Abstract
(1) Background: The insect male accessory gland (MAG) produces seminal fluid components crucial for male reproduction, analogous to the mammalian prostate. While some insect MAGs exhibit binucleate epithelial cells for luminal volume plasticity, the diversity of cellular arrangements and their functional implications across insects remain largely unknown. (2) Methods: We investigated the cellular architecture of MAG epithelia in various shore bug species (infraorder Leptopodomorpha, Hemiptera) and their mechanisms of multinucleation and potential MAG volume regulation. (3) Results: The MAG epithelia of shore bugs comprise a small number of large, plastic syncytial cells with varying nuclear numbers. We hypothesize that these syncytia facilitate effective MAG volume expansion post-eclosion. Uniquely, MAG shrinkage involves the localized contraction of limited muscle fibers, unlike the systematic contraction of circular muscles in most other insects. We further describe sequential cell fusion during the nymphal stage as the mechanism of multinucleation. (4) Conclusions: The unique syncytial organization of Leptopodomorpha MAG epithelia represents an evolutionary divergence from typical binucleate or mononucleate structures in other insects; it is likely that this enables distinct mechanisms for reproductive fluid storage and evacuation. This study highlights the evolutionary diversity of male reproductive organ morphology and function within insects.
1. Introduction
Exocrine glands, which secrete various substances externally, often exhibit binucleate cells when a large quantity of material needs to be supplied. Examples include mammary gland cells in mice Mus musculus Linnaeus, 1758 (Muridae: Rodentia), salivary gland cells in the assassin bug Rhodnius prolixus Stål, 1859 (Hemiptera: Reduviidae), and male accessory gland (MAG) cells in the fruit fly Drosophila melanogaster Meigen, 1830 (Diptera: Drosophilidae) [1,2,3]. While an increased number of nuclei might seem to suggest greater transcriptional activity and thus higher substance production, it is often observed that cell mass increases proportionally with nuclear number and does not necessarily lead to enhanced substance production [4]. Furthermore, if the goal were simply increased transcription, DNA amplification alone would suffice, implying that binucleation incurs the cost of dividing the nucleus. Therefore, an alternative hypothesis proposes that binucleation enhances cell pliability. This idea is not unreasonable, considering that various muscle cells are commonly multinucleated. Indeed, in D. melanogaster males, the morphological plasticity of the male accessory gland (MAG) between contraction and distension was predicted to be 4% lower in polyploid mononucleate cells compared to binucleate epithelial cells, and 23% lower in paired diploid mononucleate cells with the same ploidy, without significant alteration in gene expression levels [4]. Furthermore, in MAGs temporarily overexpressing the fizzy-related gene during development, where most binucleate cells are replaced by these mononucleate cells, the contraction rate drops to approximately 70% [5]. This strongly suggests that binucleation contributes more to increasing the storage and release capacity of secretions rather than their synthesis.
The insect MAG is considered to be an exocrine gland analogous to the mammalian prostate, responsible for producing and secreting seminal fluid components other than sperm [6,7]. Its roles are diverse, including sperm activation, nutritional provisioning to females, female behavioral control, and producing physiologically active substances to gain an advantage in sperm competition [8,9,10,11,12,13,14,15]. For example, in D. melanogaster, a mutation in the gene for Sex Peptide, a 36-amino acid exocrine peptide synthesized in the MAG, almost completely abolishes its ability to suppress female re-mating 48 h post-copulation, a behavior typically inhibited when mated with wild-type males [16]. Peptides with similar functions have been identified in other insects. Head peptide-1, a 10-amino acid peptide from the MAG of the dengue mosquito Aedes aegypti Linnaeus, 1762 (Diptera, Culicidae), can prevent the paternity of subsequently mated males by up to 38% [17,18]. Similarly, Maccessin, a 56-amino acid peptide recently discovered in the brown planthopper Nilaparvata lugens Stål, 1854 (Hemiptera, Delphacidae), significantly reduces female re-mating, while its knockdown significantly increases it [19]. Generally, it is believed that delivering more of these substances to the female increases male reproductive success [3,20,21], and the MAG is presumed to have undergone various functional evolutions to achieve this. Hemiptera, alongside Drosophila mentioned above, is a distinctive taxonomic group that frequently exhibits binucleation or multinucleation in MAGs [22,23]. However, since both groups have undergone homoplasious evolution, their binucleation may not serve the same purpose. In Hemiptera, elongated binucleate cells can form structures suited for a slender lumen or a distended storage organ in the MAG by altering the nuclear position arrangement [22]. Changes in nuclear arrangement to form larger MAGs from the small, confined space of the nymphal stage have also been identified [23]. Regarding multinucleation, systematic observations are lacking, although some old information is available on Dysdercus fasciatus Signoret, 1861 (Pyrrhocoridae) [24].
In this study, through comparative observations of MAGs from various taxa, we discovered that a unique form of multinucleated MAGs is widely established in species belonging to the infraorder Leptopodomorpha, including Saldula and Teloleuca. We also observed that these MAGs increase in size from the nymphal stage to the mature adult stage, in addition to the peculiar morphology of their outer muscle cells and their mechanism of syncytium formation. We then examined the characteristics of these MAGs in comparison to those of other species. The organization of Hemipteran MAGs is not uniform, making this an interesting and unique morphological study.
2. Materials and Methods
2.1. Shore Bug Species Used in This Study
For comparative studies between evolutionary lineages, it was necessary to use live materials for protein staining, as shown below. For this purpose, the following five species, which are easy to obtain in Japan and can be readily used for observation, were chosen:
- Saldula pallipes Fabricius, 1794 (Hemiptera, Saldidae) (Figure 1A): Takao, Tokyo, Japan, 22 May 2018, by TAY (ID: yA4, aTK10, and aTK14). Suwa, Nagano, Japan, 8 September 2018, and 12 May 2020, by JY/KT (ID: aSW03).
- Teloleuca kusnezowi Lindberg, 1934 (Hemiptera, Saldidae) (Figure 2B): Hamamasu, Hokkaido, Japan, 10 June 2018; 24 June 2021; and 21 June 2022, by TAY/AK/RH (for nymphs) (ID: aH54, aH56, aH85, and aH2051). Hamamasu, Hokkaido, Japan, 14 July 2020, by RN/KT (for adults) (ID: tX0).
- Macrosaldula violacea Cobben, 1985 (Hemiptera, Saldidae) (Figure 2C): Sapporo, Hokkaido, Japan, 16 July 2020, by RNR/KT (ID: tH05 and aH2012).
- Salduncula decempunctata Miyamoto, 1963 (Hemiptera, Saldidae) (Figure 2D): Ishigaki, Okinawa, Japan, 23 June 2019, by JY (ID: y1–2, y1–3, and aIG11).
- Corallocoris satoi Miyamoto, 1963 (Hemiptera, Omaniidae) (Figure 2E): Nago, Okinawa, Japan, 30 June 2019, by MO/NI/KT (ID: t1.10 and aOK2501).
2.2. Husbandry of T. kusnezowi Nymphs and Adults
The collected T. kusnezowi nymphs were reared at 25 °C on 1% agar medium S-6 (Ina Food Industry Co., Ltd., Nagano, Japan). This medium contained 5% of the isotonic beverage Pocari Sweat (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) as a mineral source and 0.1% methyl parahydroxybenzoate (Sigma-Aldrich Co. LLC, St Louis, MO, USA) as an antifungal agent. Commercially available frozen chironomid larvae (Kyorin Co., Ltd., Hyogo, Japan) were fed to the T. kusnezowi nymphs and adults several times per week.
2.3. Dissection, Optical Microscopy, and Fixation of MAGs
Whole reproductive organs were dissected from the abdomens of adult bugs or nymphs in standard phosphate-buffered saline (PBS). Dissections were performed using fine forceps (Dumont #5, Fine Science Tools, Foster City, CA, USA) and Vannas spring scissors (#15002-08, Fine Science Tools) under a binocular microscope (Leica Microsystems, Wetzlar, Germany). The excised organs were photographed using a VHX-2000 digital microscope (Keyence, Tokyo, Japan). Subsequently, the organs were fixed in ultra-pure formaldehyde (16%, Polysciences, Warrington, PA, USA) diluted 4-fold (final 4%) with PBS (pH 7.4) for 20–60 min with intermittent mixing at room temperature.
2.4. Immunofluorescence of MAG
After fixation, the organs were thoroughly washed in PBT (PBS containing 0.1% Triton X-100; #12967, Nacalai Tesque, Japan). The following reagents were used for tissue staining: DAPI (0.1 mg/mL, 4′,6-diamidino-2-phenylindole, #D8417, Sigma-Aldrich, St. Louis, MO, USA) for nuclear staining. Rhodamine-labeled phalloidin (#R415, Thermo Fisher Scientific Inc., Waltham, MA, USA) was used at a 1:500 dilution for F-actin staining. A 1:200 dilution of rabbit anti-p-H3 antibody (#05-806, Sigma-Aldrich) was used to stain phospho-histone H3 on chromosomes in the mitotic phase. Additionally, a 1:200 dilution of Alexa Fluor 647-labeled anti-rabbit IgG (#111-605-003, Jackson ImmunoResearch, West Grove, PA, USA) was used. Staining procedures followed previously described methods [25].
2.5. Laser Confocal Microscopy
Laser confocal microscopic images of the optical tissue sections were acquired using FV1000, FV3000 (Olympus, Tokyo, Japan), or Digital-Eclipse C1Si (Nikon, Tokyo, Japan) systems. For overall views of MAGs, multiple image pieces were stitched together using FV31S-SW version 2.6.1.243 software (Evident Scientific, Tokyo, Japan).
2.6. Transmission Electron Microscopy (TEM)
Fixation: Samples were fixed overnight at 4 °C in a solution containing 2% paraformaldehyde (PFA) and 2% glutaraldehyde (GA) in 0.1 M cacodylate buffer (pH 7.4). Following primary fixation, samples were washed three times for 30 min each in 0.1 M cacodylate buffer, and then postfixed in 2% osmium tetroxide (OsO4) in 0.1 M cacodylate buffer at 4 °C for 2 h. Dehydration: Samples were dehydrated through a graded ethanol series: 50% and 70% ethanol for 30 min each at 4 °C, followed by 90% ethanol for 30 min at room temperature (RT). Dehydration was completed with three 30 min changes in 100% ethanol at RT. Infiltration: Infiltration was performed by immersing samples twice in propylene oxide (PO) for 30 min each. Samples were then transferred to a 70:30 mixture of PO and Quetol-812 resin (Nisshin EM Co., Tokyo, Japan) for 1 h, after which the tubes were uncapped to allow PO to volatilize overnight. Embedding and Polymerization: Samples were then transferred to fresh 100% resin and polymerized at 60 °C for 48 h. Ultra-thin Sectioning and Staining: Ultra-thin sections (70 nm) were cut from the polymerized resin blocks using a diamond knife on an ultramicrotome (Ultracut UCT; Leica, Vienna, Austria) and mounted on copper grids. Sections were stained with 2% uranyl acetate at RT for 15 min, washed with distilled water, and then counterstained with Lead stain solution (Sigma-Aldrich Co., Tokyo, Japan) at RT for 3 min. Observation and Imaging: Grids were observed using a transmission electron microscope (JEM-1400Plus; JEOL Ltd., Tokyo, Japan) operated at an acceleration voltage of 100 kV. Digital images (3296 × 2472 pixels) were captured with a CCD camera (EM-14830RUBY2; JEOL Ltd., Tokyo, Japan).
2.7. Nucleotide Sequencing of mtCOI and Phylogenetic Analysis
To support the taxonomic identification of naturally collected bugs, DNA samples were prepared from a few legs or a part of the carcass of each bug using a NucleoSpin DNA Insect DNA extraction kit (Macherey-Nagel, Düren, Germany). To obtain representative barcode sequences, mitochondrial cytochrome oxidase subunit I (mtCOI) gene fragments were amplified by polymerase chain reaction (PCR) using LCO1490 and HCO2198 primers [26,27]. The PCR conditions were as follows: denaturation at 94 °C for 30 s, annealing at 45–55 °C for 90 s, extension at 72 °C for 60 s, and 40 cycles. The thermostable DNA polymerase used here was EmeraldAmp PCR Master Mix (Takara Bio Inc., Kusatsu, Japan). If this did not work, we performed another PCR using the primers tRWF1 and LepR1 [28,29] under the following conditions: denaturation at 98 °C for 10 s, annealing at 48 °C for 30 s, extension at 72 °C for 30 s, and 30 cycles. The thermostable DNA polymerase used here was ExTaq (Takara Bio Inc., Kusatsu, Japan). Nucleotide sequences of the PCR products were analyzed by Eurofins Genomics Japan (Tokyo, Japan).
Using the obtained nucleotide sequences of four Saldid species, we constructed a multiple alignment across the 606 nucleotide segment using the default parameters of ClustalW in the program MEGA-X [30]. Based on this alignment, a phylogenetic tree was conducted using the Maximum Likelihood method. The bootstrap tests were performed with 1000 replicates.
3. Results
3.1. Discovery of Syncytial Epithelium in the MAG of Saldula pallipes
While observing the diversity of MAG morphology across various insect species, we focused on specimens from the infraorder Leptopodomorpha. In mature male Saldula pallipes (Figure 1A), a pair of spindle-shaped MAGs, smaller than the testes, originates near the confluence with the seminal vesicle (Figure 1B). Confocal microscopic observation of this epithelium revealed a remarkable morphology: numerous nuclei were present within irregularly shaped regions enclosed by cell membranes, visualized by F-actin detection (Figure 1C,C’). These multiple nuclei appeared to be arranged in a horizontal plane, seemingly maximizing the volume of the MAG cavity (Figure 1C’’,C’’’). Conversely, the typical circular muscles which usually contract the MAG during copulation were absent in this species’ MAGs. Instead, several irregularly shaped longitudinal muscles were attached to the MAG surface, with their fibers not contacting all cells (Figure 1C’’’’,G).

Figure 1.
Syncytial epithelium in MAGs of Saldula pallipes. (A) Habitus of a dissected adult male. (B) Whole male reproductive system. EG: External genitalia; MAG: male accessory gland; SV: seminal vesicle; TE: testis. (C–C’’’’) Laser confocal images of the MAG. (C,C’) Superficial views; (C’’,C’’’) sectional views; (C’,C’’’) are magnified images of the boxed areas in (C,C’’), respectively. (C’’’’) Outer muscles. Magenta indicates DNA staining with DAPI; green indicates F-actin staining with phalloidin (also applicable to (E,F)). (D) and (D’): A representative TEM image of an ultra-thin section of the MAG epithelium. Nuclei are pseudocolored in magenta. (D’) is a magnified image of the boxed area in (D). No plasma membranes were observed between the two nuclei. (E) An example of adjacent multinucleated populations showing distinct properties (outlined with broken lines). The left population is composed of low-density nuclei with weakly stained DNA, whereas the right population is composed of high-density nuclei with strongly stained DNA, suggesting synchronous chromatin condensation within the single population. (F) An example of apoptotic nuclei surrounded by a plasma membrane (asterisk). All nuclei within a single population synchronously show nuclear fragmentation. (G) Schematic diagram of outer muscles of Saldula’s and general MAGs.
Concerned that not all cell membranes might be visible under F-actin staining, as some cell membranes in various tissues had sparse F-actin lining, we examined cross-sections of the epithelium using TEM (Figure 1D,D’ and Supplementary Figure S1). We studied a total of 13 cells within a single MAG, showing endoplasmic reticulum membranes between the numerous nuclei but no cell membranes whatsoever, strongly suggesting that these cells are multinucleate. The irregular number of nuclei per cell, ranging from 9 to 41, suggested that these were not coenocytes formed by endomitosis or nuclear division, but rather syncytia formed by cell fusion, as subsequently demonstrated. Detailed observations of individual cells revealed instances where nuclear spacing and chromatin condensation were synchronized among the multiple nuclei within a single cell but differed between adjacent cells (Figure 1E). Additionally, in cells undergoing apoptosis, all nuclei within the multinucleated cell exhibited synchronized nuclear fragmentation (Figure 1F). These observations are consistent with the interpretation that multiple nuclei share a common cytoplasm.
3.2. Diversity of MAG Epithelial Multinucleation Across Various Taxa in Leptopodomorpha
We investigated the evolutionary diversity of this unique syncytial epithelium. Figure 2A illustrates the phylogenetic relationships of five genera based on our mtCOI sequence analysis, although it is not entirely consistent with the published results [31,32]. We compared the tissue and cellular morphologies among representative species of these genera. Teloleuca shared many characteristics with Saldula, including the overall external appearance of the internal reproductive organs, the multinucleated state of the MAG epithelia, and the arrangement of muscle fibers, differing only slightly in the aspect ratio of MAG morphology (approximately 2 in Teloleuca and 3 in Saldula) (Figure 2B’–B’’’’). T. kusnezowi, the species used from this genus, was larger in individual and organ size (Figure 2B,B’) than S. pallipes and the following species, and its MAG contained an overwhelmingly greater number of cells, making it suitable for subsequent developmental studies due to the ease of observing cell division and fusion events.
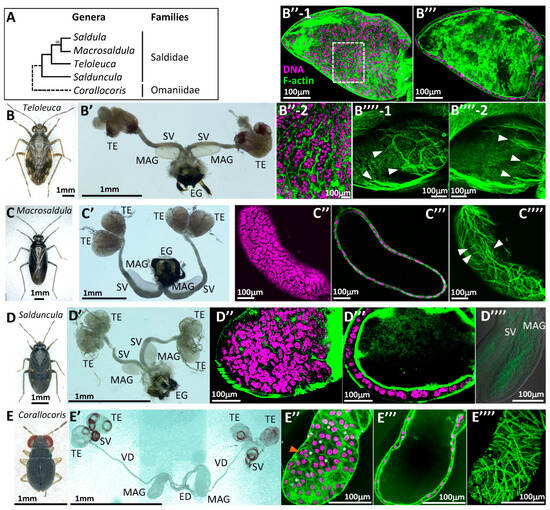
Figure 2.
Comparison of epithelial MAG morphology among various taxa in Leptopodomorpha. (A) Summary of phylogenetic relationships of five genera belonging to two Leptopodomorpha families according to our analysis of mtCOI nucleotide sequences. A numeral at the node between the Saldula and Macrosaldula branches denotes bootstrap support. (B–B’’’’) Teloleuca kusnezowi. (C–C’’’’) Macrosaldula violacea. (D–D’’’’) Salduncula decempunctata. (E–E’’’’) Corallocoris satoi. (B–E) Habitus of dissected adult males. (B’–E’) Whole male reproductive organs. ED: Ejaculatory duct; EG: external genitalia; MAG: male accessory gland; SV: seminal vesicle; TE: testis; VD: vas deferens. (B’’–E’’) Superficial laser confocal images of MAG epithelia. Magenta indicates DNA staining with DAPI; green indicates F-actin staining with phalloidin (also applicable to the following photos); (B’’-2) is a magnified image of the boxed area in (B’’-1), showing an extremely irregular cell shape. Asterisks in E’’ indicate trinucleate epithelial cells. An orange arrowhead in E’’ indicates a trinucleate cell, where all nuclei show similarly small diameters and condensed chromatin. (B’’’–E’’’) Sectional views of MAG epithelia. (B’’’’–E’’’’) Outer muscles. (B’’’’-1,B’’’’-2) show different sides of the surface in the same MAG. White arrowheads indicate the termini of muscle fibers.
In Macrosaldula violacea (Figure 2C), the MAG was more elongated (aspect ratio of approx. 4), and it had simple testicular follicles (two pairs per each side), showing differences from the previous two species (Figure 2C’). The MAG of M. violacea was composed of a syncytial epithelium with nuclei arranged in a planar fashion, similar to the aforementioned species (Figure 2C’’,C’’’), but the number of nuclei per cell was not as high as in the previous two species. While the outer muscle fibers were not as sparse as in the previous two species, the circular muscles were still poor, and thin longitudinal muscles were present (Figure 2C’’’’). Salduncula decempunctata (Figure 2D), similarly to Macrosaldula, had two pairs of testicular follicles per each side and small MAGs (approx. 250 μm) with fewer cells. However, various features were fundamentally consistent with the preceding three species (Figure 2D’–D’’’’).
Among the specimens examined, an intertidal dwarf bug Corallocoris satoi (Figure 2E) was the only species that did not belong to Saldidae. In this species, the seminal vesicle was located directly beneath the two pairs of testicular follicles per each side, and a long vas deferens reached the ejaculatory duct, where it merged with the MAG, which had a narrow, tubular base (Figure 2E’). This morphology was distinct from the Saldidae species mentioned above. The MAG epithelia consisted of a population of binucleate cells, but, uniquely, trinucleate cells were frequently interspersed (approximately 30%), showing a multinucleated state not observed in other species (Figure 2E’’,E’’’). The outer muscles resembled the standard circular muscles found in other insects, but their arrangement and spacing were not as orderly as in other insects, exhibiting some similarities with the Saldidae species discussed above (Figure 2E’’’’).
3.3. Expansion of MAG Luminal Volume During Adult Maturation
In Aphelocheirus vittatus, a benthic water bug species within Heteroptera, the MAG rapidly increases in size after adult eclosion due to feeding, similar to many other insects. During this process, the binucleate cell population forming the MAG increases the luminal volume by changing the angle of the binucleate epithelial surface from vertical to horizontal [23]. Therefore, we investigated whether the arrangement of nuclei within the MAG syncytia of Saldidae changes during maturation.
Our results showed that in both Saldula (Figure 3A,A’) and Teloleuca (Figure 3B,B’), the multiple nuclei contained within a single syncytium of a teneral adult were not arranged in a horizontal plane like those in a mature adult. Instead, they were highly stacked towards the lumen of the MAG. This stacking reached a maximum of about five nuclear layers in Saldula and about seven nuclear layers in Teloleuca. On the other hand, the outer muscle fibers, which were sparsely present in mature adults, were only found in considerable numbers on one side of the dorsoventral surface in teneral adults (Figure 3B’’-1,B’’-2). However, their thickness was extremely poor, differing in appearance from the standard circular muscles of other insects.

Figure 3.
Arrangement of multiple nuclei of MAG epithelial cells during expansion of MAGs after feeding. (A,A’) Saldula pallipes. (B–B’’) Teloleuca kusnezowi. (A,B) Superficial views. (A’,B’) Sectional views. (B’’-1,B’’-2) Outer muscles on each side of the MAG. Magenta indicates DNA staining with DAPI; green indicates F-actin staining with phalloidin. (C) Schematic representation of the transverse sections of the MAG before and after feeding in Saldula and Teloleuca. Diameters of the MAG cavity (right green) on confocal sections increase 8 times (Saldula) and 9 times (Teloleuca) after growth due to feeding. Green segments on the circumference of the MAGs represent fragmented outer muscles. (D) Similar schematic representation in the case of Aphelocheirus according to the reference. The diameter of the MAG cavity increases 4 times after growth due to feeding. The green continuous circumferences of the MAGs represent the outer circular muscles.
We predict that if the highly stacked nuclei spread into a horizontal plane with maturation due to feeding, the cross-sectional area of the cell, and consequently the volume of the MAG lumen, would significantly increase (Figure 3C). Indeed, measuring the diameter of the cavity in Figure 1C’’, Figure 2B’’’ and Figure 3A’,B’ showed an 8.1-fold increase in Saldula (266 μm/33 μm) and a 9.1-fold increase in Teloleuca (578 μm/63 μm) between teneral and mature adults. For comparison, the same measurement in Aphelocheirus (mentioned above) showed a 4.3-fold increase (144 μm/34 μm) (Figure 3D). Therefore, multinucleation is predicted to be an effective method for expanding cavity volume during growth.
3.4. Mechanism of Multinucleation in Teloleuca MAG Epithelia
To investigate how multinucleation is achieved in this syncytial epithelium, we dissected final-instar nymphs of T. kusnezowi, which have the highest total nuclear count (Figure 4A,B). We confirmed that these nymphs were the same species as the adults based on their mtCOI nucleotide sequences.
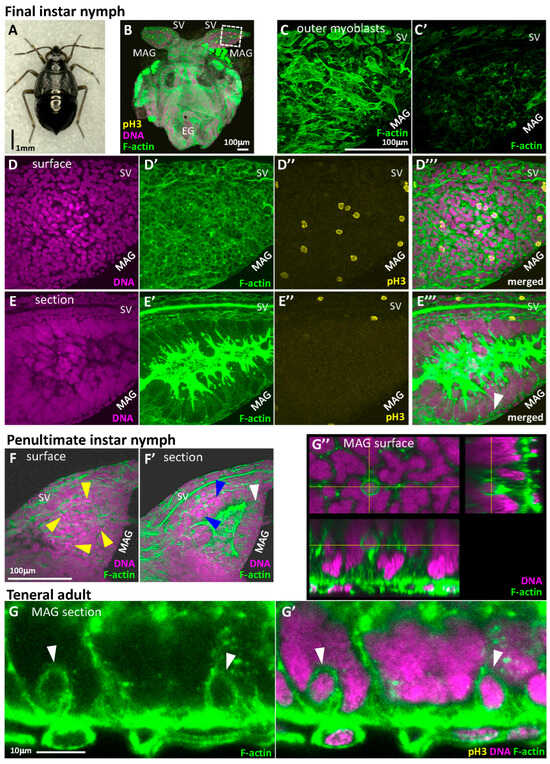
Figure 4.
Syncytium formation by consecutive cell fusion in nymphal MAG epithelia of T. kusnezowi. (A) Habitus of a dissected final-instar male nymph. (B) A laser confocal image of developing male reproductive organs. The boxed area in the developing MAG and SV is magnified in (C–E’’’). EG: External genitalia; MAG: male accessory gland; SV: seminal vesicle. Magenta indicates DNA staining with DAPI; green indicates F-actin staining with phalloidin; yellow indicates phospho-Histone H3 antibody staining (also applicable to the following photos). (C,C’) Outer myoblasts of MAG. (C,C’) show different sides of the same MAG. One side of the MAG surface is temporarily paved with myoblasts while the other side is not. (D–D’’’) Superficial views. (E–E’’’) Sectional views. (D,E) DNA staining with DAPI. (D’,E’) F-actin staining with phalloidin. Unlike the adult MAGs, the surface of the nymphal developing MAGs is covered with a mono- or stratified epithelia composed of diploid cells, while syncytial cells are present inside, as in adult MAGs. (D’’,E’’) Nuclei in mitotic phase stained with phospho-Histone H3 (pH3) antibody. Mitotic nuclei can only be seen in the outer epithelium composed of diploid cells but not in all syncytia. (D’’’,E’’’) Merged images with three colors. The white arrowhead in (E’’’) indicates the diploid cell just prior to cell fusion. (F) and (F’) Superficial (F) and sectional (F’) views in the developing MAG of a penultimate-instar nymph. Yellow arrowheads in F indicate the condensed chromosomes aligned on metaphase plates shown in the proliferative epithelium with diploid cells. In (F’), multinucleation can already be observed although the numbers of nuclei per cell are fewer (blue arrowheads). A diploid cell just prior to cell fusion can also be observed at this stage (white arrowhead). (G–G’’) One-by-one cell fusion from the diploid cell epithelium to large internal syncytia in teneral adults, in which mitotic-phase cells are no longer observed. (G) F-actin staining. (G’) Merged with DNA and F-actin staining. White arrowheads in (G,G’) indicate the diploid cells just incorporated into the syncytia. (G’’) The 3D images of a fusing cell inside the syncytial cell. Circular F-actin staining indicates the fusing cell. The bottom and right images in (G’’) are optically reconstituted sections of the yellow axes from the Z-stack collection of multiple confocal images.
The outer muscles of these nymphal MAGs differed from those of mature adults; myoblasts almost completely covered only one side of the dorsoventral surfaces (Figure 4C), which can be understood as a precursor stage to the muscle distribution in teneral adults (Figure 2B’’’’-1,B’’’’-2). The epithelial cells also differed from those of mature adults. In addition to the multinucleated epithelium already present in mature adults (though with fewer nuclei), another epithelium, composed of cells each with a single diploid nucleus, covered the outer side (Figure 4D,D’,E). This was not necessarily a monolayer; in some areas (especially on the side contacting the seminal vesicle), it formed a stratified epithelium (Figure 4E’).
To identify the locations of dividing nuclei, we used an anti-phospho-Histone H3 antibody to detect nuclei in the mitotic phase. Interestingly, dividing nuclei were found to be localized and scattered within the outer diploid cell layer (Figure 4D,D’’,E,E’’). While the irregular number of nuclei per syncytium in Teloleuca (17–39) suggested that multinucleation occurred via cell fusion, nuclear division within the multinucleated cells was never observed despite multinucleation being ongoing. This suggests that multinucleation progresses through cells fusing one at a time from the outer proliferating layer. Indeed, round-shaped interphase cells often detached from the outer cell layer on the inner surface of the diploid cell layer (white arrowhead in Figure 4E’’’), which were considered to be undergoing fusion. This proliferation and fusion were also observed in the previous instar (Figure 4F,F’), but it was not possible to determine when this phenomenon began, as obtaining younger nymphs is challenging (we did not succeed in hatching eggs after overwintering.). After adult eclosion, proliferating cells were no longer found, but cell fusion continued until the external diploid cell layer disappeared (Figure 4G,G’). During this period, the MAG epithelial structure became simpler, making cell fusion easier to observe. The diploid cells undergoing fusion at interfaces are thought to lose the hexagonal shape and apicobasal polarity typically found in single-layer epithelial cells, becoming rounded from all perspectives (Figure 4G’’). Therefore, if the numerous and widely distributed round cells in the nymphal diploid epithelial cell layer (Figure 4D’) are not in the mitotic phase, they are considered to be cells undergoing fusion.
4. Discussion
4.1. Evolutionary Diversity of MAG Syncytia in Hemiptera
The syncytial epithelia of the male accessory gland (MAG) reported in this study are a common feature in Saldidae but are rare in other taxa. While the MAG epithelia of Heteroptera are typically composed of binucleate cells [22,23], the first instance of a MAG where all cells were stable multinucleate cells was discovered over 40 years ago through TEM observations of Dysdercus fasciatus [24]. This is considered the earliest record of broad multinucleation in Hemipteran MAG epithelia. However, the number of nuclei per cell in D. fasciatus was only 3–5, significantly fewer than the 9–41 nuclei in S. pallipes or 17–39 in T. kusnezowi reported in this study. Furthermore, the complex, branched morphology of MAGs in D. fasciatus differs greatly from the simple, sac-like structure of Saldidae. Thus, it is unlikely that the homoplasiously evolved multinucleation in these two groups serves the same purpose.
We also found scattered multinucleate cells within the binucleate cell populations of MAGs across a wide range of Heteropteran suborders, including Enicocephalomorpha, Cimicomorpha, and Pentatomomorpha (JY: unpublished results). However, we hypothesize that these are likely unavoidable errors that occur when MAG epithelial binucleation is achieved by cell fusion, as seen in the bed bug Cimex lectularius Linnaeus, 1758 [22]. This mechanism is distinctly different from the active multinucleation observed in Saldidae. Conversely, the widespread presence of this latent capacity for multinucleation through cell fusion across diverse taxa is likely the foundation upon which the advanced multinucleation of all MAG cells in Saldidae evolved. It is likely that the MAGs of intertidal dwarf bugs Corallocoris are intermediates in this evolutionary pathway, consisting primarily of binucleate cells but with a high frequency (approximately 30%) of trinucleate cells interspersed (Figure 2E’’).
4.2. Possible Function of Syncytial MAGs
The high degree of multinucleation in Saldidae MAG epithelia is undoubtedly an efficient mechanism for the development from a contracted MAG in teneral adults to a distended MAG in mature adults. However, if this were the optimal method for MAG substance storage and expulsion, it would have evolved in many insects. The fact that it has not suggests that this specialized method comes with certain constraints. It is likely that one such constraint is the degeneration of the outer muscles associated with this syncytial epithelium. Due to the significant size difference between immature and mature states (Figure 3C), the circular muscles adhering to the epithelium undergo degeneration beyond their elastic limit, resulting in only a few irregular longitudinal muscles remaining in mature adults (Figure 1C’’’’ and Figure 2B’’’’–D’’’’). This implies that these few muscle fibers would struggle to achieve the high degree of MAG contraction normally brought about by the ubiquitous circular muscles found in most insects, which are essential for expelling as much content as possible during copulation. Indeed, the muscle fibers do not reach all cells.
Therefore, it is possible that this specialized MAG is a method only usable by insects that do not require a large volume of seminal fluid per copulation. While insects often exist with accessory glands larger than their testes, the MAGs of various Saldidae species are less than half the size of their testes (Figure 1B and Figure 2B’–D’) and do not appear to occupy a large volume within the abdominal cavity. On the other hand, we have observed that male Teloleuca can copulate three times in succession immediately after a previous copulation. This suggests that this type of MAG may have the characteristic or constraint of being adapted for continuous copulation involving small amounts of seminal fluid.
4.3. Mechanism of Syncytium Formation
One could hypothesize that syncytia formation occurs through a process similar to binucleation in the bed bug Cimex, where numerous mononucleate cells proliferate, arrest their cell cycle, and then synchronously fuse to achieve multinucleation (Figure 5F–J, [22]). However, in such a scenario, the number of nuclei that can be accommodated within the limited volume of the MAG anlage would be restricted, making it difficult to develop a large MAG.

Figure 5.
Comparison of cell morphologies and bi- or multinucleation processes among three hemipteran taxa. (A–E) Aphelocheirus. (F–J) Cimex. (K–N) Teloleuca. The developmental stages proceed from left to right. Cells to focus on are highlighted. In Aphelocheirus, a clear surge of the final M-phase occurs just before the final molting, and cytokinesis is skipped. In Cimex, the cell cycles seem to be random with a low mitotic index, and the fusion interface between the adjacent cells disappears synchronously just before the final molting. In Teloleuca, one-by-one cell fusion from the diploid cell epithelium to large internal syncytia occurs continuously after at least the penultimate-instar nymphal stage.
Teloleuca adopts a unique mechanism (Figure 5K–N) where the outer surrounding epithelium, composed of diploid cells, proliferates and then sends the newly formed cells one by one into the inner multinucleated cell, thereby stacking nuclei inwards. This allows nuclei to be packed into a small volume and is considered a more rational developmental strategy for enhancing MAG expansion efficiency. While MAG development does not occur in penultimate-instar Cimex nymphs [22], in Teloleuca, MAG development is presumed to begin earlier to allow a longer period of nuclear delivery to the syncytia (Figure 4F,F’). The observed cell fusion process is unusual as it differs from that of Cimex, in which the septa between adjacent cells disappear [22]. However, similar phenomena where cells are engulfed intact are also known, such as the entosis (a type of cell cannibalism) of uterine epithelial cells by syncytiotrophoblast cells seen during implantation in mammals [33].
4.4. Development and Characteristics of Outer Muscles
Another interesting observation is the nature of the myoblast population in the nymphal stage. While restricted to only one of the two dorsoventral sides of the MAG, it spreads out to cover the outer surface (Figure 4C,C’). After adult eclosion, it temporarily exhibits an appearance similar to circular muscles (Figure 3B’’-1) before subsequently degenerating (Figure 2B’’’’-1,2B’’’’-2). The elimination of a structure that was initially formed is often observed in the development of vestigial organs. This muscle degeneration appears to occur randomly, with the number and shape of the remaining muscle fibers varying completely among individual adults. However, a common feature is that most circular muscles do not remain, while many longitudinal or poorly oriented muscle fibers persist, with their termini becoming apparent (Figure 2B’’’’-1,2B’’’’-2).
Consequently, during copulation, the MAG is expected to be pulled in the proximodistal direction, causing the cells to deform as if compressed in that direction (Figure 2B’’-2) and thereby reducing the luminal volume. The degree of shape change flexibility in these multinucleated cells is astonishing; such irregularly shaped cells are not found in the MAG epithelia of other taxonomic groups. Conversely, if these syncytia maintained the hexagonal morphology common to typical epithelia, the overall plasticity of the MAG would be poor, making it difficult for a few muscle fibers to contract the entire MAG. Therefore, it is highly probable that these few muscle fibers and flexible syncytia only function rationally when they are present together.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17070481/s1. Figure S1: TEM images of ultra-thin sections of 12 cells showing multiple nuclei in the MAG epithelium from Teloleuca kusnezowi. Nuclei are pseudocolored in magenta. No plasma membranes were observed between the two nuclei. Scale bar at right bottom photo is 2 m and applicable to all photos.
Author Contributions
Conceptualization, T.A.-Y.; methodology, K.T. and T.I.; software, K.T., J.Y. and T.A.-Y.; validation, T.A.-Y.; formal analysis, T.A.-Y.; investigation, K.T. and J.Y. and R.N.; resources, K.T., J.Y., R.N. and T.A.-Y.; data curation, K.T. and T.A.-Y.; writing—original draft preparation, T.A.-Y.; writing—review and editing, K.T.; supervision, T.A.-Y.; project administration, T.A.-Y.; funding acquisition, T.A.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by Subsidies for Private School Facilities (2019) from MEXT and MEXT-Supported Program for the Private University Research Branding Project 2016–2020 (*Ministry of Education, Culture, Sports, Science and Technology).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank R. Harada, N. Ito, A. Kirioka, and M. Otani for their assistance in collecting insect materials from wild habitats. We also thank Y. Ishihara at Tokai Electron Microscopy, Inc. for his TEM observations.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MAG | Male Accessory Gland |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| mtCOI | Mitochondrial cytochrome oxidase subunit I |
| TEM | Transmission Electron Microscopy |
References
- Rios, A.C.; Fu, N.Y.; Jamieson, P.R.; Pal, B.; Whitehead, L.; Nicholas, K.R.; Lindeman, G.J.; Visvader, J.E. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun. 2016, 7, 11400. [Google Scholar] [CrossRef]
- Borella Marfil Anhê, A.C.; Maia Godoy, R.S.; Nacif-Pimenta, R.; Barbosa, W.F.; Lacerda, M.V.; Monteiro, W.M.; Costa Secundino, N.F.; Paolucci Pimenta, P.F. Microanatomical and secretory characterization of the salivary gland of the Rhodnius prolixus (Hemiptera, Reduviidae, Triatominae), a main vector of Chagas disease. Open Biol. 2021, 11, 210028. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.J.; Akerkar, G.A.; Ard, R.L.; Gonzalez, C.; Wolfner, M.F. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech. Dev. 1992, 38, 33–40. [Google Scholar] [CrossRef]
- Taniguchi, K.; Kokuryo, A.; Imano, T.; Minami, R.; Nakagoshi, H.; Adachi-Yamada, T. Binucleation of Drosophila Adult Male Accessory Gland Cells Increases Plasticity of Organ Size for Effective Reproduction. Biol. Syst. 2012, 1, e101. [Google Scholar] [CrossRef]
- Taniguchi, K.; Kokuryo, A.; Imano, T.; Nakagoshi, H.; Adachi-Yamada, T. Binucleation of Accessory Gland Lobe Contributes to Effective Ejection of Seminal Fluid in Drosophila melanogaster. Zool. Sci. 2018, 35, 446–458. [Google Scholar] [CrossRef]
- Kaulenas, M. Insect Accessory Reproductive Structures: Function, Structure, and Development; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 31. [Google Scholar]
- Hopkins, B.R.; Allen, S.E.; Avila, F.W.; Wolfner, M.F. Male Reproductive Glands and Their Secretions in Insects, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Chen, P.S.; Stumm-Zollinger, E.; Aigaki, T.; Balmer, J.; Bienz, M.; Bohlen, P. A male accessory gland peptide that regulates reproductive behavior of female D. Melanogaster. Cell 1988, 54, 291–298. [Google Scholar] [CrossRef]
- Aigaki, T.; Fleischmann, I.; Chen, P.-S.; Kubli, E. Ectopic expression of sex peptide alters reproductive behavior of female D. Melanogaster. Neuron 1991, 7, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T. Seminal fluid-mediated fitness traits in Drosophila. Heredity 2001, 87, 511–521. [Google Scholar] [CrossRef]
- Gwynne, G.T. Katydids and Bush-Crickets: Reproductive Behavior and Evolution of the Tettigoniidae; Cornell University Press: Ithaca, NA, USA, 2001. [Google Scholar]
- Lewis, S.M.; Cratsley, C.K.; Rooney, J.A. Nuptial gifts and sexual selection in photinus fireflies. Integr. Comp. Biol. 2004, 44, 234–237. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Kapahi, P.; Anderson, D.J.; Benzer, S. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr. Biol. 2006, 16, 692–696. [Google Scholar] [CrossRef]
- Ravi Ram, K.; Wolfner, M.F. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 2007, 47, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hayashi, F.; Lavine, L.C.; Yang, D. Is diversification in male reproductive traits driven by evolutionary trade-offs between weapons and nuptial gifts? Proc. Biol. Sci. 2015, 282, 20150247. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kubli, E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2003, 100, 9929–9933. [Google Scholar] [CrossRef] [PubMed]
- Naccarati, C.; Audsley, N.; Keen, J.N.; Kim, J.-H.; Howell, G.J.; Kim, Y.-J.; Isaac, R.E. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and transferred to the female during copulation. Peptides 2012, 34, 150–157. [Google Scholar] [CrossRef]
- Duvall, L.B.; Basrur, N.S.; Molina, H.; McMeniman, C.J.; Vosshall, L.B. A peptide signaling system that rapidly enforces paternity in the Aedes aegypti mosquito. Curr. Biol. 2017, 27, 3734–3742. e3735. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Zhang, N.; Bu, R.-T.; Nässel, D.R.; Gao, C.-F.; Wu, S.-F. A novel male accessory gland peptide reduces female post-mating receptivity in the brown planthopper. PLoS Genet. 2025, 21, e1011699. [Google Scholar] [CrossRef]
- Bangham, J.; Chapman, T.; Partridge, L. Effects of body size, accessory gland and testis size on pre-and postcopulatory success in Drosophila melanogaster. Anim. Behav. 2002, 64, 915–921. [Google Scholar] [CrossRef]
- Wigby, S.; Sirot, L.K.; Linklater, J.R.; Buehner, N.; Calboli, F.C.; Bretman, A.; Wolfner, M.F.; Chapman, T. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 2009, 19, 751–757. [Google Scholar] [CrossRef]
- Takeda, K.; Yamauchi, J.; Miki, A.; Kim, D.; Leong, X.Y.; Doggett, S.L.; Lee, C.Y.; Adachi-Yamada, T. Binucleation of male accessory gland cells in the common bed bug Cimex lectularius. Sci. Rep. 2019, 9, 6500. [Google Scholar] [CrossRef]
- Takeda, K.; Yamauchi, J.; Adachi-Yamada, T. Morphological and developmental traits of the binucleation of male accessory gland cells in the benthic water bug, Aphelocheirus vittatus (Hemiptera: Aphelochiridae). J. Insect Sci. 2020, 20, 18. [Google Scholar] [CrossRef]
- Awiti, L.R.S. A strange multinuclear condition in the epithelial cells of the mesadenial accessory reproductive gland of Dysdercus fascjatus SIGNORET. Insect Sci. Appl. 1981, 2, 167–173. [Google Scholar]
- Takeda, K.; Okumura, T.; Taniguchi, K.; Adachi-Yamada, T. Adult Intestine Aging Model. Adv. Exp. Med. Biol. 2018, 1076, 11–23. [Google Scholar] [CrossRef]
- Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Matzen da Silva, J.; Creer, S.; Dos Santos, A.; Costa, A.C.; Cunha, M.R.; Costa, F.O.; Carvalho, G.R. Systematic and evolutionary insights derived from mtDNA COI barcode diversity in the Decapoda (Crustacea: Malacostraca). PLoS ONE 2011, 6, e19449. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Park, D.-S.; Suh, S.-J.; Oh, H.-W.; Hebert, P.D. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genom. 2010, 11, 1–7. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, Y.; Ren, D. New shore bug (Hemiptera, Heteroptera, Saldidae) from the Early Cretaceous of China with phylogenetic analyses. ZooKeys 2011, 130, 185–198. [Google Scholar]
- Wu, Y.-F.; Liu, X.; Zhang, F.; Wang, J.-J. Complete mitochondrial genome of Saldoida armata Horváth, 1911 (Heteroptera: Saldidae) and phylogenetic analysis. Mitochondrial DNA Part B 2024, 9, 1341–1344. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Dey, S.K. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015, 11, 358–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).