Temporally Determinate, but Spatially Consistent Breeding Performance of Lesser Spotted Eagle (Clanga pomarina) Along the Southern Periphery of Its Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Design of the Study
2.3. Data Analysis
3. Results
3.1. Overall Breeding Parameters
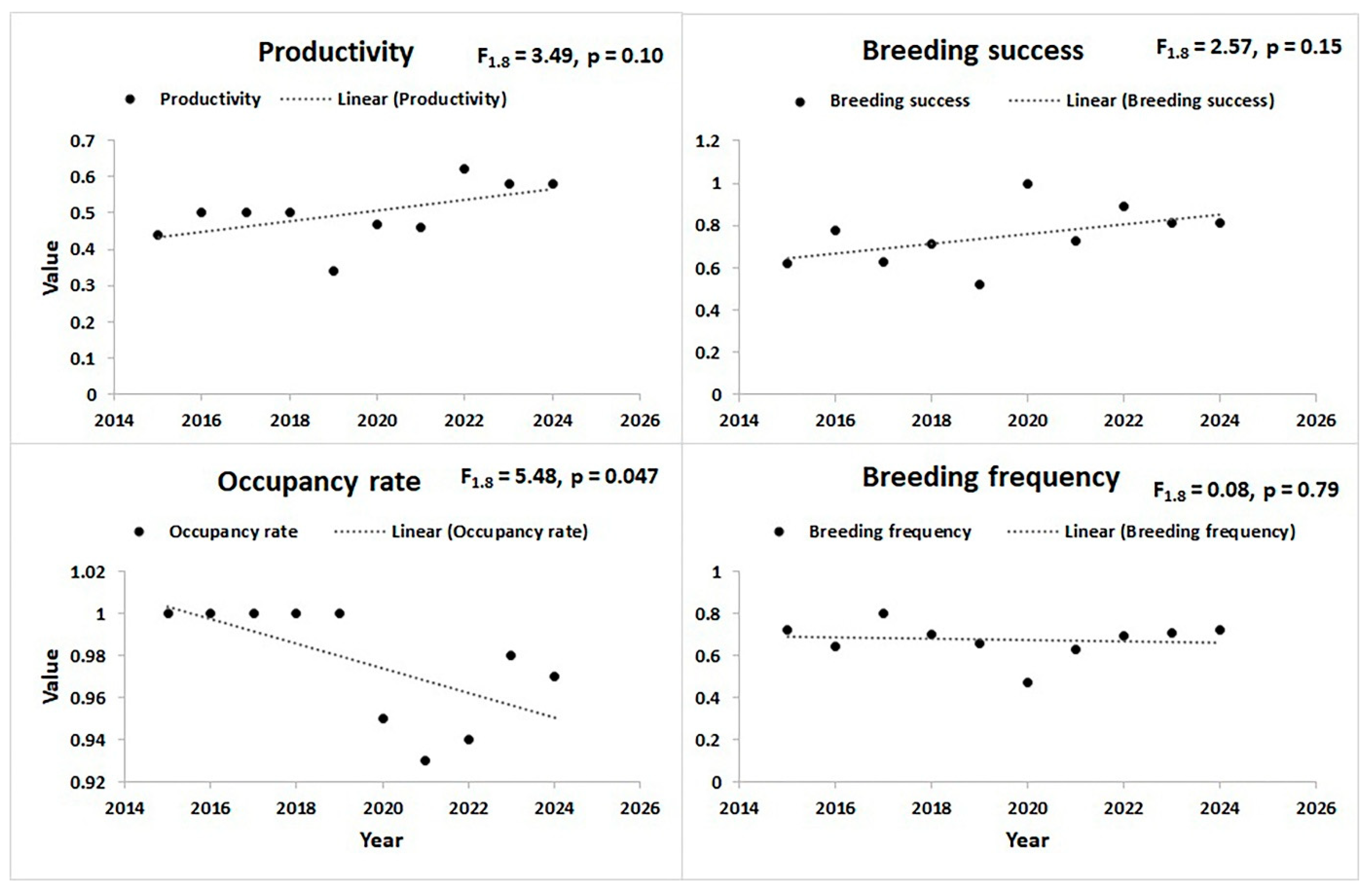
3.2. Temporal Patterns of Breeding Performance
3.3. Regional Patterns of Breeding Performance
3.4. Breeding Performance vs. Breeding Density
4. Discussion
4.1. Breeding Parameters: Variation in Space and Time
4.2. Breeding Parameters and Effect of Density
4.3. Conservation Recommendation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demerdzhiev, D. Breeding parameters and factors infuencing the reproduction of an expanding Long-legged Buzzard (Buteo rufnus) population under high breeding density conditions. J. Ornithol. 2022, 163, 405–415. [Google Scholar] [CrossRef]
- Margalida, A.; Mañosa, S.; Gonzalez, L.M.; Ortega, E.; Sanchez, R.; Oria, J. Breeding of non-adults and effect of age on productivity in the Spanish Imperial Eagle Aquila adalberti. Ardea 2008, 96, 173–180. [Google Scholar] [CrossRef]
- Newton, I. Population Ecology of Raptors; T. and A.D. Poyser: Berkhamsted, UK, 1979. [Google Scholar]
- Väli, Ü. Factors limiting reproductive performance and nestling sex ratio in the Lesser Spotted Eagle Aquila pomarina at the northern limit of its range: The impact of weather and prey abundance. Acta Ornithol. 2012, 47, 157–168. [Google Scholar] [CrossRef]
- Väli, Ü. Sex ratio of Lesser Spotted Eagle Aquila pomarina nestlings in good and poor breeding years. Bird Study 2004, 51, 189–191. [Google Scholar] [CrossRef]
- Buechley, E.R.; Santangeli, A.; Girardello, M.; Neate-Clegg, M.H.C.; Oleyar, D.; McClure, J.W.C.; Şekercioğlu, Ç.H. Global raptor research and conservation priorities: Tropical raptors fall prey to knowledge gaps. Divers. Distrib. 2019, 25, 856–869. [Google Scholar] [CrossRef]
- Donàzar, J.A.; Cortés-Avizanda, A.; Fargallo, J.A.; Margalida, A.; Moleón, M.; Morales-Reyes, Z.; Moreno-Opo, R.; Pérez-García, J.M.; Sánchez-Zapata, J.A.; Zuberogoitia, I.; et al. Roles of raptors in a changing world: From flagships to providers of key ecosystem services. Ardeola 2016, 63, 181–234. [Google Scholar] [CrossRef]
- Bergmanis, U.; Petrinš, A.; Cĩrulis, V.; Matusiak, J.; Kuze, J. Lesser spotted eagle Aquila pomarina in Latvia—Current status, endangerment and perspectives. Popul. Greifvogel Eulenarten 2006, 5, 95–115. [Google Scholar]
- Väli, Ü.; Tuvi, J.; Sein, G. Agricultural land use shapes habitat selection, foraging and reproductive success of the Lesser Spotted Eagle Clanga pomarina. J. Ornithol. 2017, 158, 841–850. [Google Scholar] [CrossRef]
- Treinys, R.; Bergmanis, U.; Väli, Ü. Strong territoriality and weak density-dependent reproduction in Lesser Spotted Eagles Clanga pomarina. Ibis 2017, 159, 343–351. [Google Scholar] [CrossRef]
- Lõhmus, A.; Väli, Ü. The effects of habitat quality and female size on the productivity of the Lesser Spotted Eagle Aquila pomarina in the light of the alternative prey hypothesis. J. Avian Biol. 2004, 35, 455–464. [Google Scholar] [CrossRef]
- Treinys, R.; Dementavičius, D. Productivity and diet of Lesser Spotted Eagle (Aquila pomarina) in Lithuania in 2001–2003. Acta Zool. Litu. 2004, 14, 83–87. [Google Scholar] [CrossRef]
- Bergmanis, U.; Petrinš, A.; Strazds, M. The number, distribution and breeding results of the lesser spotted eagle Aquila pomarina in Latvia. Acta Ornithoecol. 2001, 4, 305–319. [Google Scholar]
- Langgemach, T.; Böhner, J. Modellierung der Populationsdynamik des Schreiadlers Aquila pomarina in Brandenburg: Welchen Effekt haben Jahre mit extrem niedriger Reproduktion? Vogelwelt 2011, 132, 93–100. [Google Scholar]
- BirdLife International. Species Factsheet: Lesser Spotted Eagle Clanga pomarina. 2021. Available online: https://datazone.birdlife.org/species/factsheet/lesser-spotted-eagle-clanga-pomarina (accessed on 3 July 2025).
- Demerdzhiev, D.; Dobrev, D.; Arkumarev, V.; Terziev, N.; Georgiev, G. Distribution, Abundance and Breeding Performance of Lesser Spotted Eagle Clanga pomarina Brehm, 1831 (Aves: Accipitridae) in Southeast Bulgaria. Acta Zool. Bulg. Suppl. 2019, 14, 15–33. [Google Scholar]
- Demerdzhiev, D.; Popgeorgiev, G.; Dobrev, D.; Arkumarev, V.; Terziev, N. Habitat Requirements of the Lesser Spotted Eagle Clanga pomarina Brehm, 1831 (Aves: Accipitridae) at the Southern Periphery of the Distribution Range (Southeast Bulgaria). Acta Zool. Bulg. Suppl. 2019, 14, 35–65. [Google Scholar]
- NIMH. Bulgaria’s Changing Climate—Data and Analyses; Marinova, T., Bocheva, L., Eds.; National Institute of Meteorology and Hydrology, BAS: Sofia, Bulgaria, 2023; p. 106. [Google Scholar]
- Demerdzhiev, D.A.; Stoychev, S.S.; Dobrev, D.D.; Spasov, S.D.; Oppel, S. Studying the demographic drivers of an increasing Imperial Eagle population to inform conservation management. Biod. Conser. 2015, 24, 627–639. [Google Scholar] [CrossRef]
- Katzner, T.; Bragin, E.; Knick, S.; Smith, A. Spatial structure in the diet of Eastern Imperial Eagle Aquila heliaca in Kazakhstan. J. Avian Biol. 2006, 37, 594–600. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Dobrev, D.; Popgeorgiev, G.; Stoychev, S. Landscape alteration affects the demography of an endangered avian predator by reducing the habitat quality. Avian Res. 2022, 13, 100030. [Google Scholar] [CrossRef]
- Sarà, M.; Di Vittorio, M. Factors influencing the distribution, abundance and nestsite selection of an endangered Egyptian vulture (Neophron percnopterus) population in Sicily. Anim. Conserv. 2003, 6, 317–328. [Google Scholar] [CrossRef]
- Bergmanis, U.; Auninš, A.; Petrinš, A.; Cĩrulis, V.; Granãts, J.; Opermanis, O.; Soms, A. Population size, dynamics and reproduction success of the lesser spotted Eagle (Aquila pomarina) in Latvia. Slovak Raptor J. 2015, 9, 45–54. [Google Scholar] [CrossRef][Green Version]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 12. 2013. Available online: www.statsoft.com (accessed on 24 October 2022).
- Väli, Ü. The Lesser Spotted Eagle and its conservation in Estonia. Hirundo Suppl. 2003, 6, 1–66. [Google Scholar]
- Väli, Ü.; Bergmanis, U. Apparent survival rates of adult Lesser Spotted Eagle Clanga pomarina estimated by GPS-tracking, colour rings and wing-tags. Bird Study 2017, 64, 104–107. [Google Scholar] [CrossRef]
- Bergmanis, U.; Amerika, K.; Väli, Ü.; Treinys, R. Nest site selection and turnover patterns in support of conservation decisions: Case study of the lesser spotted eagle in the core area of its global population. For. Ecol. Manag. 2019, 448, 67–75. [Google Scholar] [CrossRef]
- Treinys, R. Habitat Use and Population Status of the Lesser Spotted Eagle Aquila Pomarine on the North-Western Periphery of the Distribution Range. Summary of Doctoral Dissertation, Vilnius University, Vilnius, Lithuania, 2009; p. 32. [Google Scholar]
- Dementavičius, D.; Rumbutis, S.; Vaitkuvienė, D.; Dagys, M.; Treinys, R. No adverse effects on Lesser Spotted Eagle breeding in an area of high White-tailed Eagle density. J. Ornithol. 2019, 160, 453–461. [Google Scholar] [CrossRef]
- Ivanovsky, V. Notes on the breeding ecology of Spotted Eagles Aquila clanga and A. pomarina in Byelorussia. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 297–299. [Google Scholar]
- Rodziewicz, M. The status, range and breeding success of the Lesser Spotted Ealge Aquila pomarina in Poland. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 291–295. [Google Scholar]
- Mirski, P.; Cenian, Z.; Wojciak, J.; Zawadzka, D.; Lontkowski, J.; Stój, M. Lesser Spotted Eagle National Action Plan for Poland; Draft Version; Eagle Conservation Committee: Olsztyn, Poland, 2013; p. 118. [Google Scholar]
- Zbyryt, A.; Kapowicz, E.; Kapowicz, R.; Zub, K. Number, density and breeding success of the Lesser Spotted Eagle Clanga pomarina in the Knyszyn forest (NE Poland) in 1999–2015. Polonica 2016, 57, 237–247, (In Polish with English Summary). [Google Scholar] [CrossRef]
- Scheller, W.; Bergmanis, U.; Meyburg, B.-U.; Furkert, B.; Knack, A.; Rriper, S. Raum-Zeit-Verhalten des Schreiadlers (Aquila pomarina). Acta Ornithoecol. 2001, 4, 75–236. [Google Scholar]
- Böhner, J.; Langgemach, T. Warum kommt es auf jeden einzelnen Schreiadler Aquila pomarina in Brandenburg an? Ergebnisse einer Populationsmodellierung. Vogelwelt 2004, 125, 271–281. [Google Scholar]
- Švehlik, J.; Meyburg, B.U. Gelegegröße und Bruterfolg des Schreiadlers (Aquila pomarina) und des Kaiseradlers (Aquila heliaca) in den ostslowakischen Karpaten 1966–1978. J. Ornithol. 1979, 120, 406–415. [Google Scholar] [CrossRef]
- Dravecký, M.; Maderič, B.; Kicko, J.; Danko, Š.; Karaska, D.; Mihók, J.; Guziová, Z. Reproductive success, selected nest characteristics and the effectiveness of establishing protection zones of the lesser spotted eagle (Aquila pomarina) population in Slovakia. Slovak. Raptor J. 2015, 9, 127–145. [Google Scholar] [CrossRef][Green Version]
- Haraszthy, L.; Bagyura, J.; Szitta, T. Zur Biologie des Schreiadlers Aquila pomarina in Ungarn. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 305–312. [Google Scholar]
- Abuladze, A. Lesser Spotted Eagle Aquila pomarina in Transcaucasia. Acta Ornithoecol. 2001, 4, 321–324. [Google Scholar]
- Abuladze, A. Lesser Spotted Eagle Aquila pomarina in Georgia. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 349–355. [Google Scholar]
- Väli, Ü.; Belik, V.P.; Babkin, I.G. The Lesser Spotted Eagle Aquila pomarina in the North Caucasus, Russian Federation: Taxonomic status, genetic diversity, breeding density and nest site characteristics. Sandgrouse 2009, 31, 122–127. [Google Scholar]
- Vlachos, C.; Papageorgiou, N. Breeding biology and feeding of the Lesser Spotted Eagle Aquila pomarina in Dadia forest, North-Eastern Greece. In Eagle Studies; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 337–347. [Google Scholar]
- Väli, Ü.; Rohtla, F. Apparent survival and population turnover in a long-lived generalist raptor: A comparison of estimation methods. Basic Appl. Ecol. 2025, 82, 28–34. [Google Scholar] [CrossRef]
- Ferrer, M.; Newton, I.; Casado, E. How to test different density-dependent fecundity hypotheses in an increasing or stable population. J. Anim. Ecol. 2006, 75, 111–117. [Google Scholar] [CrossRef]
- Sergio, F.; Blas, J.; Baos, R.; Forero, M.G.; Donázar, J.A.; Hiraldo, F. Short- and long term consequences of individual and territory quality in a long-lived bird. Oecologia 2009, 160, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Demerdzhiev, D.A.; Dobrev, D.D.; Boev, Z.N. Grassland Alterations Do Not Affect Breeding Success, but Can Explain Dietary Shifts of a Generalist Raptor Species. Diversity 2023, 15, 422. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Boev, Z.; Dobrev, D.; Terziev, N.; Nedyalkov, N.; Stoychev, S.; Petrov, T. Diet of Eastern Imperial Eagle (Aquila heliaca) in Bulgaria: Composition, distribution and variation. Biodivers. Data J. 2022, 10, e77746. [Google Scholar] [CrossRef] [PubMed]
- Miltschev, B.; Menzel, J. Rupfung eines weiblichen Schreiadlers Aquila pomarina durch einen Bubo bubo. Ornithol. Mitteilungen 2012, 64, 21–23. [Google Scholar]
- Catry, I.; Franco, A.; Sutherland, W. Landscape and weather determinants of prey availability: Implications for the Lesser Kestrel Falco naumanni. Ibis 2012, 154, 111–123. [Google Scholar] [CrossRef]
- Topercer, J.; Maderič, B. Nest-platform, nest-tree, nest-site, home-range and landscape characteristics of the Lesser Spotted Eagle (Clanga pomarina) in relation to its breeding performance in East Carpathians. For. Ecol. Manag. 2022, 520, 120350. [Google Scholar] [CrossRef]
- Sagarin, R.D.; Gaines, S.D.; Gaylord, B. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol. Evol. 2006, 21, 524–530. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998; 597p. [Google Scholar] [CrossRef]
- Wingfield, J.C. Influence of weather on reproduction. J. Exp. Zool. 1984, 232, 589–594. [Google Scholar] [CrossRef]
- Elkins, N. Weather and Bird Behaviour, 3rd ed.; Poyser: London, UK, 2004; 276p. [Google Scholar]
- Ferrer, M.; Donázar, J.A. Density-dependent fecundity by habitat heterogeneity in an increasing population of Spanish Imperial Eagles. Ecology 1996, 77, 69–74. [Google Scholar] [CrossRef]
- Fretwell, S.D. Populations in Seasonal Environments; Princeton University Press: Princeton, NJ, USA, 1972. [Google Scholar]
- Fretwell, S.D.; Lucas, H.L. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1969, 19, 16–36. [Google Scholar] [CrossRef]

| Year | No. of Visited Territories | No. of Monitored Occupied Territories | No. of Monitored Breeding Pairs |
|---|---|---|---|
| 2015 | 27 | 18 | 13 |
| 2016 | 47 | 28 | 18 |
| 2017 | 46 | 30 | 24 |
| 2018 | 44 | 30 | 21 |
| 2019 | 43 | 35 | 23 |
| 2020 | 47 | 43 | 20 |
| 2021 | 81 | 59 | 37 |
| 2022 | 50 | 39 | 27 |
| 2023 | 48 | 38 | 27 |
| 2024 | 46 | 36 | 26 |
| TOTAL | 479 | 356 | 236 |
| Country | Breeding Parameter | Period | References | |||
|---|---|---|---|---|---|---|
| Productivity (Fledglings/Occupied Territories) | Breeding Success (Fledglings/Incubated Pairs) | Breeding Frequency (Incubated Pairs/Occupied Territories) | Occupancy Rate (Occupied Territories/Monitored Territories) | |||
| Estonia | 0.62 | 1981–2002 | [25] | |||
| Estonia | 0.56 | 0.78 | 0.69 | 1992–2009 | [4] | |
| Estonia | 0.69 | 1999–2002 | [5] | |||
| Estonia | 0.44 | 2002–2010 | [10] | |||
| Estonia | 0.67 | 2004–2006 2010–2012 | [26] | |||
| Latvia | 0.58 | 0.79 | 0.68 | 1985–1996 | [13] | |
| Latvia | 0.58 | 1988–2003 | [8] | |||
| Latvia | 0.49 | 0.74 | 0.66 | 1988–2014 | [23] | |
| Latvia | 0.43 | 2002–2010 | [10] | |||
| Latvia | 0.83 | 2006–2017 | [27] | |||
| Lithuania | 0.61 | 2001–2003 | [12] | |||
| Lithuania | 0.60 | 2001–2006 | [28] | |||
| Lithuania | 0.65 | 2002–2010 | [10] | |||
| Lithuania | 0.90 | 2012–2017 | [29] | |||
| Belarus | 0.76 | 1981–1991 | [30] | |||
| Poland | 0.63 | 1988–1991 | [31] | |||
| Poland | 0.69 | ?? | [32] | |||
| North-Eastern Poland | 0.54 | 0.81 | 1999–2015 | [33] | ||
| Eastern Germany | 0.51 | 0.78 | 1994–1997 | [34] | ||
| Eastern Germany | 0.65 | ?? | [35] | |||
| Slovakia | 0.53 | 0.77 | 1966–1978 | [36] | ||
| Slovakia | 0.51 | 0.69 | 0.75 | 2011–2014 | [37] | |
| Hungary | 0.68 | ?? | [38] | |||
| Eastern Georgia/Western Azerbaijan | 0.94 | 1.06 | 1982–1992 | [39] | ||
| Eastern Georgia | 0.98 | 1.09 | 1982–1988 | [40] | ||
| Northern Caucasus, Russia | 0.71 | 2007 | [41] | |||
| Northern Greece | 0.67 | 1985–1987 | [42] | |||
| South-Eastern Bulgaria | 0.50 | 0.67 | 0.74 | 2014–2018 | [16] | |
| Bulgaria | 0.50 | 0.75 | 0.67 | 0.98 | 2015–2024 | This study |
| Parameter | Factor Effect | β2 | Std. Err. | Lower CL | Upper CL | df | p |
|---|---|---|---|---|---|---|---|
| Occupancy rate | Year | 0.29 | 0.14 | 0.02 | 0.55 | 1.415 | 0.04 |
| Productivity | Year | −0.06 | 0.04 | −0.14 | 0.02 | 1.337 | 0.12 |
| Breeding success | Year | −0.12 | 0.06 | −0.23 | −0.003 | 1.226 | 0.04 |
| Breeding frequency | Year | −0.01 | 0.04 | −0.09 | 0.08 | 1.337 | 0.86 |
| Occupancy rate | Region | 0.19 | 0.11 | −0.02 | 0.41 | 1.425 | 0.08 |
| Productivity | Region | 0.02 | 0.04 | −0.07 | 0.10 | 1.346 | 0.68 |
| Breeding success | Region | 0.09 | 0.06 | −0.02 | 0.20 | 1.235 | 0.12 |
| Breeding frequency | Region | −0.05 | 0.05 | −0.15 | 0.04 | 1.346 | 0.27 |
| Occupancy rate | Density | −0.75 | 0.39 | −1.51 | 0.01 | 1.424 | 0.054 |
| Productivity | Density | 0.25 | 0.12 | 0.02 | 0.48 | 1.345 | 0.03 |
| Breeding success | Density | 0.15 | 0.16 | −0.16 | 0.47 | 1.234 | 0.34 |
| Breeding frequency | Density | 0.27 | 0.12 | 0.02 | 0.51 | 1.345 | 0.03 |
| Region | N | Occupancy Rate | Std. Err. | No. | Productivity | Std. Err. | No. | Breeding Success | Std. Err. | No. | Breeding Frequency | Std. Err. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suha Reka river | 31 | 0.97 | 0.03 | 25 | 0.76 | 0.09 | 21 | 0.90 | 0.07 | 25 | 0.84 | 0.07 |
| Strandzha Mnt. | 25 | 1.00 | 25 | 0.48 | 0.10 | 19 | 0.63 | 0.11 | 25 | 0.76 | 0.09 | |
| Dervent Heights | 199 | 0.99 | 0.01 | 164 | 0.46 | 0.04 | 101 | 0.74 | 0.04 | 164 | 0.62 | 0.04 |
| Rusenski Lom | 22 | 1.00 | 19 | 0.58 | 0.12 | 11 | 1.00 | 19 | 0.58 | 0.12 | ||
| Batova river | 7 | 1.00 | 7 | 1.00 | 0.22 | 7 | 1.00 | 0.22 | 7 | 1.00 | ||
| Sakar Mnt. | 66 | 0.91 | 0.04 | 48 | 0.46 | 0.07 | 30 | 0.73 | 0.08 | 48 | 0.63 | 0.07 |
| Eastern Balkan | 25 | 1.00 | 21 | 0.62 | 0.11 | 18 | 0.72 | 0.11 | 21 | 0.86 | 0.08 | |
| Gorata ridge | 9 | 1.00 | 7 | 0.71 | 0.18 | 6 | 0.83 | 0.17 | 7 | 0.86 | 0.14 | |
| Byala reka river | 20 | 0.95 | 0.05 | 15 | 0.33 | 0.13 | 12 | 0.42 | 0.15 | 15 | 0.80 | 0.11 |
| Tundzha river | 23 | 0.96 | 0.04 | 17 | 0.47 | 0.12 | 12 | 0.67 | 0.14 | 17 | 0.71 | 0.11 |
| TOTAL | 427 | 348 | 237 | 348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demerdzhiev, D.; Dobrev, D.; Delchev, A.; Iliev, M.; Georgiev, G.; Terziev, N.; Angelov, I.; Arkumarev, V. Temporally Determinate, but Spatially Consistent Breeding Performance of Lesser Spotted Eagle (Clanga pomarina) Along the Southern Periphery of Its Distribution. Diversity 2025, 17, 566. https://doi.org/10.3390/d17080566
Demerdzhiev D, Dobrev D, Delchev A, Iliev M, Georgiev G, Terziev N, Angelov I, Arkumarev V. Temporally Determinate, but Spatially Consistent Breeding Performance of Lesser Spotted Eagle (Clanga pomarina) Along the Southern Periphery of Its Distribution. Diversity. 2025; 17(8):566. https://doi.org/10.3390/d17080566
Chicago/Turabian StyleDemerdzhiev, Dimitar, Dobromir Dobrev, Atanas Delchev, Mihail Iliev, Georgi Georgiev, Nikolay Terziev, Ivaylo Angelov, and Volen Arkumarev. 2025. "Temporally Determinate, but Spatially Consistent Breeding Performance of Lesser Spotted Eagle (Clanga pomarina) Along the Southern Periphery of Its Distribution" Diversity 17, no. 8: 566. https://doi.org/10.3390/d17080566
APA StyleDemerdzhiev, D., Dobrev, D., Delchev, A., Iliev, M., Georgiev, G., Terziev, N., Angelov, I., & Arkumarev, V. (2025). Temporally Determinate, but Spatially Consistent Breeding Performance of Lesser Spotted Eagle (Clanga pomarina) Along the Southern Periphery of Its Distribution. Diversity, 17(8), 566. https://doi.org/10.3390/d17080566








