Identification of Auchenorrhyncha Nymphs Using DNA Barcoding and Phylogenetic Analysis of the Most Common Genera Collected in Olive Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Morphology Identification
2.3. DNA Extraction, Amplification and Sequencing
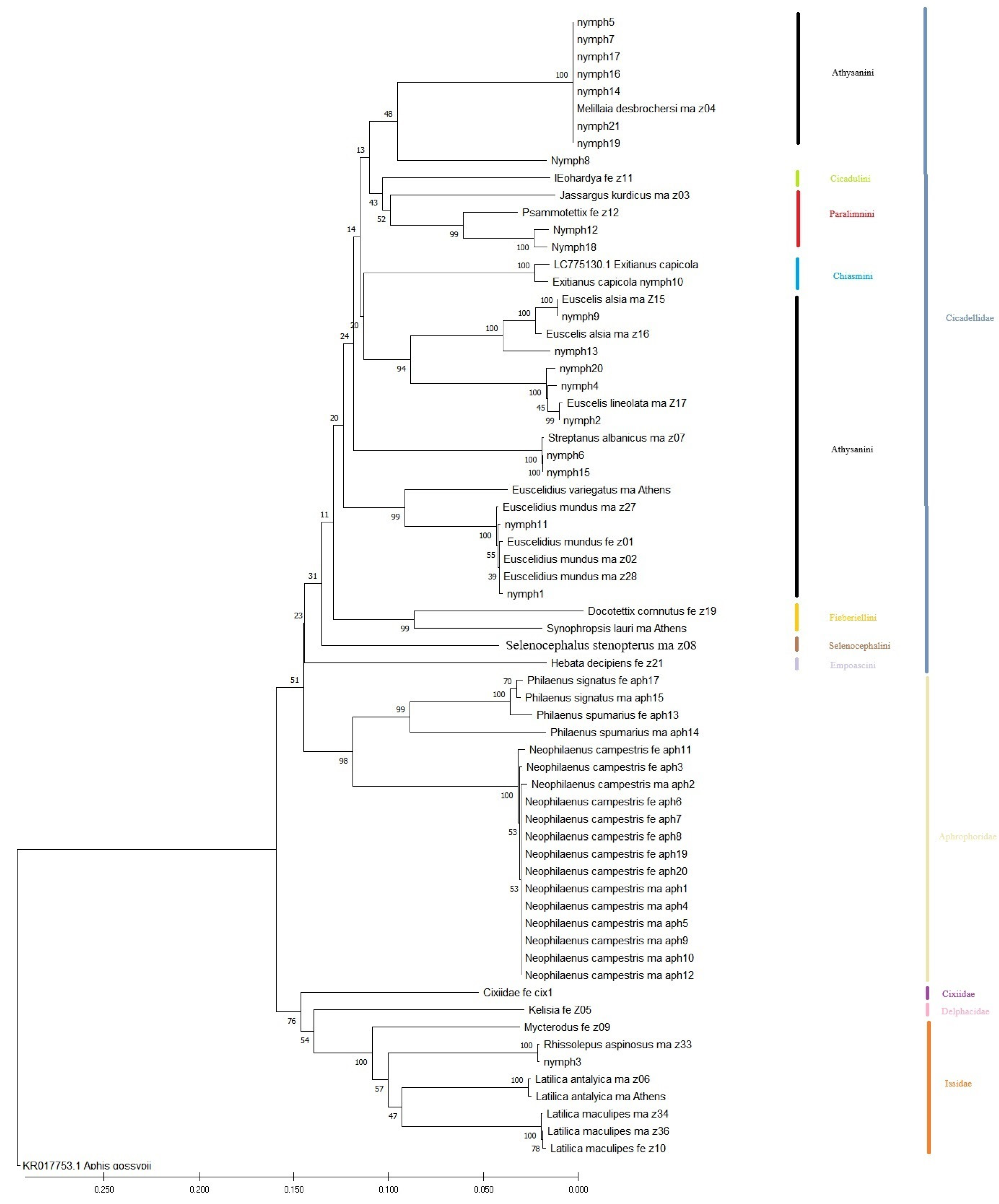
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ben Moussa, I.E.B.; Mazzoni, V.; Valentini, F.; Yaseen, T.; Lorusso, D.; Speranza, S.; Digiaro, M.; Varvaro, L.; Krugner, R.; D’Onghia, A.M. Seasonal Fluctuations of Sap-Feeding Insect Species Infected by Xylella fastidiosa in Apulian Olive Groves of Southern Italy. J. Econ. Entomol. 2016, 109, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Saponari, M.; Boscia, D.; Nigro, F.; Martelli, G.P. Identification of Dna Sequences Related to Xylella fastidiosa in Oleander, Almond and Olive Trees Exhibiting Leaf Scorch Symptoms in Apulia (Southern Italy). J. Plant Pathol. 2013, 95, 668. [Google Scholar] [CrossRef]
- Saponari, M.; Loconsole, G.; Cornara, D.; Yokomi, R.K.; De Stradis, A.; Boscia, D.; Bosco, D.; Martelli, G.P.; Krugner, R.; Porcelli, F. Infectivity and Transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 2014, 107, 1316–1319. [Google Scholar] [CrossRef] [PubMed]
- Chuche, J.; Sauvion, N.; Thiéry, D. Mixed Xylem and Phloem Sap Ingestion in Sheath-Feeders as Normal Dietary Behavior: Evidence from the Leafhopper Scaphoideus titanus. J. Insect Physiol. 2017, 102, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Elbeaino, T.; Yaseen, T.; Valentini, F.; Ben Moussa, I.E.; Mazzoni Valerio, V.; D’Onghia, A.M. Identification of Three Potential Insect Vectors of Xylella fastidiosa in Southern Italy. Phytopathol. Mediterr. 2014, 53, 328–332. [Google Scholar] [CrossRef]
- Purcell, A.H. The Ecology of Bacterial and Mycoplasma Plant Diseases Spread by Leafhoppers and Planthoppers; John Wiley & Sons: Hoboken, NJ, USA, 1985; pp. 351–380. [Google Scholar]
- Pires, A.C.; Marinoni, L. DNA barcoding and traditional taxonomy unified through Integrative Taxonomy: A view that challenges the debate questioning both methodologies. Biota Neotrop. 2010, 10, 339–346. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B Biol. Sci. 2003, 270 (Suppl. 1), S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaei, M.; Janzen, D.H.; Burns, J.M.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. USA 2006, 103, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, A.; Haszprunar, G.; Hebert, P.D.N. DNA Barcoding the Geometrid Fauna of Bavaria (Lepidoptera): Successes, Surprises, and Questions. PLoS ONE 2011, 6, e17134. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Ôhira, H.; Murase, Y.; Moriyama, A.; Kumazawa, Y. DNA Barcoding of Japanese Click Beetles (Coleoptera, Elateridae). PLoS ONE 2015, 10, e0124857. [Google Scholar] [CrossRef] [PubMed]
- Pentinsaari, M.; Anderson, R.; Borowiec, L.; Bouchard, P.; Brunke, A.; Douglas, H.; Smith, A.; Hebert, P. DNA barcodes reveal 63 overlooked species of Canadian beetles (Insecta, Coleoptera). ZooKeys 2019, 894, 53–150. [Google Scholar] [CrossRef] [PubMed]
- Ács, Z.; Challis, R.J.; Bihari, P.; Blaxter, M.; Hayward, A.; Melika, G.; Csóka, G.; Pénzes, Z.; Pujade-Villar, J.; Nieves-Aldrey, J.-L.; et al. Phylogeny and DNA barcoding of inquiline oak gallwasps (Hymenoptera: Cynipidae) of the Western Palaearctic. Mol. Phylogenet. Evol. 2010, 55, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Taeger, A.; Morinière, J.; Liston, A.; Blank, S.M.; Kramp, K.; Kraus, M.; Schmidt, O.; Heibo, E.; Prous, M.; et al. Identification of sawflies and horntails (Hymenoptera, ‘Symphyta’) through DNA barcodes: Successes and caveats. Mol. Ecol. Resour. 2017, 17, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, C.S.; Hebert, P.D.N.; Kevan, P.G.; Packer, L. DNA barcoding a regional bee (Hymenoptera: Apoidea) fauna and its potential for ecological studies. Mol. Ecol. Resour. 2009, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Gwiazdowski, R.A.; Foottit, R.G.; Maw, H.E.L.; Hebert, P.D.N. The Hemiptera (Insecta) of Canada: Constructing a Reference Library of DNA Barcodes. PLoS ONE 2015, 10, e0125635. [Google Scholar] [CrossRef] [PubMed]
- Foottit, R.G.; Maw, H.E.L.; Von Dohlen, C.D.; Hebert, P.D.N. Species identification of aphids (Insecta: Hemiptera: Aphididae) through DNA barcodes. Mol. Ecol. Resour. 2008, 8, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Foottit, R.G.; Maw, E.; Hebert, P.D.N. DNA Barcodes for Nearctic Auchenorrhyncha (Insecta: Hemiptera). PLoS ONE 2014, 9, e101385. [Google Scholar] [CrossRef] [PubMed]
- Gopurenko, D.; Fletcher, M.; Löcker, H.; Mitchell, A. Morphological and DNA barcode species identifications of leafhoppers, planthoppers and treehoppers (Hemiptera: Auchenorrhyncha) at Barrow Island. Rec. West. Aust. Mus. Suppl. 2013, 83, 253–285. [Google Scholar] [CrossRef]
- Park, D.-S.; Foottit, R.; Maw, E.; Hebert, P.D.N. Barcoding Bugs: DNA-Based Identification of the True Bugs (Insecta: Hemiptera: Heteroptera). PLoS ONE 2011, 6, e18749. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, R.; Niedringhaus, R. The Plant- and Leafhoppers of Germany (Frund); WABV: Scheeßel, Germany, 2009. [Google Scholar]
- Dmitriev, D.A.; Anufriev, G.A.; Bartlett, C.R.; Blanco-Rodríguez, E.; Borodin, O.I.; Cao, Y.-H.; Deitz, L.L.; Dietrich, C.H.; Dmitrieva, M.O.; El-Sonbati, S.A.; et al. World Auchenorrhyncha Database. TaxonPages. 2022. Available online: https://hoppers.speciesfile.org (accessed on 15 May 2025).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic Amplification of β-Globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, I.; Evangelou, V.; Papoutsis, L.; Bouga, M.; Emmanouil, N. Molecular taxonomy of the genus Physokermes (Hemiptera: Coccidae) species in Greece, based on mtDNA sequencing data. J. Apic. Res. 2018, 57, 479–483. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; deWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef] [PubMed]
- Roslin, T.; Somervuo, P.; Pentinsaari, M.; Hebert, P.D.N.; Agda, J.; Ahlroth, P.; Anttonen, P.; Aspi, J.; Blagoev, G.; Blanco, S.; et al. A molecular-based identification resource for the arthropods of Finland. Mol. Ecol. Resour. 2022, 22, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, V.; Lytra, I.; Krokida, A.; Antonatos, S.; Georgopoulou, I.; Milonas, P.; Papachristos, D.P. Insights into the Diversity and Population Structure of Predominant Typhlocybinae Species Existing in Vineyards in Greece. Insects 2023, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, D. General Morphology of Leafhopper nymphs of the subfamily Deltocephalinae (Hemiptera: Cicadellidae). Acta Entomol. Slov. 2002, 10, 65–82. [Google Scholar]
- Dmitriev, D. Nymphs of some Nearctic leafhoppers (Homoptera, Cicadellidae) with description of a new tribe. ZooKeys 2009, 29, 13–33. [Google Scholar] [CrossRef]
- Antonatos, S.; Papachristos, D.P.; Kapantaidaki, D.E.; Lytra, I.C.; Varikou, K.; Evangelou, V.I.; Milonas, P. Presence of Cicadomorpha in Olive Orchards of Greece with Special Reference to Xylella fastidiosa Vectors. J. Appl. Entomol. 2020, 144, 1–11. [Google Scholar] [CrossRef]
- Tsagkarakis, A.E.; Afentoulis, D.G.; Matared, M.; Thanou, Z.N.; Stamatakou, G.D.; Kalaitzaki, A.P.; Tzobanoglou, D.K.; Goumas, D.; Trantas, E.; Zarboutis, I.; et al. Identification and Seasonal Abundance of Auchenorrhyncha with a Focus on Potential Insect Vectors of Xylella fastidiosa in Olive Orchards in Three Regions of Greece. J. Econ. Entomol. 2018, 111, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Thanou, Z.; Stamouli, M.; Magklara, A.; Theodorou, D.; Stamatakou, G.; Konidis, G.; Koufopoulou, P.; Lyberopoulos, C.; Tribonia, S.; Vetsos, P.; et al. Faunistic Study of Auchenorrhyncha in Olive Orchards in Greece, Including First Records of Species. Agronomy 2024, 14, 2792. [Google Scholar] [CrossRef]
- Nickel, H.J. The Leafhoppers and Planthoppers of Germany (Hemiptera, Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects; Pensoft Publishers: Sofia, Bulgaria, 2003; pp. 30–174. [Google Scholar]
- Gnezdilov, V.M.; Drosopoulos, S. Review of the subgenus Semirodus Dlabola of the genus Mycterodus Spinola (Homoptera: Issidae). Ann. Société Entomol. Fr. 2004, 40, 235–241. [Google Scholar] [CrossRef][Green Version]
- Dlabola, J. Neue griechische ZIkadenarten der Fam. Cixiidae, Issidae und CIcadellidae (Homoptera, Auchenorrhyncha). Acta Faun. Entomol. Musei Natl. Pragae 1980, 16, 5–13. [Google Scholar]
- Bosco, D.; Alma, A.; Arzone, A. Studies on Population Dynamics and Spatial Distribution of Leafhoppers in Vineyards (Homoptera: Cicadellidae). Ann. Appl. Biol. 1997, 130, 1–11. [Google Scholar] [CrossRef]
- Guglielmino, A.; Modola, F.; Scarici, E.; Speranza, S.; Buckle, C. The Auchenorrhyncha Fauna (Insecta, Hemiptera) of Villa Lante, Bagnaia (Italy): A Study of an Urban Ecosystem. Bull. Insectol. 2015, 68, 239–253. [Google Scholar]
- Guglielmino, A.; Bückle, C. Remarks on the composition of the Auchenorrhyncha fauna in some moist areas in Southern Apulia (Italy). Biodivers. J. 2015, 6, 309–322. [Google Scholar]
- Dlabola, J. Fortsetzung der Erganzungen zur Issiden-Taxonomie von Anatolien, Iran und Griechenland (Hom. Auch). Acta Entomol. Musei Natl. Pragae 1982, XXXVIII, 113–169. [Google Scholar]
- Gnezdilov, V.M. New combinations and data on distribution for some Mediterranean Issidae (Homoptera, Fulgoroidea). Zool. Inst. 2004, 13, 80. [Google Scholar] [CrossRef]
- Abt, I.; Derlink, M.; Mabon, R.; Virant-Doberlet, M.; Jacquot, E. Integrating multiple criteria for the characterization of Psammotettix populations in European cereal fields. Bull. Entomol. Res. 2018, 108, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, D.; Monaghan, M.T.; Vogler, A.P. DNA-based taxonomy for associating adults and larvae in multi-species assemblages of chafers (Coleoptera: Scarabaeidae). Mol. Phylogenetics Evol. 2007, 44, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Weigand, H.; Beermann, A.J.; Čiampor, F.; Costa, F.O.; Csabai, Z.; Duarte, S.; Geiger, M.F.; Grabowski, M.; Rimet, F.; Rulik, B.; et al. DNA barcode reference libraries for the monitoring of aquatic biota in Europe: Gap-analysis and recommendations for future work. Sci. Total Environ. 2019, 678, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dietrich, C.H.; Zahniser, J.N.; Dmitriev, D.A. Dense sampling of taxa and characters improves phylogenetic resolution among deltocephaline leafhoppers (Hemiptera: Cicadellidae: Deltocephalinae). Syst. Entomol. 2022, 47, 430–444. [Google Scholar] [CrossRef]
- Gnezdilov, V.M.; Konstantinov, F.V.; Bodrov, S.Y. New insights into the molecular phylogeny and taxonomy of the family Issidae (Hemiptera: Auchenorrhyncha: Fulgoroidea). Proc. Zool. Inst. RAS 2020, 324, 146–161. [Google Scholar] [CrossRef][Green Version]
- Albre, J.; Gibernau, M. Diversity and Temporal Variations of the Hemiptera Auchenorrhyncha Fauna in the Ajaccio Region (France, Corsica). Ann. Soc. Entomol. Fr. 2019, 55, 497–508. [Google Scholar] [CrossRef]
- Kapantaidaki, D.E.; Antonatos, S.; Evangelou, V.; Papachristos, D.P.; Milonas, P. Genetic and endosymbiotic diversity of Greek populations of Philaenus spumarius, Philaenus signatus and Neophilaenus campestris, vectors of Xylella fastidiosa. Sci. Rep. 2021, 11, 3752. [Google Scholar] [CrossRef] [PubMed]
- Seabra, S.G.; Rodrigues, A.S.B.; Silva, S.E.; Neto, A.C.; Pina-Martins, F.; Marabuto, E.; Thompson, V.; Wilson, M.R.; Yurtsever, S.; Halkka, A.; et al. Population structure, adaptation and divergence of the meadow spittlebug, Philaenus spumarius (Hemiptera, Aphrophoridae), revealed by genomic and morphological data. PeerJ 2021, 9, e11425. [Google Scholar] [CrossRef] [PubMed]


| Morphotype (na) 1 | Species | Lak | Kal | Nuf | Sweep Net |
|---|---|---|---|---|---|
| 1 | Philaenus signatus (Melichar, 1896) | - | - | - | 4 |
| 2 | Philaenus spumarius (Linnaeus, 1758) | 10 | 4 | 4 | 66 |
| 3 | Neophilaenus (Neophilaenulus) campestris (Fallén, 1805) | 18 | 1 | 1 | 87 |
| 4 | Anoscopus albifrons (Linnaeus, 1758) | 7 | - | - | - |
| 5 | Allygus sp. | - | 1 | 1 | - |
| 6 | Anoplotettix sp. | 1 | 2 | - | - |
| 7 | Anoplotettix fuscovenosus (Ferrari, 1882) | 1 | - | - | - |
| 8 | Balclutha sp. | - | 8 | 1 | - |
| 9 | Balclutha frontalis (Ferrari, 1882) | 3 | 16 | 2 | 3 |
| 10 | Balclutha saltuella (Kirschbaum, 1868) | - | 2 | - | 1 |
| 11 | Cicadulina (Cicadulina) bipunctata (Melichar, 1904) | 2 | 119 | - | 5 |
| 12 | Docotettix cornutus (Ribaut, 1948) | 13 | 8 | 5 | - |
| 13 | Eohardya fraudulenta (Horváth, 1903) | - | 1 | - | 9 |
| 14 | Euscelidius mundus (Haupt, 1927) | 2 | 71 | - | 54 |
| 15 | Euscelis alsia (Ribaut, 1952) | 3 | 71 | 17 | 1 |
| 16 | Euscelis (Euscelis) lineolata (Brullé, 1832) | 23 | 143 | 11 | 404 |
| 17 | Exitianus capicola (Stål, 1855) | 1 | 46 | 3 | 25 |
| 18 | Goniagnathus (Goniozygotes) bolivari (Melichar, 1907) | - | - | 1 | 1 |
| 19 | Goniagnathus (Goniagnathus) brevis (Herrich-Schäffer, 1835) | 1 | 1 | 1 | 3 |
| 20 | Jassargus (Pontojargus) kurdicus (Remane & Schulz, 1976) | - | - | - | 8 |
| 21 | Macrosteles sp. | - | 2 | - | - |
| 22 | Macrosteles quadripunctulatus (Kirschbaum, 1868) | - | 8 | - | 1 |
| 23 | Macrosteles ramosus (Ribaut, 1952) | - | 17 | - | - |
| 24 | Macrosteles sexnotatus (Fallén, 1806) | - | 3 | - | - |
| 25 | Maiestas schmidtgeni (Wagner, 1939) | 2 | 15 | 6 | 14 |
| 26 | Melillaia desbrochersi (Lethierry, 1889) | 1 | - | - | 32 |
| 27 | Mocydiopsis sp. | 1 | - | - | - |
| 28 | Neoaliturus (Circulifer) haematoceps (Mulsant & Rey, 1855) | 1 | 5 | - | - |
| 29 | Neoaliturus (Neoaliturus) fenestratus (Herrich-Schäffer, 1834) | - | 7 | - | 11 |
| 30 | Opsius stactogalus (Fieber, 1866) | - | - | 2 | - |
| 31 | Orosius orientalis (Matsumura, 1914) | - | 10 | - | - |
| 32 | Phlepsius intricatus (Herrich-Schäffer, 1838) | - | 10 | 3 | 1 |
| 33 | Psammotettix sp. | - | 22 | 5 | - |
| 34 | Psammotettix alienus (Dahlbom, 1850) | 11 | 181 | 3 | 73 |
| 35 | Psammotettix confinis (Dahlbom, 1850) | - | 2 | - | - |
| 36 | Proceps acicularis (Mulsant & Rey, 1855) | 1 | - | - | 1 |
| 37 | Selenocephalus stenopterus (Signoret, 1880) | 8 | 15 | 19 | 31 |
| 38 | Streptanus (Streptanulus) albanicus (Horváth, 1916) | 1 | - | - | 54 |
| 39 | Synophropsis lauri (Horváth, 1897) | - | 1 | - | - |
| 40 | Thamnotettix zelleri (Kirschbaum, 1868) | 78 | 6 | 153 | 1 |
| 41 | Anaceratagallia (Anaceratagallia) glabra (Dmitriev, 2020) | 6 | 46 | 7 | 15 |
| 42 | Anaceratagallia (Anaceratagallia) ribauti (Ossiannilsson, 1938) | - | 20 | 2 | - |
| 43 | Austroagallia sinuata (Mulsant & Rey, 1855) | 4 | 69 | 1 | 13 |
| 44 | Megopthalmus scabripennis (Edwards, 1915) | 1 | 5 | 3 | - |
| 45 | Anzygina honiloa (Kirkaldy, 1906) | 1 | - | - | - |
| 46 | Arboridia sp. | 3 | 6 | 2 | - |
| 47 | Assymetrasca decedens (Paoli, 1932) | - | 53 | 3 | - |
| 48 | Eupteryx (Eupteryx) gyaurdagica (Dlabola, 1957) | 45 | 6 | 3 | - |
| 49 | Eupteryx (Eupteryx) insulana (Ribaut, 1948) | - | 3 | - | - |
| 50 | Fruticidia sp. | 1 | 2 | - | - |
| 51 | Hauptidia (Hauptidia) provincialis (Ribaut, 1931) | 1 | 1 | - | - |
| 52 | Hebata sp. | 13 | 16 | 11 | - |
| 53 | Hebata (Alboneurasca) decipiens (Paoli, 1930) | - | 216 | 12 | 6 |
| 54 | Lindbergina cretica (Asche, 1980) | 3 | - | - | - |
| 55 | Ribautiana cruciata (Ribaut, 1931) | - | 7 | 2 | - |
| 56 | Zygina sp. | - | 1 | 1 | - |
| 57 | Zygina (Zygina) roseipennis (Tollin, 1851) | - | - | 2 | - |
| 58 | Zyginidia adamczewskii (Dworakowska, 1970) | - | - | 1 | - |
| 59 | Zyginidia pullula (Boheman, 1845) | 3 | 145 | 10 | 10 |
| 60 | Reptalus sp. | - | 1 | 1 | - |
| 61 | Laodelphax striatellus (Fallén, 1826) | - | 10 | - | 11 |
| 62 | Toya (Metadelphax) propinqua (Fieber, 1866) | 2 | 5 | - | 36 |
| 63 | Kelisia sp. | - | - | - | 9 |
| 64 | Kelisia ribauti (Wagner, 1938) | - | - | - | 13 |
| 65 | Kelisia sabulicola (Wagner, 1952) | - | - | - | 2 |
| 66 | Phantia subquadrata (Herrich-Schäffer, 1838) | 1 | 5 | 1 | 3 |
| 67 | Agalmatium flavescens (Olivier, 1792) | 1 | - | - | - |
| 68 | Latilica antalyica (Dlabola, 1986) | 4 | - | - | - |
| 69 | Latilica maculipes (Melichar, 1906) | - | - | - | 5 |
| 70 | Mycterodus sp. | - | - | - | 2 |
| 71 | Rhissolepus aspinosus (Dlabola, 1982) | - | - | - | 8 |
| Morphotype (nn) 1 | Genus | Species | N. (Malaise) | N. (Sweep Net) | Name of Sequence |
|---|---|---|---|---|---|
| 1 | Euscelis | lineolata | 0 | 4 | nymph4 |
| 2 | Euscelidius | mundus | 0 | 16 | nymph1, nymph11 |
| 3 | Euscelis | lineolata, alsia | 25 | 130 | nymph2, nymph9, nymph20 |
| 4 | - | - | 6 | 0 | failed |
| 5 | Psammotettix | - | 0 | 3 | nymph12, nymph18 |
| 6 | Melillaia | desbrochersi | 0 | 48 | nymph5, nymph7, nymph17, nymph19, nymph21 |
| 7 | Hecalus | glaucescens | 0 | 1 | - |
| 8 | Melillaia | desbrochersi | 3 | 2 | nymph16 |
| 9 | Rhissolepus | aspinosus | 1 | 34 | nymph3 |
| 10 | - | - | 3 | 548 | failed |
| 11 | - | - | 0 | 11 | nymph8 |
| 12 | Euscelis | alsia | 0 | 6 | nymph13 |
| 13 | Streptanus | albanicus | 0 | 22 | nymph6 |
| 14 | Melillaia | desbrochersi | 0 | 7 | nymph14 |
| 15 | Cicadella | viridis | 0 | 1 | - |
| 16 | Streptanus | albanicus | 0 | 2 | nymph15 |
| 17 | Maiestas | schmidtgeni | 0 | 2 | - |
| 18 | Opsius | stactogalus | 0 | 6 | - |
| 19 | Exitianus | capicola | 0 | 1 | nymph10 |
| Total | 38 | 844 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanou, Z.; Bouga, M.; Papadoulis, G.; Tsagkarakis, A. Identification of Auchenorrhyncha Nymphs Using DNA Barcoding and Phylogenetic Analysis of the Most Common Genera Collected in Olive Fields. Diversity 2025, 17, 496. https://doi.org/10.3390/d17070496
Thanou Z, Bouga M, Papadoulis G, Tsagkarakis A. Identification of Auchenorrhyncha Nymphs Using DNA Barcoding and Phylogenetic Analysis of the Most Common Genera Collected in Olive Fields. Diversity. 2025; 17(7):496. https://doi.org/10.3390/d17070496
Chicago/Turabian StyleThanou, Zoi, Maria Bouga, Georgios Papadoulis, and Antonios Tsagkarakis. 2025. "Identification of Auchenorrhyncha Nymphs Using DNA Barcoding and Phylogenetic Analysis of the Most Common Genera Collected in Olive Fields" Diversity 17, no. 7: 496. https://doi.org/10.3390/d17070496
APA StyleThanou, Z., Bouga, M., Papadoulis, G., & Tsagkarakis, A. (2025). Identification of Auchenorrhyncha Nymphs Using DNA Barcoding and Phylogenetic Analysis of the Most Common Genera Collected in Olive Fields. Diversity, 17(7), 496. https://doi.org/10.3390/d17070496








