Impact of Climate Change on the Distribution of Cinnamomum malabatrum (Laurales—Lauraceae), a Culturally and Ecologically Important Species of Malabar, Western Ghats, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
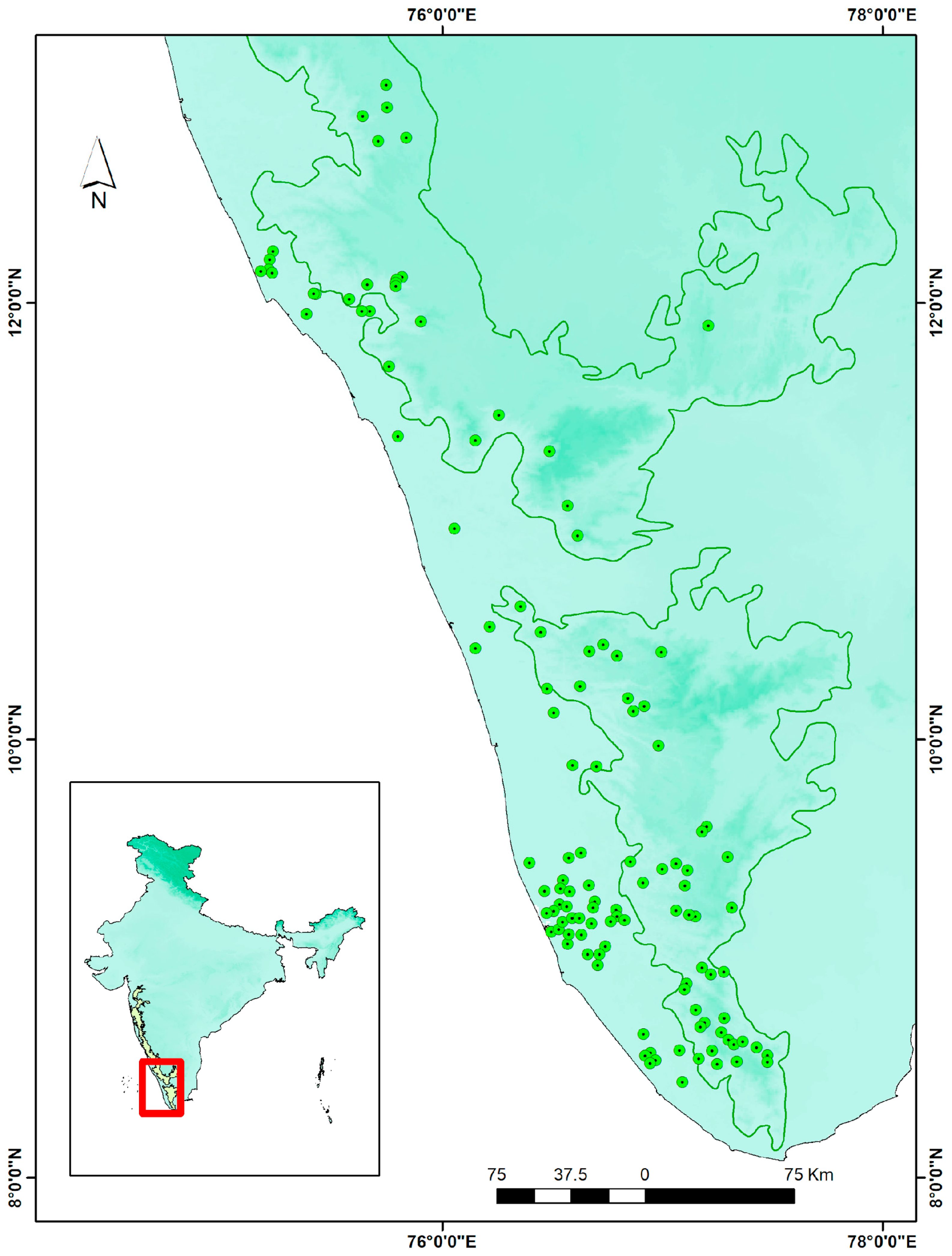
2.2. Species Occurrence and Collection of Field Data
2.3. Elimination of Sampling Biases via Spatial Thinning
2.4. Occurrence Data and the Fundamental Niche
2.5. Environmental Variables
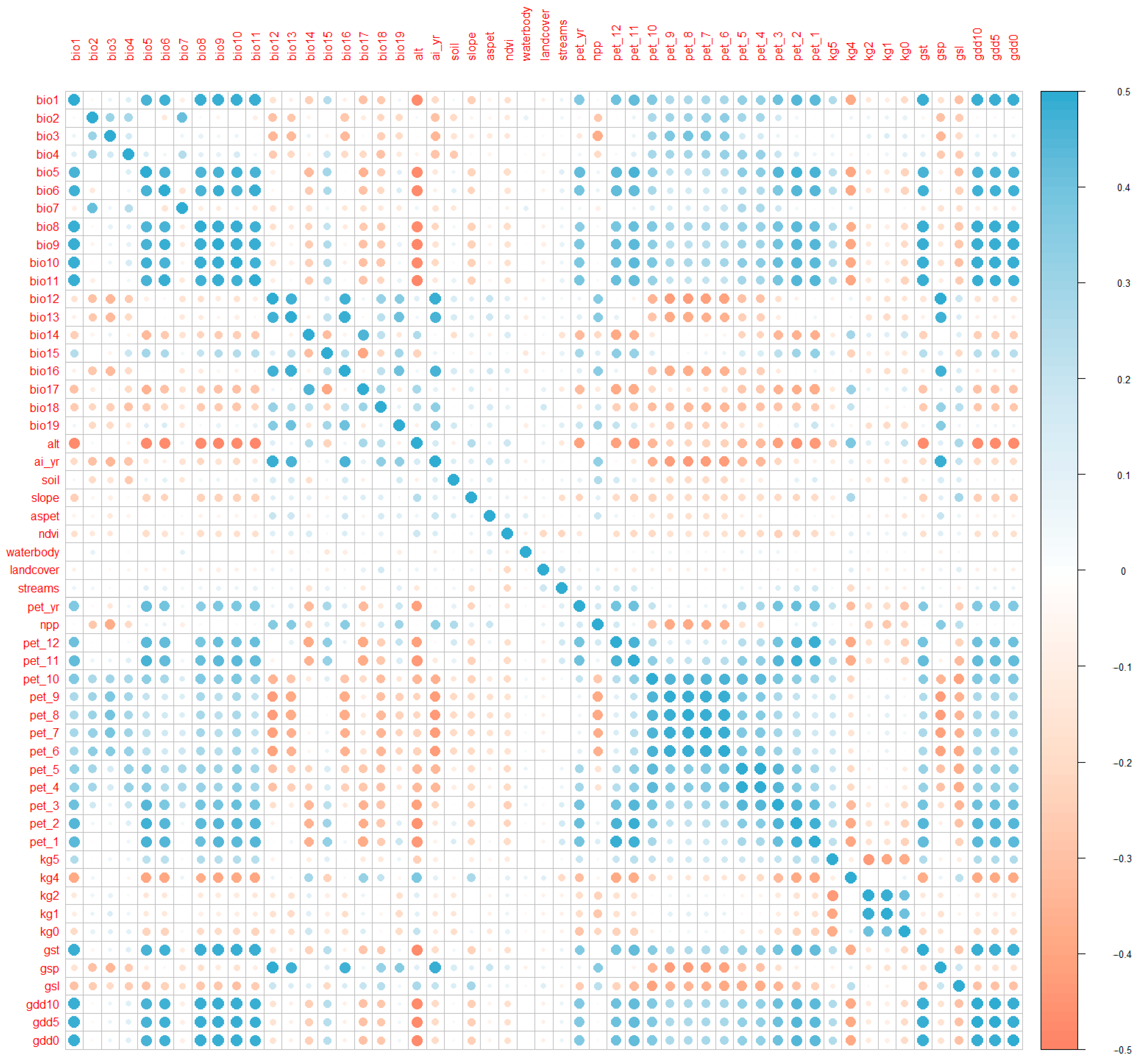
2.6. Multicollinearity
2.7. Shared Socioeconomic Pathways
2.8. MaxEnt Modeling
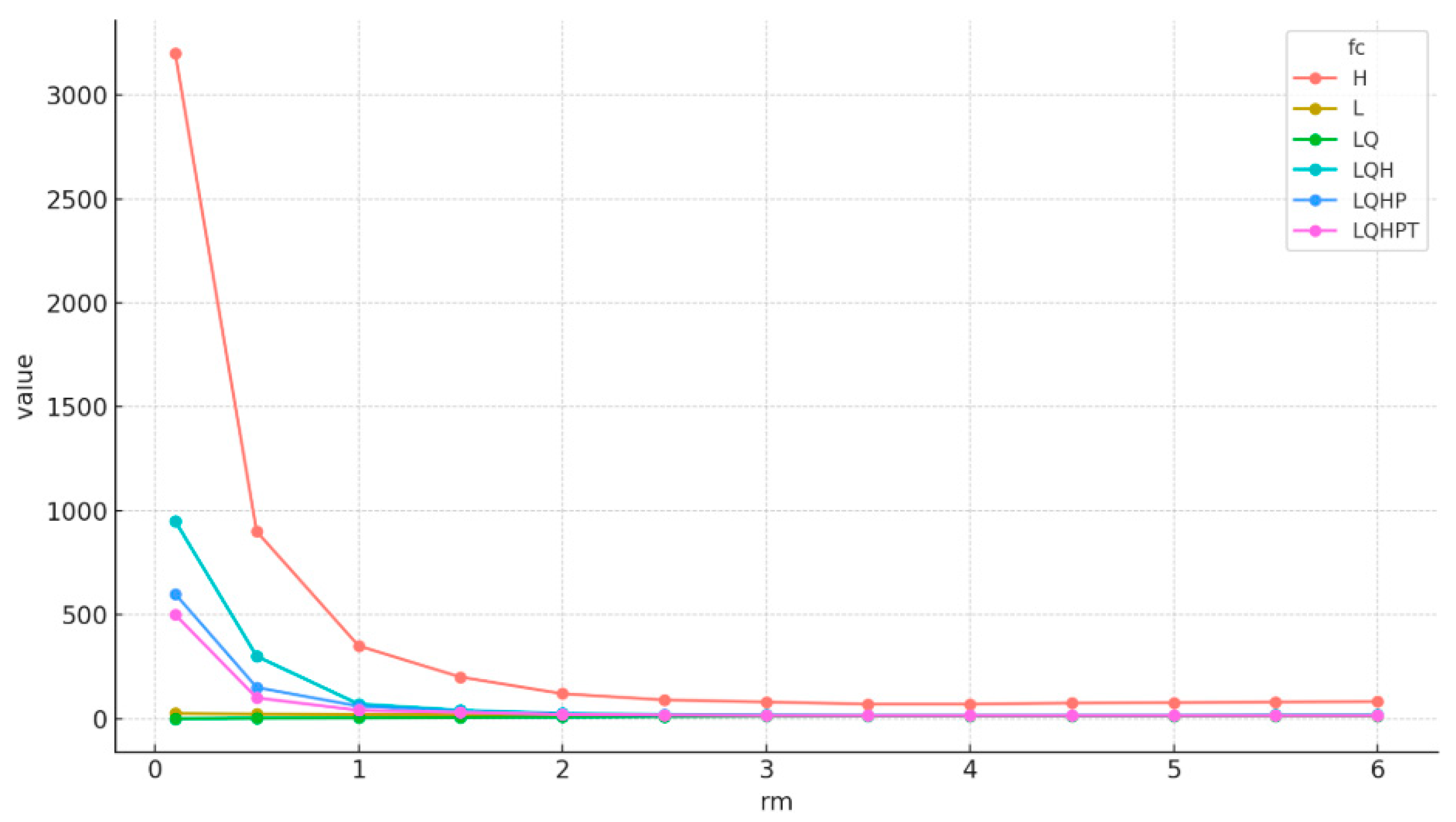
2.9. Justification for Variable-to-Occurrence Ratio in MaxEnt Modeling
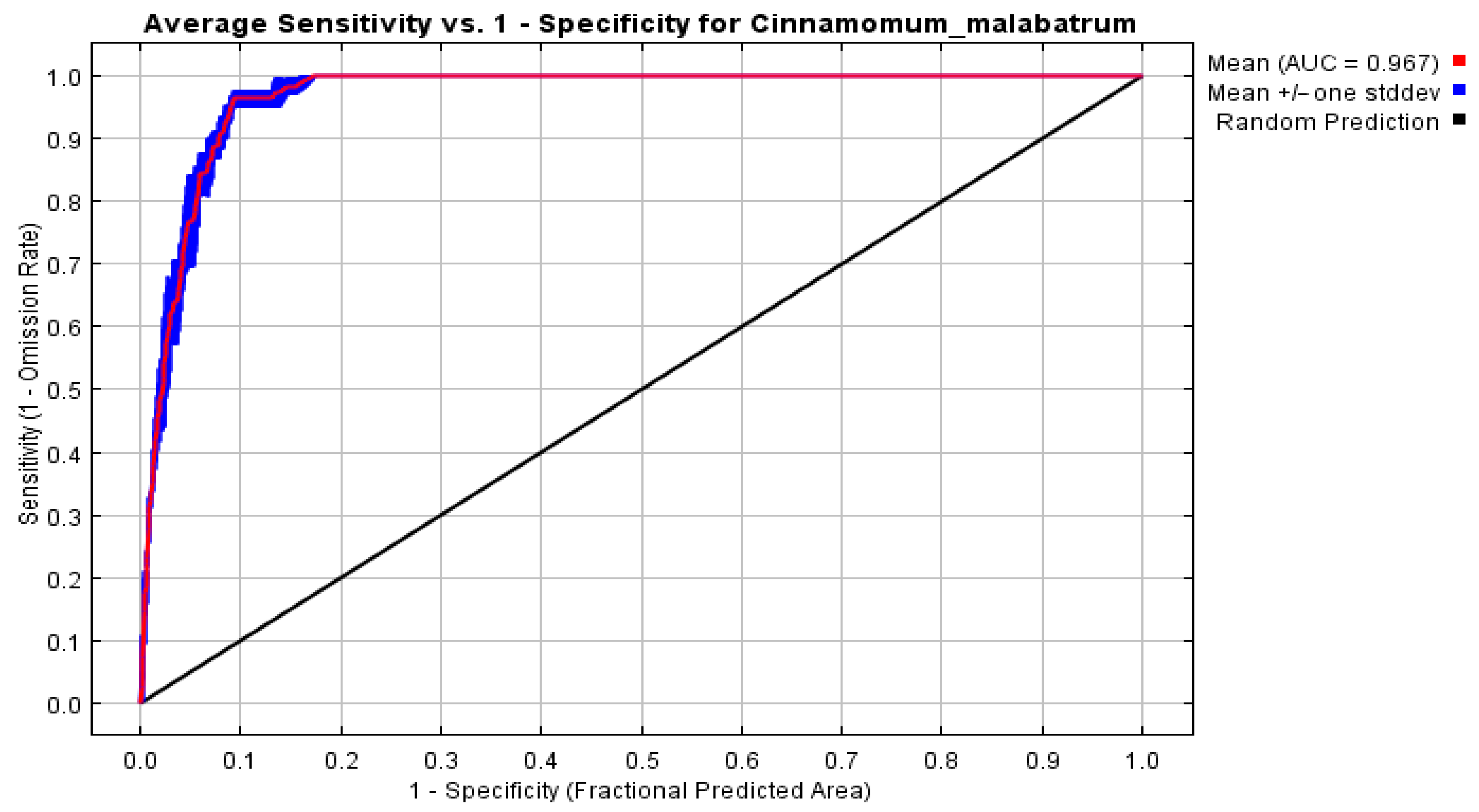
2.10. Model Validation
2.11. Preparation of Species Distribution and Range Shift Maps
3. Results and Discussion
3.1. Optimization and Relative Contributions of the Environmental Variables
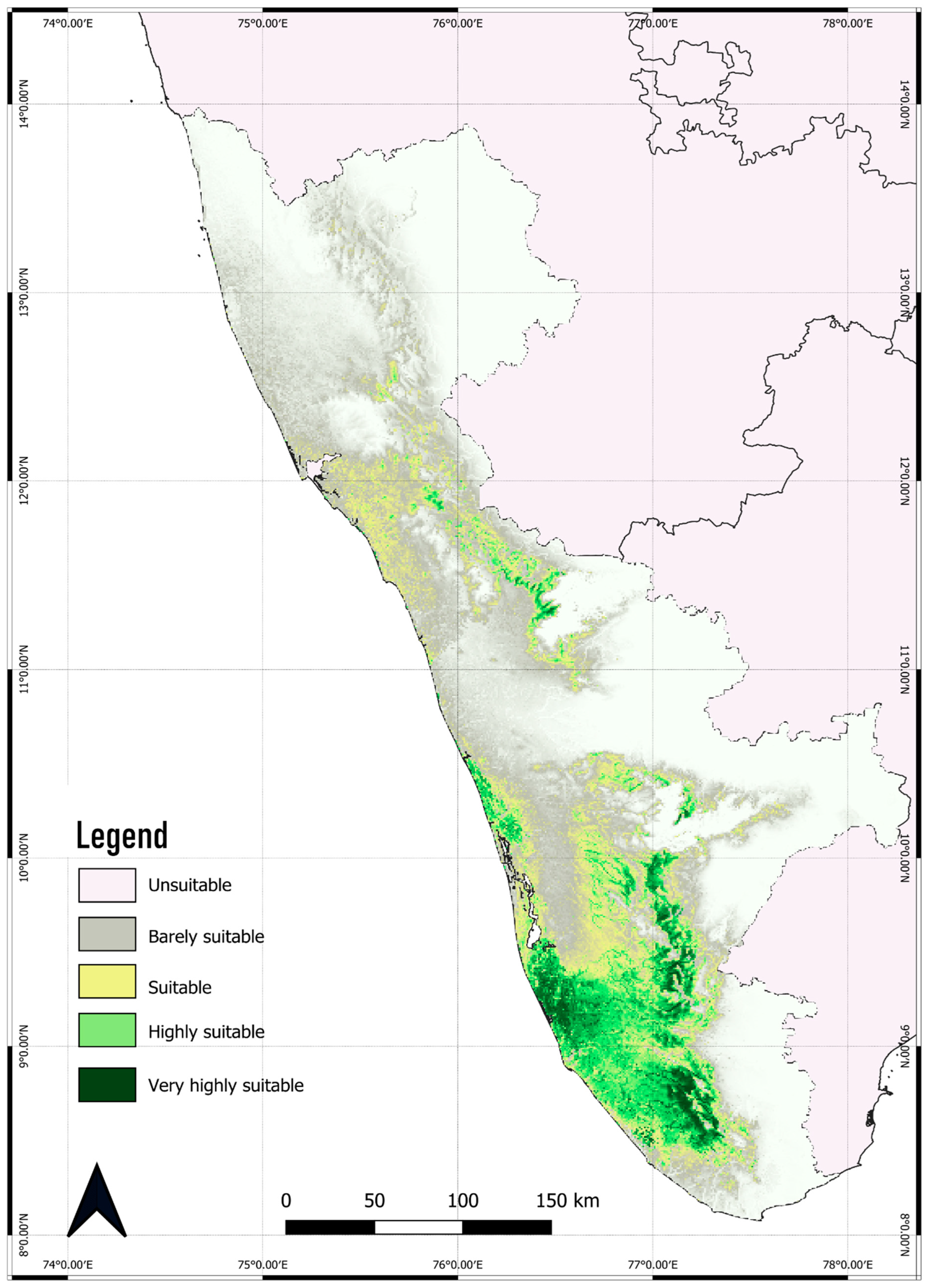
3.2. Current Distribution and Range of C. malabatrum
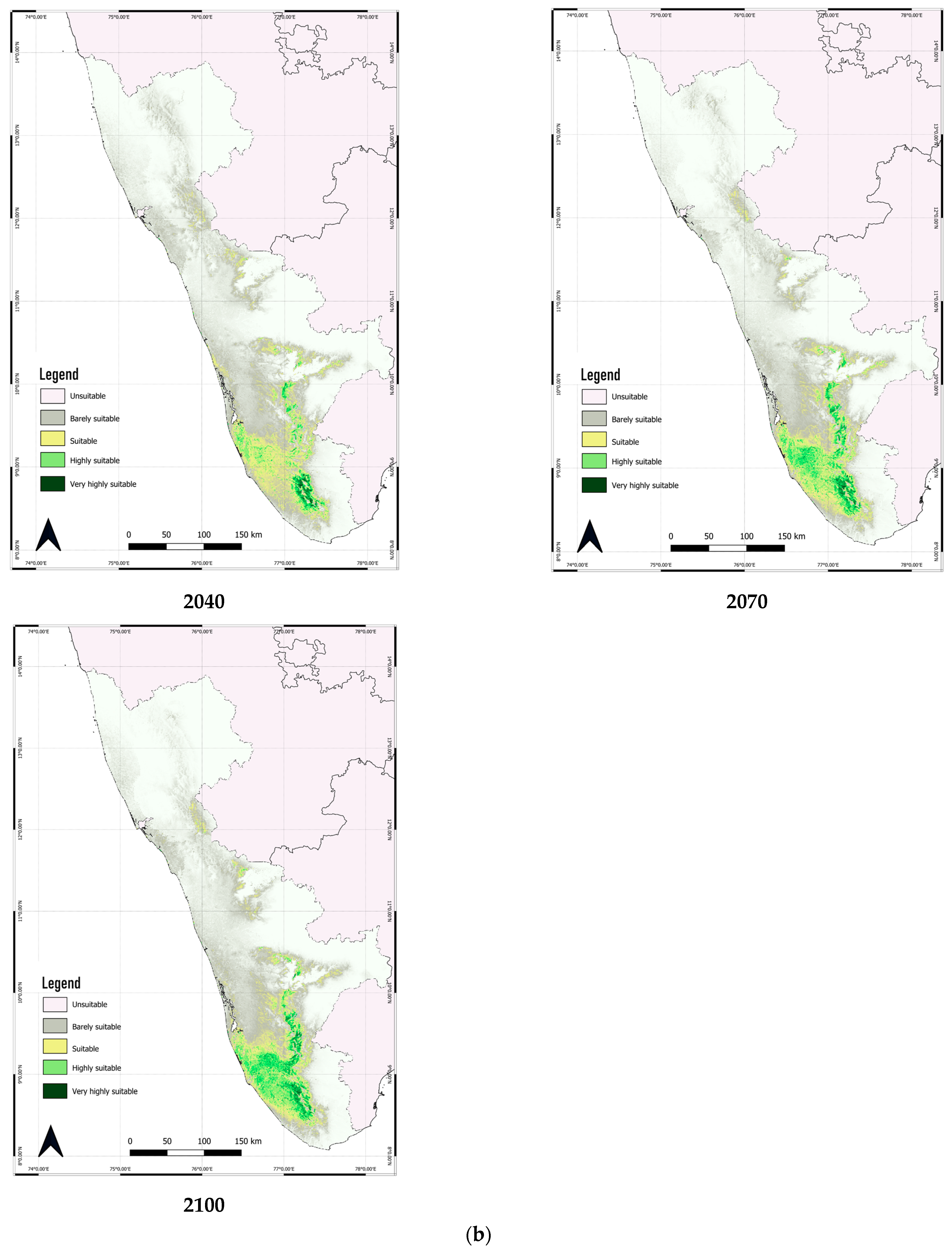
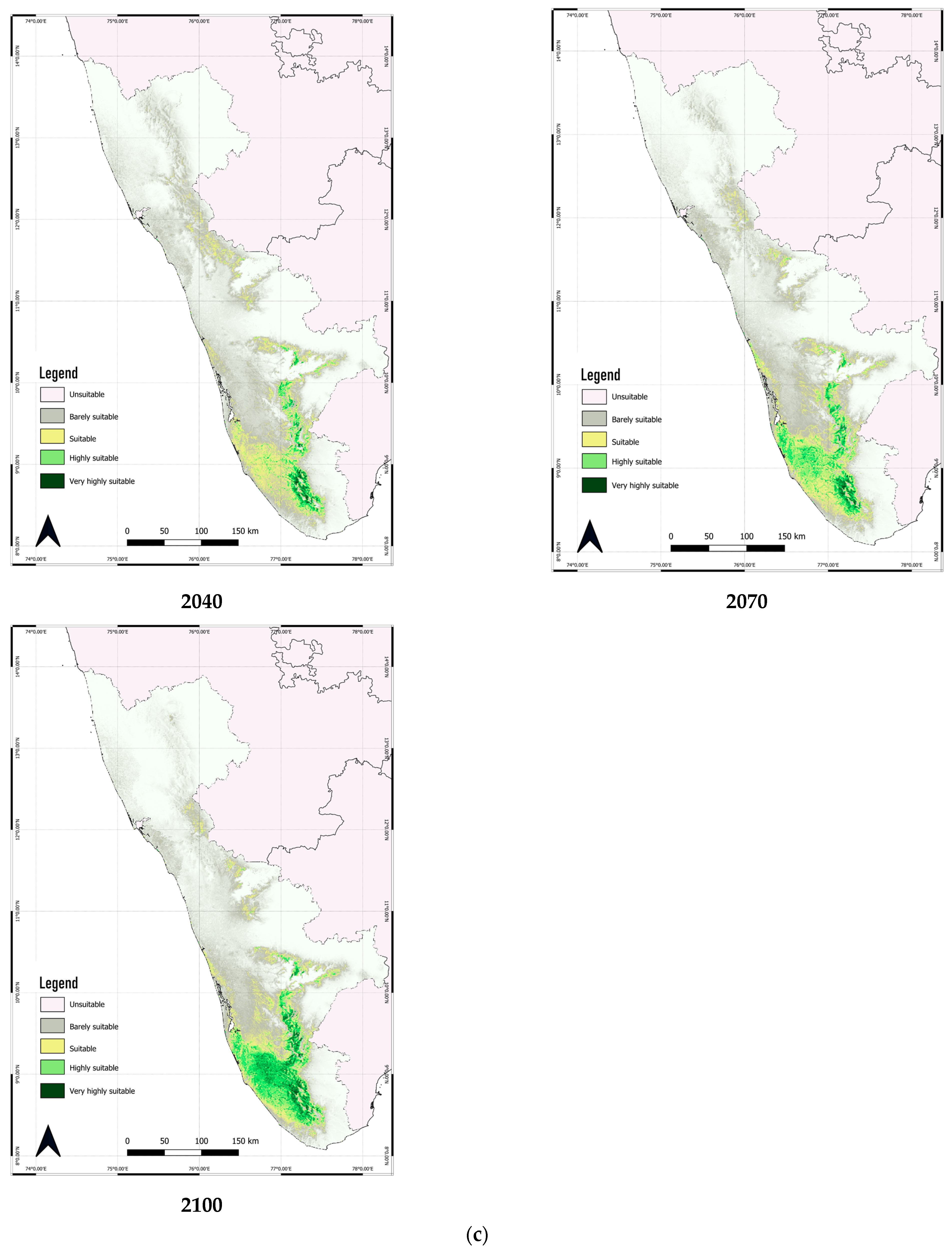
3.3. Predicted Distribution of C. malabatrum
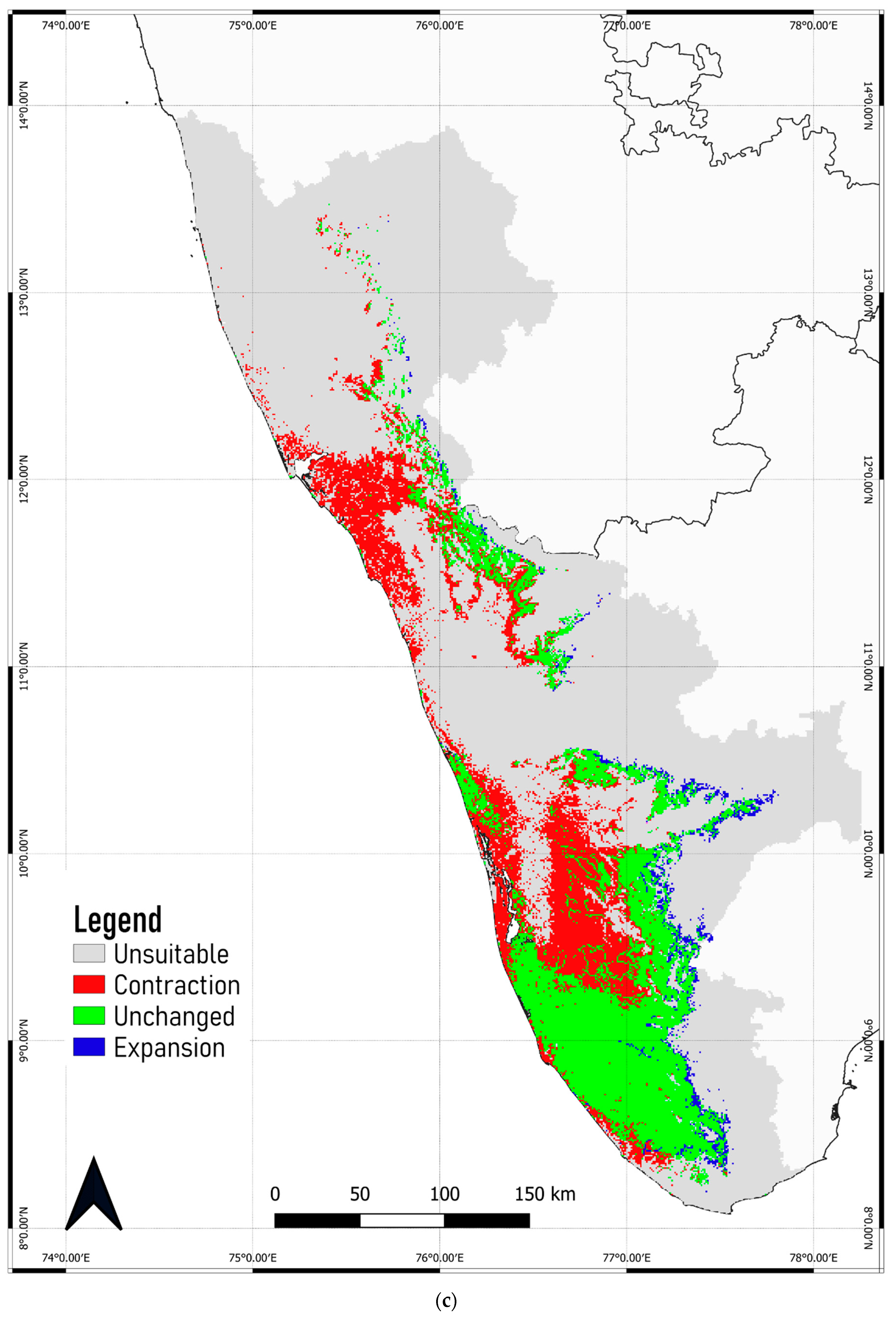
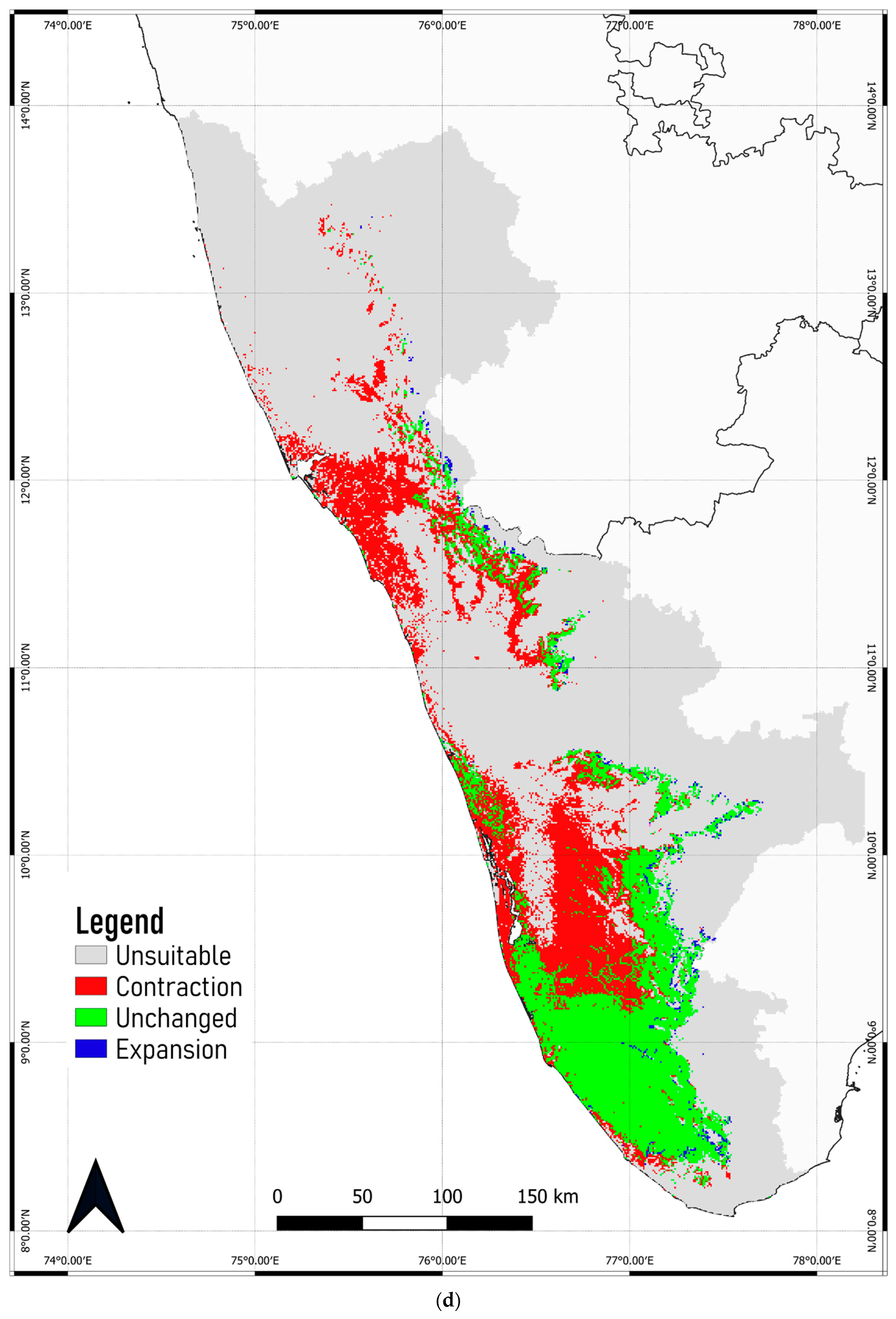
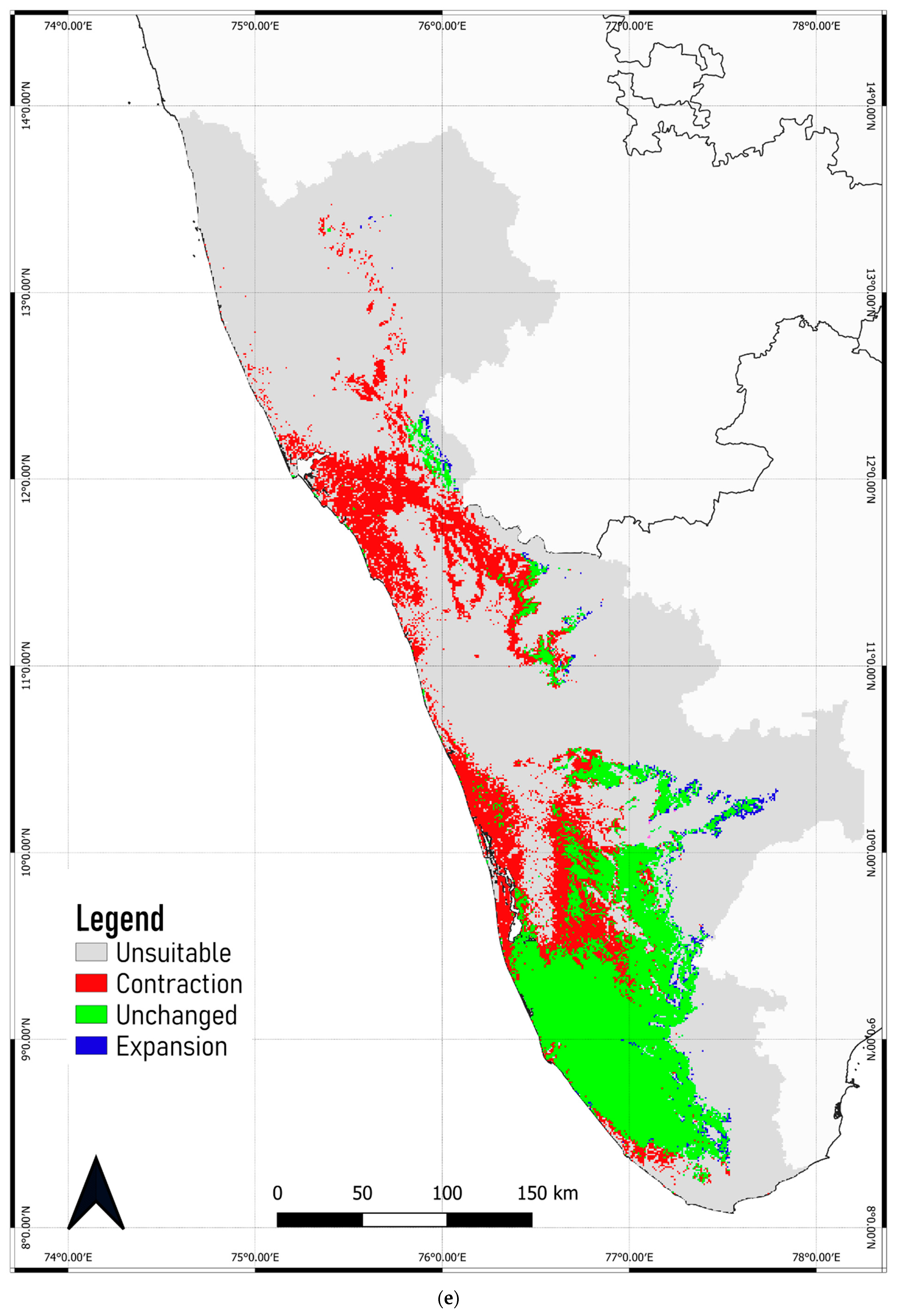
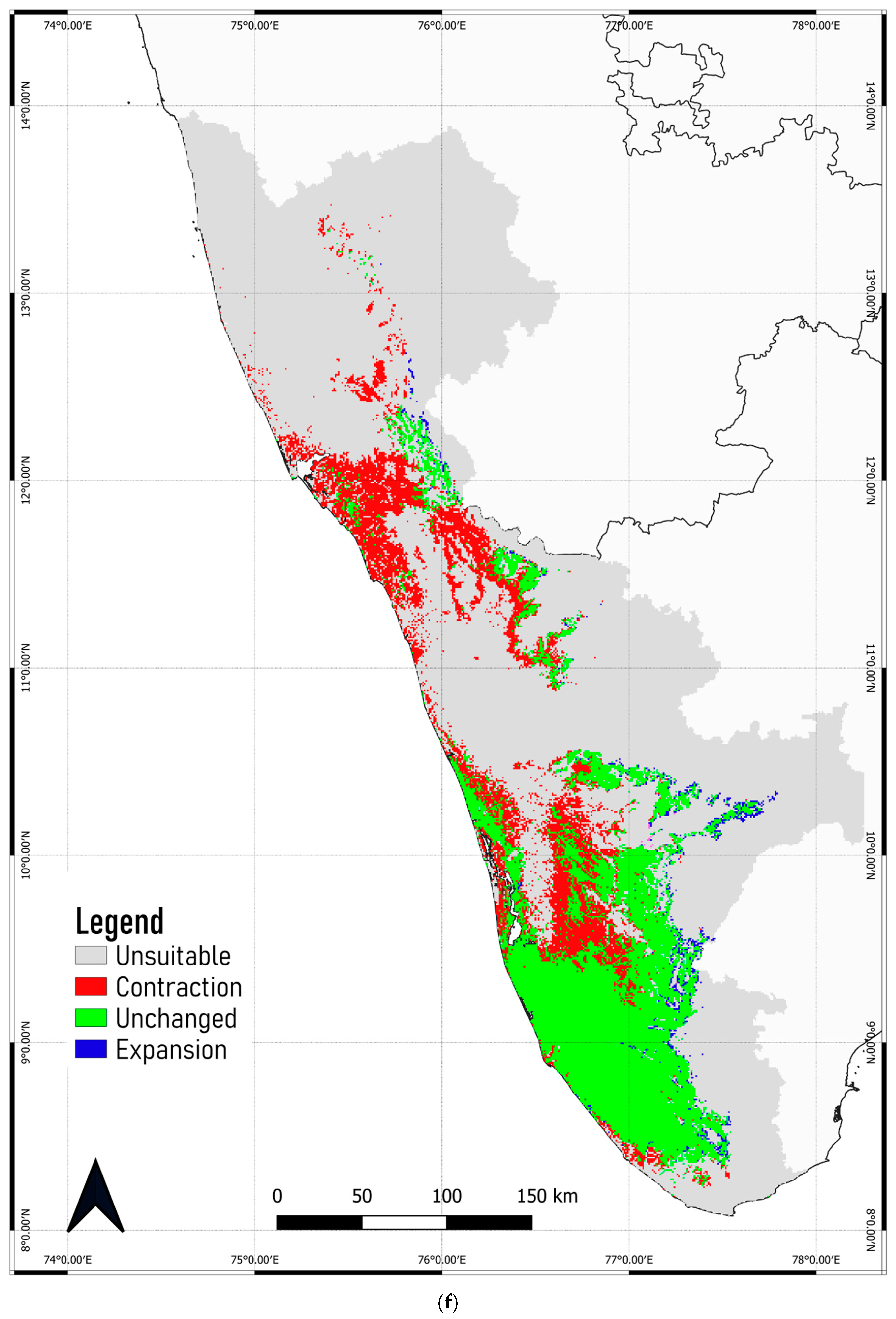
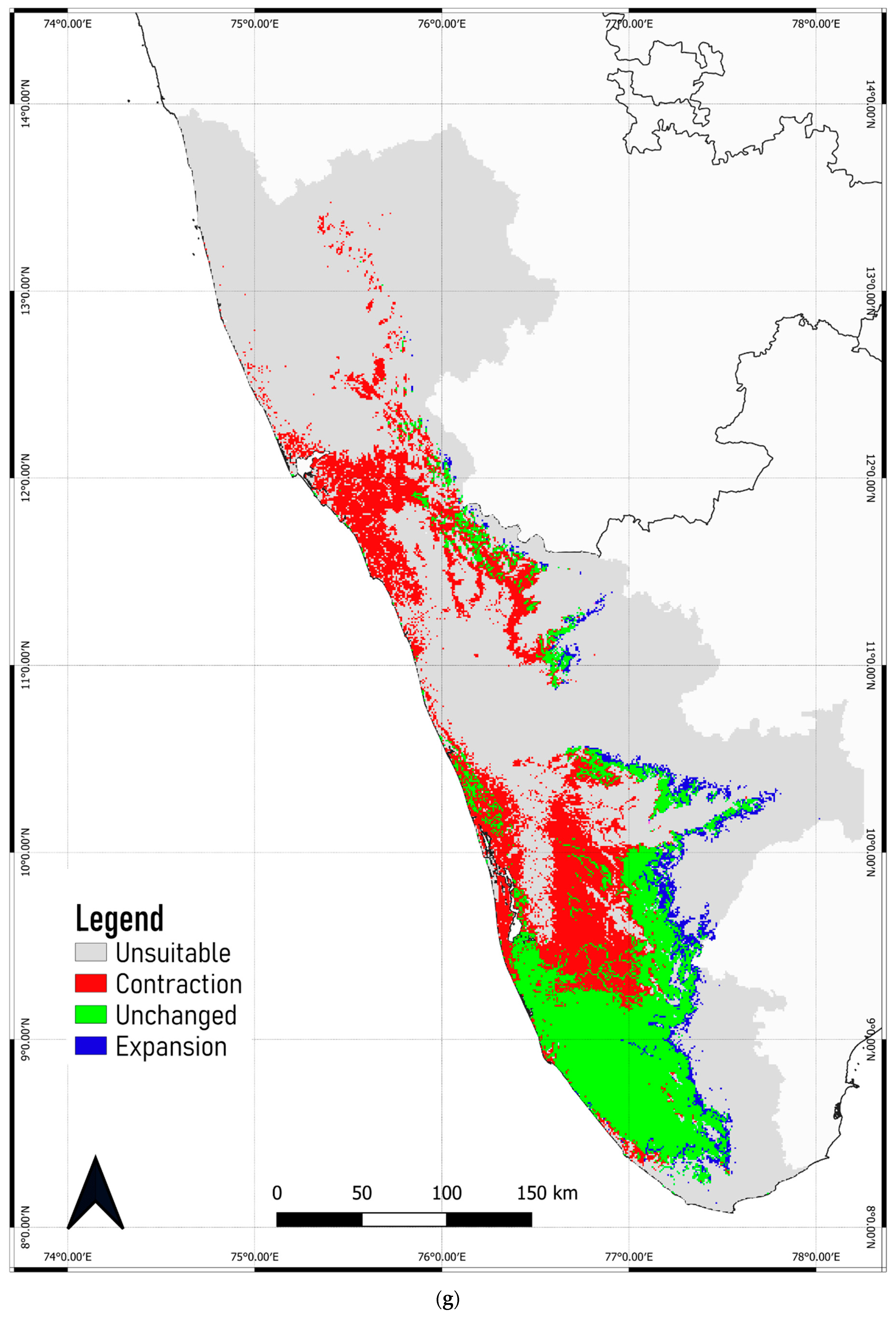
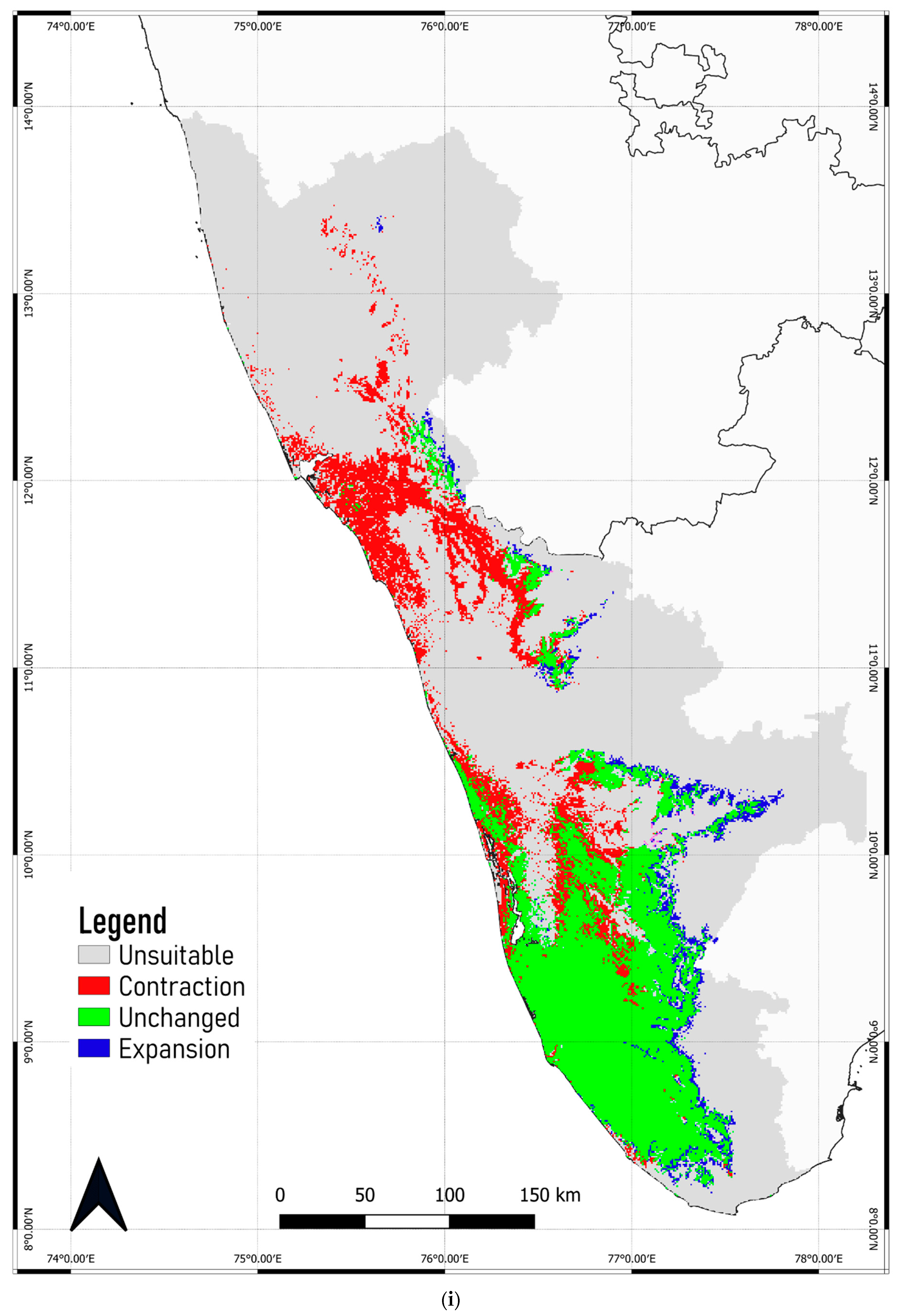
3.4. Predicted Shift in the Range of C. malabatrum
4. Adaptation Strategies for the Conservation of C. malabatrum
- (a)
- The relatively widespread distribution of C. malabatrum (as opposed to other species with limited suitability and endemicity within WG) in the Malabar midlands and coastal plains is unique. These are also the regions where the maximum range contraction is projected to occur across scenarios and time periods. Therefore, any conservation strategy for C. malabatrum should focus on Malabar midlands and coastal plains because conservation efforts here may prove more accessible in some respects.
- (b)
- Informal protected areas and traditions (such as sacred groves) may be as valuable as protected reserves in conserving biodiversity, as suggested by [122].
- (c)
- Cinnamomum malabatrum is most widely reported within the Malabar plains in the ‘sacred groves’ of Kerala, a type of Indigenous Community Conserved Area, as mentioned in Section 3.2. These areas face challenges in Kerala, primarily due to anthropogenic pressure for urbanization and land-use conversion. The improved management and protection of these resources could aid in conserving not only C. malabatrum but also threatened and endangered species, such as Myristica.
- (d)
- Globally, fragmentation due to land-use conversion significantly impacts tropical tree species. Although the Malabar plains are densely populated, C. malabatrum is at risk of habitat loss due to deforestation, land degradation, and commercial exploitation. This study confirms that precipitation during the driest month is a key ecological variable that stresses C. malabatrum.
- (e)
- In a study from Nicaragua related to tropical dry forest restoration, irrigation and fertilization were positively correlated with seedling quality and were suggested to improve posttransplant results [123]. A similar study in a seasonally moist forest in Peru revealed that irrigation improved the survival and growth of young seedlings and the growth of older seedlings. These strategies may also benefit our study area, for example, through the social forestry department in Kerala.
- (f)
- The relationships of indigenous and tribal communities with their surrounding ecosystems and the importance of traditional knowledge, beliefs, and practices for biodiversity conservation have been well documented over the past few decades [121]. The economic importance of C. malabatrum to the Kattunaikka tribe has been mentioned previously [25]. We suggest new policy initiatives to initiate and improve local markets and institutions in SWG. Furthermore, indigenous tribal groups must be included as key stakeholders, which could help conserve the species.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Agasthyamalai Biosphere Reserve |
| AIC | Akaike Information Criterion |
| Am | Tropical Monsoon Climate |
| Aw | Tropical Savanna Climate |
| AUC | Area Under the Curve (Receiver Operating Characteristic) |
| Bio4 | Temperature Seasonality |
| Bio17 | Precipitation of the driest month |
| BHUVAN | A geospatial platform developed by the Indian Space Research Organization (ISRO) |
| C. agasthyamalayanum | Cinnamomum agasthyamalayanum |
| C. gamblei | Cinnamomum gamblei |
| C. goaense | Cinnamomum goaense |
| C. heyneanum | Cinnamomum heyneanum |
| C. litseaefolium | Cinnamomum litseaefolium |
| C. malabatrum | Cinnamomum malabatrum |
| C. nilagiricum | Cinnamomum nilagiricum |
| CHR | Cardiom Hill Reserves |
| CC | Climate Change |
| CD–ROM | Compact Disk Read-Only Memory |
| CHELSA | Climatologies at High Resolution for the Earth’s Land Surface Areas |
| BIOCLIM | refers to a dataset that provides climate variables at high spatial resolution. |
| Cloglog | Complementary log-log |
| CMIP–5 | Coupled Model Intercomparison Project Phase 5 |
| CMIP–6 | Coupled Model Intercomparison Project Phase 6 |
| DEM | Digital Elevation Model maps |
| ENMeval | Ecological NMiche Modeling evaluation R package |
| FAO | Food and Agriculture Organization database |
| GFDL ESM–4 | Geophysical Fluid Dynamics Laboratory Earth System Model Version 4 |
| IPCC | Intergovernmental Panel on Climate Change |
| ISRO | Indian Space Research Organization |
| IUCN | International Union for Conservation of Nature |
| Kappa | Cohen’s kappa |
| KGCC | Köppen–Geiger Climate Classification |
| Kg0 | Köppen–Geiger Climate Classification for tropical rainforests without a dry season |
| Kg1 | Köppen–Geiger Climate Classification without dry summer and dry winter subclassifications for tropical savanna |
| Kg2–kg5 | Köppen–Geiger Climate Classifications for other subtypes |
| Landcover | Land Cover Data |
| MIP | Model Intercomparison Project |
| MRI ESM–2 | Meteorological Research Institute Earth System Model Version 2 |
| NAWS | New Amarambalam Wildlife Sanctuary |
| NDVI | Normalized difference vegetation index |
| NND | Nearest Neighbor Distance |
| NTFP | Non-Timber Forest Produce |
| NWG | Northern Western Ghats |
| PCOS | Polycystic Ovary Syndrome |
| Pet_4 | Monthly Potential Evapotranspiration in April |
| PNP | Periyar National Park |
| PTR | Periyar Tiger Reserve |
| QGIS 3.16 | Quantum Geographic Information System version 3.28 |
| r | Correlation Coefficient |
| RCP | Regional Concentration Pathways |
| ROC | Receiver Operating Characteristic |
| SDM | Species Distribution Model |
| SSF | Social Forestry Department |
| SSP | Shared Socioeconomic Pathways |
| SSP—1 | Shared Socioeconomic Pathway 1 |
| SSP—3 | Shared Socioeconomic Pathway 3 |
| SSP—5 | Shared Socioeconomic Pathway 5 |
| SWG | Southern Western Ghats |
| TSS | True Skill Statistics |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| WG | Western Ghats |
| W/m2 | Radiative Forcing (watts per square meter) |
References
- IPCC. Climate Change 2022–Impacts, Adaptation and Vulnerability; Cambridge University Press (CUP): Cambridge, UK, 2023. [Google Scholar]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2021, 29, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Nunez, S.; Arets, E.; Alkemade, R.; Verwer, C.; Leemans, R. Assessing the impacts of climate change on biodiversity: Is below 2 °C enough? Clim. Change 2019, 154, 351–365. [Google Scholar] [CrossRef]
- Segan, D.B.; Murray, K.A.; Watson, J.E. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Obura, D. The Kunming-Montreal Global Biodiversity Framework: Business as usual or a turning point? One Earth 2023, 6, 77–80. [Google Scholar] [CrossRef]
- Reddy, C.S.; Jha, C.S.; Dadhwal, V.K. Earth Observations based Conservation Prioritization in Western Ghats, India. J. Geol. Soc. India 2018, 92, 562–567. [Google Scholar] [CrossRef]
- Anitha, K.; Joseph, S.; Chandran, R.J.; Ramasamy, E.; Prasad, S.N. Tree species diversity and community composition in a human-dominated tropical forest of Western Ghats biodiversity hotspot, India. Ecol. Complex. 2010, 7, 217–224. [Google Scholar] [CrossRef]
- Varikoden, H.; Revadekar, J.V.; Kuttippurath, J.; Babu, C.A. Contrasting trends in southwest monsoon rainfall over the Western Ghats region of India. Clim. Dyn. 2018, 52, 4557–4566. [Google Scholar] [CrossRef]
- Pramanik, M.; Paudel, U.; Mondal, B.; Chakraborti, S.; Deb, P. Predicting climate change impacts on the distribution of the threatened Garcinia indica in the Western Ghats, India. Clim. Risk Manag. 2018, 19, 94–105. [Google Scholar] [CrossRef]
- Sen, S.; Gode, A.; Ramanujam, S.; Ravikanth, G.; Aravind, N.A. Modeling the impact of climate change on wild Piper nigrum (Black Pepper) in Western Ghats, India using ecological niche models. J. Plant Res. 2016, 129, 1033–1040. [Google Scholar] [CrossRef]
- Sony, R.; Sen, S.; Kumar, S.; Sen, M.; Jayahari, K. Niche models inform the effects of climate change on the endangered Nilgiri Tahr (Nilgiritragus hylocrius) populations in the southern Western Ghats, India. Ecol. Eng. 2018, 120, 355–363. [Google Scholar] [CrossRef]
- Sreekumar, E.; Nameer, P. A MaxEnt modelling approach to understand the climate change effects on the distributional range of White-bellied Sholakili Sholicola albiventris (Blanford, 1868) in the Western Ghats, India. Ecol. Inform. 2022, 70, 101702. [Google Scholar] [CrossRef]
- Sreekumar, E.R.; Nameer, P.O. Impact of Climate Change on Two High-Altitude Restricted and Endemic Flycatchers of The Western Ghats, India. Curr. Sci. 2021, 121, 1335–1342. [Google Scholar] [CrossRef]
- Raman, S.; Shameer, T.T.; Sanil, R.; Usha, P.; Kumar, S. Protrusive influence of climate change on the ecological niche of endemic brown mongoose (Herpestes fuscus fuscus): A MaxEnt approach from Western Ghats, India. Model. Earth Syst. Environ. 2020, 6, 1795–1806. [Google Scholar] [CrossRef]
- Das, M.L.; Bondada, S.; Rajesh, K.; Subrahmanyam, S. Climate change impacts on habitat suitability of Cinnamomum travancoricum (Lauraceae), a critically endangered endemic vascular plant in the Western Ghats, India. Isr. J. Ecol. Evol. 2023, 1, 1–19. [Google Scholar] [CrossRef]
- Subrahmanyam, S.; Das, M.L.; Kumara, H.N. Climate Change Projections of Current and Future Distributions of the Endemic Loris lydekkerianus (Lorinae) in Peninsular India. In Exploring Synergies and Trade-offs between Climate Change and the Sustainable Development Goals; Springer: Berlin, Germany, 2021. [Google Scholar]
- Anoop, N.; Babu, S.; Nagarajan, R.; Sen, S. Identifying suitable reintroduction sites for the White-rumped Vulture (Gyps bengalensis) in India’s Western Ghats using niche models and habitat requirements. Ecol. Eng. 2020, 158, 106034. [Google Scholar] [CrossRef]
- Priti, H.; Aravind, N.; Shaanker, R.U.; Ravikanth, G. Modeling impacts of future climate on the distribution of Myristicaceae species in the Western Ghats, India. Ecol. Eng. 2016, 89, 14–23. [Google Scholar] [CrossRef]
- Kostermans, A. A Note on Two Species of Cinnamomum (Lauraceae) Described in Hortus Indicus Malabaricus. In Botany and history of Hortus Malabaricus; Oxford IBH: New Delhi, India, 1980; pp. 163–167. [Google Scholar]
- van Rheede, H.; Casearium, J.; Syen, A. Hortus Indicus Malabaricus Vol 1; Ametelodami: Amsterdam, The Netherlands, 1685. [Google Scholar]
- Hooker, J.D. Flora of British India, Vol. V; Reeve & Co.: London, UK, 1886. [Google Scholar]
- Gamble, J.S. Flora of the Presidency of Madras, Vol. II; Botanical Survey of India: Calcutta, India, 1925.
- Berkes, F. Community conserved areas: Policy issues in historic and contemporary context. Conserv. Lett. 2009, 2, 20–25. [Google Scholar] [CrossRef]
- Narayanan, M.R.; Mithunlal, S.; Sujanapal, P.; Kumar, N.A.; Sivadasan, M.; Alfarhan, A.H.; Alatar, A. Ethnobotanically Important Trees and Their Uses by Kattunaikka Tribe in Wayanad Wildlife Sanctuary, Kerala, India. J. Med. Plants Res. 2011, 5, 604–612. [Google Scholar]
- Duguta, T.; Cheriyan, B.V. An Introduction and Various Phytochemical Studies of Cinnamomum Malabatrum: A Brief Review. Pharmacogn. J. 2021, 13, 792–797. [Google Scholar] [CrossRef]
- Kuttithodi, A.M.; Narayanankutty, A.; Visakh, N.U.; Job, J.T.; Pathrose, B.; Olatunji, O.J.; Alfarhan, A.; Ramesh, V. Chemical Composition of the Cinnamomum malabatrum Leaf Essential Oil and Analysis of Its Antioxidant, Enzyme Inhibitory and Antibacterial Activities. Antibiotics 2023, 12, 940. [Google Scholar] [CrossRef] [PubMed]
- Soumya, V.; Deepa, S.; Thachil, K.; Saravanan, J.; Hariprasad, R. GC-MS analysis and in silico docking of constituents of Cinnamomum malabatrum against CYP450 17α and CYP450 19 (Aromatase)- Key targets for hyperandrogenism. Drug Res. 2023, 73, 441–447. [Google Scholar] [CrossRef]
- Ananthakrishnan, R.; SanthoshKumar, E.S.; Rameshkumar, K.B. Comparative Chemical Profiles of Essential Oil Constituents of Eight Wild Cinnamomum Species from the Western Ghats of India. Nat. Prod. Commun. 2018, 13, 1934578. [Google Scholar] [CrossRef]
- Shylaja, M.; Ravindran, P.; Babu, K.N. Other Useful Species of Cinnamomum. In Cinnamon and Cassia; CRC Press: Boca Raton, FL, USA, 2003; pp. 346–371. [Google Scholar]
- Qi, S.; Luo, W.; Chen, K.-L.; Li, X.; Luo, H.-L.; Yang, Z.-Q.; Yin, D.-M. The Prediction of the Potentially Suitable Distribution Area of Cinnamomum mairei H. Lév in China Based on the MaxEnt Model. Sustainability 2022, 14, 7682. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Z.; Li, Z.; Liu, Y.; Fang, S. Predictive Modeling of Suitable Habitats for Cinnamomum Camphora (L.) Presl Using Maxent Model under Climate Change in China. Int. J. Environ. Res. Public Health 2019, 16, 3185. [Google Scholar] [CrossRef]
- Costion, C.M.; Simpson, L.; Pert, P.L.; Carlsen, M.M.; Kress, W.J.; Crayn, D. Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biol. Conserv. 2015, 191, 322–330. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994–2004) at the GLORIA* master site Schrankogel, Tyrol, Austria. Glob. Change Biol. 2006, 13, 147–156. [Google Scholar] [CrossRef]
- Bucklin, D.N.; Basille, M.; Benscoter, A.M.; Brandt, L.A.; Mazzotti, F.J.; Romañach, S.S.; Speroterra, C.; Watling, J.I.; Thuiller, W. Comparing species distribution models constructed with different subsets of environmental predictors. Divers. Distrib. 2014, 21, 23–35. [Google Scholar] [CrossRef]
- Luoto, M.; Virkkala, R.; Heikkinen, R.K. The role of land cover in bioclimatic models depends on spatial resolution. Glob. Ecol. Biogeogr. 2006, 16, 34–42. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Singh, A.K. Probable Agricultural Biodiversity Heritage Sites in India: XXI. The Malabar Region. Asian Agri-Hist. 2014, 18, 311–341. [Google Scholar]
- Pascal, J.P.; Ramesh, B.R. Atlas of Endemics of the Western Ghats (India): Distribution of Tree Species in the Evergreen and Semi-Evergreen Forests; Institut Français de Pondichéry: Pondichéry, India, 1997. [Google Scholar]
- Young, W.; Weckman, G.; Holland, W. A survey of methodologies for the treatment of missing values within datasets: Limitations and benefits. Theor. Issues Ergon. Sci. 2011, 12, 15–43. [Google Scholar] [CrossRef]
- Lavoie, C. Biological collections in an ever changing world: Herbaria as tools for biogeographical and environmental studies. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 68–76. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G. Habitat suitability modelling and niche theory. J. Appl. Ecol. 2008, 45, 1372–1381. [Google Scholar] [CrossRef]
- Faurby, S.; Araújo, M.B. Anthropogenic range contractions bias species climate change forecasts. Nat. Clim. Change 2018, 8, 252–256. [Google Scholar] [CrossRef]
- Jarvie, S.; Svenning, J.-C. Using species distribution modelling to determine opportunities for trophic rewilding under future scenarios of climate change. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170446. [Google Scholar] [CrossRef] [PubMed]
- Von Wissmann, H. Die Klima- und Vegetationsgebiete Eurasiens: Begleitworte zu einer Karte der Klimagebiete Eurasiens. Z. Der Ges. Für Erdkd. Berl. 1939, 81–92. [Google Scholar]
- Thompson, L. Mapping Agricultural Production in South America. Geogr. Rev. 1922, 12, 319. [Google Scholar] [CrossRef]
- Troll, C. Karte der Jahreszeiten-Klimate der Erde. Erdkunde 1964, 18, 5–28. [Google Scholar] [CrossRef]
- Brun, P.; Zimmermann, N.E.; Hari, C.; Pellissier, L.; Karger, D.N. Global climate-related predictors at kilometer resolution for the past and future. Earth Syst. Sci. Data 2022, 14, 5573–5603. [Google Scholar] [CrossRef]
- Databasin. 30 Arc-Second DEM of Asia. 2019. Available online: https://databasin.org/datasets/366a1bef53344c02bcd7d7611d5f61f7/ (accessed on 12 November 2019).
- FAO; IIASA. Harmonized World Soil Database v1.2. 2012. Available online: http://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases (accessed on 26 February 2016).
- Chen, G.; Li, X.; Liu, X. Global land projection based on plant functional types with a 1-km resolution under socio-climatic scenarios. Sci. Data 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Dragonetti, C.; Thuiller, W.; Guégen, M.; Renaud, J.; Visconti, P.; Di Marco, M. The impact of global change on the distribution of mountain mammals and birds. Authorea 2024. [Google Scholar] [CrossRef]
- Frye, C.E.; Sayre, R.G.; Breyer, S.; Roehrdanz, P.; Elsen, P.; Butler, K.A.; Sangermano, F.; Karagulle, D.; Wolff, N.; Smyth, R.; et al. Comparison Between Distributions of 2015 and Potential 2050 World Terrestrial Ecosystems Modeled from CMIP6 SSP-RCP-Based Climate Regions and Land Cover. AGU Fall Abstracts. 2024. Available online: https://agu.confex.com/agu/agu24/meetingapp.cgi/Paper/1593625 (accessed on 12 November 2019).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Mateo, R.G.; Vanderpoorten, A.; Muñoz, J.; Laenen, B.; Désamoré, A.; Bond-Lamberty, B. Modeling Species Distributions from Heterogeneous Data for the Biogeographic Regionalization of the European Bryophyte Flora. PLoS ONE 2013, 8, e55648. [Google Scholar] [CrossRef]
- Ballesteros-Barrera, C.; Tapia-Pérez, O.; Zárate-Hernández, R.; Leyte-Manrique, A.; Martínez-Bernal, A.; Vargas-Miranda, B.; Martínez-Coronel, M.; Ortiz-Burgos, S. The Potential Effect of Climate Change on the Distribution of Endemic Anurans from Mexico’s Tropical Dry Forest. Diversity 2022, 14, 650. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A new scenario framework for climate change research: The concept of shared socioeconomic pathways. Clim. Change 2014, 122, 387–400. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Chang. 2017, 42, 169–180. [Google Scholar] [CrossRef]
- O’NEill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Saha, U.; Sateesh, M. Rainfall extremes on the rise: Observations during 1951–2020 and bias-corrected CMIP6 projections for near- and late 21st century over Indian landmass. J. Hydrol. 2022, 608, 127682. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Data from: Climatologies at high resolution for the earth’s land surface areas. Dryad Digit. Repository 2018, 4, 170122. [Google Scholar] [CrossRef]
- Dunne, J.P.; Horowitz, L.W.; Adcroft, A.J.; Ginoux, P.; Held, I.M.; John, J.G.; Krasting, J.P.; Malyshev, S.; Naik, V.; Paulot, F.; et al. The GFDL Earth System Model Version 4.1 (GFDL-ESM 4.1): Overall Coupled Model Description and Simulation Characteristics. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002015. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. The Meteorological Research Institute Earth System Model Version 2.0, MRI-ESM2.0: Description and Basic Evaluation of the Physical Component. J. Meteorol. Soc. Jpn. Ser. II 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Gupta, V.; Singh, V.; Jain, M.K. Assessment of precipitation extremes in India during the 21st century under SSP1-1.9 mitigation scenarios of CMIP6 GCMs. J. Hydrol. 2020, 590, 125422. [Google Scholar] [CrossRef]
- Patel, G.; Das, S.; Das, R. Accuracy of historical precipitation from CMIP6 global climate models under diversified climatic features over India. Environ. Dev. 2024, 50, 100998. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2006, 34, 102–117. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2015, 39, 542–552. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2021, 92, e1486. [Google Scholar] [CrossRef]
- Phillips, S.; Dudík, M.; Schapire, R. Maxent Software for Modeling Species Niches and Distributions (Version 3.3.3). Available online: https://biodiversityinformatics.amnh.org/ (accessed on 17 December 2018).
- Radosavljevic, A.; Anderson, R.P.; Araújo, M. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Hu, Y. Prediction of Potential Suitable Distribution of Liriodendron chinense (Hemsl.) Sarg. in China Based on Future Climate Change Using the Optimized MaxEnt Model. Forests 2024, 15, 988. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; McPherson, J. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Comita, L.S.; Engelbrecht, B.M.J. Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology 2009, 90, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, B.M.J.; Comita, L.S.; Condit, R.; Kursar, T.A.; Tyree, M.T.; Turner, B.L.; Hubbell, S.P. Drought sensitivity shapes species distribution patterns in tropical forests. Nature 2007, 447, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; ter Steege, H.; Lopez-Gonzalez, G.; Mendoza, A.M.; Brienen, R.; Feldpausch, T.R.; Pitman, N.; et al. Seasonal drought limits tree species across the Neotropics. Ecography 2016, 40, 618–629. [Google Scholar] [CrossRef]
- Kodandapani, N.; Cochrane, M.A.; Sukumar, R. A comparative analysis of spatial, temporal, and ecological characteristics of forest fires in seasonally dry tropical ecosystems in the Western Ghats, India. For. Ecol. Manag. 2008, 256, 607–617. [Google Scholar] [CrossRef]
- Nair, A.; Joseph, K.A.; Nair, K. Spatio-temporal analysis of rainfall trends over a maritime state (Kerala) of India during the last 100 years. Atmospheric Environ. 2014, 88, 123–132. [Google Scholar] [CrossRef]
- Thomas, J.; Prasannakumar, V. Temporal analysis of rainfall (1871–2012) and drought characteristics over a tropical monsoon-dominated State (Kerala) of India. J. Hydrol. 2016, 534, 266–280. [Google Scholar] [CrossRef]
- Vijay, A.; Sivan, S.D.; Mudbhatkal, A.; Mahesha, A. Long-Term Climate Variability and Drought Characteristics in Tropical Region of India. J. Hydrol. Eng. 2021, 26, 05021003. [Google Scholar] [CrossRef]
- Köppen, W. The thermal zones of the Earth according to the duration of hot, moderate and cold periods and to the impact of heat on the organic world. Meteorol. Z. 2011, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R. Klassifikation der Klimate nach W. Köppen. In Landolt-Börnstein—Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik, alte Serie; Springer: Berlin, Germany, 1954; Volume 3, pp. 603–607. [Google Scholar]
- Hirota, M.; Holmgren, M.; Van Nes, E.H.; Scheffer, M. Global Resilience of Tropical Forest and Savanna to Critical Transitions. Science 2011, 334, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.P.; Bowman, D.M. What controls the distribution of tropical forest and savanna? Ecol. Lett. 2012, 15, 748–758. [Google Scholar] [CrossRef]
- Oliveras, I.; Malhi, Y. Many shades of green: The dynamic tropical forest–savannah transition zones. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150308. [Google Scholar] [CrossRef] [PubMed]
- Bunyan, M.; Bardhan, S.; Jose, S. The Shola (Tropical Montane Forest)-Grassland Ecosystem Mosaic of Peninsular India: A Review. Am. J. Plant Sci. 2012, 3, 1632–1639. [Google Scholar] [CrossRef]
- Bala, P.R.; Kavil, S.P.; Tayasu, I.; Yoshimizu, C.; Thirumalai, K.; Sajeev, K.; Sukumar, R. Paleovegetation dynamics in an alternative stable states landscape in the montane Western Ghats, India. Holocene 2021, 32, 297–307. [Google Scholar] [CrossRef]
- Raja, P.; Achyuthan, H.; Farooqui, A.; Ramesh, R.; Kumar, P.; Chopra, S. Tropical Rainforest Dynamics and Palaeoclimate Implications since the late Pleistocene, Nilgiris, India. Quat. Res. 2018, 91, 367–382. [Google Scholar] [CrossRef]
- Kavil, S.P.; Bala, P.R.; Ghosh, D.; Kumar, P.; Sukumar, R. Climate Change and the Migration of a Pastoralist People c. 3500 cal. Years BP Inferred from Palaeofire and Lipid Biomarker Records in the Montane Western Ghats, India. Environ. Archaeol. 2021, 28, 192–206. [Google Scholar] [CrossRef]
- Bailey, K.M.; McCleery, R.A.; Binford, M.W.; Zweig, C. Land-cover change within and around protected areas in a biodiversity hotspot. J. Land Use Sci. 2015, 11, 154–176. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Phillips, H.R.P.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Blandon, A.; Butchart, S.H.M.; Booth, H.L.; Day, J.; et al. A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141371. [Google Scholar] [CrossRef] [PubMed]
- Robi, A.; Sujanapal, P.; Udayan, P. Cinnamomum Agasthyamalayanum Sp. Nov.(Lauraceae) from Kerala, India. Int. J. Adv. Res. 2014, 2, 1012–1016. [Google Scholar]
- Geethakumary, M.P.; Pandurangan, A.G.; Deepu, S. A new species of Cinnamomum (Lauraceae) from Nilgiris, southern Western Ghats, India. Phytotaxa 2015, 224, 283. [Google Scholar] [CrossRef]
- Geethakumary, M.P.; Deepu, S.; Pandurangan, A.G. A new species of Cinnamomum and notes on the status of C. palghatensis in Western Ghats, India. Phytotaxa 2017, 326, 252. [Google Scholar] [CrossRef]
- Geethakumary, M.P.; Pandurangan, A.G.; Santhoshkumar, E.S. Cinnamomum litseaefolium (Lauraceae)—A new distributional record for India. Rheedea 2012, 22, 127–130. [Google Scholar]
- Geethakumary, M.P.; Deepu, S.; Pandurangan, A.G. Rediscovery, extended distribution and conservation assessment of Cinnamomum goaense (Lauraceae) in the Western Ghats, India. J. Threat. Taxa 2018, 10, 12137–12139. [Google Scholar] [CrossRef]
- Kumar, E.S.S.; Geethakumary, M.P.; Pandurangan, A.G.; Shaju, T. Rediscovery of Cinnamomum heyneanum Nees (Lauraceae) -A species endemic to the Western Ghats. Indian J. For. 2003, 26, 409–411. [Google Scholar] [CrossRef]
- Chandrashekara, U.M. Conservation and Management of Sacred Groves in Kerala; Project funded by the Biodiversity Cell, Department of Forests and Wildlife, Government of Kerala; KFRI: Peechi, India, 2011. [Google Scholar]
- Warrier, K.C.; Kunhikannan, C.; Sasidharan, K.R. Endangering sacred groves of a non forested region in Kerala, India and strategies for their conservation. Indian For. 2015, 141, 832–837. [Google Scholar]
- Dutta, K.; Reddy, C.S. Geospatial Analysis of Reed Bamboo (Ochlandra travancorica) Invasion in Western Ghats, India. J. Indian Soc. Remote. Sens. 2016, 44, 699–711. [Google Scholar] [CrossRef]
- Damayanti, E.K.; Masuda, M. Implementation process of India Ecodevelopment Project and the sustainability: Lessons from Periyar Tiger Reserve in Kerala State, India. Tropics 2008, 17, 147–158. [Google Scholar] [CrossRef]
- Gubbi, S.; MacMillan, D.C. Can non-timber forest products solve livelihood problems? A case study from Periyar Tiger Reserve, India. Oryx 2008, 42, 222–228. [Google Scholar] [CrossRef]
- Salish, J.M.; Hrideek, T.K.; Sujanapal, P. Shade tree composition in the cardamom plantations of the Cardamom Hill Reserve area, Western Ghats, India. Prospect. For. Agric. 2015, 119–121. [Google Scholar]
- Alroy, J. Effects of habitat disturbance on tropical forest biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 6056–6061. [Google Scholar] [CrossRef]
- Laladhas, K.P.; Rani, J.R.; Vijaya Sree, A.S.; Nair, A.S.; Oommen, O.V. Statutory and Obligatory Responsibilities of State Biodiversity Boards for the Conservation of Indigenous Biodiversity and ABS. In Biodiversity Conservation Through Access and Benefit Sharing (ABS); Oommen, O.V., Laladhas, K.P., Nelliyat, P., Pisupati, B., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Stein, B.A.; Staudt, A.; Cross, M.S.; Dubois, N.S.; Enquist, C.; Griffis, R.; Hansen, L.J.; Hellmann, J.J.; Lawler, J.J.; Nelson, E.J.; et al. Preparing for and managing change: Climate adaptation for biodiversity and ecosystems. Front. Ecol. Environ. 2013, 11, 502–510. [Google Scholar] [CrossRef]
- Zurell, D.; Jeltsch, F.; Dormann, C.F.; Schröder, B. Static species distribution models in dynamically changing systems: How good can predictions really be? Ecography 2009, 32, 733–744. [Google Scholar] [CrossRef]
- Gadgil, M.; Berkes, F.; Folke, C. Indigenous knowledge for biodiversity conservation. Ambio 1993, 22, 151–156. [Google Scholar]
- Bhagwat, S.A.; Kushalappa, C.G.; Williams, P.H.; Brown, N.D. The Role of Informal Protected Areas in Maintaining Biodiversity in the Western Ghats of India. Ecol. Soc. 2005, 10, 8. [Google Scholar] [CrossRef]
- Lanuza-Lanuza, O.R.; Peguero, G.; Vilchez-Mendoza, S.; Casanoves, F. Effect of Irrigation and Fertilization on the Hardening of Forest Seedlings with Potential Use for Dry Tropical Forest Restoration. Rev. For. Mesoam. Kurú 2021, 18, 18–28. [Google Scholar]





| State | District Name | % Area of District Suitable for C. malabatrum | % of the Total Suitable Area of C. malabatrum Present in the District |
|---|---|---|---|
| Karnataka | Chikmagalur | 1.59% | 0.47% |
| Dakshin Kannad | 1.83% | 0.35% | |
| Hassan | 1.18% | 0.33% | |
| Kodagu | 20.29% | 3.44% | |
| Udupi | 0.13% | 0.02% | |
| Kerala | Alappuzha | 91.86% | 4.73% |
| Ernakulam | 58.19% | 5.42% | |
| Idukki | 60.26% | 12.44% | |
| Kannur | 65.31% | 7.49% | |
| Kasaragod | 6.57% | 0.53% | |
| Kollam | 98.14% | 9.76% | |
| Kottayam | 81.75% | 7.11% | |
| Kozhikode | 43.45% | 4.08% | |
| Malappuram | 17.05% | 2.47% | |
| Palakkad | 17.98% | 3.33% | |
| Pathanamthitta | 96.90% | 10.56% | |
| Thiruvananthapuram | 91.54% | 7.73% | |
| Thrissur | 32.88% | 4.00% | |
| Wayanad | 41.83% | 3.67% | |
| Tamil Nadu | Coimbatore | 6.36% | 1.95% |
| Dindigul | 2.67% | 0.67% | |
| Kanniyakumari | 35.45% | 2.33% | |
| Nilgiris | 16.79% | 1.77% | |
| Theni | 5.66% | 0.67% | |
| Tirunelveli | 16.68% | 4.67% |
| Species Name | Endemic/Non-Endemic | Prevalence Localities | Occurrence (Spatial Thin x = 10 km) |
|---|---|---|---|
| Cinnamomum malabatrum | Endemic to SWG | 122 | 56 |
| Variable Name | Expansion |
|---|---|
| bio1 | Mean annual air temperature |
| bio2 | Mean diurnal air temperature range |
| bio3 | Isothermality |
| bio4 | Temperature seasonality |
| bio5 | Mean daily maximum air temperature of the warmest month |
| bio6 | Mean daily minimum air temperature of the coldest month |
| bio7 | Annual range of air temperature |
| bio8 | Mean daily mean air temperatures of the wettest quarter |
| bio9 | Mean daily mean air temperatures of the driest quarter |
| bio10 | Mean daily mean air temperatures of the warmest quarter |
| bio11 | Mean daily mean air temperatures of the coldest quarter |
| bio12 | Annual precipitation amount |
| bio13 | The precipitation amount of the wettest month |
| bio14 | The precipitation amount of the driest month |
| bio15 | Precipitation seasonality |
| bio16 | Mean monthly precipitation amount of the wettest quarter |
| bio17 | Mean monthly precipitation amount of the driest quarter |
| bio18 | Mean monthly precipitation amount of the warmest quarter |
| bio19 | Mean monthly precipitation amount of the coldest quarter |
| soil | Categorical soil map |
| aspect | Compass direction of the slope of the terrain |
| ndvi | Normalized difference vegetation index |
| drainage | Drainage map of the terrain |
| alt | Altitude |
| waterbody | Categorical map of waterbodies |
| aridity | Aridity index |
| slope | Slope of terrain |
| pet | Annual potential evapotranspiration |
| pet_1 | Monthly potential evapotranspiration—January |
| pet_2 | Monthly potential evapotranspiration—February |
| pet_3 | Monthly potential evapotranspiration—March |
| pet_4 | Monthly potential evapotranspiration—April |
| pet_5 | Monthly potential evapotranspiration—May |
| pet_6 | Monthly potential evapotranspiration—June |
| pet_7 | Monthly potential evapotranspiration—July |
| pet_8 | Monthly potential evapotranspiration—August |
| pet_9 | Monthly potential evapotranspiration—September |
| pet_10 | Monthly potential evapotranspiration—October |
| pet_11 | Monthly potential evapotranspiration—November |
| pet_12 | Monthly potential evapotranspiration—December |
| npp | Net Primary productivity |
| landcover | Categorical map of land cover |
| gst | Mean temperature of the growing season |
| gsp | Accumulated precipitation amount on growing season days |
| gsl | Growing season length |
| gdd0 | Growing degree days heat sum above 0 °C |
| gdd5 | Growing degree days heat sum above 5 °C |
| gdd10 | Growing degree days heat sum above 10 °C |
| kg0 | Köppen–Geiger climate classification |
| kg1 | Köppen–Geiger climate classification without As/Aw distinction |
| kg2 | Köppen–Geiger climate classification [45] |
| kg3 | Köppen–Geiger climate classification [50] |
| kg4 | Köppen–Geiger climate classification [51] |
| kg5 | Köppen–Geiger climate classification [52] |
| RUN1 | RUN2 | RUN3 | AVG | |
|---|---|---|---|---|
| TSS | 0.854 | 0.823 | 0.884 | 0.85 |
| KAPPA | 0.701 | 0.716 | 0.692 | 0.70 |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| bio17 | 28 | 14.1 |
| kg1 | 24.7 | 3.4 |
| landcover | 11.9 | 1.1 |
| pet_4 | 6.7 | 1.7 |
| bio4 | 5.6 | 23.6 |
| ai_yr | 4.8 | 1 |
| npp | 2.9 | 30.4 |
| bio12 | 2.3 | 7.2 |
| slope_india | 2.1 | 0.5 |
| bio1 | 2 | 0.1 |
| pet_7 | 2 | 1.6 |
| bio18 | 1.4 | 3.4 |
| bio3 | 1.3 | 0.4 |
| pet_6 | 1.3 | 1 |
| bio2 | 1 | 2.3 |
| alt | 0.7 | 3.7 |
| kg0 | 0.5 | 1.6 |
| india_aspect | 0.4 | 1.6 |
| in_water | 0.3 | 0.5 |
| ind_ndvi | 0.2 | 0.7 |
| Habitat Type | p-Value | Current Potential Distribution (Km2) | Cinnamomum malabatrum (%) |
|---|---|---|---|
| Unsuitable habitat | 0.0–0.2 | 51,387.59 | 56.43% |
| Barely suitable habitat | 0.2–0.4 | 15,510.36 | 17.03% |
| Suitable habitat | 0.4–0.6 | 11,249.84 | 12.35% |
| Highly suitable habitat | 0.6–0.8 | 8011.03 | 8.80% |
| Very highly suitable habitat | 0.8–1.0 | 4912.16 | 5.39% |
| Total—91,070.98 | 100.00% |
| Cinnamomum malabatrum | 2040 | 2070 | 2100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Habitat Class | Current | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 |
| Unsuitable | 51,387.59 | 59,164.27 | 61,637.63 | 59,777.97 | 65,507.84 | 64,502.15 | 60,302.32 | 64,946.41 | 62,632.37 | 61,553.33 |
| Barely suitable | 15,510.36 | 16,169.58 | 15,872.00 | 16,996.57 | 13,525.94 | 12,704.01 | 15,046.71 | 12,618.02 | 13,670.93 | 12,064.17 |
| Suitable | 11,249.84 | 8801.76 | 8564.04 | 9240.97 | 7769.93 | 6641.15 | 7720.19 | 7213.55 | 7052.54 | 7649.38 |
| Highly Suitable | 8011.03 | 5376.65 | 4333.86 | 4030.38 | 3570.11 | 6067.07 | 6588.89 | 4658.42 | 6223.03 | 6302.27 |
| Very highly suitable | 4912.16 | 1558.71 | 663.44 | 1025.09 | 697.16 | 1156.60 | 1412.87 | 1634.58 | 1492.11 | 3501.82 |
| Cinnamomum malabatrum | 2040 | 2070 | 2100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Habitat Class | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 |
| Unsuitable | 15.13% | 19.95% | 16.33% | 27.48% | 25.52% | 17.35% | 26.39% | 21.88% | 19.78% |
| Barely suitable | 4.25% | 2.33% | 9.58% | −12.79% | −18.09% | −2.99% | −18.65% | −11.86% | −22.22% |
| Suitable | −21.76% | −23.87% | −17.86% | −30.93% | −40.97% | −31.38% | −35.88% | −37.31% | −32.00% |
| Highly suitable | −32.88% | −45.90% | −49.69% | −55.44% | −24.27% | −17.75% | −41.85% | −22.32% | −21.33% |
| Very highly suitable | −68.27% | −86.49% | −79.13% | −85.81% | −76.45% | −71.24% | −66.72% | −69.62% | −28.71% |
| 2040 | 2070 | 2100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range Shift Category | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 |
| Range expansion | 1100.12 | 567.34 | 1266.19 | 506.64 | 668.50 | 730.88 | 1931.31 | 459.44 | 1884.11 |
| Unsuitable | 65,849.26 | 66,382.04 | 65,678.13 | 66,437.67 | 66,275.82 | 66,213.44 | 65,018.90 | 66,489.94 | 65,060.21 |
| Unchanged | 14,578.00 | 12,938.36 | 13,009.18 | 11,511.17 | 13,171.88 | 14,953.98 | 11,538.14 | 14,221.41 | 15,512.89 |
| Range contraction | 9543.60 | 11,183.24 | 11,117.48 | 12,615.50 | 10,954.79 | 9172.68 | 12,582.62 | 9900.19 | 8613.77 |
| 2040 | 2070 | 2100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range Contraction in Kozhikode, Kannur, Kottayam, and Idukki Districts | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 | SSP1_2.6 | SSP3_7.0 | SSP5_8.5 |
| Range contraction (sq.km) | 4779.78 | 5142.22 | 5410.36 | 5698.18 | 4701.88 | 4432.92 | 5658 | 4189.38 | 3747.4 |
| % total range contraction | 51.21% | 47.03% | 49.68% | 46.13% | 43.74% | 49.36% | 45.98% | 43.23% | 44.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, M.L.; Chandran, S.; Subrahmanyam, S. Impact of Climate Change on the Distribution of Cinnamomum malabatrum (Laurales—Lauraceae), a Culturally and Ecologically Important Species of Malabar, Western Ghats, India. Diversity 2025, 17, 476. https://doi.org/10.3390/d17070476
Das ML, Chandran S, Subrahmanyam S. Impact of Climate Change on the Distribution of Cinnamomum malabatrum (Laurales—Lauraceae), a Culturally and Ecologically Important Species of Malabar, Western Ghats, India. Diversity. 2025; 17(7):476. https://doi.org/10.3390/d17070476
Chicago/Turabian StyleDas, Mukesh Lal, Sarat Chandran, and Sreenath Subrahmanyam. 2025. "Impact of Climate Change on the Distribution of Cinnamomum malabatrum (Laurales—Lauraceae), a Culturally and Ecologically Important Species of Malabar, Western Ghats, India" Diversity 17, no. 7: 476. https://doi.org/10.3390/d17070476
APA StyleDas, M. L., Chandran, S., & Subrahmanyam, S. (2025). Impact of Climate Change on the Distribution of Cinnamomum malabatrum (Laurales—Lauraceae), a Culturally and Ecologically Important Species of Malabar, Western Ghats, India. Diversity, 17(7), 476. https://doi.org/10.3390/d17070476





