Genetic Evidence Reveals Unexpected Diversity and Genetic Exchange Between White-Fringed Weevils (Coleoptera, Curculionidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Amplification and Sequencing of Mitochondrial and Nuclear Markers
2.3. ddRAD-Seq Library Preparation and SNP Genotyping
2.4. Phylogenetic Reconstruction Based on Mitochondrial, Nuclear, and Genomic Data
2.5. Network and Genetic Structure Analyses
2.6. Species Delimitation Analysis
2.7. Morphological Evaluation
3. Results
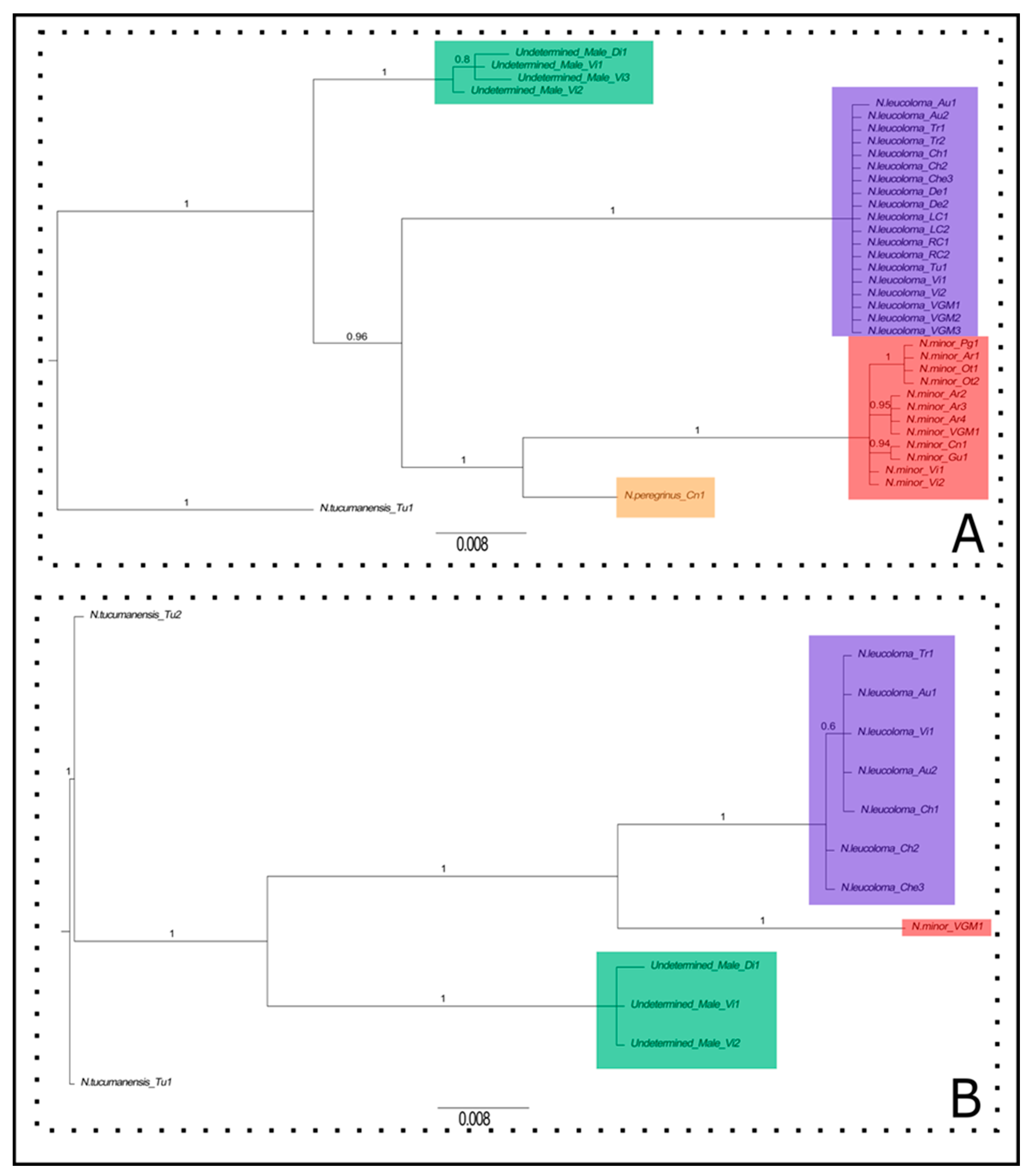
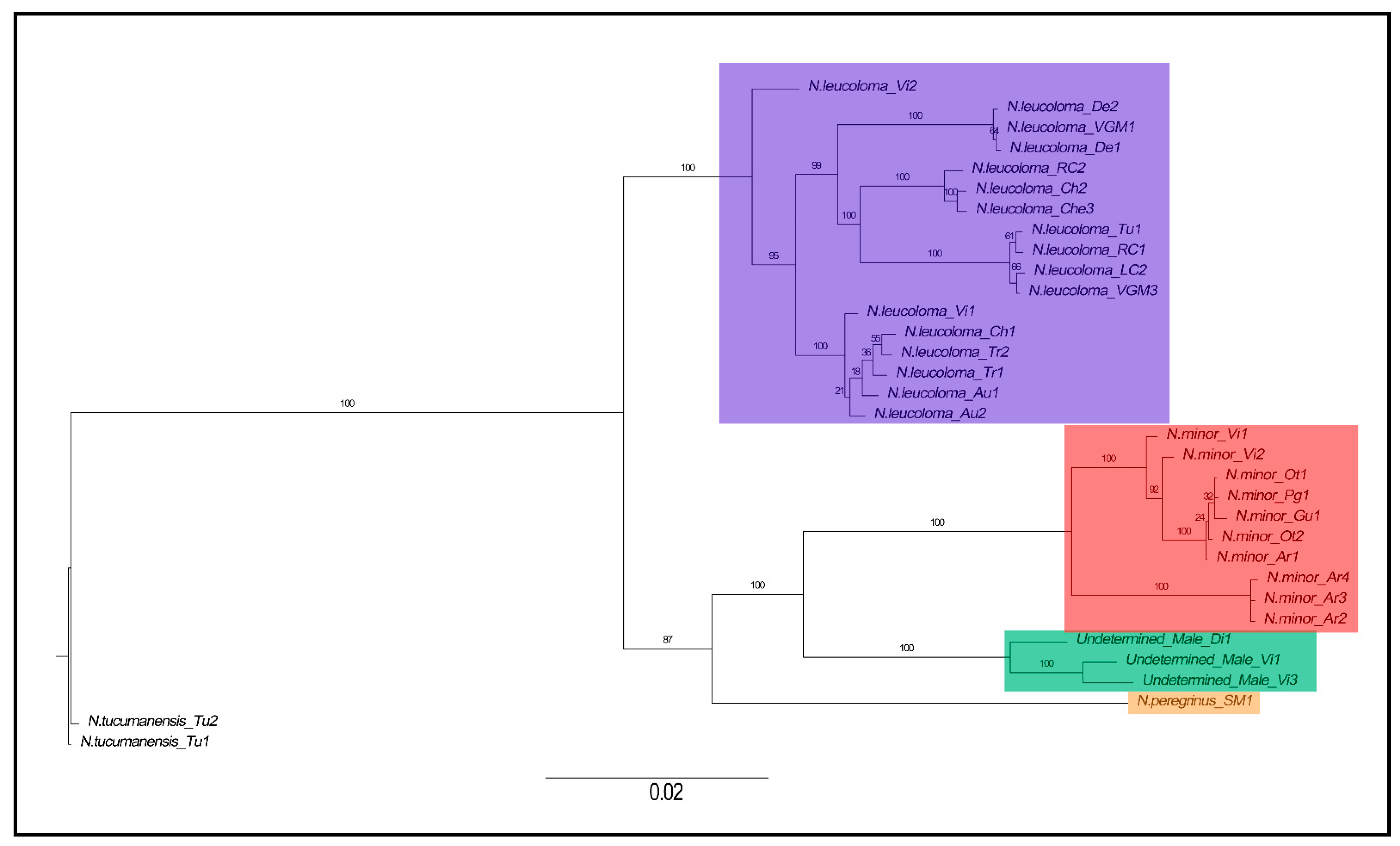
3.1. Phylogenetic Reconstruction Based on Mitochondrial, Nuclear, and Genomic Data
3.2. Network and Genetic Structure Analyses
3.3. Species Delimitation Analysis
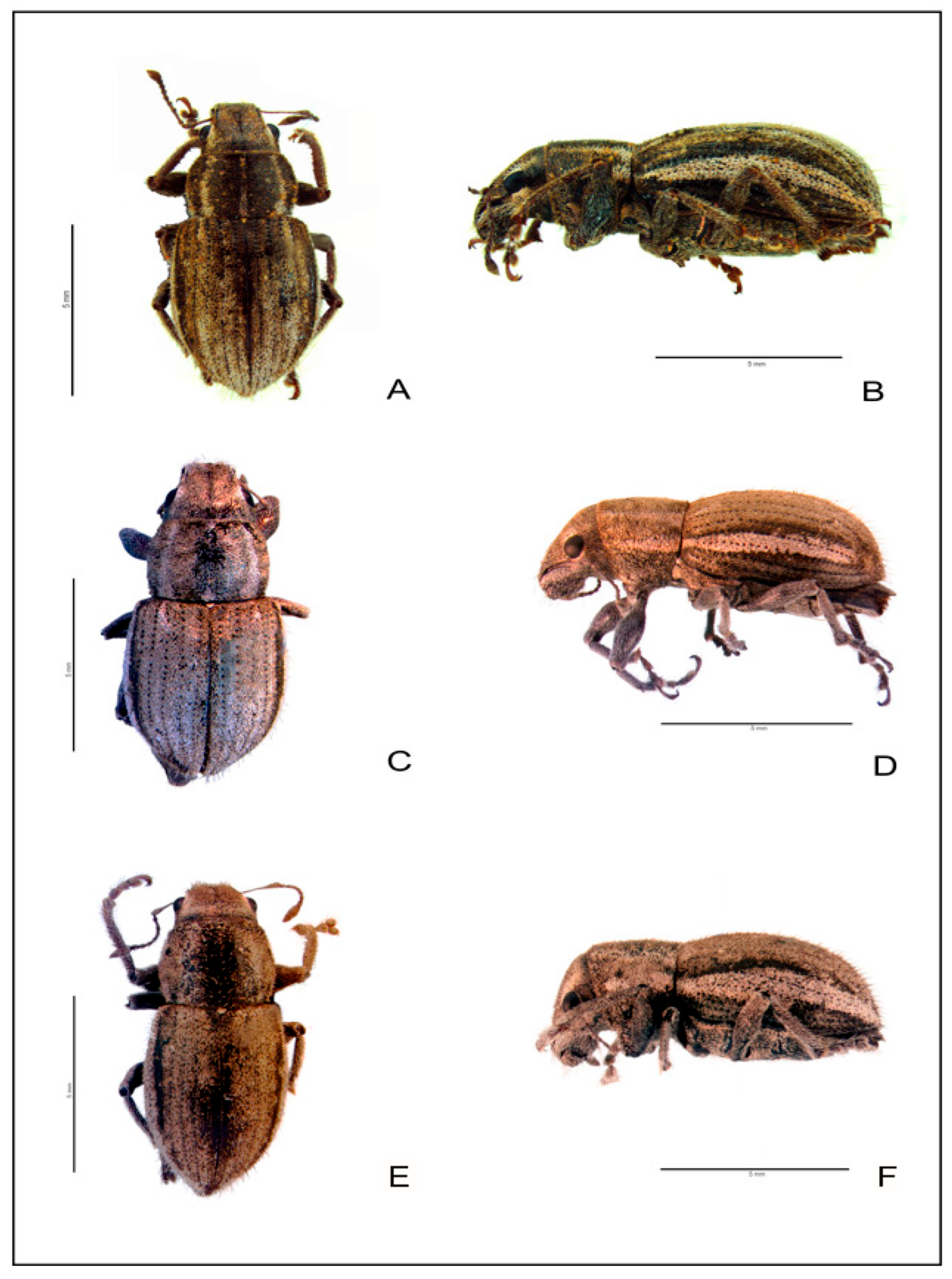
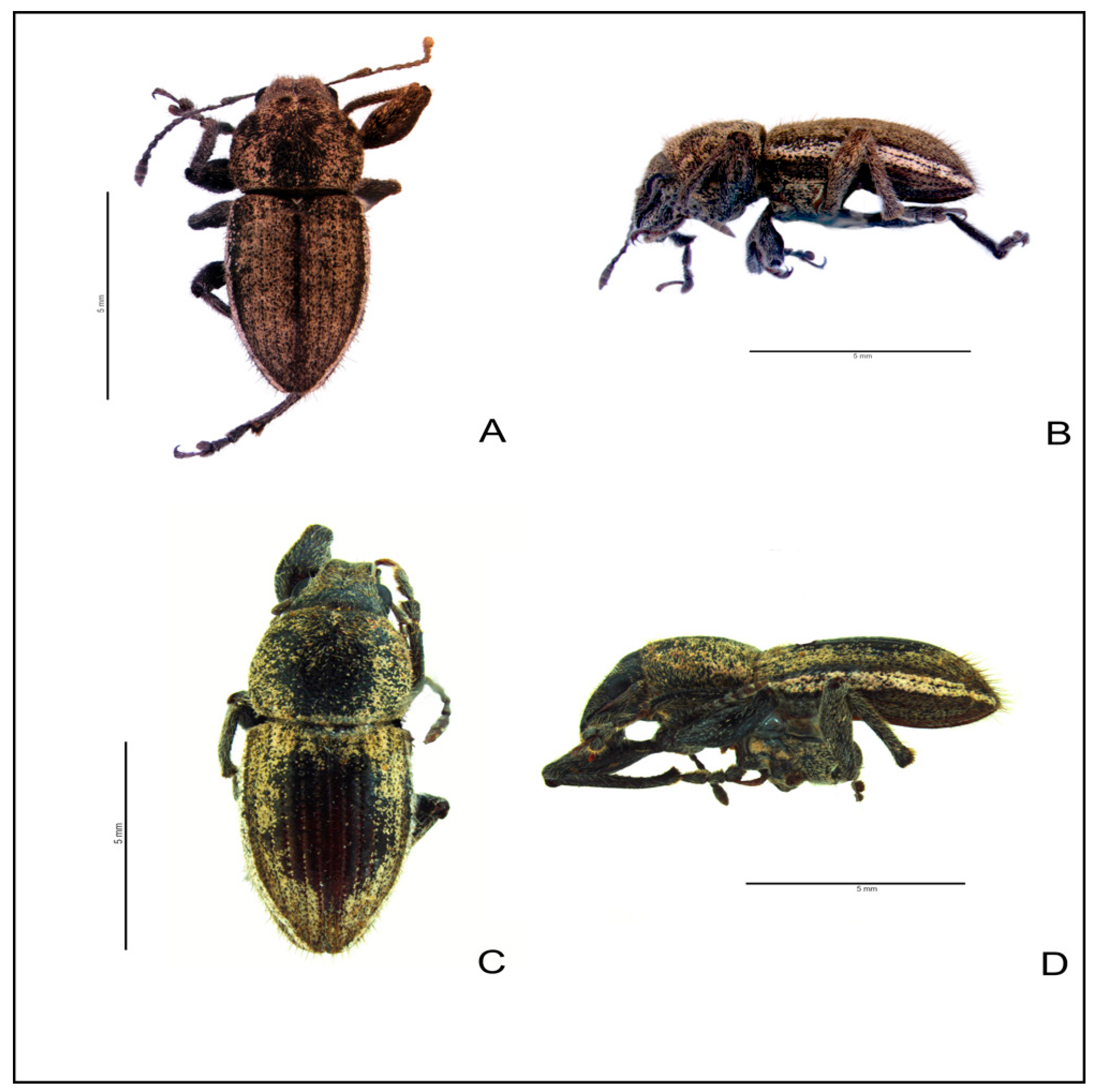
3.4. Morphological Evaluation
4. Discussion
4.1. Four Independently Evolving Lineages
4.2. Causes of Conflict
4.3. Successive Rounds of Parthenogenesis and Sexuality Shapes the Genetic Variation of Weevils in Singular Ways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanteri, A.A.; Marvaldi, A.E. Graphognathus Buchanan, a new synonym of Naupactus Dejean, and systematics of the N. leucoloma species group (Coleoptera: Curculionidae). Coleop. Bull. 1995, 49, 206–228. [Google Scholar]
- EPPO Naupactus leucoloma. EPPO Datasheets on Pests Recommended for Regulation. 2021. Available online: https://gd.eppo.int (accessed on 1 May 2025).
- Watson, R.J. Naupactus leucoloma (Coleoptera, Curculionidae): A Pest New to the United States. Fla. Entomol. 1937, 20, 1–3. [Google Scholar] [CrossRef]
- Young, H.C.; App, B.A. Biology of the white-fringed beetle (Naupactus leucoloma Boh.). USDA Bur. Entomol. Plant Quar. Circular E-464. 1939. 24p. Available online: https://www.biodiversitylibrary.org/item/216240 (accessed on 1 May 2025).
- Buchanan, L.L. The species of Pantomorus of America north of Mexico. United States Department of Agriculture. Misc. Publ. 1939, 341, 1–39. [Google Scholar]
- Buchanan, L.L. Four new species of white-fringed beetles (subgenus Graphognathus) from the southeastern part of the United States (Coleoptera: Curculionidae). Bull. Brooklyn Entomol. Soc. 1942, 37, 107–110. [Google Scholar]
- Buchanan, L.L. A correction and two new races in Graphognatus (white-fringed beetles) (Coleoptera: Curculionidae). J. Wash. Acad. Sci. 1947, 37, 19–22. [Google Scholar]
- Lanteri, A.A.; Normark, B. Parthenogenesis in the tribe Naupactini (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1995, 88, 722–731. [Google Scholar] [CrossRef]
- Guzmán, N.V.; Lanter, A.A.; Confalonieri, V.A. Colonization ability of two invasive weevils with different reproductive modes. Evol. Ecol. 2012, 26, 1371–1390. [Google Scholar] [CrossRef]
- Lanteri, A.A.; Guzmán, N.V.; del Río, M.G.; Confalonieri, V.A. Potential geographic distributions and successful invasions of parthenogenetic broad–nosed weevils (Coleoptera: Curculionidae) native to South America. Environ. Entomol. 2013, 42, 677–686. [Google Scholar] [CrossRef]
- Scataglini, M.A.; Lanteri, A.A.; Confalonieri, V.A. Phylogeny of the Pantomorus–Naupactus complex based on morphological and molecular data (Coleoptera: Curculionidae). Cladistics 2005, 21, 131–142. [Google Scholar] [CrossRef]
- del Rio, M.G.; Rodriguero, M.S.; Confalonieri, V.A.; Lanteri, A.A. Molecular and Morphological Phylogenetic Analysis of Naupactus Dejean (Curculionidae: Entiminae) and Allied Genera: The Dilemma of Classification. Diversity 2018, 10, 59–68. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Confalonieri, V.A.; Guedes, J.C.; Lanteri, A.A. Wolbachia infection in the tribe Naupactini (Coleoptera, Curculionidae): Association between thelytokous parthenogenesis and infection status. Insect Mol. Biol. 2010, 19, 631–640. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Guzmán, N.V.; Lanteri, A.A.; Confalonieri, V.A. The effect of reproductive system on invasiveness: Lessons from South American weevils. Fla. Entomol. 2019, 102, 495–500. [Google Scholar] [CrossRef]
- Mackay-Smith, A.; Dornon, K.M.; Lucier, R.; Okimoto, A.; Sousa, M.D.F.; Rodriguero, M.; Confalonieri, A.V.; Lanteri, A.A.; Sequeira, S. A Host-specific gene expression as a tool for introduction success in Naupactus parthenogenetic weevils. PLoS ONE 2021, 16, e0248202. [Google Scholar] [CrossRef]
- Sunnucks, P.; Hales, D.F. Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol. Biol. Evol. 1996, 13, 510–524. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Lanteri, A.A.; Confalonieri, V.A. Speciation in the asexual realm: Is the parthenogenetic weevil Naupactus cervinus a complex of species in statu nascendi? Mol. Phylogenet. Evol. 2013, 68, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Normark, B.B. Phylogeny and Evolution of Parthenogenesis in the Aramigus tessellatus Complex (Coleoptera: Curculionidae). Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1994. [Google Scholar]
- Vrain, T.C.; Wakarchuk, D.C.; Levesque, A.C.; Hamilton, R.I. Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 1992, 15, 563–573. [Google Scholar]
- Cherry, T.; Szalanski, A.L.; Todd, T.C.; Powers, T.O. The internal transcribed spacer region of Belonolaimus (Nemata: Belonolaimidae). J. Nematol. 1997, 29, 23–29. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Res. Spec. Ser. 1999, 41, 95–98. [Google Scholar]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Catchen, J.M.; Amores, A.; Hohenlohe, P.; Cresko, W.; Postlethwait, J.H. Stacks: Building and Genotyping Loci De Novo From Short-Read Sequences. G3 Genes Genomes Genet. 2011, 1, 171–182. [Google Scholar] [CrossRef]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef]
- Rivera-Colón, A.G.; Catchen, J. Population Genomics Analysis with RAD, Reprised: Stacks 2. In Marine Genomics, 1st ed.; Verde, C., Giordano, D., Eds.; Humana: New York, NY, USA, 2022; Volume 2498, pp. 145–168. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, C.J.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, E.S.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, R.; Serra, F.; Tárraga, J.; Medina, I.; Carbonell, J.; Pulido, L.; de María, A.; Capella-Gutíerrez, S.; Huerta-Cepas, J.; Gabaldón, T.; et al. Phylemon 2.0: A suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Res. 2011, 39, W470–W474. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Version 4.2.3; Available online: https://www.R-project.org (accessed on 1 December 2024).
- RStudio Team. RStudio: Integrated Development Environment for R; Version 2024.04.2+746; RStudio, Inc.: Boston, MA, USA, 2020. [Google Scholar]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Knaus, B.J.; Grünwald, N.J. vcfr: A package to manipulate and visualize variant call format data in R. Mol. Ecol. Res. 2017, 17, 44–53. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jost, L. GST and its relatives do not measure differentiation. Mol. Ecol. 2008, 17, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.A.; Byrne, M. Variable clonality and genetic structure among disjunct populations of Banksia mimica. Conserv. Genet. 2020, 21, 803–818. [Google Scholar] [CrossRef]
- Elias-Costa, A.J.; Confalonieri, V.A.; Lanteri, A.A.; Rodriguero, M.S. Game of clones: Is Wolbachia inducing speciation in a weevil with a mixed reproductive mode? Mol. Phylogenet. Evol. 2019, 133, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.Y.; Patterson, N.; Reich, D.; Slatkin, M. Testing for ancient admixture between closely related populations. Mol. Biol. Evol. 2011, 28, 2239–2252. [Google Scholar] [CrossRef]
- Malinsky, M.; Matschiner, M.; Svardal, H. Dsuite—Fast D-statistics and related admixture evidence from VCF files. Mol. Ecol. Resour. 2021, 21, 584–595. [Google Scholar] [CrossRef]
- Wiens, B.J.; DeCicco, L.H.; Colella, J.P. triangulaR: An R package for identifying AIMs and building triangle plots using SNP data from hybrid zones. Heredity 2025, 134, 251–262. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Baele, G.; Lemey, P.; Bedford, T.; Rambaut, A.; Suchard, M.A.; Alekseyenko, A.V. Improving the accuracy of demographic and molecular clock model comparison while accommodating phylogenetic uncertainty. Mol. Biol. Evol. 2012, 29, 2157–2167. [Google Scholar] [CrossRef]
- Grummer, J.A.; Bryson, R.W.; Reeder, T.W. Species delimitation using Bayes factors: Simulations and application to the Sceloporus scalaris species group (Squamata: Phrynosomatidae). Syst. Biol. 2014, 63, 119–133. [Google Scholar] [CrossRef]
- Kass, R.E.; Raftery, A.E. Bayes factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Wirth, S.A.; Alberghina, J.S.; Lanteri, A.A.; Confalonieri, V.A. A tale of swinger insects: Signatures of past sexuality between divergent lineages of a parthenogenetic weevil revealed by ribosomal intraindividual variation. PLoS ONE 2018, 13, e0195551. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.L. Statistical phylogeography. Annu. Rev. Ecol. Evol. Syst 2009, 40, 593–612. [Google Scholar] [CrossRef]
- Whitfield, J.B.; Lockhart, P.J. Deciphering ancient rapid radiations. Trends Ecol. Evol. 2007, 22, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Normark, B.B.; Lanteri, A.A. Incongruence between morphological and mitochondrial-DNA characters suggests hybrid origins of parthenogenetic weevil lineages (genus Aramigus). Syst. Biol. 1998, 47, 475–494. [Google Scholar] [CrossRef]
- Tomiuk, J.; Loeschcke, V. Evolution of parthenogenesis in the Otiorhynchus scaber complex. Heredity 1992, 68, 391–397. [Google Scholar] [CrossRef]
- Stenberg, P.; Lundmark, M.; Knutelski, S.; Saura, A. Evolution of clonality and polyploidy in a weevil system. Mol. Biol. Evol. 2003, 20, 1626–1632. [Google Scholar] [CrossRef]
- Stenberg, P.; Terhivuo, J.; Lokki, J.; Saura, A. Clone diversity of tetraploid Otiorhynchus scaber in Northern Europe. Hereditas 1997, 126, 169–172. [Google Scholar] [CrossRef]
- Stenberg, P.; Terhivuo, J.; Lokki, J.; Saura, A. Clone diversity in the polyploid weevil Otiorhynchus scaber. Hereditas 2000, 132, 137–142. [Google Scholar] [CrossRef]
- Stenberg, P.; Lundmark, M. Distribution, mechanisms and evolutionary significance of clonality and polyploidy in weevils. Agric. For. Entomol. 2004, 6, 259–266. [Google Scholar] [CrossRef]
- Husemann, M.; Guzmán, N.V.; Danley, P.D.; Cigliano, M.M.; Confalonieri, V.A. Biogeography of Trimerotropis pallidipennis (Acrididae: Oedipodinae): Deep divergence across the Americas. J. Biogeogr. 2013, 40, 261–273. [Google Scholar] [CrossRef]

| Specimen ID | Species Name | Sex | Sampling Season | Location | Geographical Coordinates | N | COI Haplotype (Acc. No.) | ITS1 Haplotype (Acc. No.) |
|---|---|---|---|---|---|---|---|---|
| Nl_LC | Naupactus leucoloma | female | 2007 | AR, CD, La Carlota | 33° 26′ S, 63° 18′ W | 2 | L1 (JF811695.1) | NA |
| Nl_RC | Naupactus leucoloma | female | 2007 | AR, CD, Río Cuarto | 33° 08′ S, 64° 21′ W | 2 | L1 (JF811695.1) | NA |
| Nl_Tr | Naupactus leucoloma | female | 2005 | AR, CHB, Trelew | 43° 15′ S, 65° 18′ W | 2 | L1 (JF811695.1) | LI (PV940799) |
| Nl_Vi | Naupactus leucoloma | female | 2013 | AR, ER, Victoria | 32° 26′ S, 60° 10′ W | 2 | L1 (JF811695.1) | LI (PV940799) |
| Nl_VGM | Naupactus leucoloma | female | 2013 | AR, ER, RN 12, Viale–María Grande | 31° 48′ S, 59° 56′ W | 3 | L1 (JF811695.1) | NA |
| Nl_Ch | Naupactus leucoloma | female | 2006 | AR, PBA, Chillar | 37° 18′ S, 59° 59′ W | 2 | L1 (JF811695.1) | LI (PV940799)–LII (PV940800) |
| Nl_De | Naupactus leucoloma | female | 2005 | AR, PBA, Delta | 34° 26′ S, 58 ° 33′ W | 2 | L1 (JF811695.1) | NA |
| Nl_Tu | Naupactus leucoloma | female | 2009 | AR, TC, Los Leales | 27° 12′ S, 65° 18′ W | 1 | L1 (JF811695.1) | NA |
| Nl_Au1 | Naupactus leucoloma | female | 2005 | AU, NSW, Kiama | 34° 52′ S, 150 ° 44′ E | 1 | L3 (JF811693.1) | LI (PV940799) |
| Nl_Au2 | Naupactus leucoloma | female | 2005 | AU, WA, Wheatblty | 31° 45′ S, 118° 06′ E | 1 | L1 (JF811695.1) | LI (PV940799) |
| Nl_Che | Naupactus leucoloma | female | 2006 | CH, RM, Santiago | 33° 32′ S, 70° 46′ W | 1 | L1 (JF811695.1) | LII (PV940800) |
| Nm_Cn | Naupactus minor | female | 2006 | AR, ER, Concordia | 31° 24′ S, 58° 02′ W | 1 | M3 (PV934253) | NA |
| Nm_Gu | Naupactus minor | female | 2006 | AR, ER, Gualeguay | 33°09′ S, 59°19′ W | 1 | M3 (PV934253) | NA |
| Nm_Vi | Naupactus minor | female | 2013 | AR, ER, Victoria | 32° 26′ S, 60° 10′ W | 2 | M4 (PV934255) | NA |
| Nm_VMG | Naupactus minor | female | 2013 | AR, ER, RN 12,Viale–María Grande | 31° 48′ S, 59° 56′ W | 1 | M2 (PV934256) | MI (PV940801) |
| Nm_Ar | Naupactus minor | female | 2005 | AR, PBA, Arrecifes | 34° 04′ S, 60° 07′ W | 4 | M1 (PV934254) M2 (pending) | NA |
| Nm_Ot | Naupactus minor | female | 2004 | AR, PBA, R.N. Otamendi | 34° 13′ S, 58° 54′ W | 2 | M1 (PV934254) | NA |
| Nm_Pg | Naupactus minor | female | 2005 | AR, PBA, Pergamino | 33 ° 53′ S, 60° 34′ W | 1 | M1 (PV934254) | NA |
| Np_Cn | Naupactus peregrinus | female | 2006 | AR, ER, Concordia | 31° 24′ S, 58° 02′ W | 1 | P1 (MH537935.1) | NA |
| Np_SM | Naupactus peregrinus | female | 2007 | BR, RS, Santa Maria | 9° 41′ S, 53° 48′ W | 1 | NA | NA |
| Nt_Tu | Naupactus tucumanensis | female | 2009 | AR, TC, Los Leales | 7° 12′ S, 65° 18′ W | 2 | T1 (MH537938.1) | TI (PV940804)–TII (PV940805) |
| UM_Di | Undetermined Males | male | 2013 | AR, ER, Diamante | 32° 03′ 43″ S, 60° 38′ 39″ W | 1 | N1 (PV934257) | NI (PV940802) |
| UM_Vi | Undetermined Males | male | 2013 | AR, ER, Victoria | 32° 26′ S, 60° 10′ W | 2 | N2 (PV934258)–N3 (PV934259)–N4 (PV934260) | NI (PV940802)–NII (PV940803) |
| Model | No. of Biological Units | MLE | BF | Ln BF |
|---|---|---|---|---|
| 1 | 4 | −5559.735 | - | - |
| 2 | 3 | −6789.130 | 2466.46 | 7.81 |
| 3 | 3 | −6859.158 | 2606.50 | 7.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman, N.V.; Rodriguero, M.S.; Confalonieri, V.A.; Lanteri, A.A. Genetic Evidence Reveals Unexpected Diversity and Genetic Exchange Between White-Fringed Weevils (Coleoptera, Curculionidae). Diversity 2025, 17, 561. https://doi.org/10.3390/d17080561
Guzman NV, Rodriguero MS, Confalonieri VA, Lanteri AA. Genetic Evidence Reveals Unexpected Diversity and Genetic Exchange Between White-Fringed Weevils (Coleoptera, Curculionidae). Diversity. 2025; 17(8):561. https://doi.org/10.3390/d17080561
Chicago/Turabian StyleGuzman, Noelia V., Marcela S. Rodriguero, Viviana A. Confalonieri, and Analia A. Lanteri. 2025. "Genetic Evidence Reveals Unexpected Diversity and Genetic Exchange Between White-Fringed Weevils (Coleoptera, Curculionidae)" Diversity 17, no. 8: 561. https://doi.org/10.3390/d17080561
APA StyleGuzman, N. V., Rodriguero, M. S., Confalonieri, V. A., & Lanteri, A. A. (2025). Genetic Evidence Reveals Unexpected Diversity and Genetic Exchange Between White-Fringed Weevils (Coleoptera, Curculionidae). Diversity, 17(8), 561. https://doi.org/10.3390/d17080561






