A New Species of Enicospilus Stephens, 1835 (Ichneumonidae, Ophioninae), from Southern Mexico, Parasitic on Zanola verago Cramer, 1777 (Lepidoptera, Apatelodidae), Feeding on Piper neesianum C. DC. (Piperaceae)
Abstract
1. Introduction
2. Materials and Methods
DNA Protocols
3. Results
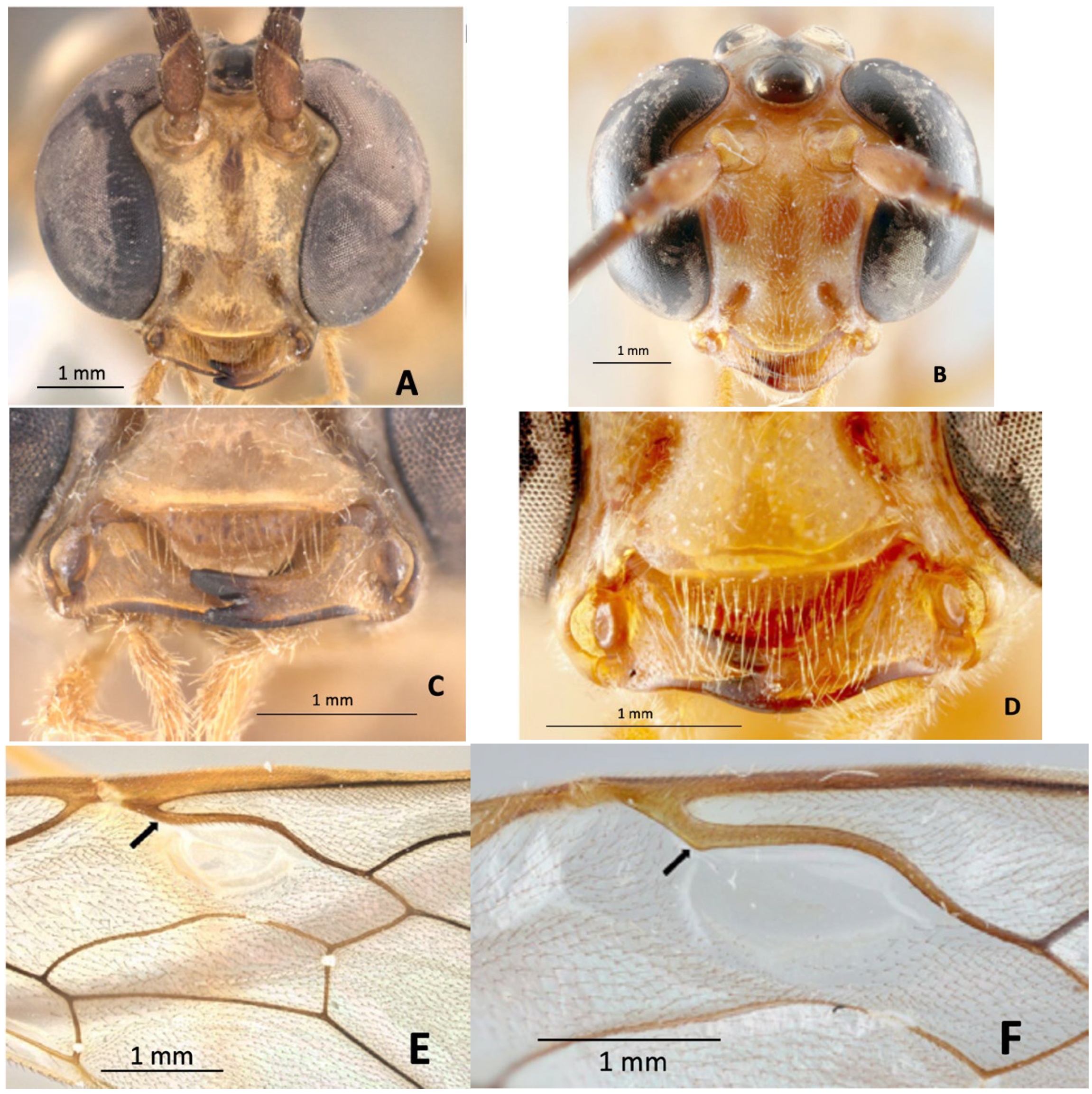
3.1. Description
3.2. Diagnosis
3.3. Etymology
3.4. Distribution
3.5. Ecology
3.6. Biology
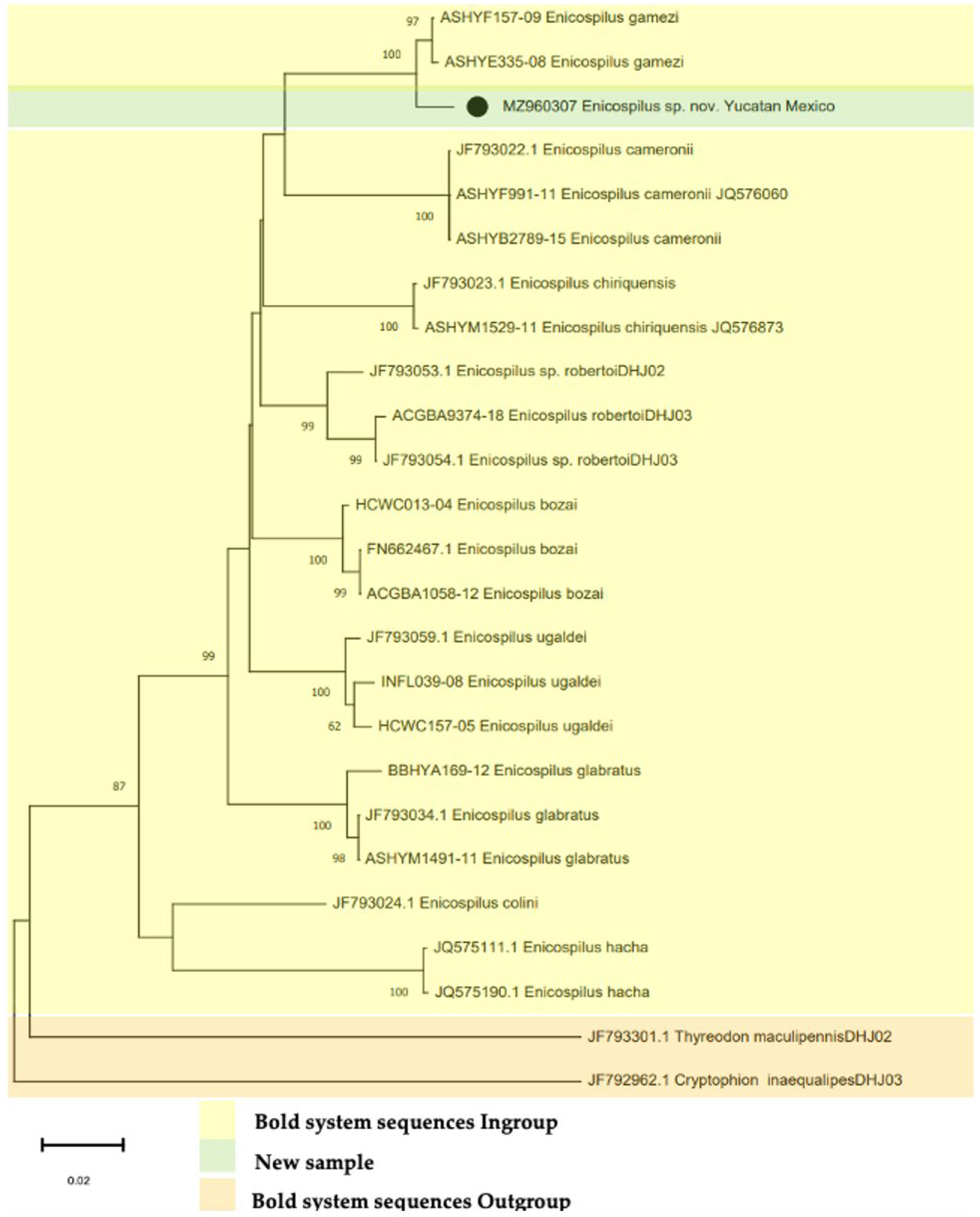
3.7. DNA Barcode
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dyer, L.A.; Smilanich, A.M.; Gompert, Z.; Forister, M.L. Insect conservation, technological traps, and the fading arts of natural history and field ecology. Curr. Opin. Insect Sci. 2024, 66, 101261. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Biodiversity research requires more boots on the ground. Nat. Ecol. Evol. 2017, 1, 1590–1591. [Google Scholar] [CrossRef]
- Price, P.W. Resource-driven terrestrial interaction webs. Ecol. Res. 2002, 17, 241–247. [Google Scholar] [CrossRef]
- Dyer, L.A.; Singer, M.S.; Lill, J.T.; Stireman, J.O.; Gentry, G.L.; Marquis, R.J.; Ricklefs, R.E.; Greeney, H.F.; Wagner, D.L.; Morais, H.C.; et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 2007, 448, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Dyer, L.A.; Singer, M.S.; Stireman, J.O.; Lill, J.T. Revisiting the evolution of ecological specialization, with emphasis on insect–plant interactions. Ecology 2012, 93, 981–991. [Google Scholar] [CrossRef]
- Singer, M.S.; Stireman, J.O. The tri-trophic niche concept and adaptive radiation of phytophagous insects. Ecol. Lett. 2005, 8, 1247–1255. [Google Scholar] [CrossRef]
- Dyer, L.A.; Forister, M.L. Challenges and advances in the study of latitudinal gradients in multitrophic interactions, with a focus on consumer specialization. Curr. Opin. Insect Sci. 2019, 32, 68–76. [Google Scholar] [CrossRef]
- Toro-Delgado, E.; Hernández-Roldán, J.; Dincă, V.; Vicente, J.C.; Shaw, M.R.; Quicke, D.L.; Vodă, R.; Albrecht, M.; Fernández-Triana, J.; Vidiella, B.; et al. Butterfly–parasitoid–hostplant interactions in Western Palaearctic Hesperiidae: A DNA barcoding reference library. Zool. J. Linn. Soc. 2022, 196, 757–774. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Ridenbaugh, R.D.; Sharanowski, B.J. Integrative taxonomy improves understanding of native beneficial fauna: Revision of the Nearctic Peristenus pallipes complex (Hymenoptera: Braconidae) and implications for release of exotic biocontrol agents. Syst. Entomol. 2017, 42, 596–608. [Google Scholar] [CrossRef]
- Poelman, E.H.; van Loon, J.J.; Dicke, M. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci. 2008, 13, 534–541. [Google Scholar] [CrossRef]
- Thierry, M.; Hrček, J.; Lewis, O.T. Mechanisms structuring host–parasitoid networks in a global warming context: A review. Ecol. Entomol. 2019, 44, 581–592. [Google Scholar] [CrossRef]
- Evans, D.M.; Kitson, J.J.; Lunt, D.H.; Straw, N.A.; Pocock, M.J. Merging DNA metabarcoding and ecological network analysis to understand and build resilient terrestrial ecosystems. Funct. Ecol. 2016, 30, 1904–1916. [Google Scholar] [CrossRef]
- Quijano-Abril, M.A.; Callejas-Posada, R.; Miranda-Esquivel, D.R. Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J. Biogeogr. 2006, 33, 1266–1278. [Google Scholar] [CrossRef]
- Bornstein, A.J. Taxonomic studies in the Piperaceae—I: The pedicellate pipers of Mexico and Central America. J. Arnold Arbor. 1989, 70, 1–55. [Google Scholar] [CrossRef]
- Ulloa Ulloa, C.P.; Acevedo-Rodríguez, S.G.; Beck, M.J.; Belgrano, R.; Bernal, P.E.; Berry, L.; Brako, M.; Celis, G.; Davidse, S.R.; Gradstein, O.; et al. An integrated assessment of the vascular plant species of the Americas. Science 2018, 358, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- HOSTS—A Database of the World’s Lepidopteran Host Plants. 2010. Available online: https://data.nhm.ac.uk/dataset/hosts (accessed on 12 August 2020).
- Janzen DHHW Philosophy, Navigation and Use of a Dynamic Database (“ACG Caterpillars SRNP”) for An Inventory of the Macrocaterpillar Fauna, Its Food Plants and Parasitoids, of the Area de Conservación Guanacaste (ACG), Northwestern Costa Rica. 2012. Available online: https://www.gdfcf.org/content/acg-species-pages-over-1000-and-counting (accessed on 21 April 2025).
- Kitching, I.; Rougerie, R.; Zwick, A.; Hamilton, C.; Laurent, R.S.; Naumann, S.; Mejia, L.B.; Kawahara, A. A global checklist of the Bombycoidea (Insecta: Lepidoptera). Biodivers. Data J. 2018, 6, e22236. [Google Scholar] [CrossRef]
- Walker, F. List of the specimens of lepidopterous insects in the collection of the British Museum. In The Turstees; British Museum: London, UK, 1855; Volume 5. [Google Scholar]
- Dyar, H.G. Descriptions of the larvae of some Lepidoptera from Mexico. Proc. Entomol. Soc. Wash. 1912, 14, 54–58. [Google Scholar]
- Ricardo-Molina, J.; Murillo-Ramos, L.; Álvarez-Pérez, P. Larvas y plantas nutricias de Lepidoptera en fragmentos de bosque seco tropical del departamento de Sucre, Colombia (Insecta: Lepidoptera). Rev. De Lepidopterol. 2019, 47, 5–24. [Google Scholar]
- Rabelo, R.S.; Dyer, L.A.; Lepesqueur, C.; Salcido, D.M.; da Silva, T.P.; Rodrigues, H.P.A.; Trindade, T.B.; Diniz, I.R.; Nascimento, A.R.; Tepe, E.J.; et al. Tritrophic interaction diversity in gallery forests: A biologically rich and understudied component of the Brazilian cerrado. Arthropod-Plant Interact. 2021, 15, 773–785. [Google Scholar] [CrossRef]
- Gauld, I.D. A survey of the Ophioninae (Hymenoptera: Ichneumonidae) of tropical Mesoamerica with special reference to the fauna of Costa Rica. Bull. Br. Mus. Nat. Hist. Entomol. Ser. 1988, 57, 1–309. [Google Scholar]
- Gauld, I.D. The species of the Enicospilus americanus complex (Hymenoptera: Ichneumonidae) in eastern North America. Syst. Entomol. 1988, 13, 31–53. [Google Scholar] [CrossRef]
- Fernández-Triana, J.L. The taxonomy and biogeography of Cuban Ophioninae (Hymenoptera: Ichneumonidae). Zootaxa 2005, 1007, 1–60. [Google Scholar] [CrossRef]
- Garcia-Garcia, A. Tres especies nuevas de Enicospilus (Ichneumonidae: Ophioninae) de Colombia. Rev. Colomb. Entomol. 2011, 37, 145–151. [Google Scholar] [CrossRef]
- Lima, A.R.; Jacobi, C.M.; Kumagai, A.F. A key to the Neotropical species of the Enicospilus ramidulus species-group (Hymenoptera: Ichneumonidae: Ophioninae), with the description of a new Brazilian species. Zootaxa 2012, 3409, 63–68. [Google Scholar] [CrossRef]
- Yu, D.S.; van Achterberg, C.V.; Horstmann, K. Taxapad 2016, Ichneumonoidea 2015. In Database on Flash-Drive; The Catalogue of Life: Nepean, ON, Canada, 2016. [Google Scholar]
- Ruíz-Cancino, E. La familia Ichneumonidae (Hymenoptera) en México. Entomol. Mex. 2015, 2, 1–13. [Google Scholar]
- Campos-Moreno, D.; Dyer, L.; Salcido, D.; Massad, T.J.; Pérez-Lachaud, G.; Tepe, E.; Whitfield, J.; Pozo, C. Importance of interaction rewiring in determining spatial and temporal turnover of tritrophic (Piper-caterpillar-parasitoid) metanetworks in the Yucatán Península, México. Biotropica 2021, 53, 1071–1081. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Dewaard, J.R.; Hebert, P.D. An inexpensive, automation friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Stigenberg, J.; Ronquist, F. Revision of the Western Palearctic Meteorini (Hymenoptera, Braconidae), with a molecular characterization of hidden Fennoscandian species diversity. Zootaxa 2011, 3084, 1–95. [Google Scholar] [CrossRef]
- Ceccarelli, F.S.; Sharkey, M.J.; Zaldívar-Riverón, A. Species identification in the taxonomically neglected, highly diverse, neotropical parasitoid wasp genus Notiospathius (Braconidae: Doryctinae) based on an integrative molecular and morphological approach. Mol. Phylogenetics Evol. 2012, 62, 485–495. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing evolutionary trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Burns, J.; Janzen, D.H.; Hajibabaei, M.; Hallwachs, W.; Hebert, P.D. DNA barcodes of closely related (but morphologically and ecologically distinct) species of skipper butterflies (Hesperiidae) can differ by only one to three nucleotides. J. Lepid. Soc. 2007, 61, 138–153. [Google Scholar]
- Janzen, D.H.; Burns, J.M.; Cong, Q.; Hallwachs, W.; Dapkey, T.; Manjunath, R.; Hajibabaei, M.; Hebert, P.D.N.; Grishin, N.V. Nuclear genomes distinguish cryptic species suggested by their DNA barcodes and ecology. Proc. Natl. Acad. Sci. USA 2017, 114, 8313–8318. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.W.H. DNA barcoding the Lepidoptera inventory of a large complex tropical conserved wildland, Area de Conservacion Guanacaste, northwestern Costa Rica. Genome 2016, 59, 641–660. [Google Scholar] [CrossRef]
- Dyer, L.A.; Miller, J.S.; Rab Green, S.B.; Gentry, G.L.; Greeney, H.F.; Walla, T.W. Caterpillars and parasitoids of the Eastern Andes in Ecuador. Available online: http://www.caterpillars.org (accessed on 8 February 2021).
- Säterberg, T.; Jonsson, T.; Yearsley, J.; Berg, S.; Ebenman, B. A potential role for rare species in ecosystem dynamics. Sci. Rep. 2019, 9, 11107. [Google Scholar] [CrossRef]
- Janzen, D.H. Ecological characterization of a Costa Rican dry forest caterpillar fauna. Biotropica 1988, 20, 120–135. [Google Scholar] [CrossRef]
- Janzen, D.H.; Gauld, I.D. Caterpillars (Lepidoptera: Saturnidae and Sphingidae) by Ichneumonid parasitoids (Hymenoptera) in Costa Rican dry forest. In Forests and Insects; Watt, A.D., Stork, N.E., Hunter, M.D., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; Volume 251. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Moreno, D.F.; Palacio, E.; Lara-Pérez, L.A.; Whitfield, J.B.; Pozo, C.; Dyer, L.A. A New Species of Enicospilus Stephens, 1835 (Ichneumonidae, Ophioninae), from Southern Mexico, Parasitic on Zanola verago Cramer, 1777 (Lepidoptera, Apatelodidae), Feeding on Piper neesianum C. DC. (Piperaceae). Diversity 2025, 17, 466. https://doi.org/10.3390/d17070466
Campos-Moreno DF, Palacio E, Lara-Pérez LA, Whitfield JB, Pozo C, Dyer LA. A New Species of Enicospilus Stephens, 1835 (Ichneumonidae, Ophioninae), from Southern Mexico, Parasitic on Zanola verago Cramer, 1777 (Lepidoptera, Apatelodidae), Feeding on Piper neesianum C. DC. (Piperaceae). Diversity. 2025; 17(7):466. https://doi.org/10.3390/d17070466
Chicago/Turabian StyleCampos-Moreno, Diego Fernando, Edgard Palacio, Luis Alberto Lara-Pérez, James B. Whitfield, Carmen Pozo, and Lee A. Dyer. 2025. "A New Species of Enicospilus Stephens, 1835 (Ichneumonidae, Ophioninae), from Southern Mexico, Parasitic on Zanola verago Cramer, 1777 (Lepidoptera, Apatelodidae), Feeding on Piper neesianum C. DC. (Piperaceae)" Diversity 17, no. 7: 466. https://doi.org/10.3390/d17070466
APA StyleCampos-Moreno, D. F., Palacio, E., Lara-Pérez, L. A., Whitfield, J. B., Pozo, C., & Dyer, L. A. (2025). A New Species of Enicospilus Stephens, 1835 (Ichneumonidae, Ophioninae), from Southern Mexico, Parasitic on Zanola verago Cramer, 1777 (Lepidoptera, Apatelodidae), Feeding on Piper neesianum C. DC. (Piperaceae). Diversity, 17(7), 466. https://doi.org/10.3390/d17070466






