Secondary Microplastics Disrupt Early Coral Development: Impacts on Brooding and Broadcast-Spawning Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Gamete Collection of O. faveolata and A. palmata
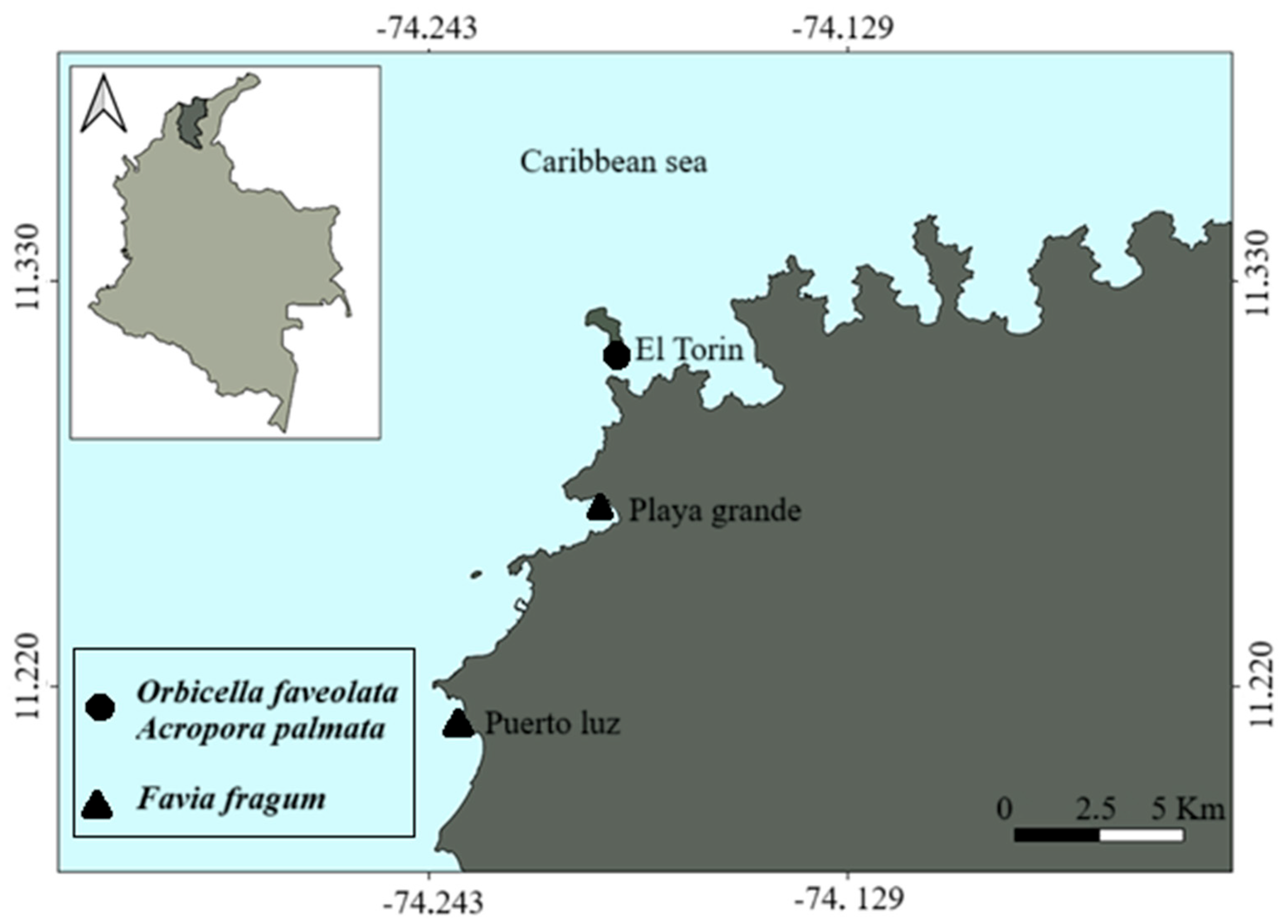
2.2. Larvae Collection of F. fragum
2.3. Collection of Crustose Coralline Algae (CCA)
2.4. Microplastics Preparation
2.5. Trace Metal Assessment
2.6. Effect of Microplastics on O. faveolata Fertilization
2.7. Effect of Microplastics on O. faveolata Embryogenesis
2.8. Effect of Microplastics on Larval Development of O. faveolata
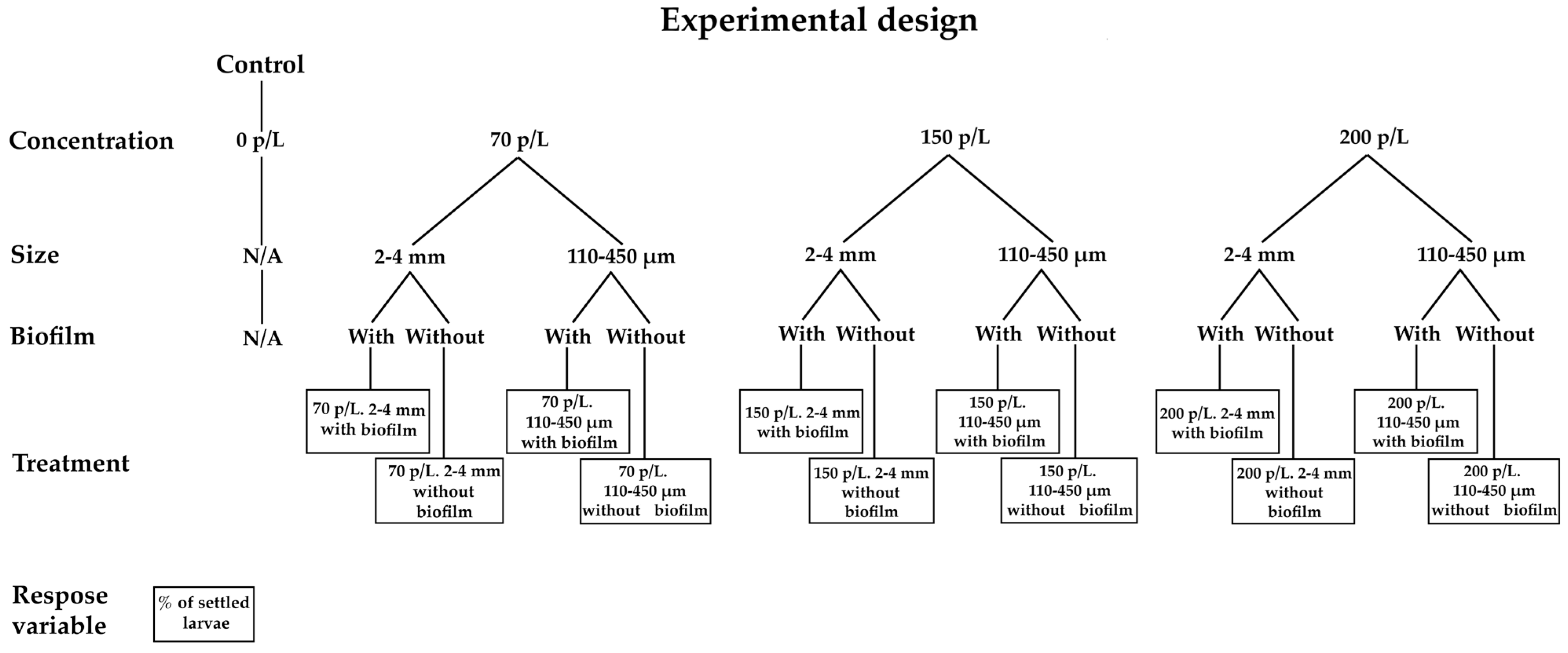
2.9. Effect of Microplastics on Larval Settlement of O. faveolata, A. palmata, and F. fragum
2.9.1. Orbicella faveolata
2.9.2. Acropora palmata
2.9.3. Favia fragum
2.9.4. Effect of Microplastics on O. faveolata Post-Settlement Survival
2.10. Data Analysis
3. Results
3.1. Trace Metal Assessment
3.2. Effect of Microplastics on O. faveolata Fertilization
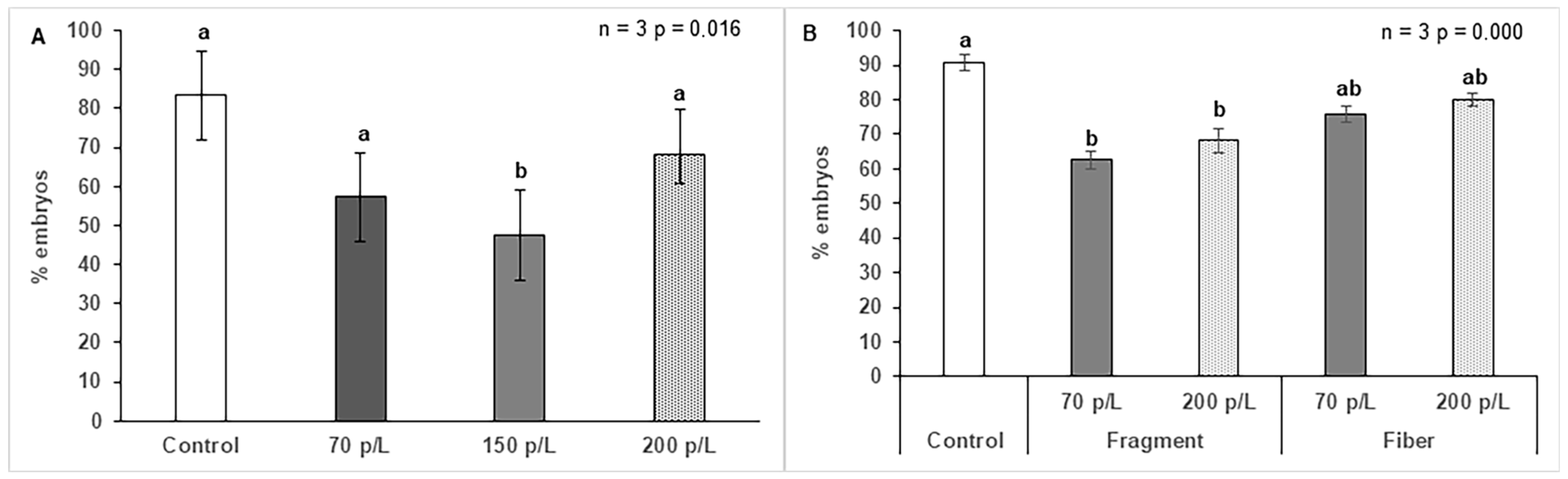
3.3. Effect of Microplastics on O. faveolata Embryogenesis
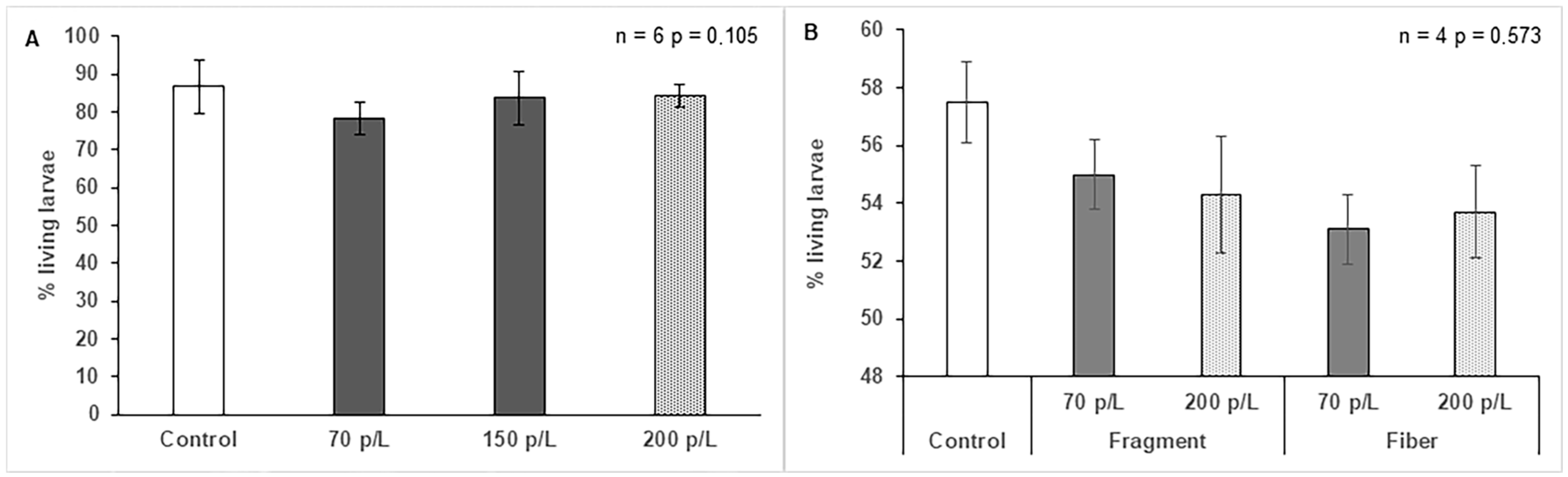
3.4. Effect of Microplastics on Larval Development of O. faveolata
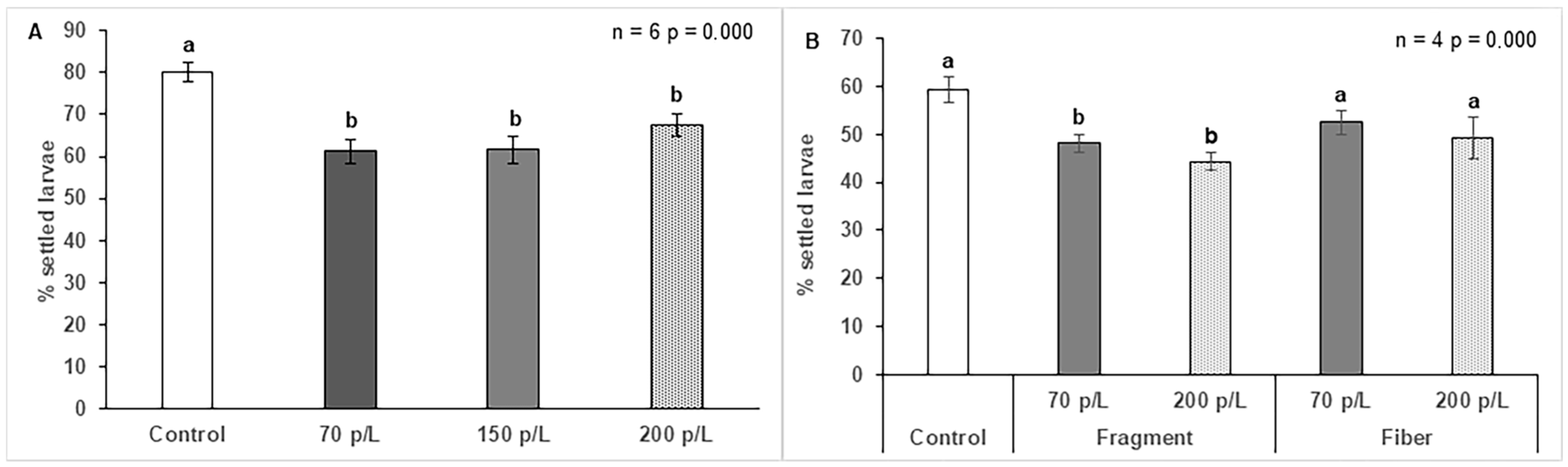
3.5. Effect of Microplastics on Larval Settlement of O. faveolata
3.6. Effect of Microplastics on A. palmata Settlement
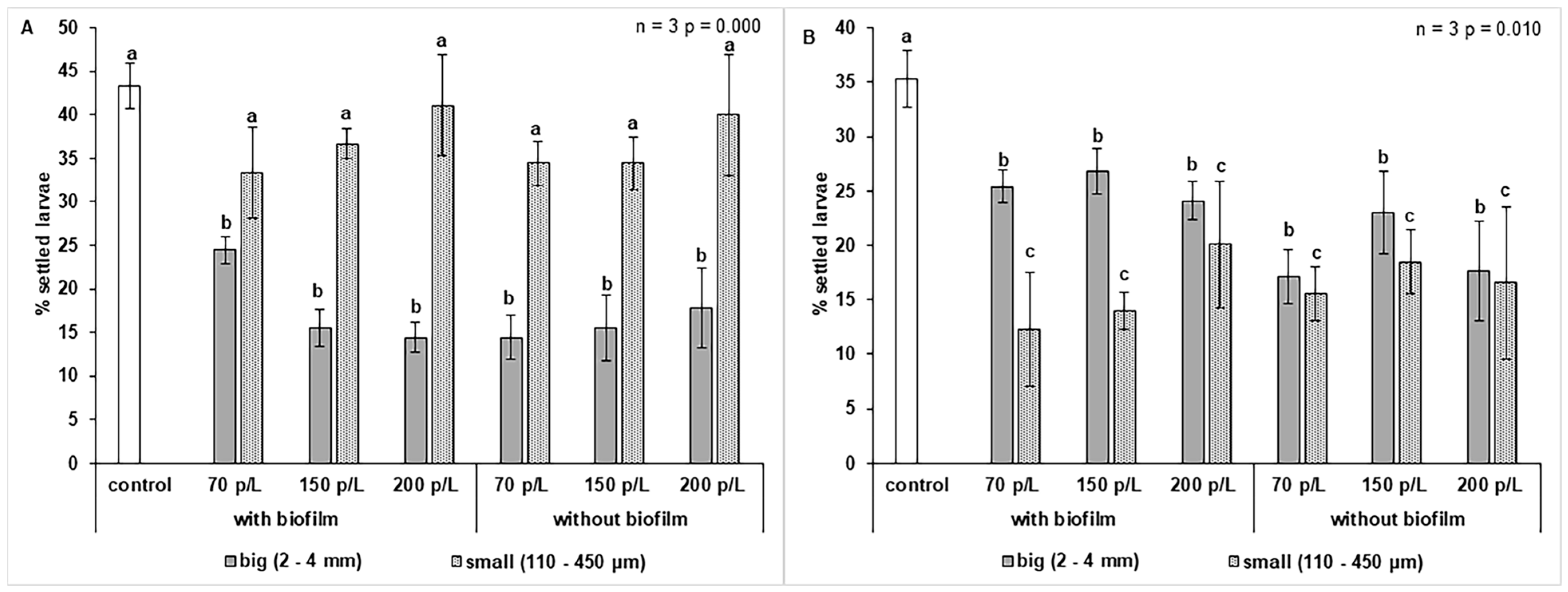
3.7. Effect of Microplastics on Larval Settlement of F. fragum
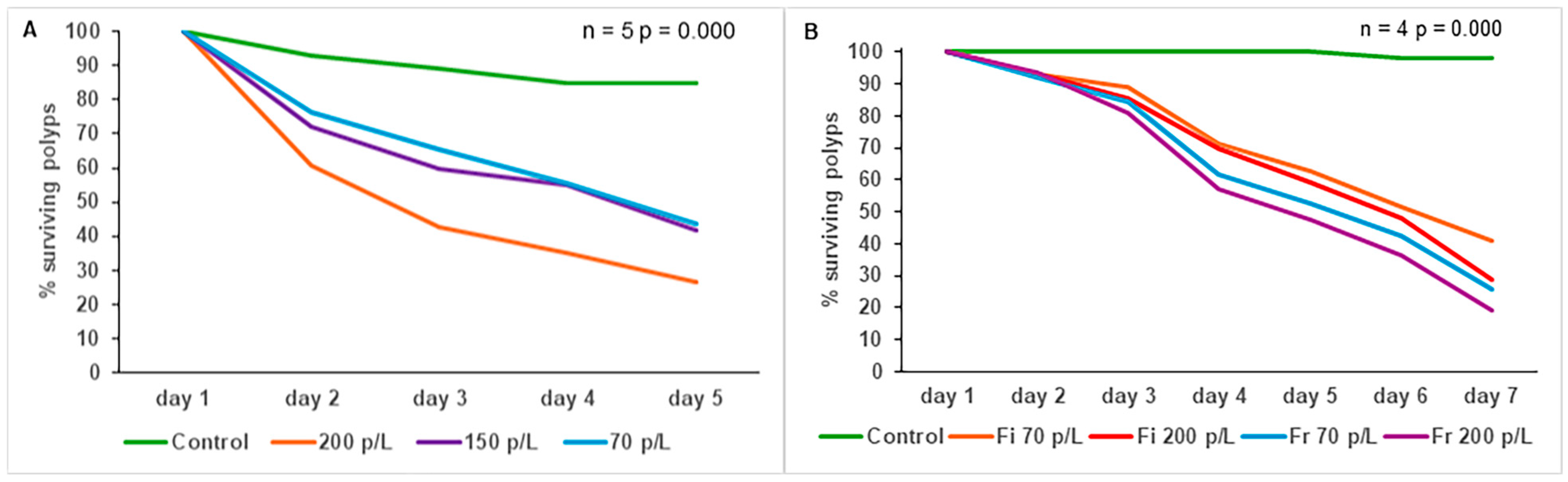
3.8. Effect of Microplastics on O. faveolata Post-Settlement Survival
4. Discussion
4.1. Trace Metal Assessment
4.2. Effect of Microplastics on O. faveolata Fertilization
4.3. Effect of Microplastics on O. faveolata Embryogenesis
4.4. Effect of Microplastics on Larval Development of O. faveolata
4.5. Effect of Microplastics on Larval Settlement of O. faveolata
4.6. Effect of Microplastics on A. palmata Settlement
4.7. Effect of Microplastics on Larval Settlement of F. fragum
4.8. Effect of Microplastics on O. faveolata Post-Settlement Survival
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Sipe, J.M.; Bossa, N.; Berger, W.; von Windheim, N.; Gall, K.; Wiesner, M.R. From bottle to microplastics: Can we estimate how our plastic products are breaking down? Sci. Total Environ. 2022, 814, 152460. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, M.; Song, B.; Deng, J.; Shen, M.; Chen, Q.; Zeng, G.; Liang, J. Microplastics in the coral reefs and their potential impacts on corals: A mini-review. Sci. Total Environ. 2021, 762, 143112. [Google Scholar] [CrossRef]
- Albaseer, S.S.; Al-Hazmi, H.E.; Kurniawan, T.A.; Xu, X.; Abdulrahman, S.A.; Ezzati, P.; Habibzadeh, S.; Hollert, H.; Rabiee, N.; Lima, E.C.; et al. Micro-plastics in water resources: Global pollution circle, possible technological solutions, legislations, and future horizon. Sci. Total Environ. 2024, 946, 173963. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Allen, A.S.; Seymour, A.C.; Rittschof, D. Chemoreception drives plastic consumption in a hard coral. Mar. Pollut. Bull. 2017, 124, 198–205. [Google Scholar] [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- e Silva, P.P.G.; Nobre, C.R.; Resaffe, P.; Pereira, C.D.S.; Gusmão, F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016, 106, 364–370. [Google Scholar] [CrossRef]
- Berry, K.L.; Epstein, H.E.; Lewis, P.J.; Hall, N.M.; Negri, A.P. Microplastic contamination has limited effects on coral fertilization and larvae. Diversity 2019, 11, 228. [Google Scholar] [CrossRef]
- Reichert, J.; Arnold, A.L.; Hoogenboom, M.O.; Schubert, P.; Wilke, T. Impacts of microplastics on growth and health of hermatypic corals are species-specific. Environ. Pollut. 2019, 254, 113074. [Google Scholar] [CrossRef] [PubMed]
- Rotjan, R.D.; Sharp, K.H.; Gauthier, A.E.; Yelton, R.; Lopez, E.M.B.; Carilli, J.; Kagan, J.C.; Urban-Rich, J. Patterns, dynamics and consequences of microplastic ingestion by the temperate coral, Astrangia poculata. Proc. R. Soc. B 2019, 286, 20190726. [Google Scholar] [CrossRef] [PubMed]
- Syakti, A.D.; Jaya, J.V.; Rahman, A.; Hidayati, N.V.; Raza’i, T.S.; Idris, F.; Trenggono, M.; Doumenq, P.; Chou, L.M. Bleaching and necrosis of staghorn coral (Acropora formosa) in laboratory assays: Immediate impact of LDPE micro-plastics. Chemosphere 2019, 228, 528–535. [Google Scholar] [CrossRef]
- Lanctôt, C.M.; Bednarz, V.N.; Melvin, S.; Jacob, H.; Oberhaensli, F.; Swarzenski, P.W.; Ferrier-Pagès, C.; Carroll, A.R.; Metian, M. Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyethylene microplastics. Environ. Pollut. 2020, 263, 114559. [Google Scholar] [CrossRef]
- Hankins, C.; Duffy, A.; Drisco, K. Scleractinian coral microplastic ingestion: Potential calcification effects, size limits, and retention. Mar. Pollut. Bull. 2018, 135, 587–593. [Google Scholar] [CrossRef]
- Sundt, P.; Schulze, P.E.; Syversen, F. Sources of Microplastic-Pollution to the Marine Environment; Report No. M-321-2015; Mepex for the Norwegian Environment Agency: Asker, Norway, 2014. [Google Scholar]
- Lee, S.; Kim, D.; Kang, K.K.; Sung, S.E.; Choi, J.H.; Sung, M.; Shin, C.H.; Jeon, E.; Kim, D.; Kim, D.; et al. Toxicity and biodistribution of fragmented polypropylene microplastics in ICR mice. Int. J. Mol. Sci. 2023, 24, 8463. [Google Scholar] [CrossRef]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Alderdice, R.; Pernice, M.; Cárdenas, A.; Hughes, D.J.; Harrison, P.L.; Boulotte, N.; Chartrand, K.; Kühl, M.; Suggett, D.J.; Voolstra, C.R. Hypoxia as a physiological cue and pathological stress for coral larvae. Mol. Ecol. 2022, 31, 571–586. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.; Labrenz, M. Marine microplastic-associated biofilms–A review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
- Feng, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qian, Z.J.; Hong, P.; Sun, S.; Li, C. Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere 2020, 252, 126565. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Chacon, E.M.; Gómez-Lemos, L.A.; Sierra-Sabalza, N.P.; Hernández-Chamorro, A.M.; Lozano-Peña, J.P.; Valcárcel-Castellanos, C.A.; Rojas, J.A. Early life history of the Caribbean coral Orbicella faveolata (Scleractinia: Merulinidae). Rev. Biol. Trop. 2020, 68, 1262–1274. [Google Scholar] [CrossRef]
- Szmant-Froelich, A.; Reutter, M.; Riggs, L. Sexual reproduction of Favia fragum (ESPER): Lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull. Mar. Sci. 1985, 37, 880–892. [Google Scholar]
- Soong, K. Colony size as a species character in massive reef corals. Coral Reefs 1993, 12, 77–83. [Google Scholar] [CrossRef]
- Gómez-Lemos, L.A.; Doropoulos, C.; Bayraktarov, E.; Kennedy, E.V.; Diaz-Pulido, G. Coralline algal metabolites induce settlement and mediate the inductive effect of epiphytic microbes on coral larvae. Sci Rep. 2018, 8, 17557. [Google Scholar] [CrossRef]
- Aoki, N.; Weiss, B.; Jézéquel, Y.; Apprill, A.; Mooney, T.A. Replayed reef sounds induce settlement of Favia fragum coral larvae in aquaria and field environments. JASA Express Lett. 2024, 4, 107701. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Kooi, M.; Law, K.L.; van Sebille, E. All is not lost: Deriving a top-down mass budget of plastic at sea. Environ Res Lett. 2017, 12, 114028. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Reichelt-Brushett, A.J.; Harrison, P.L. The effect of copper on the settlement success of larvae from the scleractinian coral Acropora tenuis. Mar. Pollut. Bull. 2001, 41, 385–391. [Google Scholar] [CrossRef]
- Woods, R.M.; Baird, A.H.; Mizerek, T.L.; Madin, J.S. Environmental factors limiting fertilisation and larval success in corals. Coral Reefs 2016, 35, 1433–1440. [Google Scholar] [CrossRef]
- Negri, A.P.; Heyward, A.J. Inhibition of coral fertilization and larval metamorphosis by tributyltin and copper. Mar. Environ. Res. 2001, 51, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Reichelt-Brushett, A.J.; Harrison, P.L. The effect of selected trace metals on the fertilization success of several scleractinian coral species. Coral Reefs 2005, 24, 524–534. [Google Scholar] [CrossRef]
- Oriji, O.G.; Nartey, V.K.; Brimah, A.K. Determination of the Polycyclic Aromatic Hydrocarbon and heavy metal contents of some Nigerian emulsion paints. Int. J. Appl. Chem. 2012, 8, 215–225. [Google Scholar]
- Nordborg, F.M.; Brinkman, D.L.; Ricardo, G.F.; Agustí, S.; Negri, A.P. Comparative sensitivity of the early life stages of a coral to heavy fuel oil and UV radiation. Sci. Total Environ. 2021, 781, 146676. [Google Scholar] [CrossRef]
- Squadrone, S.; Pederiva, S.; Bezzo, T.; Sartor, R.M.; Battuello, M.; Nurra, N.; Griglione, A.; Brizio, P.; Abete, M.C. Microplastics as vectors of metals contamination in Mediterranean Sea. Environ. Sci. Pollut. Res. 2021, 29, 29529–29534. [Google Scholar] [CrossRef]
- Zeeshan, M.; Murugadas, A.; Ghaskadbi, S.; Ramaswamy, B.R.; Akbarsha, M.A. Ecotoxicological assessment of cobalt using Hydra model: ROS, oxidative stress, DNA damage, cell cycle arrest, and apoptosis as mechanisms of toxicity. Environ. Pollut. 2017, 224, 54–69. [Google Scholar] [CrossRef]
- Wilkins, K.W.; Yew, J.Y.; Seeley, M.; Richmond, R.H. Microplastic Leachate Negatively Affects Fertilization in the Coral Montipora capitata. Integr. Comp. Biol. 2024, 64, 1131–1140. [Google Scholar] [CrossRef]
- Chiarelli, R.; Roccheri, M.C. Marine invertebrates as bioindicators of heavy metal pollution. Open J Metal. 2014, 4, 93–106. [Google Scholar] [CrossRef]
- Harrison, P.L.; Babcock, R.C.; Bull, G.D.; Oliver, J.K.; Wallace, C.C.; Willis, B.L. Mass spawning in tropical reef corals. Science 1984, 223, 1186–1189. [Google Scholar] [CrossRef]
- Heyward, A.J.; Negri, A.P. Turbulence, cleavage, and the naked embryo: A case for coral clones. Science 2012, 335, 1064. [Google Scholar] [CrossRef]
- Ricardo, G.F.; Jones, R.J.; Clode, P.L.; Humanes, A.; Negri, A.P. Suspended sediments limit coral sperm availability. Sci. Rep. 2016, 5, 18084. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Virgile, Q.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Estes, L.L.; Schweizer, M. Fibers, 4. Polyamide Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Sadiku, R.; Ibrahim, D.; Agboola, O.; Owonubi, S.J.; Fasiku, V.O.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Adekomaya, O.S.; Varaprasad, K.; et al. Automotive Components Composed of Polyolefins. In Polyolefin Fibres; Woodhead Publishing: Cambridge, UK, 2017; pp. 449–496. [Google Scholar]
- Humphrey, C.; Weber, M.; Lott, C.; Cooper, T.; Fabricius, K.J. Effects of suspended sediments, dissolved inorganic nutrients and salinity on fertilization and embryo development in the coral Acropora millepora (Ehrenberg, 1834). Coral Reefs 2008, 27, 837–850. [Google Scholar] [CrossRef]
- Ricardo, G.F.; Jones, R.J.; Clode, P.L.; Humanes, A.; Giofre, N.; Negri, A.P. Sediment characteristics influence the fertilisation success of the corals Acropora tenuis and Acropora millepora. Mar. Pollut. Bull. 2018, 135, 941–953. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Vandermeulen, J.H. Studies on reef corals. II. Fine structure of planktonic planula larva of Pocillopora damicornis, with emphasis on the aboral epidermis. Mar. Biol. 1974, 27, 239–247. [Google Scholar] [CrossRef]
- Wild, C.; Naumann, M.; Niggl, W.; Haas, A. Carbohydrate composition of mucus released by scleractinian warm- and cold-water reef corals. Aquat. Biol. 2010, 10, 41–45. [Google Scholar] [CrossRef]
- Ricardo, G.F.; Jones, R.J.; Clode, P.L.; Negri, A.P. Mucous Secretion and Cilia Beating Defend Developing Coral Larvae from Suspended Sediments. PLoS ONE 2016, 11, e0162743. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Zhou, X.; Yan, H.; Han, X.; Wu, M.; Chen, F. Research progress on the role of biofilm in heavy metals adsorption-desorption characteristics of microplastics: A review. Environ. Pollut. 2023, 336, 122448. [Google Scholar] [CrossRef] [PubMed]
- Takeda-Sakazume, A.; Honjo, J.; Sasano, S.; Matsushima, K.; Baba, S.A.; Mogami, Y.; Hatta, M. Gravitactic Swimming of the planula larva of the coral Acropora: Characterization of straightforward vertical swimming. Zool. Sci. 2022, 40, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Jiang, L.; Zhang, Y.; Sun, Y.; Zhou, G.; Lian, J.; Huang, H. Coral larval settlement and post-settlement survival facilitated by crustose coralline algae with or without living tissue. Mar. Biol. 2021, 168, 128. [Google Scholar] [CrossRef]
- Martinez, S.; Abelson, A. Coral recruitment: The critical role of early post-settlement survival. ICES J. Mar. Sci. 2013, 70, 1294–1298. [Google Scholar] [CrossRef]
- Flores, F.; Hoogenboom, M.O.; Smith, L.D.; Cooper, T.F.; Abrego, D.; Negri, A.P. Chronic exposure of corals to fine sediments: Lethal and sub-lethal impacts. PLoS ONE 2012, 7, e37795. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galindo, C.; Gómez-Lemos, L.A.; Quiroga, S.; García-Urueña, R. Secondary Microplastics Disrupt Early Coral Development: Impacts on Brooding and Broadcast-Spawning Species. Diversity 2025, 17, 468. https://doi.org/10.3390/d17070468
García-Galindo C, Gómez-Lemos LA, Quiroga S, García-Urueña R. Secondary Microplastics Disrupt Early Coral Development: Impacts on Brooding and Broadcast-Spawning Species. Diversity. 2025; 17(7):468. https://doi.org/10.3390/d17070468
Chicago/Turabian StyleGarcía-Galindo, Camilo, Luis A. Gómez-Lemos, Sigmer Quiroga, and Rocío García-Urueña. 2025. "Secondary Microplastics Disrupt Early Coral Development: Impacts on Brooding and Broadcast-Spawning Species" Diversity 17, no. 7: 468. https://doi.org/10.3390/d17070468
APA StyleGarcía-Galindo, C., Gómez-Lemos, L. A., Quiroga, S., & García-Urueña, R. (2025). Secondary Microplastics Disrupt Early Coral Development: Impacts on Brooding and Broadcast-Spawning Species. Diversity, 17(7), 468. https://doi.org/10.3390/d17070468






