marco Martinoli, Arianna Martini, Alessandra Fianchini, Luca Fontanesi, Francescantonio Gallucci, Enrico Paris, Domitilla Pulcini, Arnold Rakaj, and et al. 2025. "Fatty Acids and Fatty Acid Trophic Markers in Two Holothurian Species from the Central Mediterranean Sea" Diversity 17, no. 8: 576. https://doi.org/10.3390/d17080576
APA StyleTonachella, N., Contò, M., Martinoli, M., Martini, A., Fianchini, A., Fontanesi, L., Gallucci, F., Paris, E., Pulcini, D., Rakaj, A., Napolitano, R., & Capoccioni, F. (2025). Fatty Acids and Fatty Acid Trophic Markers in Two Holothurian Species from the Central Mediterranean Sea. Diversity, 17(8), 576. https://doi.org/10.3390/d17080576











