Variations in Fine-Root Traits of Pseudotsuga sinensis Across Different Rocky-Desertification Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Plot Establishment
2.3. Root Sample Collection and Preparation
2.4. Trait Measurements
2.5. Data Analyses
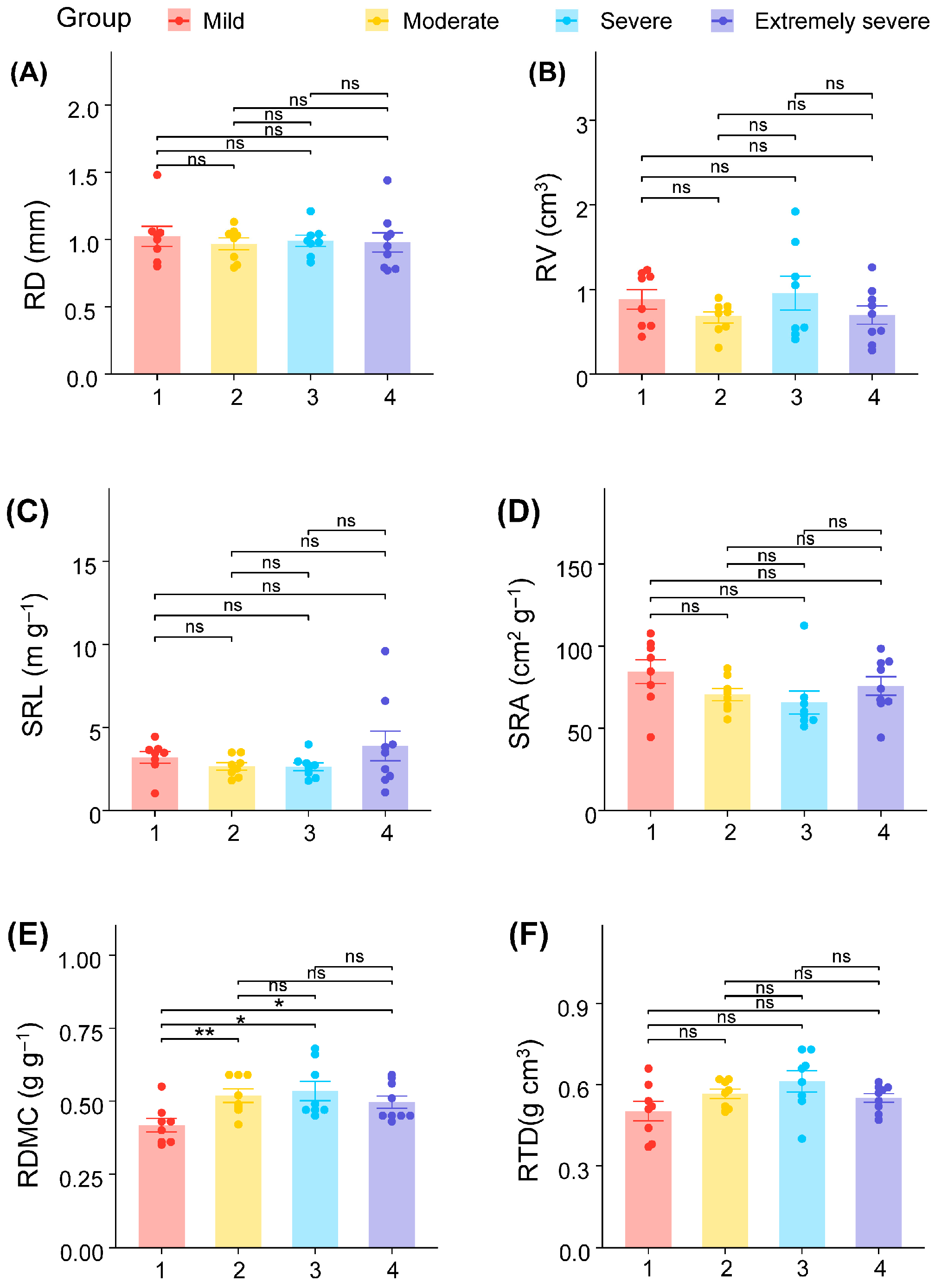
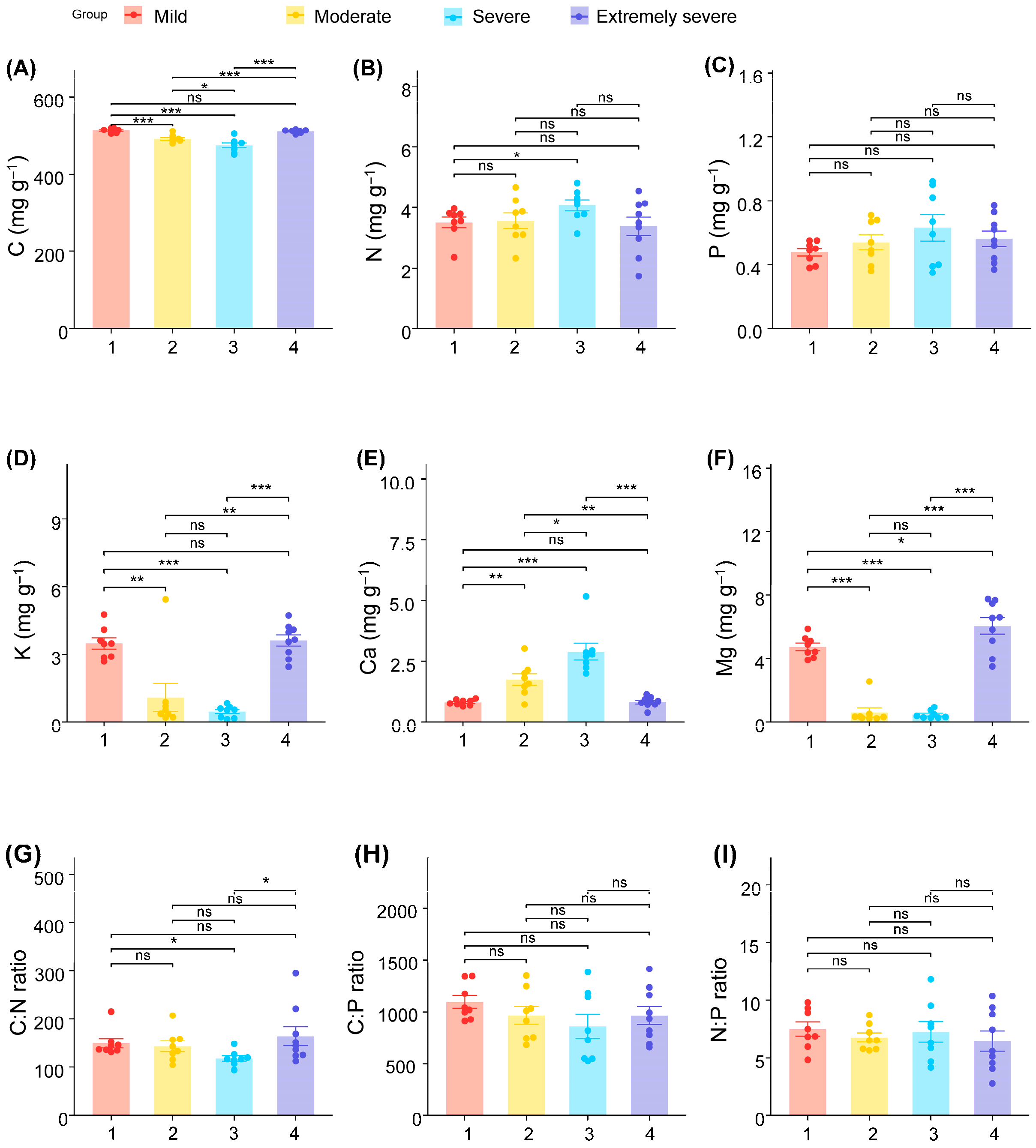
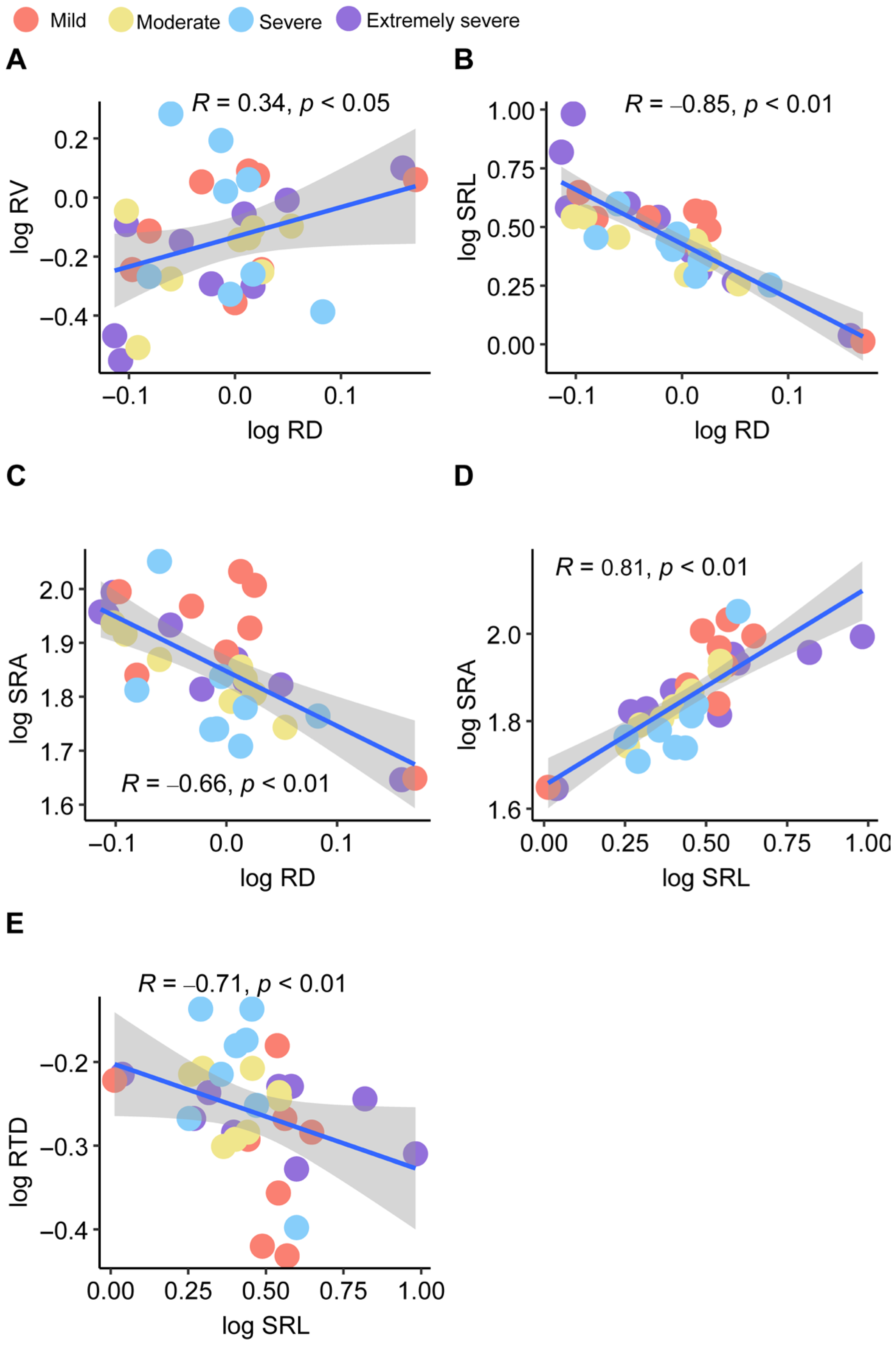
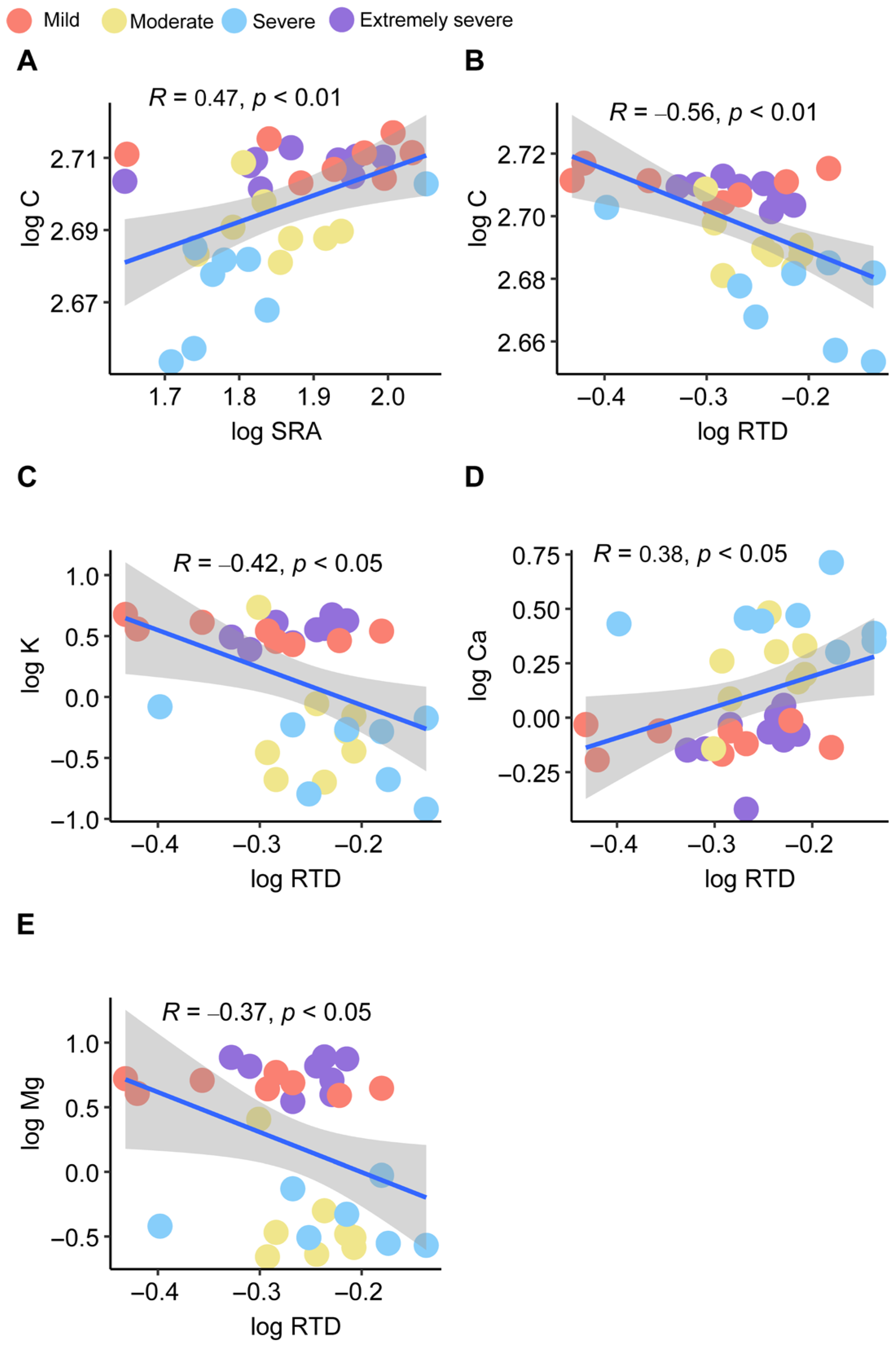
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Defrenne, C.; McCormack, M.L.; Yang, L.; Tian, D.; Luo, Y.; Hou, E.; Yan, T.; Li, Z.; Bu, W.; et al. Fine-root functional trait responses to experimental warming: A global meta-analysis. New Phytol. 2021, 230, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E. Below-ground frontiers in trait-based plant ecology. New Phytol. 2017, 213, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Cusack, D.F.; Addo-Danso, S.D.; Agee, E.A.; Andersen, K.M.; Arnaud, M.; Batterman, S.A.; Brearley, F.Q.; Ciochina, M.I.; Cordeiro, A.L.; Dallstream, C.; et al. Tradeoffs and synergies in tropical forest root traits and dynamics for nutrient and water acquisition: Field and modeling advances. Front. For. Glob. Change 2021, 4, 704469. [Google Scholar] [CrossRef]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, Y.; Wang, X.; Jin, B.; Chen, C.; Zhao, X. Contribution of litter and root to soil nutrients in different rocky desertification grasslands in a karst area. Plants 2024, 13, 2329. [Google Scholar] [CrossRef]
- Burton, A.J.; Pregitzer, K.S.; Hendrick, R.L. Relationships between fine root dynamics and nitrogen availability in Michigan northern hardwood forests. Oecologia 2000, 125, 389–399. [Google Scholar] [CrossRef]
- Addo-Danso, S.D.; Defrenne, C.E.; McCormack, M.L.; Ostonen, I.; Addo-Danso, A.; Foli, E.G.; Borden, K.A.; Isaac, M.E.; Prescott, C.E. Fine-root morphological trait variation in tropical forest ecosystems: An evidence synthesis. Plant Ecol. 2020, 221, 1–13. [Google Scholar] [CrossRef]
- Guerrero-Ramírez, N.R.; Mommer, L.; Freschet, G.T.; Iversen, C.M.; McCormack, M.L.; Kattge, J.; Poorter, H.; van der Plas, F.; Bergmann, J.; Kuyper, T.W.; et al. Global root traits (GRooT) database. Glob. Ecol. Biogeogr. 2021, 30, 25–37. [Google Scholar] [CrossRef]
- Pinno, B.D.; Wilson, S.D. Fine root response to soil resource heterogeneity differs between grassland and forest. Plant Ecol. 2013, 214, 821–829. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Miyatani, K.; Tanikawa, T.; Makita, N.; Hirano, Y. Relationships between specific root length and respiration rate of fine roots across stands and seasons in Chamaecyparis obtusa. Plant Soil 2018, 423, 215–227. [Google Scholar] [CrossRef]
- Mommer, L.; Weemstra, M. The role of roots in the resource economics spectrum. New Phytol. 2012, 195, 725–727. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef]
- Ostonen, I.; Püttsepp, Ü.; Biel, C.; Alberton, O.; Bakker, M.R.; Lõhmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Lõhmus, K.; Oja, T.; Lasn, R. Specific root area: A soil characteristic. Plant Soil 1989, 119, 245–249. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Querejeta, J.I.; Villar, R.; Pérez-Ramos, I.M.; Marañón, T.; Galán Díaz, J.; Marín, S.T.; Prieto, I. The economics spectrum drives root trait strategies in Mediterranean vegetation. Front. Plant Sci. 2021, 12, 773118. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Froehle, J.; Tilman, D.G.; Wedin, D.A.; Chapin, F.S., III. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 2001, 93, 274–285. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer Science & Business Media: Heidelberg, Germany, 2008. [Google Scholar]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Ruffel, S.; Krouk, G.; Ristova, D.; Shasha, D.; Birnbaum, K.D.; Coruzzi, G.M. Nitrogen economics of root foraging: Transitive closure of the nitrate–cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc. Natl. Acad. Sci. USA 2011, 108, 18524–18529. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Ågren, G.I. The C: N: P stoichiometry of autotrophs–theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, S.; Huang, B. Changes of vegetation features of rocky desertification process in karst area of Guizhou. J. Nanjing For. Univ. 2003, 27, 26–30. [Google Scholar]
- Liu, J.; Guo, Y.; Wang, L.; Han, D. Current status and control measures of ecological restoration in the karst rocky desertification areas of Guizhou. J. Anhui Agric. Sci. 2011, 39, 11684–11686. [Google Scholar]
- Cao, J.; Yuan, D.; Zhang, C.; Jiang, Z. Karst ecosystem constrained by geological conditions in southwest China. Earth Environ. 2004, 32, 1–8. [Google Scholar]
- Li, L.; Cai, Z.; Chen, Z.; Li, Q.; Sun, J. Extraction and analysis of temporal and spatial evolution of rocky desertification: Acase study of Weining County. J. Geomat. 2022, 47, 115–118. [Google Scholar]
- Xiong, B.; Wang, Z.; Tian, K.; Zhang, E.; Li, Z.; Li, S.; Peng, Z. Coenological characteristics of Pseudotsuga sinensis forests in Qizimei Mountains Nature Reserve. Guihaia 2017, 37, 434–441. [Google Scholar]
- Li, W.; Zhang, W.; Feng, T.; Lv, D.; Zou, S.; He, B.; Bai, X. Variations in leaf functional traits of Pseudotsuga sinensis across forests with varying levels of rocky desertification. Ecol. Evol. 2025, 15, e70916. [Google Scholar] [CrossRef]
- Li, M.; Xie, S. Study on community and population structure of Pseudotsuga sinensis forest in karst mountainous region of Guizhou Province. Tianjin Agric. Sci. 2015, 21, 150–153. [Google Scholar]
- Nong, X.; Yu, H.; Xiang, Y.; Yang, P.; Zhang, Q. Analysis of potential suitable habitat change of Pseudotsuga sinensis based on MaxEnt mode. J. Guangxi Norm. Univ. 2024, 42, 215–225. [Google Scholar]
- Li, W.J.; Feng, T.; Li, J.; He, B.; Zou, S.; Liu, P.J. The complete chloroplast genome of Pseudotsuga sinensis, a China endemic species. Mitochondrial DNA Part B 2023, 8, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, C.; Ni, J.; Zhang, Y.; Yu, L. Analysis and forecast method for maximum and minimum temperature in towns of Weining. Mid-Low Latit. Mt. Meteorol. 2019, 43, 29–35. [Google Scholar]
- Linghu, X.; Teng, M.; Wang, J. Lang cover changes and transfer characteristics in the Weining Mountains based on topographic gradients: A case study on Weining County, Guizhou Province. Geograph. Environ. Sci. 2023, 41, 74–81. [Google Scholar]
- Zang, Y.; Wei, X.; Wei, X.; Shu, Z.; Liu, T. Analysis of mechanical composition of three typical soil types in Weining County, Guizhou Province. J. Green Sci. Technol. 2020, 17–22. [Google Scholar]
- Chen, Q. Analysis of monitoring results and control measure for stony desertification in Weining County. J. Green Sci. Technol. 2023, 25, 64–67. [Google Scholar]
- Wang, K.; Yue, Y.; Chen, H.; Wu, X.; Xiao, J.; Zhang, W.; Du, H. The comprehensive treatment of karst rocky desertification and its regional restoration effects. Acta Ecol. Sin. 2019, 39, 7432–7440. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Dai, Y.; Yang, K. Temporal-spatial variation of vegetation productivity in Guizhou: A case study in Weining County. Environ. Sci. Technol. 2018, 41, 200–205. [Google Scholar]
- Li, S.; Dong, Y.X.; Wang, J.H. Re-discussion on the concept and classification of rocky desertification. Carsol. Sin. 2007, 26, 279–284. [Google Scholar]
- Gao, Y.; Yuan, Y.; Li, Q.; Kou, L.; Fu, X.; Dai, X.; Wang, H. Mycorrhizal type governs foliar and root multi-elemental stoichiometries of trees mainly via root traits. Plant Soil 2021, 460, 229–246. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, K.; Lv, S.; Niklas, K.J.; Mipam, T.D.; Crowther, T.W.; Umaña, M.N.; Zhao, Q.; Huang, H.; Reich, P.B. The scaling of fine root nitrogen versus phosphorus in terrestrial plants: A global synthesis. Funct. Ecol. 2019, 33, 2081–2094. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerling, D.J. Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Aritsara, A.N.A.; Wang, S.; Li, B.N.; Jiang, X.; Qie, Y.D.; Tan, F.S.; Zhang, Q.W.; Cao, K.F. Divergent leaf and fine root “pressure–volume relationships” across habitats with varying water availability. Plant Physiol. 2022, 190, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, H.; Yang, F.; Dai, X.; Meng, S.; Hu, M.; Kou, L.; Fu, X. Relationships between root exudation and root morphological and architectural traits vary with growing season. Tree Physiol. 2024, 44, tpad118. [Google Scholar] [CrossRef]
- Kraft, N.J.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Autumn, K.; Pugnaire, F. Evolution of suites of traits in response to environmental stress. Am. Nat. 1993, 142, S78–S92. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 1–20. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Liu, X.; Ma, K. Plant functional traits—Concepts, applications and future directions. Sci. Sin. Vitae 2015, 45, 325–339. [Google Scholar] [CrossRef]
- Hu, Q.; Sheng, M.; Yin, J.; Bai, Y. Stoichiometric characteristics of fine roots and rhizosphere soil of Broussonetia papyrifera adapted to the karst rocky desertification environment in southwest China. Chin. J. Plant Ecol. 2020, 44, 962–972. [Google Scholar] [CrossRef]
- Gondal, A.H.; Hussain, I.; Ijaz, A.B.; Zafar, A.; Ch, B.I.; Zafar, H.; Sohail, M.D.; Niazi, H.; Touseef, M.; Khan, A.A.; et al. Influence of soil pH and microbes on mineral solubility and plant nutrition: A review. Int. J. Agric. Biol. Sci. 2021, 5, 71–81. [Google Scholar]
- Wang, F.; Yan, L.; Liu, L. Research progress of plant functional traits in Karst areas. Guizhou Fore. Sci. Technol. 2025, 53, 80–86. [Google Scholar]
- Sell, M.; Rohula-Okunev, G.; Kupper, P.; Ostonen, I. Adapting to climate change: Responses of fine root traits and C exudation in five tree species with different light-use strategy. Front. Plant Sci. 2024, 15, 1389569. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Richardson, S.J.; Dickie, I.A.; Peltzer, D.A.; Coomes, D.A. Species-and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J. Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Sun, J.H.; Shi, H.L.; Chen, K.Y.; Ji, B.M.; Zhang, J. Research advances on trade-off relationships of plant fine root functional traits. Chin. J. Plant Ecol. 2023, 47, 1055. [Google Scholar] [CrossRef]
- Fort, F.; Jouany, C.; Cruz, P. Root and leaf functional trait relations in Poaceae species: Implications of differing resource-acquisition strategies. J. Plant Ecol. 2013, 6, 211–219. [Google Scholar] [CrossRef]
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat. Plants 2015, 1, 15050. [Google Scholar] [CrossRef]
- Zheng, J.; Freschet, G.T.; Tedersoo, L.; Li, S.; Yan, H.; Jiang, L.; Wang, H.; Ma, N.; Dai, X.; Fu, X.; et al. A trait-based root acquisition-defence-decomposition framework in angiosperm tree species. Nat. Commun. 2024, 15, 5311. [Google Scholar] [CrossRef]
- Hendricks, J.J.; Hendrick, R.L.; Wilson, C.A.; Mitchell, R.J.; Pecot, S.D.; Guo, D. Assessing the patterns and controls of fine root dynamics: An empirical test and methodological review. J. Ecol. 2006, 94, 40–57. [Google Scholar] [CrossRef]

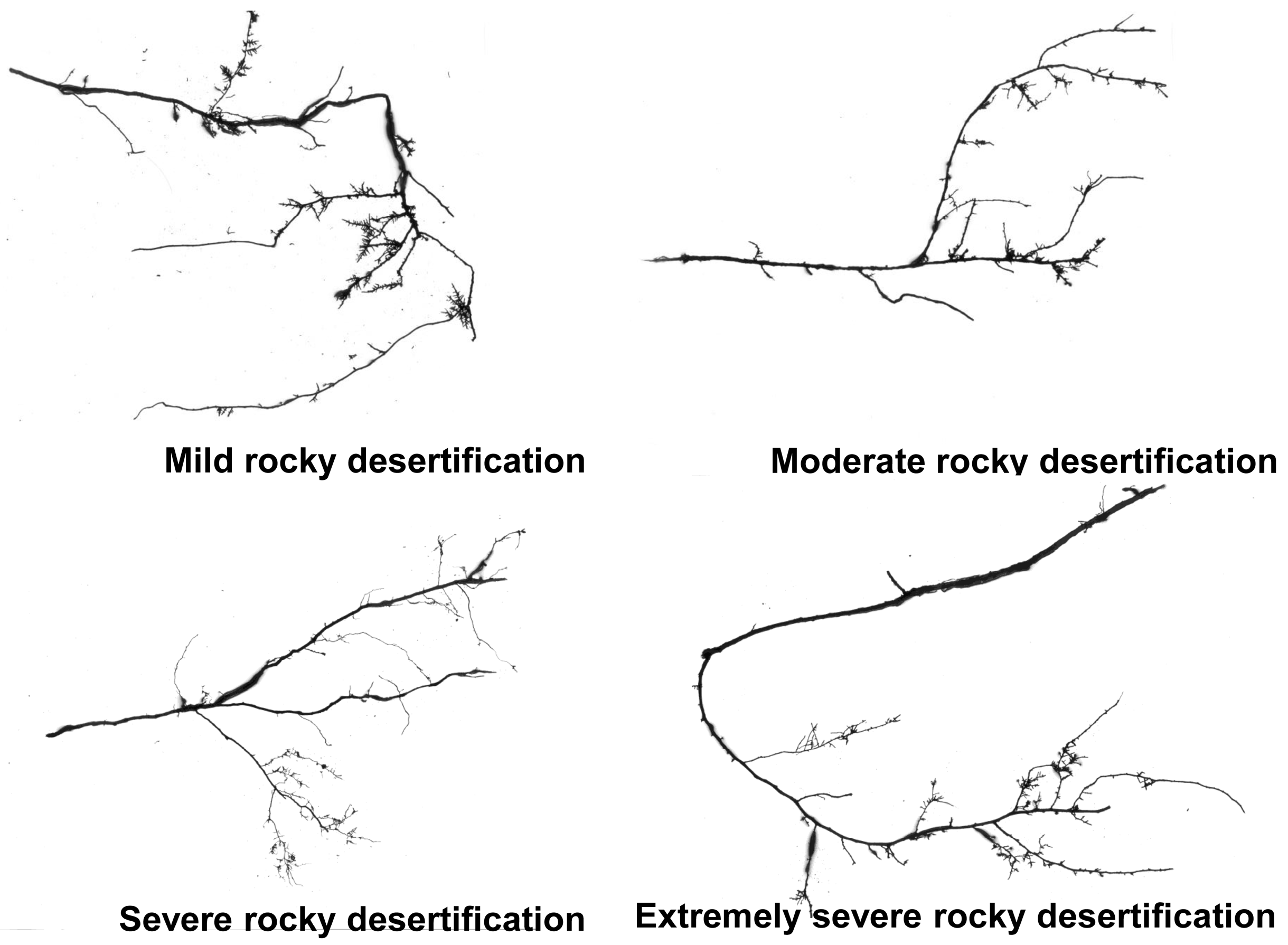


| Degree of Rocky Desertification | Rock Expose Rate (%) | Vegetation Coverage (%) | Average Soil Thickness (cm) |
|---|---|---|---|
| Mild | 30–50 | 50–70 | 30–50 |
| Moderate | 50–70 | 30–50 | 20–40 |
| Severe | 70–90 | 10–30 | <20 |
| Extremely severe | >90 | <10 | <10 |
| Traits | df | F-Value | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| RD | (3, 28) | 0.143 | [0.93, 1.06] | 0.934 |
| RV | (3, 28) | 0.982 | [0.68, 0.95] | 0.416 |
| SRL | (3, 28) | 1.12 | [2.52, 3.67] | 0.358 |
| SRA | (3, 28) | 1.660 | [67.16, 80.18] | 0.198 |
| RDMC | (3, 28) | 4.010 | [0.46, 0.52] | 0.017 |
| RTD | (3, 28) | 2.408 | [0.52, 0.59] | 0.088 |
| C | (3, 28) | 22.74 | [490.40, 504.04] | <0.001 |
| N | (3, 28) | 1.465 | [3.37, 3.89] | 0.245 |
| P | (3, 28) | 1.266 | [0.49, 0.61] | 0.305 |
| K | (3, 28) | 19.552 | [1.51, 2.74] | <0.001 |
| Ca | (3, 28) | 21.569 | [1.18, 1.94] | <0.001 |
| Mg | (3, 28) | 85.295 | [2.04, 4.01] | <0.001 |
| C/N | (3, 28) | 2.144 | [129.87, 159.04] | 0.117 |
| C/P | (3, 28) | 1.084 | [882.69, 1071.72] | 0.372 |
| N/P | (3, 28) | 0.298 | [6.28, 7.77] | 0.872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zou, S.; Lv, D.; He, B.; Bai, X. Variations in Fine-Root Traits of Pseudotsuga sinensis Across Different Rocky-Desertification Gradients. Diversity 2025, 17, 533. https://doi.org/10.3390/d17080533
Li W, Zou S, Lv D, He B, Bai X. Variations in Fine-Root Traits of Pseudotsuga sinensis Across Different Rocky-Desertification Gradients. Diversity. 2025; 17(8):533. https://doi.org/10.3390/d17080533
Chicago/Turabian StyleLi, Wangjun, Shun Zou, Dongpeng Lv, Bin He, and Xiaolong Bai. 2025. "Variations in Fine-Root Traits of Pseudotsuga sinensis Across Different Rocky-Desertification Gradients" Diversity 17, no. 8: 533. https://doi.org/10.3390/d17080533
APA StyleLi, W., Zou, S., Lv, D., He, B., & Bai, X. (2025). Variations in Fine-Root Traits of Pseudotsuga sinensis Across Different Rocky-Desertification Gradients. Diversity, 17(8), 533. https://doi.org/10.3390/d17080533






