Using Circuit Theory to Identify Important Ecological Corridors for Large Mammals Between Wildlife Refuges
Abstract
1. Introduction
2. Materials and Methods
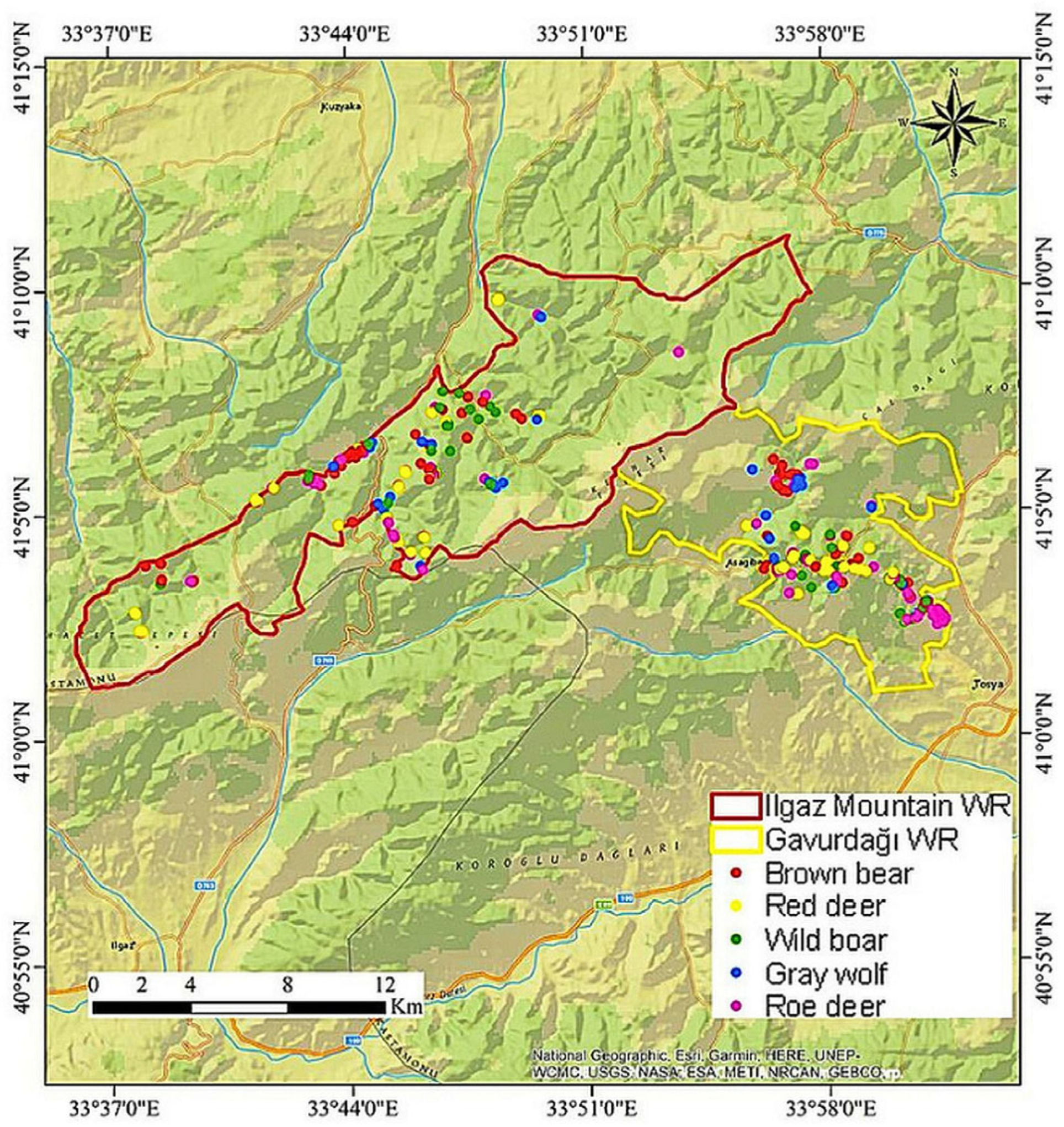
2.1. Study Area
2.2. Determination of Species
2.3. Occurrence Data
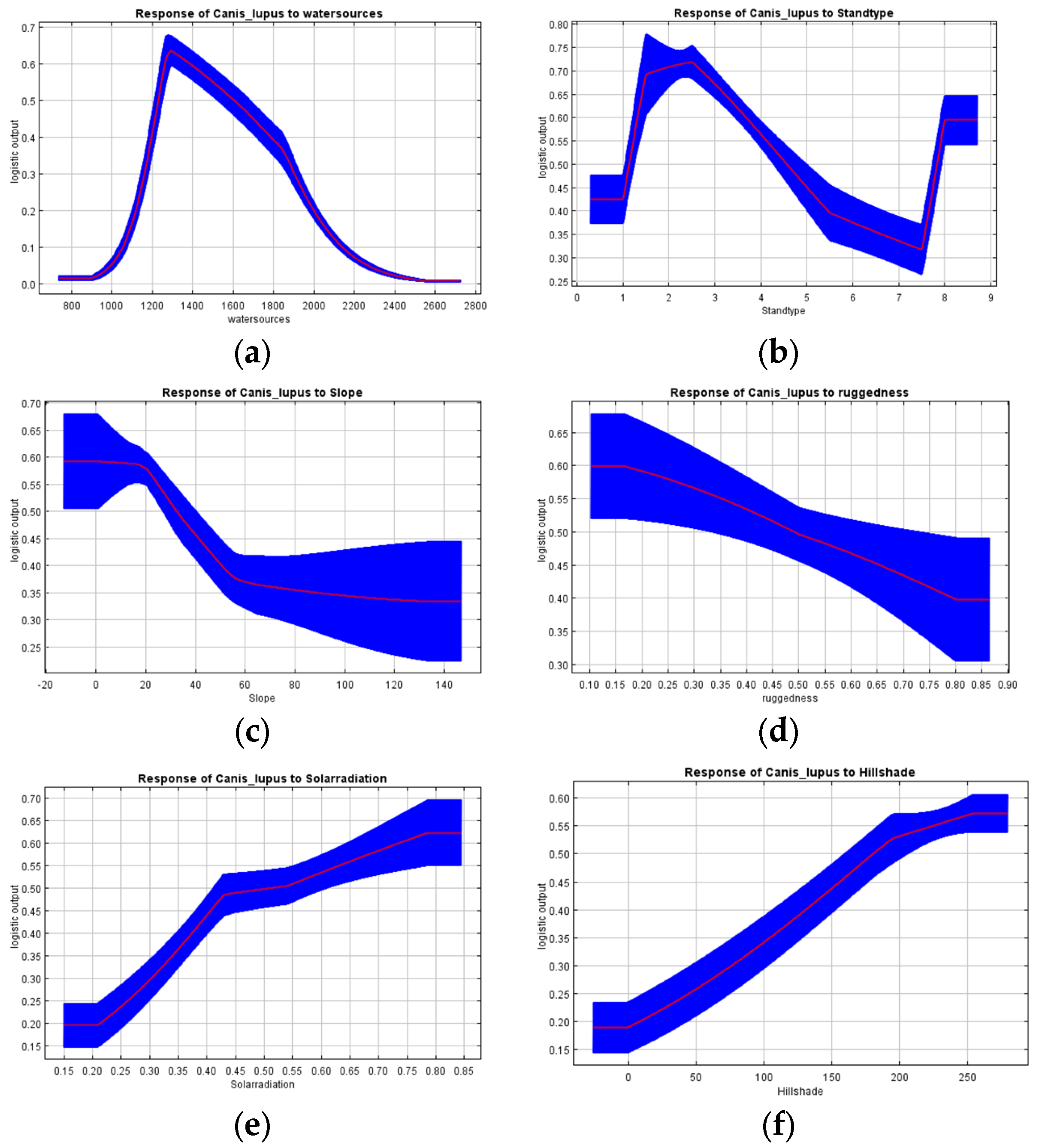
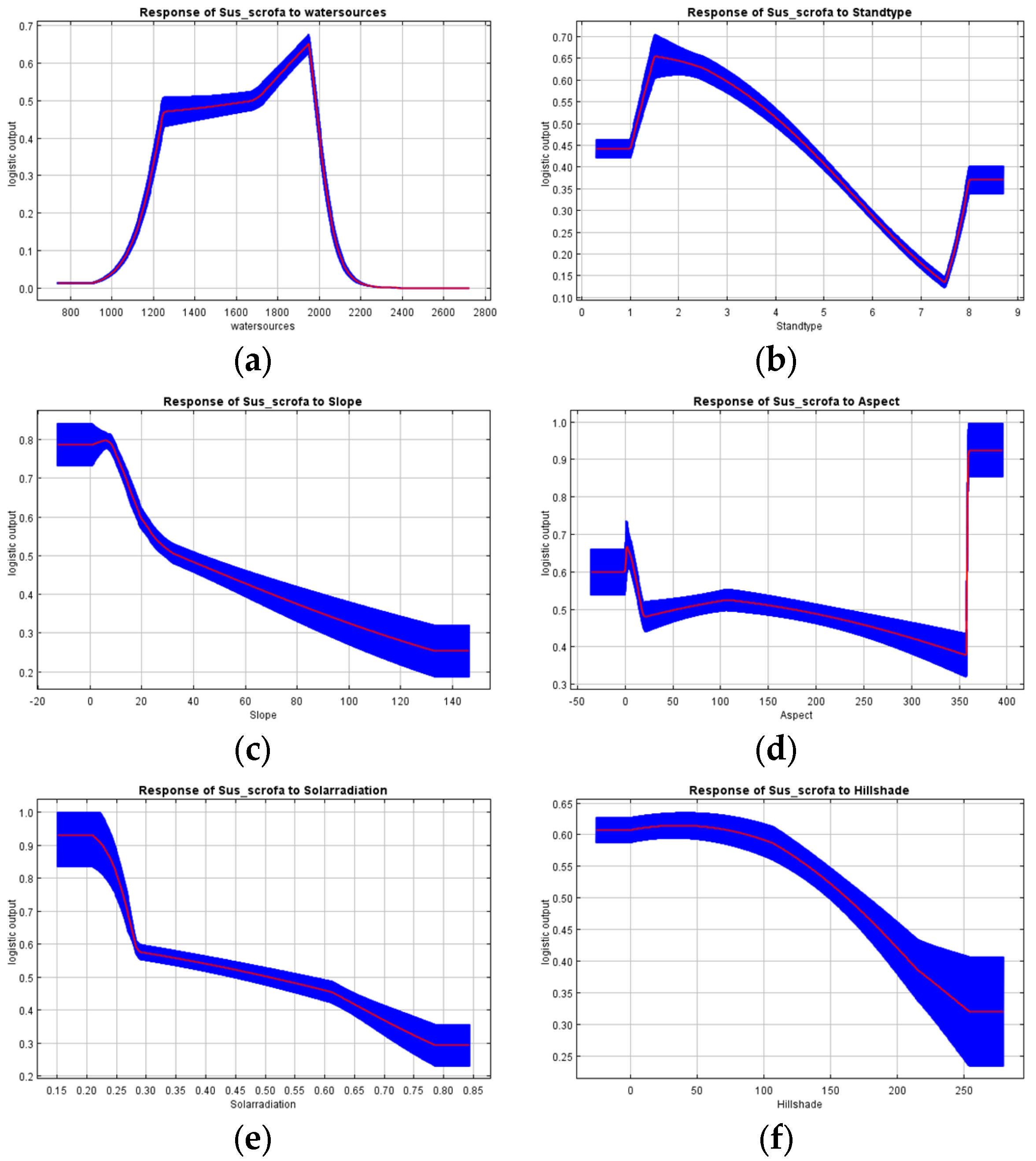
2.4. Ecological Variables
2.5. Habitat Suitability Model Development
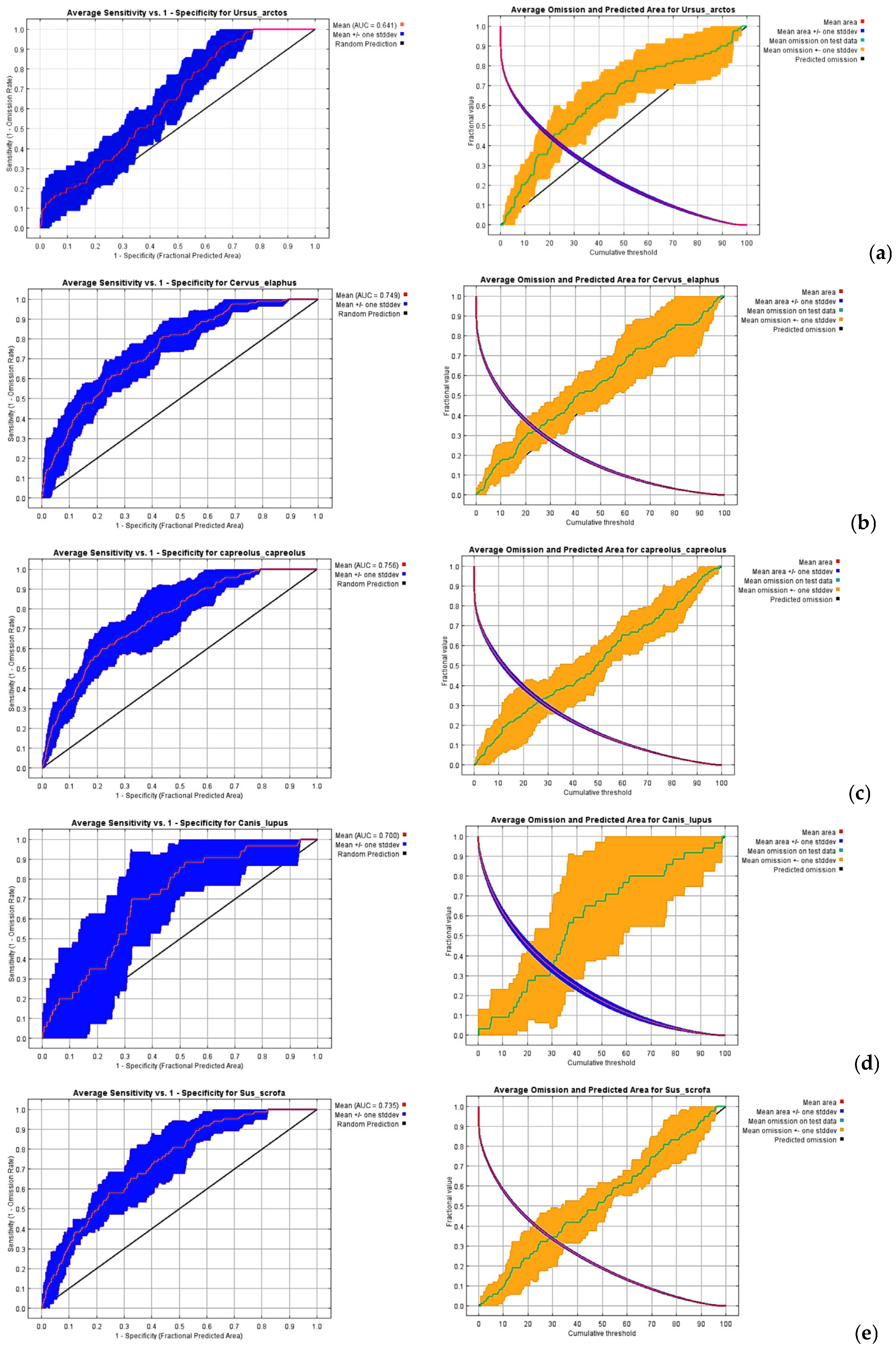
2.6. Model Validation and Analyses
2.7. Circuit Theory Method
2.8. Connectivity Model Development
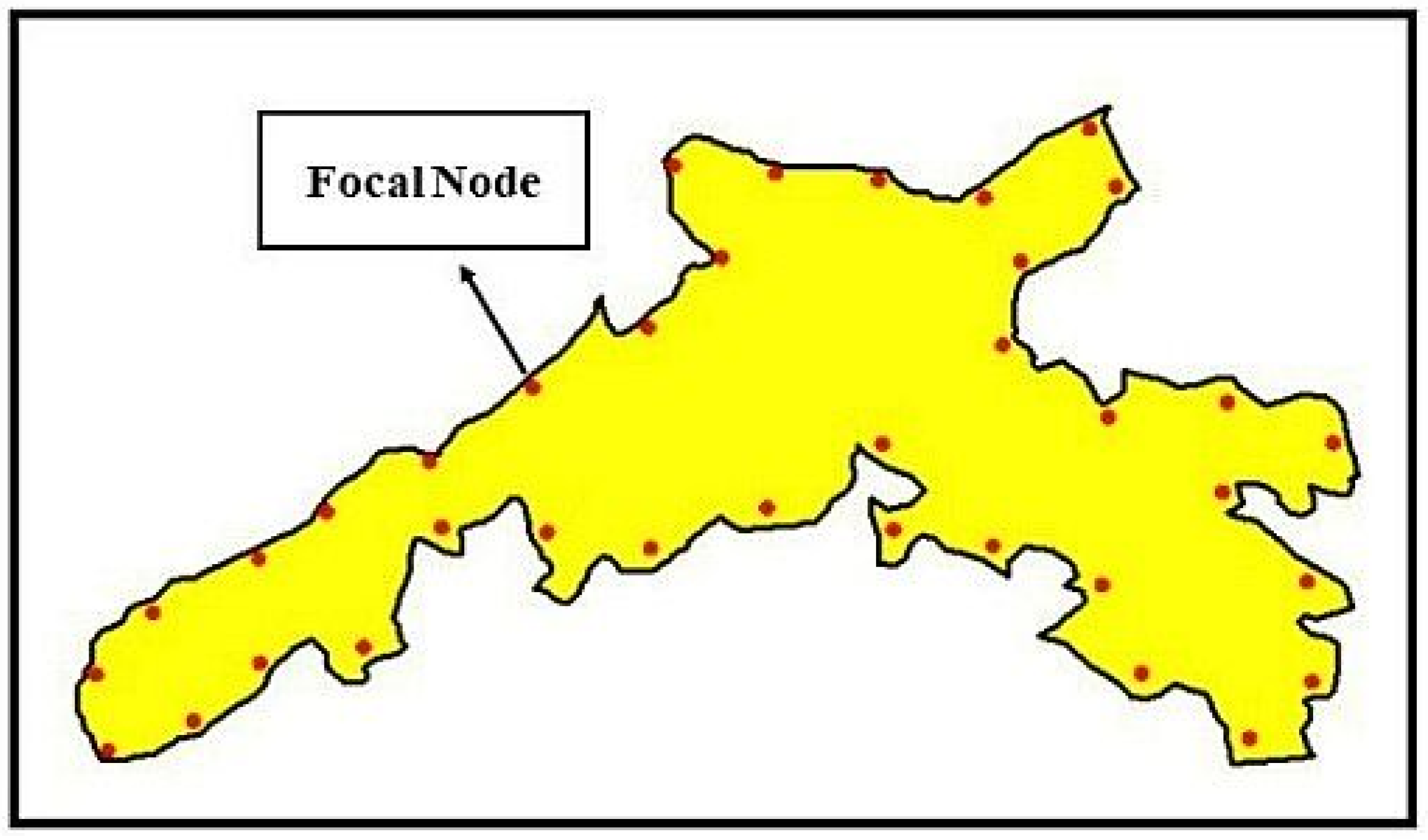
2.8.1. Determination of Focal Nodes
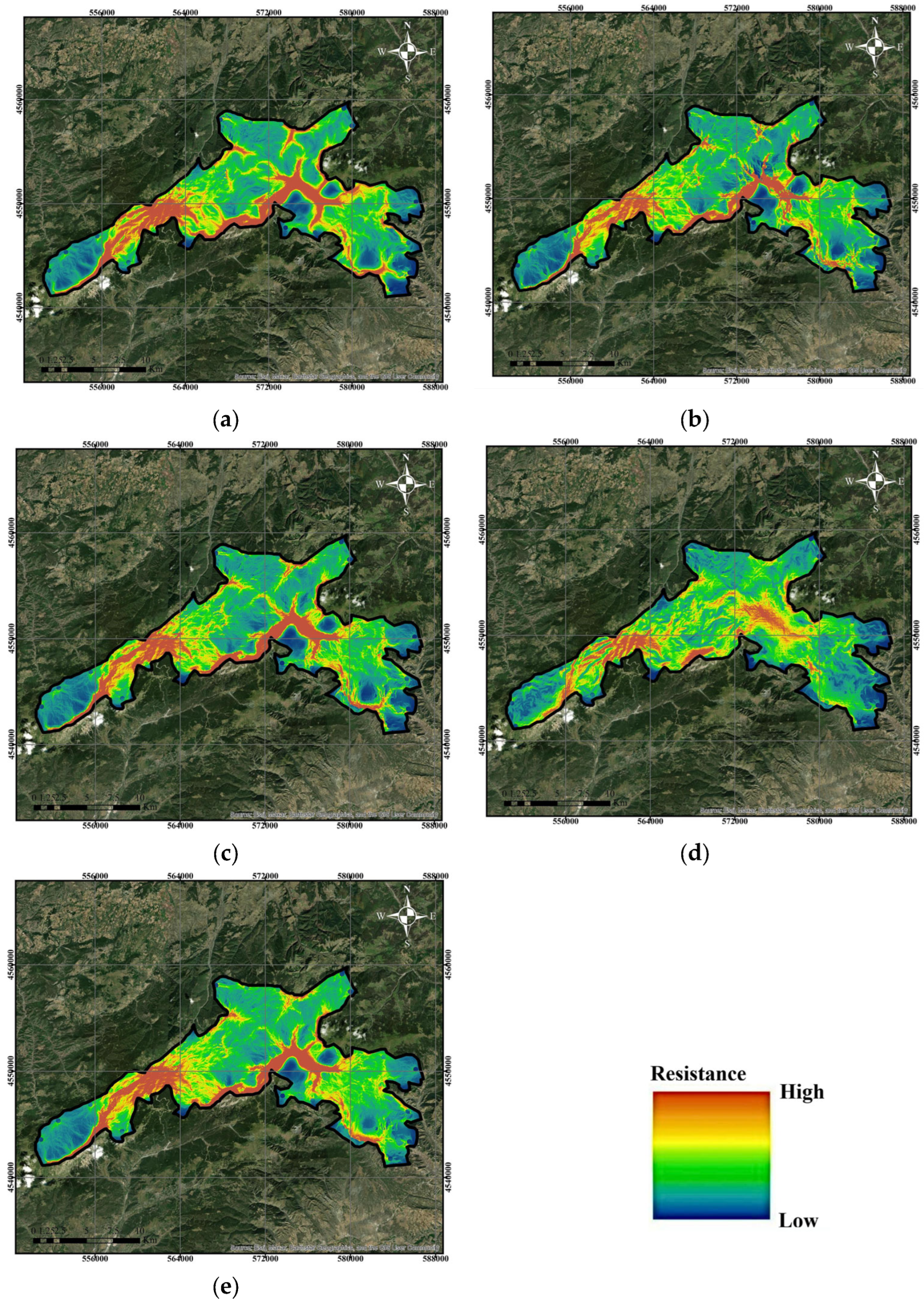
2.8.2. Determination of Resistance Surface
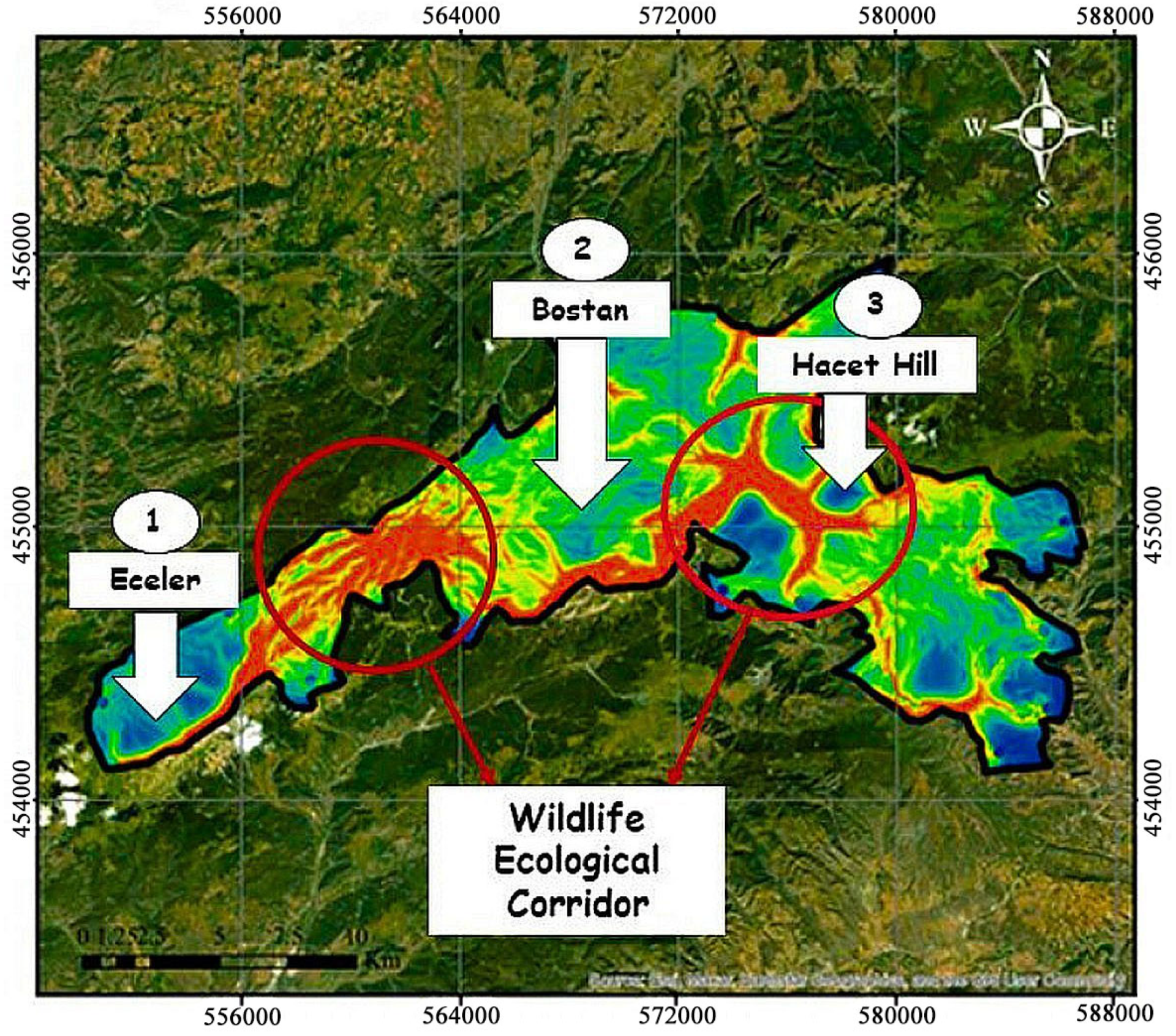
2.8.3. Determination of Pinch-Points
3. Results
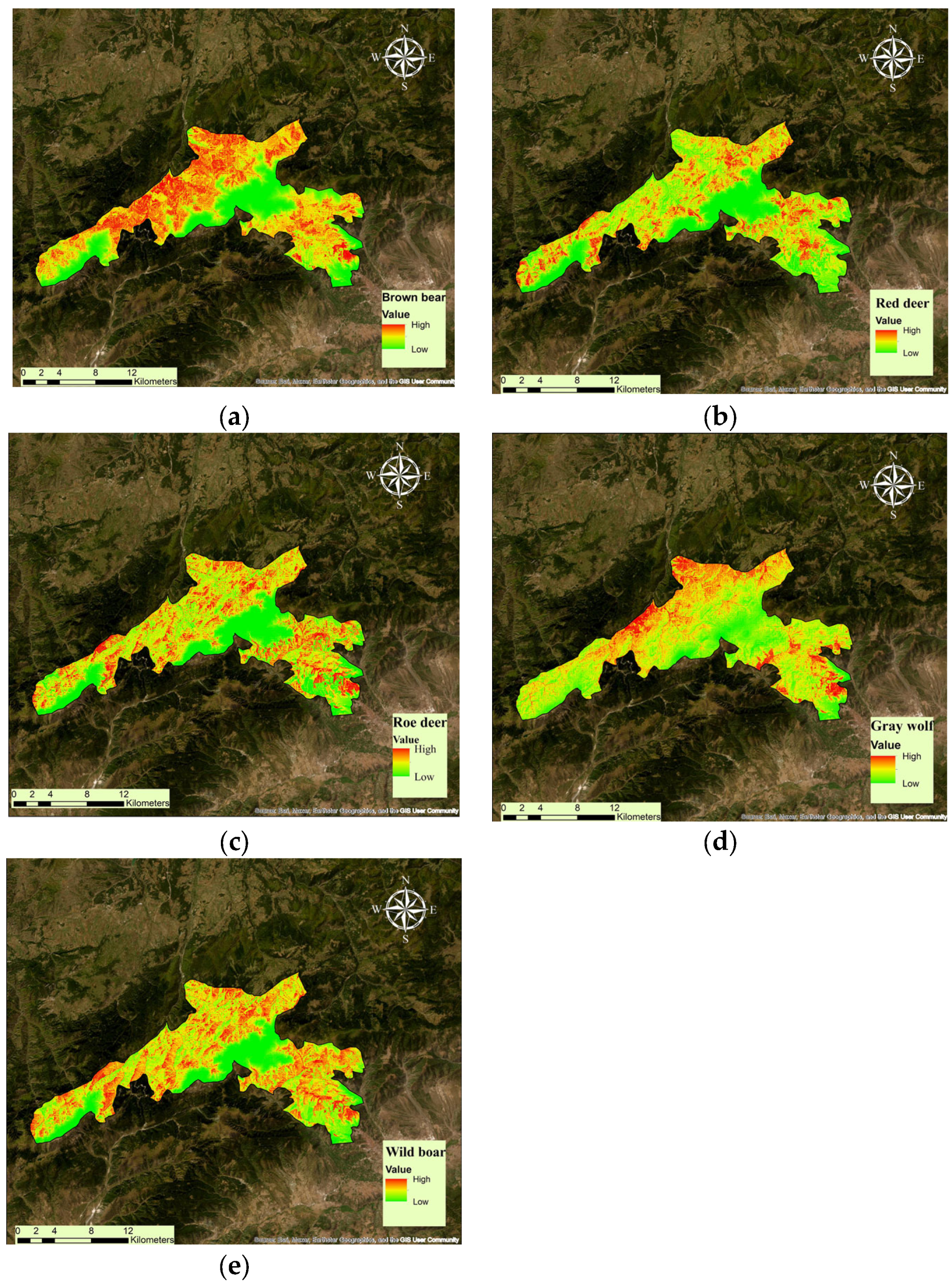
3.1. Habitat Suitability Model
3.2. Wildlife Ecological Corridor Modeling
4. Discussion
4.1. Habitat Suitability Modeling
4.2. Evaluation of Wildlife Corridors Modeling Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Aspect | Slope | Hillshade | Closeness | Standtype | Ruggedness | Solarradiation | Watersources | Road | Elevation | |
| Aspect | 1 | 0.012 | 0.516 | 0.039 | 0.028 | 0.001 | −0.013 | 0.107 | 0.019 | 0.107 |
| Slope | 0.012 | 1 | −0.131 | 0.077 | −0.106 | −0.024 | −0.014 | −0.069 | 0.054 | −0.069 |
| Hillshade | 0.516 | −0.131 | 1 | 0.165 | −0.027 | 0.003 | −0.004 | 0.141 | −0.057 | 0.141 |
| Closeness | 0.039 | 0.077 | 0.165 | 1 | −0.709 * | 0.016 | 0.007 | −0.274 | −0.309 | −0.274 |
| Standtype | 0.028 | −0.106 | −0.027 | −0.709 * | 1 | −0.003 | −0.002 | 0.348 | 0.151 | 0.348 |
| Ruggedness | 0.001 | −0.024 | 0.003 | 0.016 | −0.003 | 1 | 0.132 | 0.067 | 0.013 | 0.067 |
| Solarradiation | −0.013 | −0.014 | −0.004 | 0.007 | −0.002 | 0.1322 | 1 | 0.047 | 0.014 | 0.047 |
| Watersources | 0.107 | −0.069 | 0.141 | −0.274 | 0.348 | 0.0670 | 0.047 | 1 | 0.058 | 1.000 * |
| Road | 0.019 | 0.054 | −0.057 | −0.309 | 0.151 | 0.0130 | 0.014 | 0.058 | 1 | 0.058 |
| Elevation | 0.107 | −0.069 | 0.141 | −0.274 | 0.348 | 0.0670 | 0.047 | 1.000 * | 0.058 | 1 |




References
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.C.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Rahimi, E.; Dong, P. Identifying barriers and pinch-points of large mammal corridors in Iran. J. Environ. Stud. Sci. 2023, 13, 285–297. [Google Scholar] [CrossRef]
- Hilty, J.; Worboys, G.L.; Keeley, A.; Woodley, S.; Lausche, B.; Locke, H.; Carr, M.; Pulsford, I.; Pittock, J.; White, J.W.; et al. Guidelines for Conserving Connectivity Through Ecological Networks and Corridors; Best Practice Protected Area Guidelines Series; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Rahimi, E.; Dong, P. What are the main human pressures affecting Iran’s protected areas? J. Environ. Stud. Sci. 2022, 12, 682–691. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, H.; Liu, Y. Urban ecological corridors construction: A review. Acta Ecol. Sin. 2017, 37, 23–30. [Google Scholar] [CrossRef]
- Zhou, D.; Song, W. Identifying ecological corridors and networks in mountainous areas. Int. J. Environ. Res. Public Health 2021, 18, 4797. [Google Scholar] [CrossRef] [PubMed]
- Boakes, E.H.; McGowan, P.J.; Fuller, R.A.; Chang-qing, D.; Clark, N.E.; O’Connor, K.; Mace, G.M. Distorted views of biodiversity: Spatial and temporal bias in species occurrence data. PLoS Biol. 2010, 8, e1000385. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Cushman, S.A.; McRae, B.; Adriaensen, F.; Beier, P.; Shirley, M.; Zeller, K. Biological corridors and connectivity. Key Top. Conserv. Biol. 2013, 2, 384–404. [Google Scholar] [CrossRef]
- Gilbert-Norton, L.; Wilson, R.; Stevens, J.R.; Beard, K.H. A meta-analytic review of corridor effectiveness. Conserv. Biol. 2010, 24, 660–668. [Google Scholar] [CrossRef]
- Compton, B.W.; McGarigal, K.; Cushman, S.A.; Gamble, L.R. A resistant-kernel model of connectivity for amphibians that breed in vernal pools. Conserv. Biol. 2007, 21, 788–799. [Google Scholar] [CrossRef]
- Malakoutikhah, S.; Fakheran, S.; Soffianian, A. Applying Circuitscape Theory to identify migration corridors between Mooteh and Ghamishloo wildlife refuges in Isfahan Province-Iran. Iran. J. Appl. Ecol. 2014, 2, 77–89. [Google Scholar]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- McRae, B.H.; Beier, P. Circuit theory predicts gene flow in plant and animal populations. Proc. Natl. Acad. Sci. USA 2007, 104, 19885–19890. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.F.; Balkenhol, N.; Fortin, M.J.; McRae, B.H.; Scribner, K.I.M. Use of resistance surfaces for landscape genetic studies: Considerations for parameterization and analysis. Mol. Ecol. 2010, 19, 3576–3591. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Agnelli, P.; Bosso, L.; Ancillotto, L.; Russo, D. Body size of Italian greater horseshoe bats (Rhinolophus ferrumequinum) increased over one century and a half: A response to climate change? Mamm. Biol. 2021, 101, 1127–1131. [Google Scholar] [CrossRef]
- Lorestani, N.; Hemami, M.R.; Rezvani, A.; Ahmadi, M. Ecological niche models reveal divergent habitat use of Pallas’s cat in the Eurasian cold steppes. Ecol. Evol. 2022, 12, e9624. [Google Scholar] [CrossRef]
- Evcin, Ö. Can highway tunnel constructıon change the habitat selection of roe deer (Capreolus capreolus Linnaeus, 1758)? Environ. Monit. Assess. 2023, 195, 1410. [Google Scholar] [CrossRef]
- Ugış, A. Kastamonu-Ballıdağ Ve Kurtgirmez Mevkiilerinde Büyük Memeli Türlerin Habitat İlişkileri, Habitat Kullanımı ve Ekolojik Niş Modellemesi. Doctoral Thesis, Kastamonu University, Kastamonu, Türkiye, 2023. [Google Scholar]
- Uzun, G.; Evcin, Ö. Determination of Dispersal Corridors Used by Large Mammals Between Close Habitats. Diversity 2025, 17, 165. [Google Scholar] [CrossRef]
- Demirsoy, A. General and Türkiye Zoogeography; Meteksan: Ankara, Türkiye, 2002. [Google Scholar]
- Şekercioğlu, Ç.H.; Anderson, S.; Akçay, E.; Bilgin, R.; Can, Ö.E.; Semiz, G.; Tavşanoğlu, Ç.; Yokeş, M.B.; Soyumert, A.; İpekdal, K.; et al. Turkey’s globally important biodiversity in crisis. Biol. Conserv. 2011, 144, 2752. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. [Google Scholar]
- World Wildlife Fund (WWF). Korumazsak Kaybederiz: Sürdürülebilir Bir Türkiye İçin Korunan Alanlar Hedef: 2030’a Kadar %30. WWF Türkiye Raporu. 2021. Available online: https://www.wwf.org.tr/?10800/Surdurulebilir-Bir-Turkiye-Icin-Korunan-Alanlar (accessed on 10 March 2025).
- Convention on Biological Diversity (CBD). The Kunming-Montreal Global Biodiversity Framework, 2030 Targets, 2022. Available online: https://www.cbd.int/gbf/targets (accessed on 14 January 2025).
- Kastamonu Ilgaz Dağı Yaban Hayatı Geliştirme Sahası Yönetim ve Gelişme Planı, T.C. Orman ve Su İşleri Bakanlığı, 10. Bölge Müdürlüğü, Kastamonu Şube Müdürlüğü, Kastamonu, 2012. Available online: https://bolge10.tarimorman.gov.tr (accessed on 22 January 2023).
- Kastamonu Tosya Gavurdağı Yaban Hayatı Geliştirme Sahası Yönetim ve Gelişme Planı, T.C. Orman ve Su İşleri Bakanlığı, 10. Bölge Müdürlüğü, Kastamonu Şube Müdürlüğü, Kastamonu, 2015. Available online: https://bolge10.tarimorman.gov.tr (accessed on 22 January 2023).
- Özcan, A.U. Ekolojik Köprülerin Potansiyel Yer Seçimleri: İzmir-Çeşme Otoyolu Örneği. Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2021, 7, 146–156. [Google Scholar] [CrossRef]
- Sütünç, H.S. Evaluation of landscape connectivity between protected areas using pinch points. Turk. J. For. 2021, 22, 331–341. [Google Scholar] [CrossRef]
- Akbaba, B.; Bulut, S. Inventory of large mammal species in the Ilgaz Mountains Çankırı: A major ecological corridor in Anatolia. Hittite J. Sci. Eng. 2020, 7, 73–80. [Google Scholar] [CrossRef]
- Evcin, Ö.; Küçük, Ö.; Aktürk, E. Habitat suitability model with maximum entropy approach for European roe deer (Capreolus capreolus) in the Black Sea Region. Environ. Monit. Assess. 2019, 191, 669. [Google Scholar] [CrossRef] [PubMed]
- Oğul, A.; Tuttu, U.; Öner, M.N.; Yorulmaz, T.; Özcan, A.U. Ilgaz Dağı’nda farklı orman kuruluşlarındaki bazı yaban hayvanlarının (Classis: Mammalia) aktivitelerinin belirlenmesi. Anadolu Orman Araştırmaları Derg. 2022, 8, 29–36. [Google Scholar] [CrossRef]
- Baguette, M.; Blanchet, S.; Legrand, D.; Stevens, V.M.; Turlure, C. Individual dispersal, landscape connectivity and ecological networks. Biol. Rev. 2013, 88, 310–326. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.J.; Jiang, Z.Y.; Ni, R.P. Identifying migration routes of wild asian elephants in China based on ecological networks constructed by circuit theory model. Animals 2023, 13, 2618. [Google Scholar] [CrossRef]
- Fleishman, E.; Murphy, D.D.; Brussard, P.F. A new method for selection of umbrella species for conservation planning. Ecol. Appl. 2000, 10, 569–579. [Google Scholar] [CrossRef]
- Roberge, J.M.; Angelstam, P.E.R. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Ertürk, A. Anadolu Canis lupus L. 1758 (Kurt) Türünün Alansal Ekolojisi ve Populasyon Yapısının Araştırılması. Doctoral Thesis, Hacettepe University, Ankara, Türkiye, 2017. [Google Scholar]
- Süel, H. Brown bear (Ursus arctos) habitat suitability modelling and mapping. Appl. Ecol. Environ. Res. 2019, 17, 4245–4255. [Google Scholar] [CrossRef]
- Oruç, M.S.; Mert, A.; Özdemir, İ. Modelling Habitat Suitability For Red Deer (Cervus elaphus L.) Using Environmental Variables In Çatacık Region. Bilge Int. J. Sci. Technol. Res. 2017, 1, 135–142. [Google Scholar]
- Küçük, Ö.; Uslu, N. Sinop-Bozburun Yaban Hayatı Koruma Alanında Yaban Domuzu (Sus scrofa L.) Sayımı. Kastamonu Orman Fakültesi Derg. 2004, 4, 45–57. [Google Scholar]
- Stachowicz, I.; Ferrer-Paris, J.R.; Sánchez-Mercado, A. Leveraging limited data from wildlife monitoring in a conflict affected region in Venezuela. Sci. Rep. 2024, 14, 1673. [Google Scholar] [CrossRef] [PubMed]
- Murie, O.J.; Elbroch, M. A Field Guide to Animal Tracks; Houghton Mifflin Harcourt: Boston, MA, USA, 2005. [Google Scholar]
- Evcin, Ö. Monitoring the annual change of fauna in Ilgaz Mountain Wildlife Reserve by using biodiversity indices. J. Biometry Stud. 2022, 2, 7–14. [Google Scholar] [CrossRef]
- McKaughan, J.E.; Stephens, P.A.; Hill, R.A. Estimating mesocarnivore abundance on commercial farmland using distance sampling with camera traps. Ecol. Solut. Evid. 2023, 4, e12229. [Google Scholar] [CrossRef]
- Özcan, A.U.; Aytaş, İ. Peyzaj direnç değişimlerinin ekolojik bağlantılar üzerine etkileri: Çankırı örneği. Bartın Orman Fakültesi Derg. 2020, 22, 979–992. [Google Scholar] [CrossRef]
- Zhou, G.; Huan, Y.; Wang, L.; Lan, Y.; Liang, T.; Shi, B.; Zhang, Q. Linking ecosystem services and circuit theory to identify priority conservation and restoration areas from an ecological network perspective. Sci. Total Environ. 2023, 873, 162261. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e01486. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.R.; Kaboli, M.; Shabani, F. MaxEnt brings comparable results when the input data are being completed; Model parameterization of four species distribution models. Ecol. Evol. 2023, 13, e9827. [Google Scholar] [CrossRef]
- Farashi, A.; Alizadeh-Noughani, M. Basic Introduction to Species Distribution Modelling. In Ecosystem and Species Habitat Modeling for Conservation and Restoration; Springer Nature: Singapore, 2023. [Google Scholar] [CrossRef]
- Pollock, L.J.; O’connor, L.M.; Mokany, K.; Rosauer, D.F.; Talluto, M.V.; Thuiller, W. Protecting biodiversity (in all its complexity): New models and methods. Trends Ecol. Evol. 2020, 35, 1119–1128. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Lee-Yaw, J.; McCune, J.L.; Pironon, S.; Sheth, S.N. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
- Liang, D.; Bailey, H.; Hoover, A.L.; Eckert, S.; Zarate, P.; Alfaro-Shigueto, J.; Mangel, J.C.; Campos, N.P.; Davilla, J.Q.; Baturen, D.S.; et al. Integrating telemetry and point observations to inform management and conservation of migratory marine species. Ecosphere 2023, 14, e4375. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, G.; Bu, W.; Gao, Y. Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y.; et al. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Burnham, K.P.; Anderson, D.R. Practical Use of the İnformation-Theoretic Approach; Springer: New York, NY, USA, 1998; pp. 75–117. [Google Scholar] [CrossRef]
- Lewis, J.S.; Farnsworth, M.L.; Burdett, C.L.; Theobald, D.M.; Gray, M.; Miller, R.S. Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep. 2017, 7, 44152. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García Marquéz, J.R.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V.; Obolenskaya, E.V. Species-distribution modeling: Advantages and limitations of its application. 1. General approaches. Biol. Bull. Rev. 2021, 11, 254–264. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zeng, H. Application of the MaxEnt model in improving the accuracy of ecological red line identification: A case study of Zhanjiang, China. Ecol. Indic. 2022, 137, 108767. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological niches and geographic distributions (MPB-49). In Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Nor, A.N.M.; Rafaai, N.H.; Abdullah, S.A. Using Circuit Theory, Connectivity Analysis and Least-Cost Path to Model the Potential Ecological Corridors of Malayan Tapir (Tapirus indicus) at Chini-Bera Forest Complex in Pahang, Peninsular Malaysia. Sains Malays. 2023, 52, 3343–3355. [Google Scholar] [CrossRef]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B.; et al. Circuit-theory applications to connectivity science and conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef]
- McRae, B.H. Pinchpoint Mapper Connectivity Analysis Software, Version 2.0; The Nature Conservancy: Seattle, WA, USA, 2012; Available online: http://www.circuitscape.org/linkagemapper (accessed on 8 April 2023).
- McRae, B.H.; Shah, V.B.; Mohapatra, T.K. Circuitscape User’s Guide; The University of California, Santa Barbara. Circuitscape 4 User Guide; The Nature Conservancy: Seattle, WA, USA, 2009. [Google Scholar]
- Pelletier, D.; Clark, M.; Anderson, M.G.; Rayfield, B.; Wulder, M.A.; Cardille, J.A. Applying circuit theory for corridor expansion and management at regional scales: Tiling, pinch points, and omnidirectional connectivity. PLoS ONE 2014, 9, e84135. [Google Scholar] [CrossRef]
- Gracanin, A.; Mikac, K.M. Evaluating modelled wildlife corridors for the movement of multiple arboreal species in a fragmented landscape. Landsc. Ecol. 2023, 38, 1321–1337. [Google Scholar] [CrossRef]
- Belote, R.T.; Dietz, M.S.; McRae, B.H.; Theobald, D.M.; McClure, M.L.; Irwin, G.H.; McKinley, P.S.; Gage, J.A.; Aplet, G.H. Identifying corridors among large protected areas in the United States. PLoS ONE 2016, 11, e0154223. [Google Scholar] [CrossRef]
- Nor, A.N.M.; Corstanje, R.; Harris, J.A.; Grafius, D.R.; Siriwardena, G.M. Ecological connectivity networks in rapidly expanding cities. Heliyon 2017, 3, e00325. [Google Scholar] [CrossRef]
- McRae, B.H.; Kavanagh, D.M. Linkage Mapper Connectivity Analysis Software; The Nature Conservancy: Seattle, WA, USA, 2011; Available online: http://www.circuitscape.org/linkagemapper (accessed on 8 April 2023).
- Zeller, K.A.; McGarigal, K.; Beier, P.; Cushman, S.A.; Vickers, T.W.; Boyce, W.M. Sensitivity of landscape resistance estimates based on point selection functions to scale and behavioral state: Pumas as a case study. Landsc. Ecol. 2014, 29, 541–557. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.; Shan, L.; Xiao, F. Constructing and optimizing urban ecological network in the context of rapid urbanization for improving landscape connectivity. Ecol. Indic. 2021, 132, 108319. [Google Scholar] [CrossRef]
- Kang, J.; Li, C.; Li, M.; Zhang, T.; Zhang, B. Identifying priority areas for conservation in the lower Yellow River basin from an ecological network perspective. Ecosyst. Health Sustain. 2022, 8, 2105751. [Google Scholar] [CrossRef]
- Keeley, A.T.; Beier, P.; Keeley, B.W.; Fagan, M.E. Habitat suitability is a poor proxy for landscape connectivity during dispersal and mating movements. Landsc. Urban Plan. 2017, 161, 90–102. [Google Scholar] [CrossRef]
- Wade, A.A.; McKelvey, K.S.; Schwartz, M.K. Resistance-Surface-Based Wildlife Conservation Connectivity Modeling: Summary of Efforts in the United States and Guide for Practitioners; United States Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2015. [Google Scholar]
- Elliot, N.B.; Cushman, S.A.; Macdonald, D.W.; Loveridge, A.J. The devil is in the dispersers: Predictions of landscape connectivity change with demography. J. Appl. Ecol. 2014, 51, 1169–1178. [Google Scholar] [CrossRef]
- Dutta, T.; Sharma, S.; McRae, B.H.; Roy, P.S.; DeFries, R. Connecting the dots: Mapping habitat connectivity for tigers in central India. Reg. Environ. Change 2016, 16, 53–67. [Google Scholar] [CrossRef]
- Meza Mori, G.; Cotrina-Sanchez, A.; Bandopadhyay, S.; Rojas-Briceño, N.B.; Torres Guzmán, C.; Coronel Castro, E.; Oliva, M. Does climate change impact the potential habitat suitability and conservation status of the national bird of Peru (Rupicola peruvianus)? Biodivers. Conserv. 2023, 32, 2323–2344. [Google Scholar] [CrossRef]
- Tao, Q.; Gao, G.; Xi, H.; Wang, F.; Cheng, X.; Ou, W.; Tao, Y. An integrated evaluation framework for multiscale ecological protection and restoration based on multi-scenario trade-offs of ecosystem services: Case study of Nanjing City, China. Ecol. Indic. 2022, 140, 108962. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Y.; Liu, Y.; Du, Y.; Meersmans, J.; Qiu, S. Linking ecosystem services and circuit theory to identify ecological security patterns. Sci. Total Environ. 2018, 644, 781–790. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Qiao, H.; Soberón, J.; Peterson, A.T. No silver bullets in correlative ecological niche modeling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 2015, 6, 1126–1136. [Google Scholar] [CrossRef]
- Mech, L.D.; Boitani, L. Wolf Social Ecology; USGS Northern Prairie Wildlife Research Center 318: Jamestown, ND, USA, 2003. [Google Scholar]
- Abaigar, T.; Del Barrio, G.; Vericad, J.R. Habitat preference of wild boar (Sus scrofa L., 1758) in a mediterranean environment. Indirect Evaluation by Signs. Mammalia 2009, 58, 201–210. [Google Scholar] [CrossRef]
- Sawaya, M.A.; Ramsey, A.B.; Ramsey, P.W. American black bear thermoregulation at natural and artificial water sources. Ursus 2017, 27, 129–135. [Google Scholar] [CrossRef]
- Sun, X.; Long, Z.; Jia, J. Identifying core habitats and corridors for giant pandas by combining multiscale random forest and connectivity analysis. Ecol. Evol. 2022, 12, e8628. [Google Scholar] [CrossRef] [PubMed]
- Acarer, A.; Mert, A. 21st century climate change threatens on the brown bear. Cerne 2024, 30, e-103305. [Google Scholar] [CrossRef]
- Oğurlu, İ. Habitat Use of Red Deer (Cervus elaphus L.) in Çatacik Forest. Turk. J. Zool. 1996, 20, 427–435. [Google Scholar] [CrossRef]
- Erdtmann, D.; Keuling, O. Behavioural patterns of free roaming wild boar in a spatiotemporal context. PeerJ 2020, 8, e10409. [Google Scholar] [CrossRef]
- Gordigiani, L.; Viviano, A.; Brivio, F.; Grignolio, S.; Lazzeri, L.; Marcon, A.; Mori, E. Carried away by a moonlight shadow: Activity of wild boar in relation to nocturnal light intensity. Mammal Res. 2022, 67, 39–49. [Google Scholar] [CrossRef]
- Kuter, N. Ilgaz Dağı Milli Parkı’nın Orman Peyzajı Ve Estetiği Açısından Değerlendirilmesi. Turk. J. For. 2008, 9, 36–47. [Google Scholar]
- DeFries, R.; Parashar, S.; Neelakantan, A.; Clark, B.; Krishnaswamy, J. Landscape Connectivity For Wildlife and Water: The State of the Literature. Curr. Landsc. Ecol. Rep. 2023, 8, 149–158. [Google Scholar] [CrossRef]
- Shaw, A.K. Causes and consequences of individual variation in animal movement. Mov. Ecol. 2020, 8, 12. [Google Scholar] [CrossRef]
- Epaphras, A.M.; Gereta, E.; Lejora, I.A.; Ole Meing’ataki, G.E.; Ng’umbi, G.; Kiwango, Y.; Mwangomo, E.; Semanini, F.; Vitalis, L.; Balozi, J.; et al. Wildlife water utilization and importance of artificial waterholes during dry season at Ruaha National Park, Tanzania. Wetl. Ecol. Manag. 2008, 16, 183–188. [Google Scholar] [CrossRef]
- Chakuya, J.; Mandisodza-Chikerema, R.; Ngorima, P.; Malunga, A. Water sources during drought period in a savanna wildlife ecosystem, northern Zimbabwe. Geol. Ecol. Landsc. 2023, 7, 323–328. [Google Scholar] [CrossRef]
- Chynoweth, M.; Coban, E.; Altin, Ç.; Şekercioğlu, Ç. Human-wildlife conflict as a barrier to large carnivore management and conservation in Turkey. Turk. J. Zool. 2016, 40, 972–983. [Google Scholar] [CrossRef]
- Khosravi, R.; Hemami, M.R. Identifying landscape species for ecological planning. Ecol. Indic. 2019, 99, 140–148. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Yi, S. An integrated approach to constructing ecological security patterns and identifying ecological restoration and protection areas: A case study of Jingmen, China. Ecol. Indic. 2022, 137, 108723. [Google Scholar] [CrossRef]


| Ecological Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Water sources | 53.3 | 39.8 |
| Stand type | 19.7 | 12.5 |
| Slope | 12.1 | 14 |
| Aspect | 8.2 | 13.2 |
| Solar radiation | 3.7 | 4.8 |
| Hillshade | 2.9 | 15.6 |
| Ruggedness | 0.1 | 0.1 |
| Ecological Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Water sources | 45 | 50.3 |
| Stand type | 30.4 | 23.3 |
| Slope | 10.1 | 11.8 |
| Aspect | 6.3 | 7.5 |
| Hillshade | 3 | 1.9 |
| Solar radiation | 3 | 3.7 |
| Ruggedness | 1.8 | 1.4 |
| Ecological Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Stand type | 40.2 | 32.3 |
| Water sources | 32.3 | 25.7 |
| Slope | 17.4 | 23.9 |
| Aspect | 4 | 3.6 |
| Hillshade | 3.4 | 10.4 |
| Solar radiation | 2 | 3 |
| Ruggedness | 0.7 | 1 |
| Ecological Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Water sources | 47.4 | 45.3 |
| Stand type | 22.3 | 24.1 |
| Aspect | 10.1 | 3.5 |
| Hillshade | 8.6 | 7.8 |
| Slope | 7.8 | 14.5 |
| Solar radiation | 2.7 | 3.8 |
| Ruggedness | 1.2 | 1 |
| Ecological Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Water sources | 53.3 | 39.8 |
| Stand type | 19.7 | 12.5 |
| Slope | 12.1 | 14 |
| Aspect | 8.2 | 13.2 |
| Solar radiation | 3.7 | 4.8 |
| Hillshade | 2.9 | 15.6 |
| Ruggedness | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalleci, B.; Evcin, Ö. Using Circuit Theory to Identify Important Ecological Corridors for Large Mammals Between Wildlife Refuges. Diversity 2025, 17, 542. https://doi.org/10.3390/d17080542
Kalleci B, Evcin Ö. Using Circuit Theory to Identify Important Ecological Corridors for Large Mammals Between Wildlife Refuges. Diversity. 2025; 17(8):542. https://doi.org/10.3390/d17080542
Chicago/Turabian StyleKalleci, Büşra, and Özkan Evcin. 2025. "Using Circuit Theory to Identify Important Ecological Corridors for Large Mammals Between Wildlife Refuges" Diversity 17, no. 8: 542. https://doi.org/10.3390/d17080542
APA StyleKalleci, B., & Evcin, Ö. (2025). Using Circuit Theory to Identify Important Ecological Corridors for Large Mammals Between Wildlife Refuges. Diversity, 17(8), 542. https://doi.org/10.3390/d17080542







