New Insights in the Helicellini Ihering, 1909 with Description of Kherattolactea gen. nov. and the First Record of Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 from Algeria
Abstract
1. Introduction
2. Material and Methods
2.1. Molecular Analyses
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequences Alignment and Diversity
2.4. Phylogenetic Analyses
2.5. Description of New Taxa
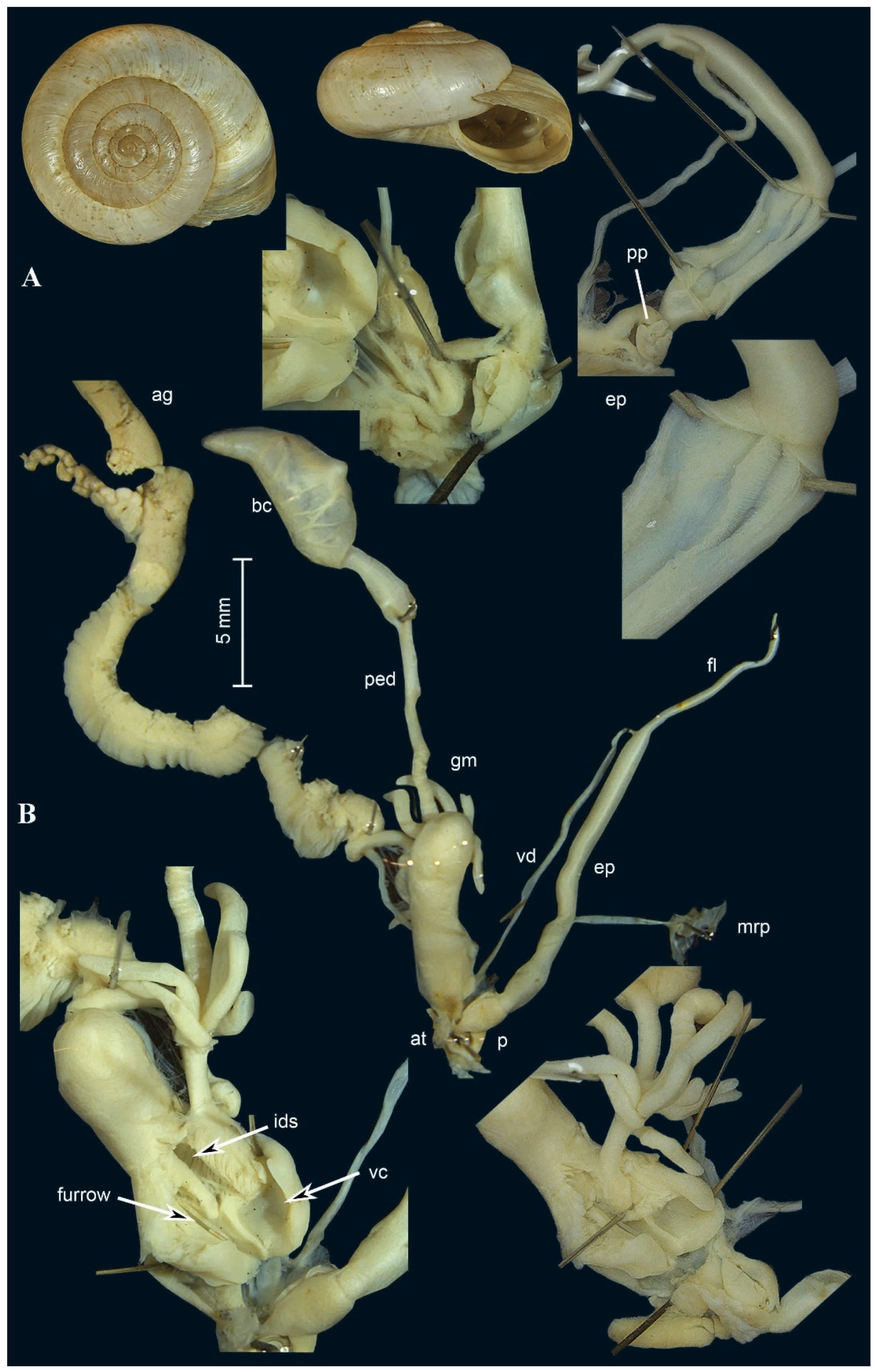
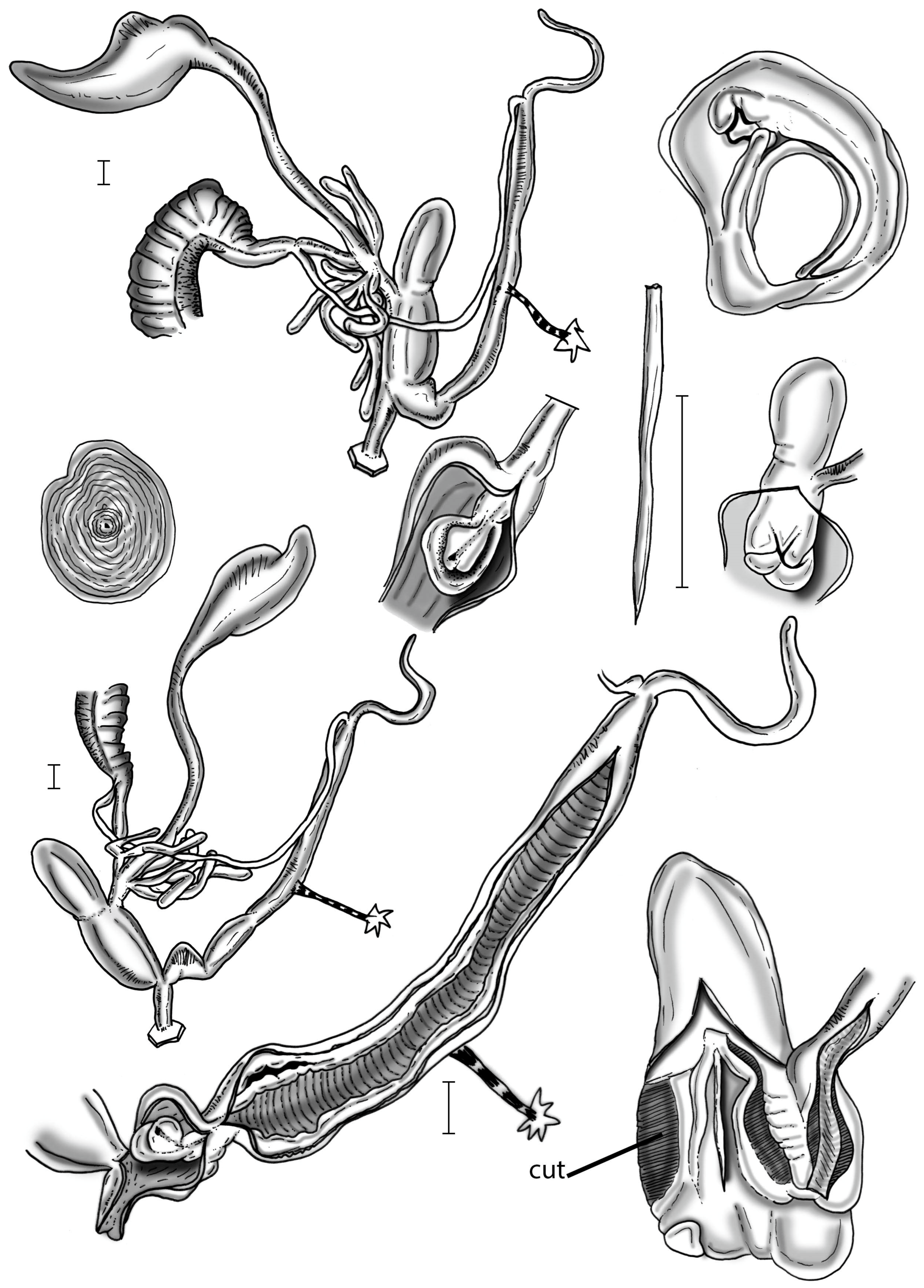
2.6. Morphological Investigation
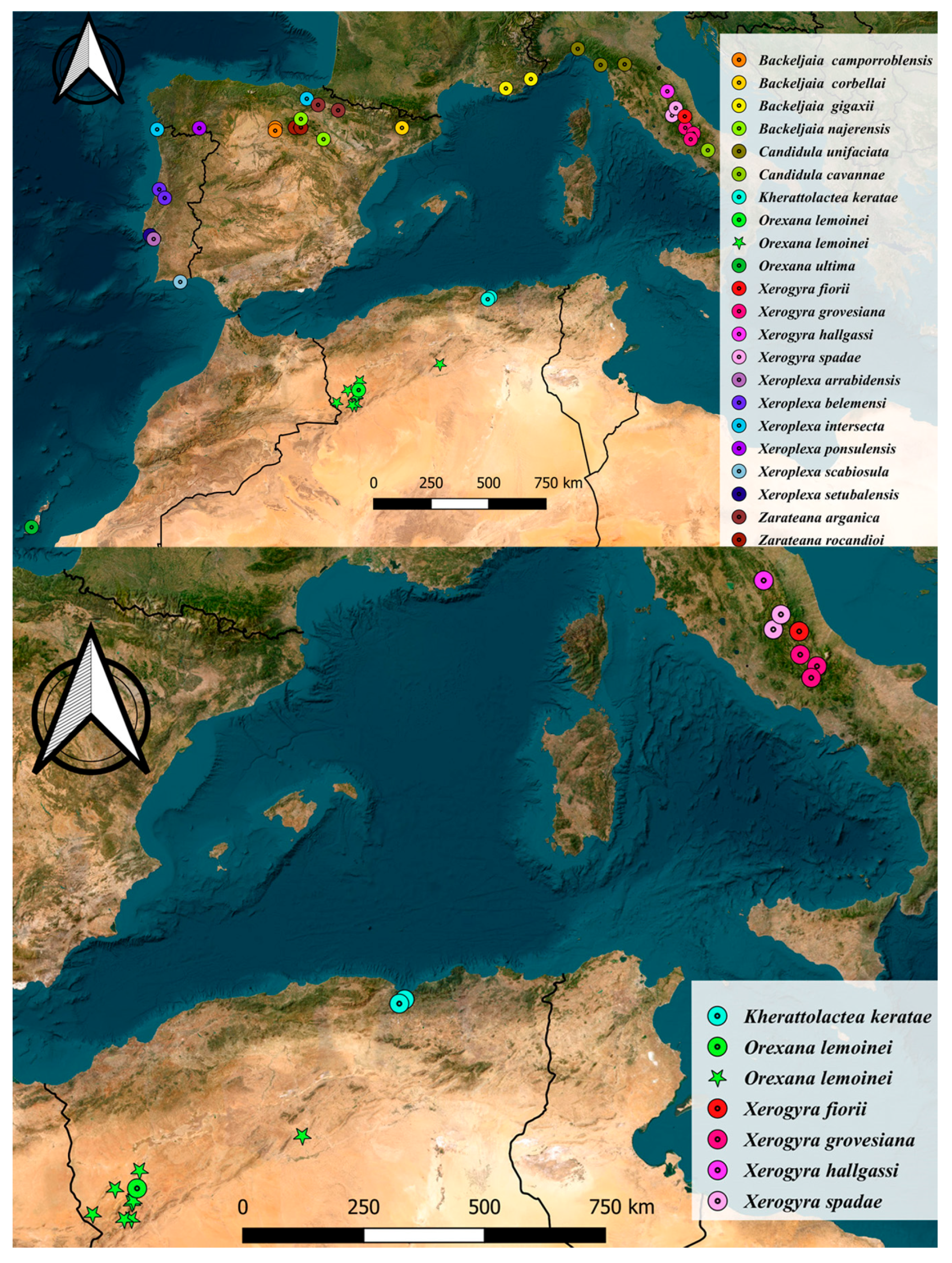
3. Results
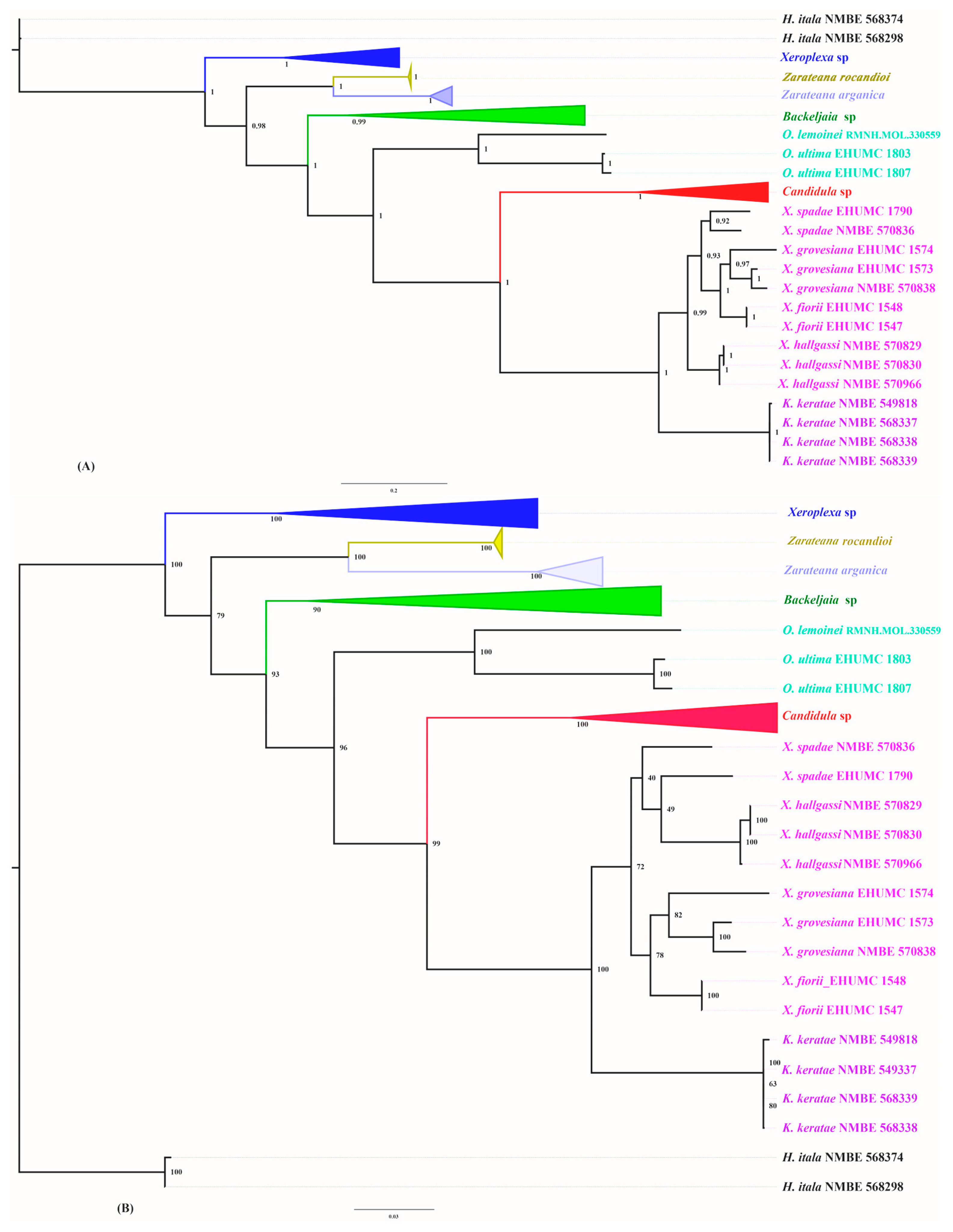
3.1. Phylogenetic Analysis
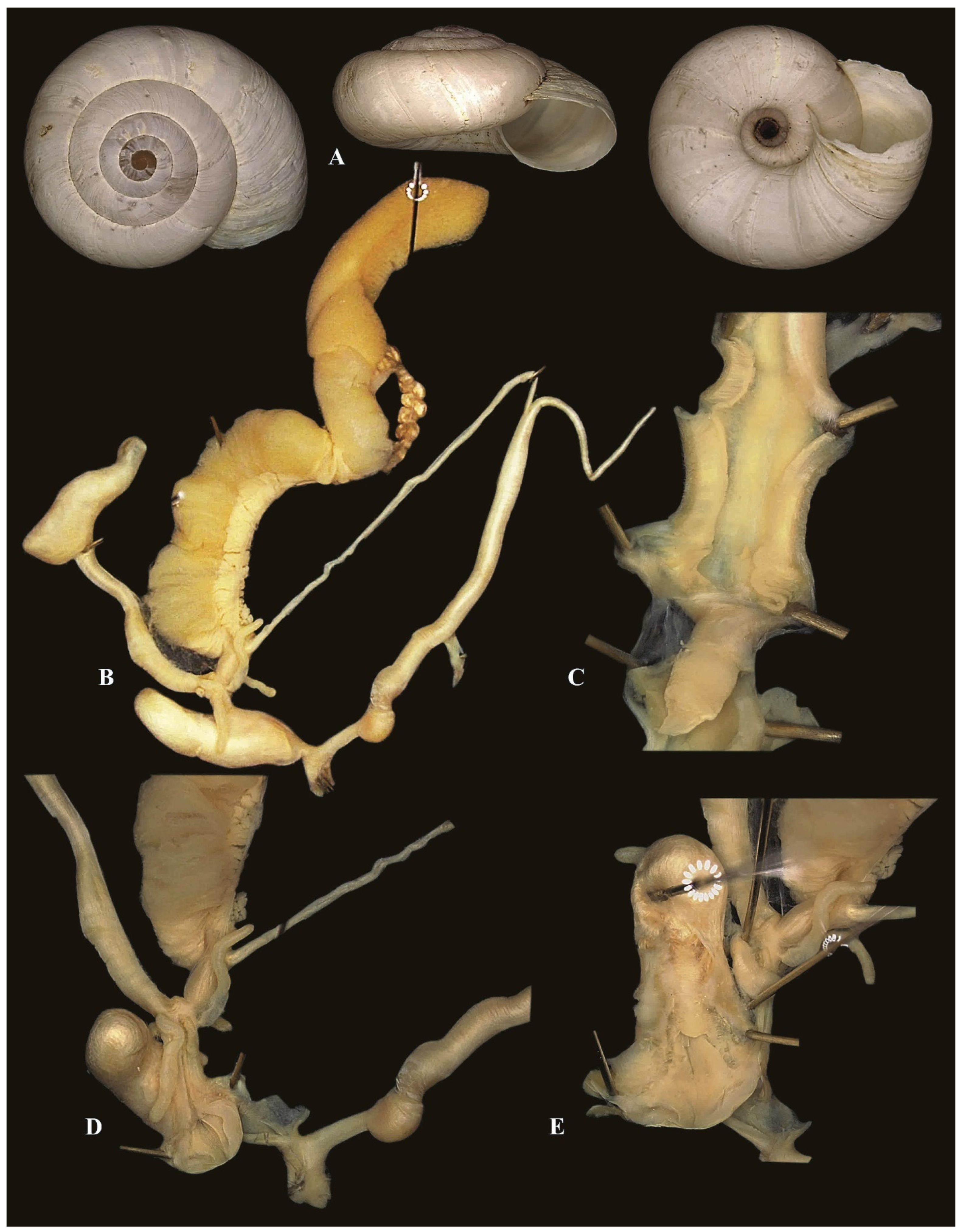
3.2. Molecular Description of New Taxa
- Kherattolactea nov. gen. Ezzine, Bouaziz, De Mattia & Neubert
- Type species: Xerophila keratae Kobelt, 1892 [16].
- Xerogyra hallgassi nov. sp. De Mattia, Ezzine & Neubert.
- Orexana lemoinei (Kobelt, 1882) [43] nov. comb.
3.3. Systematic Implications
- Kherattolactea gen. nov. Ezzine, Bouaziz, De Mattia & Neubert
- Kherattolactea keratae (Kobelt, 1892) [16]
- Xerogyra Monterosato, 1892
- Xerogyra fiorii (Alzona & Alzona Bisacchi, 1938) [42]
- Xerogyra grovesiana (Paulucci, 1881) [41]
- Xerogyra spadae (Calcara, 1845) [40]
- Xerogyra hallgassi De Mattia, Ezzine & Neubert n. sp.
- Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 [14]
- Orexana lemoinei (Kobelt, 1882) [43]
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations of Museum’s Acronyms
| MHNG-BGT | Museum d’Histoire Naturelle de Genève, coll. Bourguignat |
| NMBE | Naturhistorisches Museum der Burgergemeinde Bern |
| SMF | Research Institute Senckenberg, Frankfurt am Main, Germany |
| RMNH-MOL | Naturalis, Leiden |
References
- Rosenbaum, G.; Lister, G.S.; Duboz, C. Reconstruction of the tectonic evolution of the western Mediterranean since the Oligocene. Reconstructi+C2:C51on of the evolution of the Alpine-Himalayan Orogen. J. Virtual Explor. 2002, 8, 107–130. [Google Scholar] [CrossRef]
- Fischer, P. Manuel de Conchyliologie et de Paléontologie Conchyliologique, ou Histoire Naturelle des Mollusques Vivants et Fossiles Suivi d’un Appendice sur les Brachiopodes par D. P. Oehlert Avec 23 Planches Contenant 600 Figures Dessinées par S. P. Woodward; Published in 11 parts (fascicules), xxiv + 1369 pp., 23 pls. [21 September 1880, 1, 1–112; 16 March 1881, 2, 113–192; 28 July 1881, 3, 193–304; 5 May 1882, 4, 305–416; 21 February 1883, 5, 417–512; 20 December 1883, 6, 513–608; 30 June 1884, 7, 609–688; 29 January 1885, 8, 689–784; 31 August 1885, 9, 785–896; 30 April 1886, 10, 897–1008; 15 June 1887, 11, 1009–1369]; librairie F. Savy: Paris, France, 1880–1887. [Google Scholar]
- Pfenninger, M.; Véla, E.; Jesse, R.; Arantzazu-Elejalde, M.; Liberto, F.; Magnin, F.; Martínez-Ortí, A. Temporal speciation pattern in the western Mediterranean genus Tudorella P. Fischer, 1885 (Gastropoda, Pomatiidae) supports the Tyrrhenian vicariance hypothesis. Mol. Phylogenet. Evol. 2010, 54, 427–436. [Google Scholar] [CrossRef]
- Bourguignat, J.-R. Prodrome de la malacologie terrestre et fluviatile de la Tunisie. In Exploration scientifique de la Tunisie, Publiée Sous les Auspices du Ministère de l’Instruction Publique; Imprimerie Nationale: Paris, France, 1887; p. 166. [Google Scholar]
- Razkin, O.; Gómez-Moliner, B.J.; Prieto, C.E.; Martínez-Ortí, A.; Arrébola, J.R.; Muñoz, B.; Chueca, L.J.; Madeira, M.J. Molecular phylogeny of the western Palaearctic Helicoidea (Gastropoda, Stylommatophora). Mol. Phylogenet. Evol. 2015, 83, 99–117. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae Per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Tomus I. Editio decima, reformata; L. Salvii: Holmiae, Sweden, 1758; Volume 1, p. 824. [Google Scholar]
- Held, F. Notizen über die Weichthiere Bayerns; Isis (Oken); Leipzig, Germany, 1837–1838; 1837, 30, 303–309; 1838, 30, 901–919. Available online: https://biodiversitylibrary.org/page/27411580 (accessed on 1 June 2025).
- Bouaziz-Yahiatene, H.; Pfarrer, B.; Medjdoub-Bensaad, F.; Neubert, E. Revision of Massylaea Möllendorff, 1898 (Stylommatophora, Helicidae). ZooKeys 2017, 694, 109–133. [Google Scholar] [CrossRef]
- Ezzine, I.K.; Pfarrer, B.; Dimassi, N.; Said, K.; Neubert, E. At home at least: The taxonomic position of some north African Xerocrassa species (Pulmonata, Geomitridae). ZooKeys 2017, 712, 1–27. [Google Scholar] [CrossRef][Green Version]
- Ezzine, I.K.; Dimassi, N.; Pfarrer, B.; Said, K.; Neubert, E. New records of the endemic Sicilian land snail species Marmorana (Murella) muralis (O. F. Müller, 1774) from the north of Tunisia (Pulmonata, Gastropoda). ZooKeys 2018, 775, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Sadouk, G.; Ramdini, R.; Medjdoub-Bensaad, F.; Bouaziz-Yahiatene, H. Diversity and ecology of terrestrial gastropods of the Kabylia region (northernAlgeria). Ekológia 2023, 42, 248–256. [Google Scholar] [CrossRef]
- von Ihering, V. System und Verbreitung der Heliciden—Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wien. Holzhausen Wien 1909, 59, 420–455. [Google Scholar]
- Kobelt, W. Catalog der im Europäischen Faunengebiet Lebenden Binnenconchylien; Mit besonderer Berücksichtigung der in Rossmässler’s Sammlung enthaltenen Arten; TH. Fischer: Cassel, Germany, 1871; Volume 1–16, pp. 1–150. [Google Scholar]
- Chueca, L.J.; Gómez-Moliner, B.J.; Madeira, M.J.; Pfenninger, M. Molecular phylogeny of Candidula (Geomitridae) land snails inferred from mitochondrial and nuclear markers reveals the polyphyly of the genus. Mol. Phylogenet. Evol. 2018, 118, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Monterosato, T.A. di. Molluschi terrestri delle isole adiacenti alla Sicilia. Atti della Reale Accademia di Scienze, Lettere e Belle Arti di Palermo. 1892, 2, 1–33. [Google Scholar]
- Kobelt, W.; Rossmässler, E.A. Iconographie der Land-Süßwasser-Mollusken mit vorzüglicher Berücksichtigung der europäischen noch nicht abgebildeten Arten. 1892–1893: 1892, (2) 6 (1/2), 1–48; 1892, (2) 6 (3/4), 49–80; 1892, 161–170; 1893, (2) 6 (5/6), 81–102, 171–180.
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Palumbi, S.; Martin, A.; Romano, S.; McMillan, W.; Stine, O.; Grabowski, G. The Simple Fool’s Guide to PCR, 2nd ed.; University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
- Wade, C.M.; Mordan, P.B. Evolution within the gastropod molluscs; using the ribosomal RNA gene-cluster as an indicator of phylogenetic relationships. J. Molluscan Stud. 2000, 66, 565–570. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 7, 1–7. [Google Scholar] [CrossRef]
- Xia, X.; Lemey, P. Assessing substitution saturation with DAMBE. In The Phylogenetic Handbook; Lemey, P., Salemi, M., Vandamme, A.M., Eds.; Cambridge University Press: Cambridge, CA, USA, 2009; pp. 615–630. [Google Scholar] [CrossRef]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Xia, X. DAMBE6: New tools for microbial genomics, phylogenetics, and molecular evolution. J. Hered. 2017, 108, 431–437. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J. Tracer v.1.5. 2007. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 31 July 2025).
- Rambaut, A. Fig. Tree: V 1.4.0. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 July 2025).
- Jörger, K.M.; Schrödl, M. How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. Zool. 2013, 10, 59. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP: Phylogenetic Analysis Using Parsimony (and Other Methods); Version 4.0 Beta 10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Kluge, A.G.; Farris, J.S. Quantitative Phyletics and the Evolution of Anurans. Syst. Zool. 1969, 18, 1–32. [Google Scholar] [CrossRef]
- Farris, J.S. The Retention Index and the Rescaled Consistency Index. Cladistics 1989, 5, 417–419. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open-Source Geospatial Foundation. 2020. Available online: http://qgis.org (accessed on 1 July 2025).
- Ortiz de Zárate, Y.; López, A. Observaciones anatómicas y posición sistemática de varios helícidos españoles. III. Bol. R. Soc. Esp. Hist. Nat. Bil. 1950, 48, 21–85. [Google Scholar]
- Servain, G. Etude sur les Mollusques Recueillis en Espagne et au Portugal; Saint-Germain, Imprimerie D. Bardin: France, 1880; pp. 1–172. Available online: https://www.biodiversitylibrary.org/page/46056557 (accessed on 30 June 2025).
- Mousson, A. Révision de la faune malacologique des Canaries. Neue Denkschr. Schweiz. Naturf. Ges. 1872, 25, 1–176. [Google Scholar]
- Calcara, P. Cenno sui molluschi viventi e fossili della Sicilia da servire da supplimento ed insieme di critiche osservazioni all’opera di R.A. Philippi 1845, 1–66, Tavola 1–4. [Google Scholar]
- Paulucci, M. Contributo alla fauna malacologica italiana. Specie raccolte dal Dott. G. Cavanna negli anni 1878, 1879, 1880, cpon elenco delle conchiglie Abruzzesi e descrizione di due nuove Succinea. Bull. Della Soc. Malacol. Ital. 1881, 7, 69–180. [Google Scholar]
- Alzona, C.; Alzona Bisacchi, J. Malacofauna italica. Genova. (Quinto al Mare), 1936–1940: 1936, I., Xx-xx; 1938, 93–128; 1939, 29–152; 1940, 153–170.
- Kobelt, W. Diagnosen neuer Arten. Jahrb. der Deutsch. Malakozool. Ges. 1882, 9, 68–72. [Google Scholar]
- Müller, O.F. Vermivm terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum, et testaceorum, non marinorum. In Succincta historia; Voluminis Imi pars altera. index [1–8]; Havniæ & Lipsiæ. (Heineck & Faber): 1774, 14–60.
- Schlüter, F. Kurzgefasstes Systematisches Verzeichniss Meiner Conchyliensammlung Nebst Andeutung Aller bis Jetzt von mir bei Halle Gefundenen Land-und Flussconchylien; Gebauersche Buchdruckerei: Halle, Germany, 1838; vii+40p, Available online: http://biodiversitylibrary.org/page/11544080 (accessed on 1 June 2025).
- Terver, A.P. Catalogue des Mollusques Terrestres et Fluviatiles, Observés Dans les Possessions Françaises au Nord de L’Afrique, Savy: Lyon, France, 1839; 1–39.
- Schileyko, A. Treatise on recent terrestrial pulmonate molluscs, part 13. Helicidae, Pleurodontidae, Polygyridae, Ammonitellidae, Oreohelicidae, Thysanophoridae. Ruthenica 2006, 13, 1765–1906. [Google Scholar]
- Schileyko, A.A. Taxonomic status, phylogenetic relations and system of the Helicoidea sensu lato (Pulmonata). Arch. Für Molluskenkd. Band 1991, 126, 187–236. [Google Scholar] [CrossRef]
- Manganelli, G.; Giusti, F. Notulae Malacologicae, XXXVIII. A new Hygromiidae from the Italian Apennines and notes on the genus Cernuella and related taxa (Pulmonata: Helicoidea). Boll. Malacol. Milano. 1987, 23, 327–380. [Google Scholar]
- Schileyko, A.A. Treatise on recent terrestrial pulmonate molluscs, part 14. Helicodontidae, Ciliellidae, Hygromiidae. Ruthenica 2006, 13 (Suppl. 2), 1907–2047. [Google Scholar]
- Fiorentino, V.; Manganelli, G.; Giusti, F.; Ketmaier, V. Recent expansion and relic survival: Phylogeography of the land snail genus Helix (Mollusca, Gastropoda) from south to north Europe. Mol. Phyl. Evol. 2016, 98, 358–372, ISSN 1055–7903. [Google Scholar] [CrossRef] [PubMed]
- Berrilli, E.; Biondi, M.; Garzia, M.; D’Alessandro, P.; Salvi, D. Apennine–Pyrenees disjunct distribution: An unusual biogeographic pattern revealed in flea beetles of the Longitarsus candidulus species-group (Coleoptera, Chrysomelidae). Curr. Zool. 2024, 70, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Beck, H. Index Molluscorum Praesentis aevi Musei Principis Augustissimi Christiani Frederici. Hafniae, 1837, 124; 1838, 1–100; 1837, 101–124; Appendix: 1–8. Available online: https://biodiversitylibrary.org/page/32058074 (accessed on 1 June 2025).
- Hausdorf, B. Über zwei Candidula-Arten von der südlichen Balkanhalbinsel (Gastropoda: Hygromiidae). Arch. Molluskenkd. 1991, 120, 119–129. [Google Scholar] [CrossRef]
- Wagner, A.J. Studien zur Molluskenfauna der Balkanhalbinsel mit besonderer Berücksichtigung Bulgariens und Thraziens, nebst monographischer Bearbeitung einzelner Gruppen—Prace Zoologiczne Polskiego Panstwowego Muzeum Przyrod-niczego. Ann. Zool. Mus. Polon. Hist. Nat. 1928, 6, 263–399. [Google Scholar]
- Soós, L. Csiki Ernö állattani kutatásai Albániabán. XIII. Puhates tüek. Explorationes zoologicae ab E. Csiki in Albania peractae. XIII. Mollusca. A Magyar Tudományos Akadémia Balkán-kutatásainak Tudományos Eredményei. I. Kötet 1924, 13, 177–197. [Google Scholar]








| Marker | Primer Name | Primer Sequence |
|---|---|---|
| COI [17] | LCO1490 | 5′-GGTCAACAAATCATAAAGATATTGG-3′ |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | |
| 16S [18] | 16S F | 5′-CGGCCGCCTGTTTATCAAAAACAT-3′ |
| 16S R | 5′-GGAGCTCCGGTTTGAACTCAGATC-3′ | |
| ITS2 [19] | LSU1 | 5′-CTAGCTGCGAGAATTAATGTGA-3′ |
| LSU3 | 5′-ACTTTCCCTCACGGTACTTG-3′ | |
| 28S [19] | LSU-2 | 5′-GGGTTGTTTGGGAATGCAGC-3′ |
| LSU-4 | 5′-GTTAGACTCCTTGGTCCGTC-3′ |
| Xerogyra hallgassi n. sp. | Xerogyra fiorii | Xerogyra spadae | Xerogyra grovesiana | Kherattolactea keratae | |
|---|---|---|---|---|---|
| Number of whorls | 5 ½ ± ½ (n = 11) | 5 ¼ ± ⅓ (n = 18) | 5 ⅓ ± ⅓ (n = 21) | 4 ¾ ± ½ (n = 15) | 5 ⅓ ± ⅓ (n = 6) |
| Average diameter | 16.8 ± 0.3 (n = 11) | 11.8 ± 0.4 (n = 18) | 9.2 ± 0.1 (n = 21) | 8.9 ± 0.2 (n = 15) | 18.5 ± 0.4 (n =4) |
| D/H | 2 (n = 11) | 1.8 (n = 18) | 1.5 (n = 21) | 2.2 (n = 15) | 1.8 (n = 4) |
| Shell’s surface | radial fine growth striae | radial fine growth striae | radial fine growth striae | radial ribs | radial fine growth striae |
| Bands along last whorl | none | very weak but visible | very weak but visible | None | weak to strong |
| Last whorl | not keeled | not keeled | not keeled | Keeled | not keeled |
| Umbilicus shape | remarkably eccentric | slightly eccentric | concentric | slightly eccentric | concentric |
| D/U | >⅓ | ⅓ | ¼ | ¼ | ⅙ |
| Aperture’s shape | broadly oval | roundish | roundish | subquadrangular | broadly oval |
| Aperture’s internal callous | very mild to absent | mild | strong | absent | mild to strong |
| Flagellum/epiphallus | 0.74 (n = 4) | 0.72 (n = 3) | 0.91 (n = 3) | 2.8 (n = 3) | 0.8 (n = 2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezzine, I.K.; Bouaziz-Yahiatene, H.; De Mattia, W.; Neubert, E. New Insights in the Helicellini Ihering, 1909 with Description of Kherattolactea gen. nov. and the First Record of Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 from Algeria. Diversity 2025, 17, 550. https://doi.org/10.3390/d17080550
Ezzine IK, Bouaziz-Yahiatene H, De Mattia W, Neubert E. New Insights in the Helicellini Ihering, 1909 with Description of Kherattolactea gen. nov. and the First Record of Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 from Algeria. Diversity. 2025; 17(8):550. https://doi.org/10.3390/d17080550
Chicago/Turabian StyleEzzine, Issaad Kawther, Houria Bouaziz-Yahiatene, Willy De Mattia, and Eike Neubert. 2025. "New Insights in the Helicellini Ihering, 1909 with Description of Kherattolactea gen. nov. and the First Record of Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 from Algeria" Diversity 17, no. 8: 550. https://doi.org/10.3390/d17080550
APA StyleEzzine, I. K., Bouaziz-Yahiatene, H., De Mattia, W., & Neubert, E. (2025). New Insights in the Helicellini Ihering, 1909 with Description of Kherattolactea gen. nov. and the First Record of Orexana Chueca, Gómez-Moliner, Madeira & Pfenninger, 2018 from Algeria. Diversity, 17(8), 550. https://doi.org/10.3390/d17080550








