Remarkable Stability of Uropodina (Acari: Mesostigmata) Communities in Artificial Microhabitats: A Case Study of Bird Nest Boxes in Bory Tucholskie National Park
Abstract
1. Introduction
2. Material and Methods
Data Analysis Methods
3. Results
3.1. Comparison of the Species Composition and Abundance of Uropodina Communities
3.2. Sex Ratio and Age Structure of the Surveyed Uropodina Species
3.3. The Abundance of Mites in Relation to Nest Box Bedding Material
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mänd, R.; Tilgar, V.; Lõhmus, A.; Leivits, A. Providing nest boxes for hole-nesting birds—Does habitat matter? Biodivers. Conserv. 2005, 14, 1823–1840. [Google Scholar] [CrossRef]
- Gutowski, J.M.; Bobiec, A.; Pawlaczyk, P.; Zub, K. Drugie Życie Drzewa; WWF: Warszawa, Poland, 2004; pp. 1–245. [Google Scholar]
- Thompson, E.K.; Keenan, R.J.; Kelly, L.T. The use of nest boxes to support bird conservation in commercially managed forests: A systematic review. For. Ecol. Manag. 2023, 550, 121504. [Google Scholar] [CrossRef]
- Mänd, R.; Leivits, A.; Leivits, M.; Rodenhouse, N.L. Provision of nestboxes raises the breeding density of Great Tits Parus major equally in coniferous and deciduous woodland. Ibis 2009, 151, 487–492. [Google Scholar] [CrossRef]
- Hansell, M.H. Bird Nests and Construction Behaviour; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Hansell, M.H. Animal Architecture; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Biddle, L.E.; Dickinson, A.M.; Broughton, R.E.; Gray, L.A.; Bennett, S.L.; Goodman, A.M.; Deeming, D.C. Construction materials affect the hydrological properties of bird nests. J. Zool. 2019, 309, 161–171. [Google Scholar] [CrossRef]
- Deeming, D.C.; Campion, E. Simulated rainfall reduces the insulative properties of bird nests. Acta. Ornithol. 2018, 53, 91–97. [Google Scholar] [CrossRef]
- Hilton, G.M.; Hansell, M.H.; Ruxton, G.D.; Reid, J.M.; Monaghan, P.; Brittingham, M. Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 2004, 121, 777–787. [Google Scholar] [CrossRef]
- Deeming, D.C.; Mainwaring, M.C. Functional properties of nests. In Nests, Eggs and Incubation: New Ideas About Avian Reproduction; Deeming, D.C., Reynolds, S.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 29–49. [Google Scholar]
- Boyes, D.H. Natural history of Lepidoptera associated with bird nests in Mid-Wales. Entomol. Rec. J. Var. 2018, 130, 249–259. [Google Scholar]
- Boyes, D.H.; Lewis, O.T. Ecology of Lepidoptera associated with bird nests in Mid-Wales. Ecol. Entomol. 2019, 44, 1–10. [Google Scholar] [CrossRef]
- Broughton, R.K.; Hebda, G.; Maziarz, M.; Smith, K.W.; Smith, L.; Hinsley, S.A. Nest-site competition between bumblebees (Bombidae), social wasps (Vespidae) and cavity-nesting birds in Britain and the Western Palearctic. Bird Study 2015, 62, 427–437. [Google Scholar] [CrossRef]
- Jaworski, T.; Gryz, J.; Krauze-Gryz, D.; Plewa, R.; Bystrowski, C.; Dobosz, R.; Horák, J. My home is your home: Nest boxes for birds and mammals provide habitats for diverse insect communities. Insect Conserv. Divers. 2022, 15, 461–469. [Google Scholar] [CrossRef]
- Krištofík, J.; Mašán, P.; Šustek, Z.; Nuhličková, S. Arthropods (Acarina, Coleoptera, Siphonaptera) in nests of hoopoe (Upupa epops) in Central Europe. Biologia 2013, 68, 155–161. [Google Scholar] [CrossRef]
- McComb, W.C.; Noble, R.E. Invertebrate use of natural tree cavities and vertebrate nest boxes. Am. Midl. Nat. 1982, 107, 163–172. [Google Scholar] [CrossRef]
- Nordberg, S. Biologisch-ökologische Untersuchungen über die Vogelnidicolen. Acta Zool. Fenn. 1936, 21, 1–168. [Google Scholar]
- Tajovský, K.; Mock, A.; Krumpál, M. Millipedes (Diplopoda) in birds’ nests. Eur. J. Soil Biol. 2001, 37, 321–323. [Google Scholar] [CrossRef]
- Turienzo, P.; Di Iorio, O.; Mahnert, V. Global checklist of pseudoscorpions (Arachnida) found in birds’ nests. Rev. Suisse Zool. 2010, 117, 557–598. [Google Scholar]
- Woodroffe, G.E. An ecological study of the insects and mites in the nests of certain birds in Britain. Bull. Entomol. Res. 1953, 44, 739–772. [Google Scholar] [CrossRef]
- Pilskog, H.E.; Solhøy, T.; Gwiazdowicz, D.J.; Grytnes, J.-A.; Coulson, S.J. Invertebrate Communities Inhabiting Nests of Migrating Passerine, Wild Fowl and Sea Birds Breeding in the High Arctic, Svalbard. Polar Biol. 2014, 37, 981–998. [Google Scholar] [CrossRef]
- Bajerlein, D.; Błoszyk, J.; Gwiazdowicz, D.J.; Ptaszyk, J.; Halliday, B. Community structure and dispersal of mites (Acari, Mesostigmata) in nests of the white stork (Ciconia ciconia). Biologia 2006, 61, 525–530. [Google Scholar] [CrossRef]
- Błoszyk, J.; Gwiazdowicz, D.J.; Kupczyk, M.; Książkiewicz-Parulska, Z. Parasitic mesostigmatid mites (Acari)—Common inhabitants of the nest boxes of starlings (Sturnus vulgaris) in a Polish urban habitat. Biologia 2016, 71, 1034–1037. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J.; Matysiak, K. Roztocze (Acari, Mesostigmata) wybranych mikrośrodowisk Parku Narodowego “Bory Tucholskie”. Acta Sci. Pol. Silvarum 2004, 3, 17–24. [Google Scholar]
- Gwiazdowicz, D.J.; Błoszyk, J.; Bajerlein, D.; Halliday, R.B.; Mizera, T. Mites (Acari: Mesostigmata) inhabiting nests of the white-tailed sea eagle Haliaeetus albicilla (L.) in Poland. Entomol. Fenn. 2006, 17, 366–372. [Google Scholar] [CrossRef]
- Mašán, P.; Fenda, P.; Krištofík, J.; Halliday, B. A review of the ectoparasitic mites (Acari: Dermanyssoidea) associated with birds and their nests in Slovakia, with notes on identification of some species. Zootaxa 2014, 3893, 77–100. [Google Scholar] [CrossRef]
- Solarz, K.; Szilman, P.; Szilman, E. Allergenic mites associated with bird nests in Poland (Astigmata: Pyroglyphidae, Acaridae, Glycyphagidae). In Ecology and Evolution of the Acari; Series Entomologica; Bruin, J., van der Geest, L.P.S., Sabelis, M.W., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 651–656. [Google Scholar]
- Roy, L.; Bouvier, J.C.; Lavigne, C.; Galès, M.; Buronfosse, T. Impact of pest control strategies on the arthropodofauna living in bird nests built in nestboxes in pear and apple orchards. Bull. Entomol. Res. 2013, 103, 458–465. [Google Scholar] [CrossRef]
- Błoszyk, J.; Bajerlein, D.; Gwiazdowicz, D.J.; Halliday, R.B.; Dylewska, M. Uropodine mite communities (Acari: Mesostigmata) in birds’ nests in Poland. Belg. J. Zool. 2006, 136, 145–153. [Google Scholar]
- Błoszyk, J.; Hebda, G.; Adamski, Z.; Zacharyasiewicz, M. Redescription of Chiropturopoda nidiphila Wiśniewski & Hirschmann (Acari: Uropodina) from a woodpecker’s tree holes, including all development stages and first notes on its ecology. Syst. Appl. Acarol. 2021, 26, 1867–1899. [Google Scholar] [CrossRef]
- Błoszyk, J.; Olszanowski, Z. Materiały do znajomości fauny roztoczy gniazd i budek lęgowych ptaków. II. Różnice w liczebności i składzie gatunkowym populacji Uropodina (Acari, Anactinotrichida) budek lęgowych na Mierzei Wiślanej na podstawie dwuletnich obserwacji. Przegląd. Zool. 1986, 30, 63–66. [Google Scholar]
- Błoszyk, J.; Wendzonka, J.; Kulczak, M.; Lubińska, K.; Napierała, A. Bird nesting boxes as a specific artificial microenvironment increasing biodiversity of mites from the suborder Uropodina (Acari: Mesostigmata): A case study of Bory Tucholskie National Park. Exp. Appl. Acarol. 2024, 93, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, R.; Indykiewicz, P.; Olszewski, A.; Tobółka, M. Mites Living in the Nests of the White Stork and Black Stork in Microhabitats of the Forest Environment and Agrocenoses. Animals 2023, 13, 3189. [Google Scholar] [CrossRef]
- Kamczyc, J.; Teodorowicz, E.; Gwiazdowicz, D.J. Mites (Acari, Mesostigmata) in the Red-Backed Shrike Lanius collurio and Great Grey Shrike Lanius excubitor Nests. Acta Sci. Pol. Silv. Colendar. Rat. Ind. Lignaria 2011, 10, 37–42. [Google Scholar]
- Mašán, P.; Krištofík, J. Mites and ticks (Acarina: Mesostigmata et Ixodida) from the nests of Riparia riparia L. in South Slovakia. Biologia 1993, 48, 155–162. [Google Scholar]
- Mašán, P.; Krištofík, J. Mesostigmatid mites (Acarina, Mesostigmata) in the nests of penduline tit (Remiz pendulinus). Biologia 1995, 50, 481–485. [Google Scholar]
- Napierała, A.; Maziarz, M.; Hebda, G.; Broughton, R.K.; Rutkowski, T.; Zacharyasiewicz, M.; Błoszyk, J. Lack of specialist nidicoles as a characteristic of mite assemblages inhabiting nests of the ground-nesting wood warbler, Phylloscopus sibilatrix (Aves: Passeriformes). Exp. Appl. Acarol. 2021, 84, 149–170. [Google Scholar] [CrossRef]
- Błoszyk, J.; Rutkowski, T.; Wojtaszyn, G.; Książkiewicz-Parulska, Z.; Zacharyasiewicz, M.; Napierała, A. Leiodinychus orbicularis (C.L. Koch, 1839) in bat boxes in Poland. Eur. J. Biol. Res. 2020, 10, 150–155. [Google Scholar] [CrossRef]
- Cramp, S.; Brooks, D.J. Handbook of the Birds of Europe, the Middle East and North Africa. In The Birds of the Western Palearctic, Vol. VI: Warblers; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Karg, W. Acari (Acarina) Milben, Unterordnung Parasitiformes (Anactinochaeta). Uropodina Kramer, Schildkrötenmilben. In Die Tierwelt Deutschlands und der Angrenzenden Meeresteile; Senglaub, K., Hannemann, H.J., Schumann, H., Eds.; Jena: Thuringia, Germany, 1989; pp. 1–203. [Google Scholar]
- Błoszyk, J. Geograficzne i Ekologiczne Zróżnicowanie Zgrupowań Roztoczy z Kohoryty Uropodina (Acari: Mesostigmata) W Polsce: Uropodina Lasów Grądowych (Carpinion Betuli); Kontekst: Poznań, Poland, 1999; pp. 1–245. [Google Scholar]
- Mašán, P. Mites of the Cohort Uropodina (Acarina, Mesostigmata) in Slovenska. Annot. Zool. Bot. 2001, 223, 1–320. [Google Scholar]
- Napierała, A.; Mądra, A.; Leszczyńska-Deja, K.; Gwiazdowicz, D.J.; Gołdyn, B.; Błoszyk, J. Community structure variability of Uropodina mites (Acari: Mesostigmata) in nests of the common mole, Talpa europaea, in Central Europe. Exp. Appl. Acarol. 2016, 68, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Botero-Delgadillo, E.; Orellana, N.; Serrano, D.; Poblete, Y.; Vásquez, R.A. Interpopulation variation in nest architecture in a secondary cavity-nesting bird suggests site-specific strategies to cope with heat loss and humidity. Auk 2017, 134, 281–294. [Google Scholar] [CrossRef]
- Slagsvold, T. Experiments on clutch size and nest size in passerine birds. Oecologia 1989, 80, 297–302. [Google Scholar] [CrossRef]
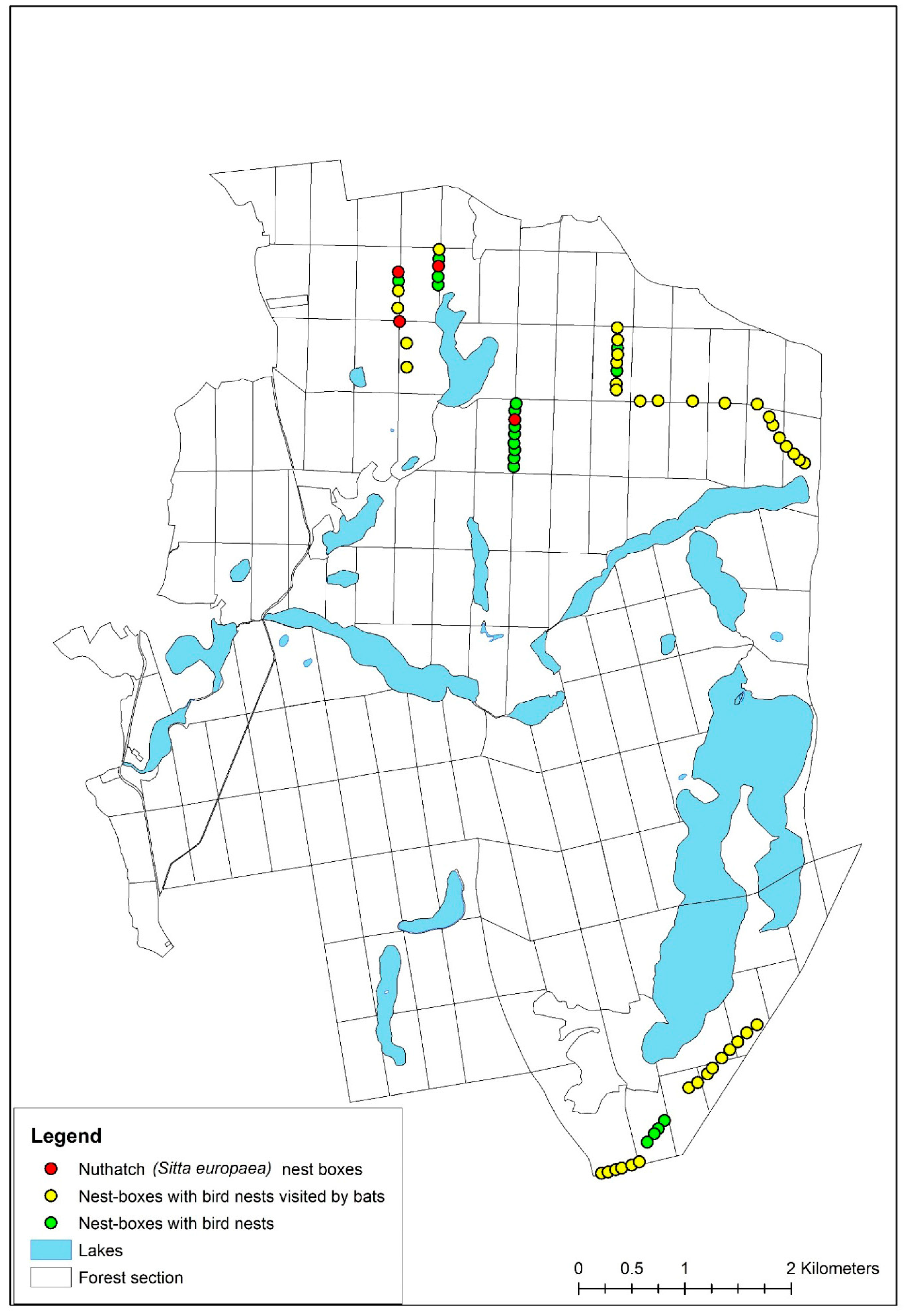
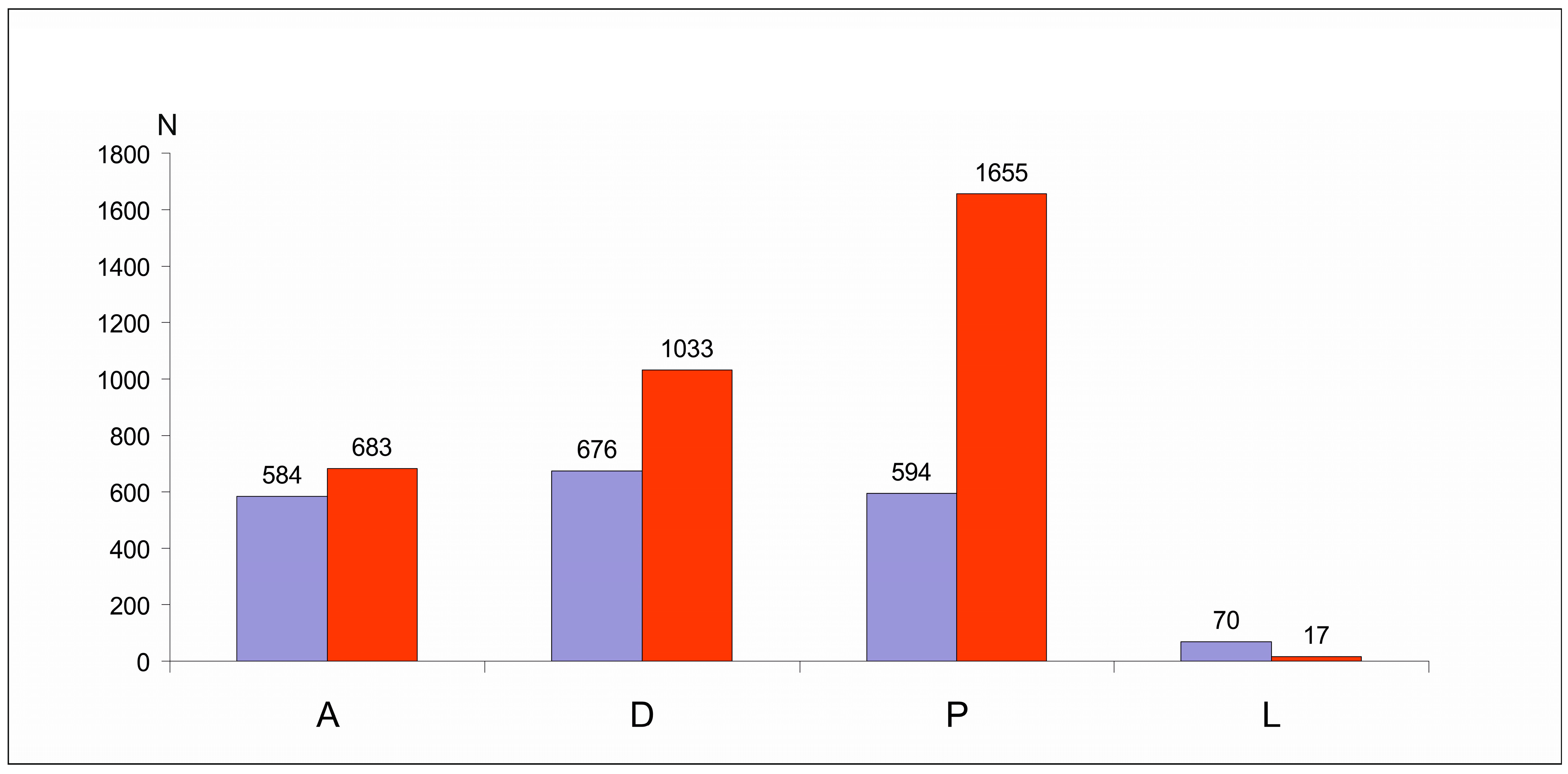
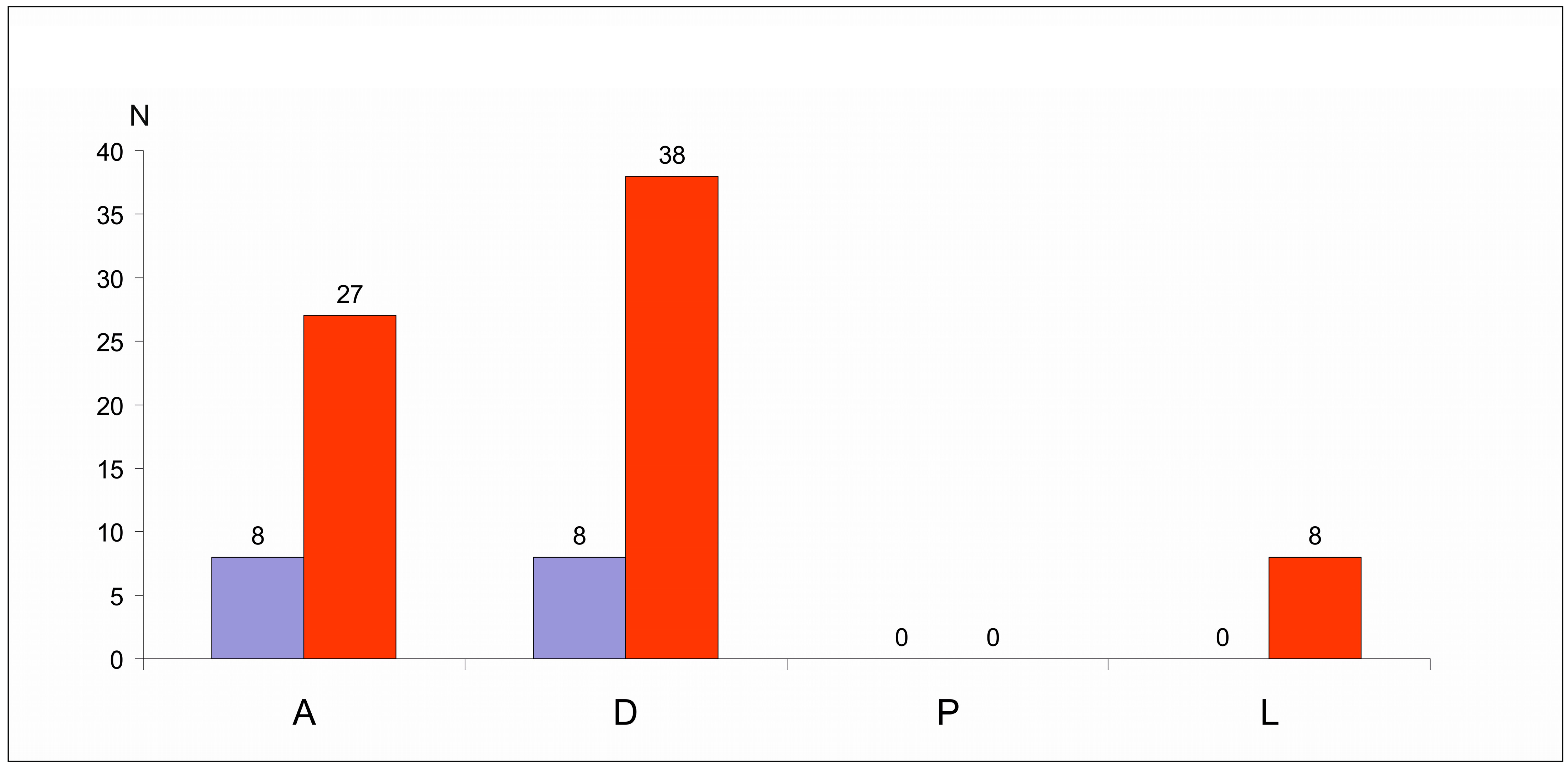
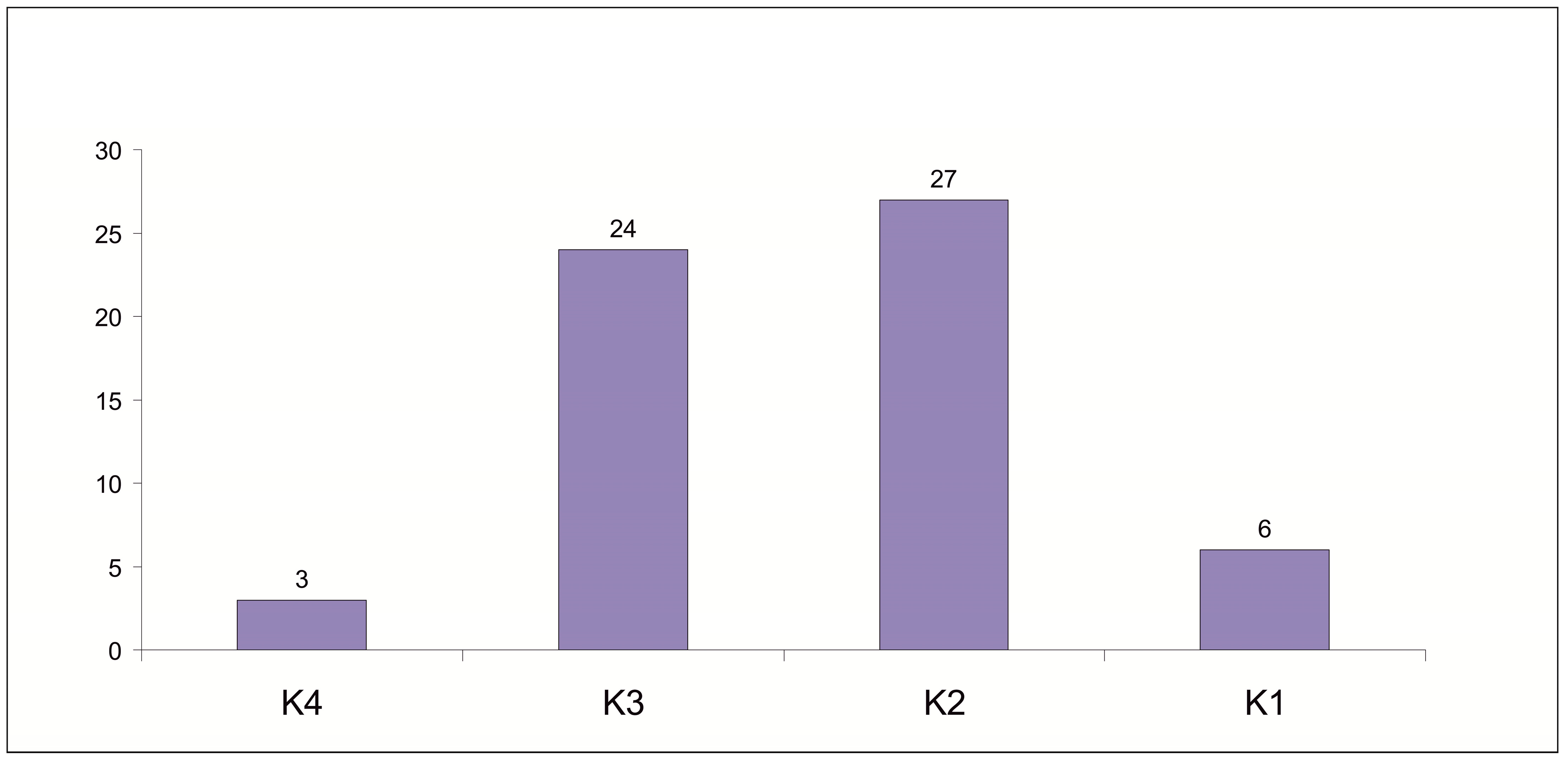
| Species | N | D% | F% | Ave ± SD |
|---|---|---|---|---|
| 2023 | ||||
| Leiodinychus orbicularis (C.L. Koch, 1839) | 1924 | 99.2 | 44.2 | 56.6 ± 183.8 |
| Chiropturopoda nidiphila Wisniewski & Hirschmann, 1993 | 16 | 0.8 | 7.8 | 2.7 ± 2.7 |
| 2024 | ||||
| L. orbicularis | 3388 | 97.9 | 51.7 | 109.3 ± 303.6 |
| Ch. nidiphila | 73 | 2.1 | 5.0 | 24.3 ± 40.4 |
| Kruskal–Wallis test value (H) | H(3, N = 274) = 36.7; p < 0.001 | |||||
| 1–2 | 1–3 | 1–4 | 2–3 | 2–4 | 3–4 | |
| Dunn’s test | ns | *** | *** | *** | *** | ns |
| Kruskal–Wallis test value (H) | H(3, N= 822) = 36.47; p < 0.001 | |||||
| 1–2 | 1–3 | 1–4 | 2–3 | 2–4 | 3–4 | |
| Dunn’s test | *** | ns | *** | *** | ns | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulczak, M.; Wendzonka, J.; Lubińska, K.; Napierała, A.; Błoszyk, J. Remarkable Stability of Uropodina (Acari: Mesostigmata) Communities in Artificial Microhabitats: A Case Study of Bird Nest Boxes in Bory Tucholskie National Park. Diversity 2025, 17, 544. https://doi.org/10.3390/d17080544
Kulczak M, Wendzonka J, Lubińska K, Napierała A, Błoszyk J. Remarkable Stability of Uropodina (Acari: Mesostigmata) Communities in Artificial Microhabitats: A Case Study of Bird Nest Boxes in Bory Tucholskie National Park. Diversity. 2025; 17(8):544. https://doi.org/10.3390/d17080544
Chicago/Turabian StyleKulczak, Marta, Jacek Wendzonka, Karolina Lubińska, Agnieszka Napierała, and Jerzy Błoszyk. 2025. "Remarkable Stability of Uropodina (Acari: Mesostigmata) Communities in Artificial Microhabitats: A Case Study of Bird Nest Boxes in Bory Tucholskie National Park" Diversity 17, no. 8: 544. https://doi.org/10.3390/d17080544
APA StyleKulczak, M., Wendzonka, J., Lubińska, K., Napierała, A., & Błoszyk, J. (2025). Remarkable Stability of Uropodina (Acari: Mesostigmata) Communities in Artificial Microhabitats: A Case Study of Bird Nest Boxes in Bory Tucholskie National Park. Diversity, 17(8), 544. https://doi.org/10.3390/d17080544








