The Genus Tegonotus Nalepa (Acariformes: Eriophyidae: Phyllocoptinae): Description of a New Species and Key to Valid Species †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Updated Diagnosis
3.2. New Species
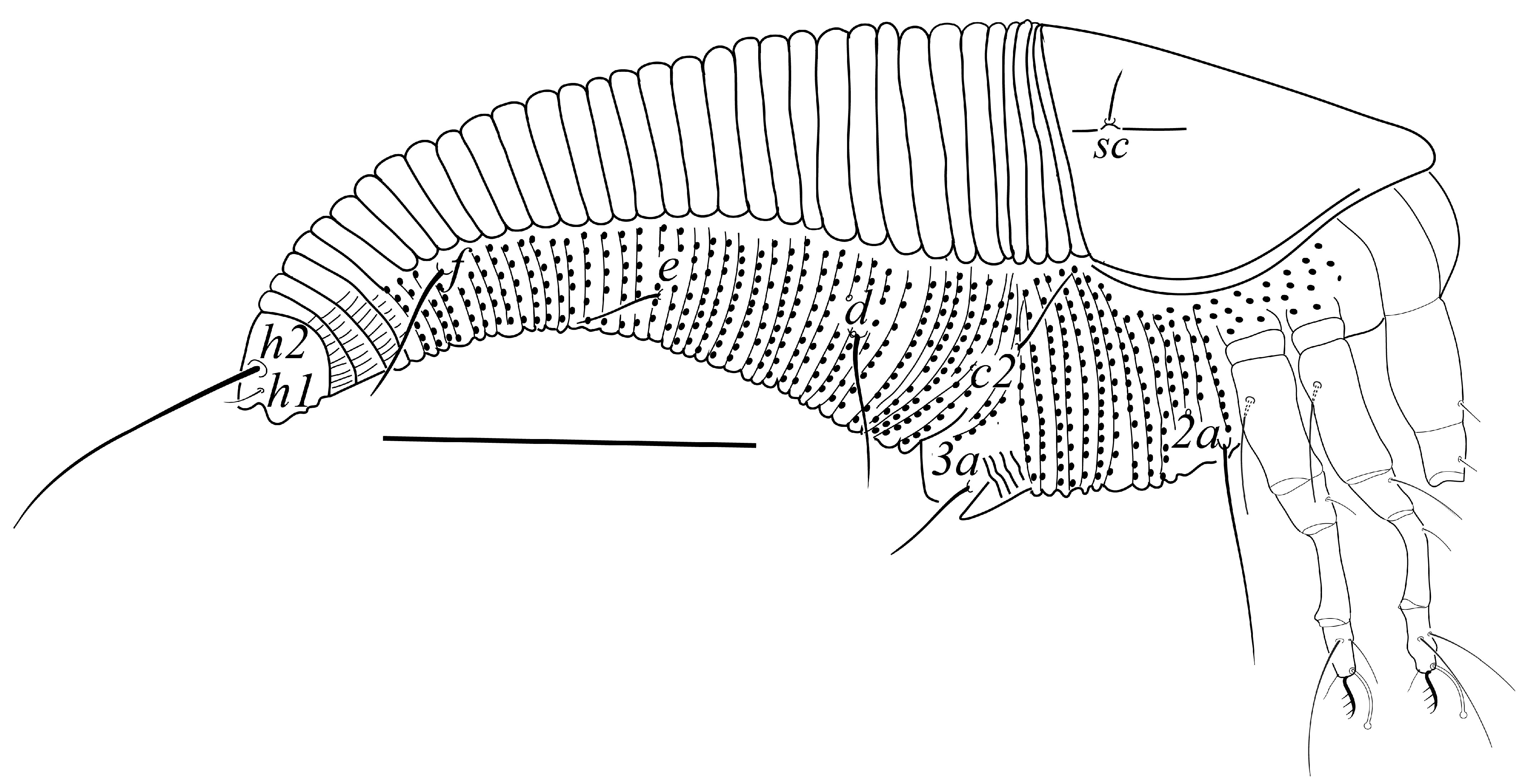
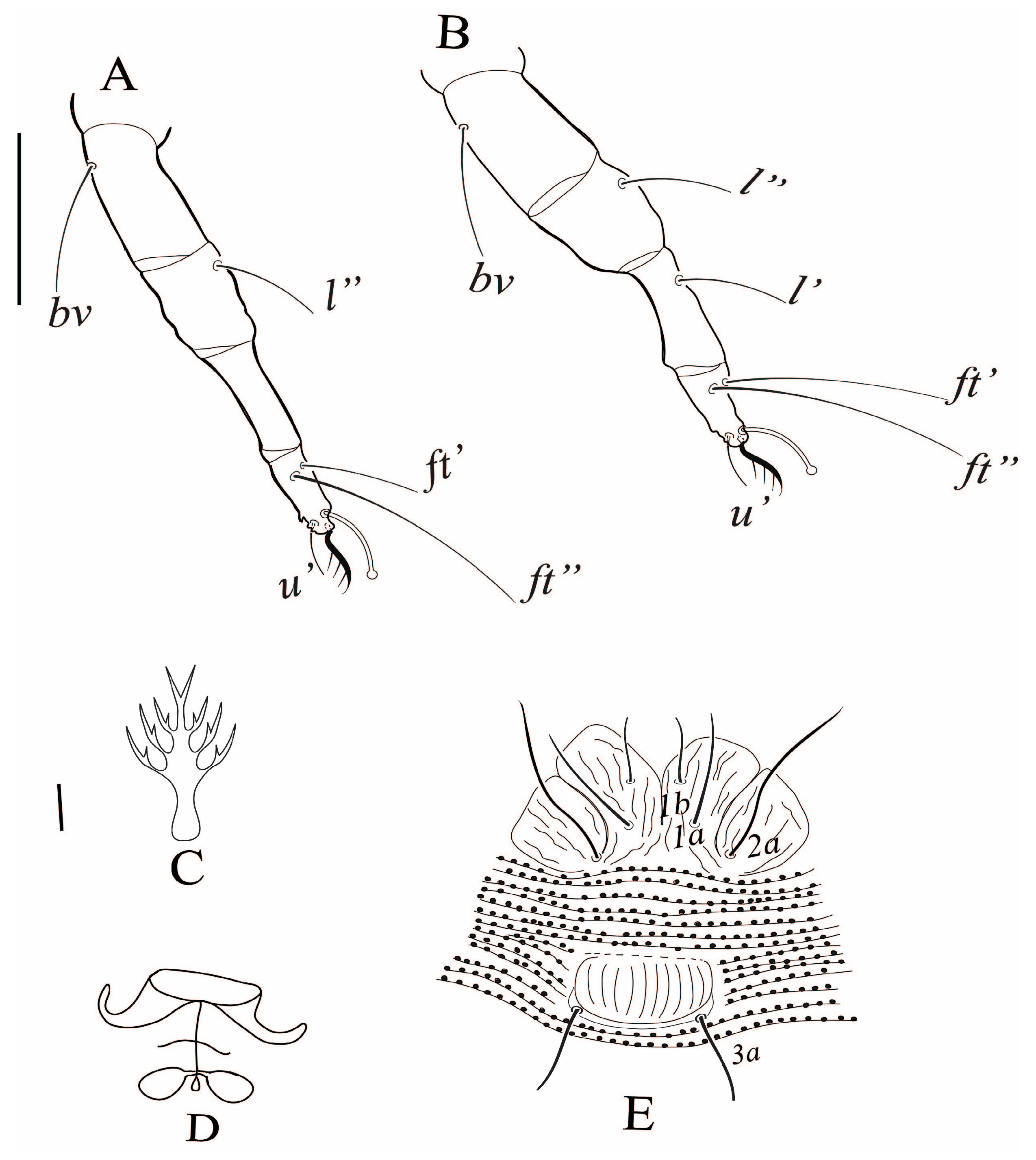
3.2.1. Tegonotus saudiensis sp. nov.
3.2.2. Identification Key for the Species of the Genus Tegonotus (47 Species)
- 1.
- Setae h1 absent……………………………………..…..………..………………………..…2
- -
- Setae h1 present…………………………………..……………..…………………….…...21
- 2.
- Female genital cover flap without striae…………………………….................................3
- -
- Female genital cover flap with striae…….………………….…………..………………...4
- 3.
- Opisthosomal dorsal semiannuli laterally with prominent pointed projections ……………………………………………………………………….....T. trouessarti Nalepa
- -
- Opisthosomal dorsal semiannuli laterally with rounded lobe-like projections…………………………………………………………...T. actinidiae Wei, Wang et Li
- 4.
- Female genital cover flap with a single row of striae………………….………...……...8
- -
- Female genital cover flap with two rows of striae……………………………….……….5
- 5.
- Coxisternal plates smooth………………..….T. bhutanensis Chakrabarti et Chakrabarti
- -
- Coxisternal plates with lines/small dashes…………………………………..…………...6
- 6.
- Prodorsal shield with only admedian lines……………………………………………….. ………………………………………..T. caricus Elhalawany, Mohamed, et Ueckermann
- -
- Prodorsal shield with median and submedian broken lines ……………………..……7
- 7.
- Anterior and lateral edges of prodorsal shield granulates…………………………… ………………………………………………T. microcarpa Ren, Guan, Tan, Yang et Wang
- -
- Anterior and lateral edges of prodorsal shield without granules…………………….……………………………………………………………………….T. camphori (Barké et Davis)
- 8.
- Opisthosomal dorsal semiannuli laterally with prominent pointed projections……9
- -
- Opisthosomal dorsal semiannuli laterally with prominent rounded projection…….10
- 9.
- Prodorsal shield with three small spines on anterior margin of frontal lobe, empodium 7-rayed……………………………………………T. gutierrezi Boczek et Natcheff
- -
- Prodorsal shield without spines on anterior margin of frontal lobe, empodium 4-rayed……………………………………………………………….T. acutilobus (Nalepa)
- 10.
- Prodorsal shield with depressed hollow pattern or complete network of cells……...11
- -
- Prodorsal shield with lines…………………………………….....………………………12
- 11.
- Prodorsal shield with depressed hollow pattern, seta on genu II present…………... …………………………………………………………………..T. canaris Xu, Chen et Xue
- -
- Prodorsal shield with complete network of cells, seta on genu II absent….............… ……………………………………………………………….T. bassius Das et Chakrabarti
- 12.
- Prodorsal shield with many irregular broken lines..……….......T. beihaiensis Wei et Xie
- -
- Prodorsal shield with prominent median, or admedian or submedian lines………. 13
- 13.
- Prodorsal shield with median line………..…………..……….…..….……………..…...14
- -
- Prodorsal shield without median line…………………………...……............................15
- 14.
- Coxisternal plates with lines; empodium 5-rayed……………......................………….. ……………………………………………………..T. paramangiferae Huang, An et Huang
- -
- Coxisternal plates without lines, empodium 6-rayed…..............................……………. ……………………………………………..T. jambolensis Mondal, Ghosh et Chakrabarti
- 15.
- Admedian lines of the prodorsal shield medially connected by a transverse line...... ……………………………………………………………T. albus Xue, Han, Song et Hong
- -
- Admedian lines of the prodorsal shield not medially connected by a transverse line………….........................................................................................................................16
- 16.
- Coxisternal plates with granules or lines…….………..……..………………………… 17
- -
- Coxisternal plates without granules or lines………..……………..……………...........19
- 17.
- Prodorsal shield with admedian lines curvy, anterior and lateral edges of prodorsal shield with granules…………………………………………………….T. streblusi Boczek
- -
- Prodorsal shield with admedian or submedian lines straight, anterior and lateral edges of prodorsal shield without granules …………………………………………...18
- 18.
- Submedian line absent, admedian line very short, from basal 1/5 to 2/5…………….. …………………………………………………………………..T. exiguus Huang et Wang
- -
- Submedian line present, long and branched near the dorsal tubercle………………… ……………………………………………………….T. bengalensis Mondal et Chakrabarti
- 19.
- Empodium 7-rayed…………………………………………….T. similis Wang et Huang
- -
- Empodium 5-rayed……………………………………………………………………….20
- 20.
- Prodorsal shield with lateral submedian line, running parallel to the lateral side of the shield…………………………………..………………….T. tinctoriae Wei, Wang et Li
- -
- Prodorsal shield without lateral submedian lines….T. caryophyllatus Huang et Cheng
- 21.
- Prodorsal shield anterior frontal lobe emarginated/notched………..T. keiferi (Farkas)
- -
- Prodorsal shield anterior frontal lobe not emarginated/rounded……………………22
- 22.
- A deep cleft between prodorsal shield and opisthosoma, first semiannuli large and projecting higher than other annuli……………………………………T. collaris Nalepa
- -
- Prodorsal shield and opisthosoma not separated by a deep cleft, first semiannuli not enlarged………..………………..………………………………………………………….23
- 23.
- Opisthosomal semiannuli laterally with prominent pointed projections……………41
- -
- Opisthosomal semiannuli laterally with rounded lobes……………………………….24
- 24.
- Prodorsal shield with lines………….....………..……..……………...………………….26
- -
- Prodorsal shield without lines.…..…..…………………………………………………..25
- 25.
- Prodorsal shield entirely granulated…………………………………..T. halleriae Meyer
- -
- Prodorsal shield entirely smooth…………………………….T. buergeriani Kuang et Lin
- 26.
- Opisthosoma with first eight dorsal semiannuli broad……..T. montanus Hu et Krantz
- -
- Opisthosoma all dorsal semiannuli of similar size…………………………………….27
- 27.
- Prodorsal shield with many irregular broken lines………………T. septentrionalis Liro
- -
- Prodorsal shield with median or admedian, or submedian lines……...…….………28
- 28.
- Prodorsal shield with median line……………………………………………..…...……29
- -
- Prodorsal shield without median line………………………………...………..…..…....32
- 29.
- Median lines complete, empodium 4-rayed......................T. hispidae Wang, Wei et Yang
- -
- Median line incomplete; empodium more than 4-rayed……………….……………..30
- 30.
- Prodorsal shield laterally granulated………………T. exbucklandia Wang, Tan et Yang
- -
- Prodorsal shield laterally without granule……………………………………………..31
- 31.
- Coxisternal plates smooth, admedian line incomplete…..T. carpathicus Lewandowski
- -
- Coxisternal plates with granules, admedian line complete …………………………… …………………………………………………………...T. parabaenae Wang, Tan et Yang.
- 32.
- Prodorsal shield lines forming a network of cells…………T. fabris Han, Xue et Hong.
- -
- Prodorsal shield without network of cells……………………………………………..33
- 33.
- Prodorsal shield with granules…………..………………………………T. borealis (Liro)
- -
- Prodorsal shield without granules ………..…………………..………………………..34
- 34.
- Coxisternal plates with granules or lines………………………………………………39
- -
- Coxisternal plates smooth……………………………………………………………….35
- 35.
- Female genital coverflap indistinct/without striae……………………………………36
- -
- Female genital coverflap with distinct striae…………………………………………..37
- 36.
- Prodorsal shield frontal lobe anteriorly triangular, empodium 4-rayed……………… …………………………………………………………………………T. scoticus Roivainen
- -
- Prodorsal shield frontal lobe anteriorly broad rounded, empodium 6-rayed……….. ……………………………………………………………………………T. abietis Bagnyuk
- 37.
- Frontal lobe of prodorsal shield anteriorly emarginated/notched…………………….. …………………………………………………………….T. adamasimilis Wang et Huang
- -
- Frontal lobe of prodorsal shield anteriorly broad rounded………………………….38
- 38.
- Opisthosomal dorsal semiannuli with filamentous microtubercles, empodium 6-rayed, admedian short at the base…………………………T. pini Wang, Tang et Yang
- -
- Opisthosomal dorsal semiannuli smooth, empodium 4-rayed, admedian complete…………………………………………………….T. larii Kamali, Majidi et Akrami
- 39.
- Female genital cover flap with single row of striae…………………………………….40
- -
- Female genital cover flap with two rows of striae….T. convolvuli (Channa Basavanna)
- 40.
- Opisthosomal dorsal semiannuli without ridges, admedian line connected by a transverse line………………………………………………………………T. saudiensis sp. nov.
- -
- Opisthosomal dorsal semiannuli with longitudinal ridges, admedian line not connected by a transverse line……………………………………T. tricarinatus Flechtmann
- 41.
- Prodorsal shield with granules…………………………………………………………..42
- -
- Prodorsal shield without granules……………………….………..……….……..……..44
- 42.
- Opisthosomal dorsal semiannuli laterally with alternative projection, prodorsal shield only laterally, with few granules…………………………….T. uranomus (Keifer)
- -
- Opisthosomal dorsal semiannuli laterally with equally expanded projection, prodorsal shield medially and laterally with many granules…………………………………43
- 43.
- Opisthosomal dorsal semiannuli with median ridges, coxisternal plates smooth…………………………………………………………….T. heptacanthus (Nalepa)
- -
- Opisthosomal dorsal semiannuli with furrow; coxisternal plates granulated ………………………………………………………….T. platycaryanis Song, Xue et Hong
- 44.
- Frontal lobe of prodorsal shield anteriorly spear-shaped…………...T. occidens (Keifer)
- -
- Frontal lobe of prodorsal shield anteriorly broad, rounded………………………….45
- 45.
- Opisthosomal dorsal semiannuli laterally with alternative pointed projections; empodium 3-rayed………………………………………………………….T. eupators Huang
- -
- Opisthosomal dorsal semiannuli laterally with equally expanded projections; empodium 4- or 5-rayed…………………………………………………………………………46
- 46.
- Empodium 5-rayed ………...............................…….T. ecovagrans (Flechtmann et Davis)
- -
- Empodium 4-rayed………………….…………................................T. depressus (Nalepa)
| Species | Type Host | Type Country | Diagnostic Characters of the Genus Transferred | Comments |
|---|---|---|---|---|
| Tegonotus castanopsis [27] | Castanopsis eyrei (Champ. et Benth.) Tutcher (Fagaceae) | Hungary | Scapular tubercles and setae (sc) are located on the rear shield marginŠ | It is suggested that four Tegonotus species be assigned to the genus Shevtchenkella, based on the consistent generic characteristics, i.e., scapular tubercles and setae (sc) on or near the rear shield margin, found in their descriptions and illustrations. Shevtchenkella is the largest genus in Tegonotini, consisting of more than 85 species. Currently, it is difficult to propose a possible synonym or a closely related species for each species. |
| T. fragariae [28] | Fragaria sp. (Rosaceae) | Serbia | ||
| Tegonotus ferruginiae [25] | Acacia ferruginea DC. (Fabaceae) | India | ||
| Tegonotus toxicodendronis [26] | Toxicodendron succedaneum (L.) Kuntze (Anacardiaceae) | China | ||
| Tegonotus dubrakoni [30] | Unknown host | India | Scapular tubercles and setae (sc) set on the rear shield margin, lack of seta on genu II. | These three species have scapular tubercles and setae (sc) located on the rear shield margin, and are lacking seta on genu II. Considering other shared generic morphological characteristics, it is suggested that these three species be assigned to Neoshevtchenkella. |
| Tegonotus schleicherae [7] | Schleichera oleosa (Lour.) Oken (Sapindaceae) | India | ||
| Tegonotus litseae [7] | Litsea sp. (Lauraceae) | India | ||
| Tegonotus fisus [29] | Mangifera indica L. (Anacardiaceae) | India | Dorsal pedipalp genu seta (d) bifurcated. | Tegonotus fisus was described and illustrated with a bifurcated pedipalp genu seta (d), and was reported from M. indica (L.) (Anacardiaceae). The morphological characters of this species corresponds to the monotypic genus Vareeboona. Moreover, all morphological characteristics aligned to V. mangiferae (Keifer), which is reported on the same host plant, and widely distributed over the world. It is suggested that this species be transferred to Vareeboona, and proposed as junior synonym of V. mangiferae (Keifer). |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arabnezhad, H.; Bahar, M.; Mohammadi, H.R.; Latifian, M. Development, characterization and use of microsatellite markers for germplasm analysis in date palm (Phoenix dactylifera L.). Sci. Hortic. 2012, 134, 150–156. [Google Scholar] [CrossRef]
- Nalepa, A. Neue Phytoptiden. Anz. Kais. Akad. Wiss. Math. Nat. Kl. Wien 1890, 27, 212–213. [Google Scholar]
- Amrine, J.W., Jr.; Stasny, T.A.H.; Flechtmann, C.H.W. Revised Keys to World Genera of Eriophyoidea (Acari: Prostigmata). Indira Publishing House: West Bloomfield, MI, USA, 2003; pp. 1–244. [Google Scholar]
- Wang, C.F.; Huang, K.W. Eriophyoid Mites (Acari: Trombidiformes) from Orchid Island and Green Island, off the Southeast Coast of Taiwan, with the description of a new genus. Formos. Entomol. 2012, 32, 199–221. [Google Scholar]
- Ren, L.M.; Guan, K.H.; Tan, M.C.; Yang, J.; Wang, G. Description of four new species, two new records, and mitochondrial COI gene of eriophyoid mites from Nanning, China (Acari: Eriophyoidea). Syst. Appl. Acarol. 2023, 28, 1195–1223. [Google Scholar] [CrossRef]
- Jeppson, L.R.; Keifer, H.H.; Baker, E.W. Mites Injurious to Economic Plants; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1975; p. 614. [Google Scholar]
- Ghosh, B.; Chakrabarti, S. An account of the genus Tegonotus (Acari: Eriophyoidea) from India with description of two new species and a key to Indian species. Indian J. Acarol. 1989, 10, 35–40. [Google Scholar]
- Wang, G.Q.; Tan, M.C.; Yang, D. Three new species in the genus Tegonotus nalepa, 1890 (Acari: Eriophyidae: Tegonotini) from China. Entomotaxonomia 2013, 35, 305–312. [Google Scholar]
- Bagdasarian, A.T. Novii rod kleshchei Eriophyoidea. [A new genus of the Eriophyoidea]. Zool. Zhurnal 1978, 57, 936–939. [Google Scholar]
- Newkirk, R.A.; Keifer, H.H. Revision of types of Eriophyes and Phytoptus. In Eriophyoid Studies: C-5 Agricultural Research Service; Keifer, H.H., Ed.; USDA: Washington, DC, USA, 1971; pp. 1–10. [Google Scholar]
- Amrine, J.W., Jr.; Stasny, T.A. Catalog of the Eriophyoidea (Acarina: Prostigmata) of the World; Indira Publish House: West Bloomfield, MI, USA, 1994; pp. 1–804. [Google Scholar]
- Craemer, C. Eriophyidae (Acari) as Potential Control Agents of South African Weeds, with Descriptions of a New Species of Tegonotus nalepa. Master’s Thesis, Rand Afrikaans University, Johannesburg, South Africa, 1993; p. 162. [Google Scholar]
- Chandrapatya, A.; Konvipasruang, P.; Amrine, J.W. Six new generic names for eriophyoid mites described from Thailand, with supplement descriptions and illustrations (Acari, Eriophyoidea). Syst. Appl. Acarol. 2015, 20, 523–555. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chakrabarti, S. A new species of Tegonotus nalepa (Acari: Eriophyoidea) from Buhtan. Entomon 2007, 32, 237–240. [Google Scholar]
- Pye, D.R. New eriophyoid mites (Acari: Prostigmata: Eriophyoidea) in Britain: One new genus, four new species, 19 new records and two incursions. Zootaxa 2012, 3578, 43–68. [Google Scholar] [CrossRef]
- Mo, Y.P.; Tan, M.C.; Li, W.H.; Wang, G.Q. One new genus, two new species and two new records of Eriophyidae from Vietnam (Acari: Eriophyoidea). Int. J. Acarol. 2017, 43, 147–152. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Yin, Y.; Xue, X.F. A new genus and two new species of eriophyoid mites (Acari: Eriophyidae) from Malaysia. Syst. Appl. Acarol. 2018, 23, 2345–2358. [Google Scholar] [CrossRef]
- Tan, M.C.; Zhao, Y.Y.; Wang, G.Q. One new genus and two new species of Tegonotini (Acari: Eriophyidae) associated to Malvaceae from South China. Int. J. Acarol. 2019, 45, 335–340. [Google Scholar] [CrossRef]
- Monfreda, R.; Nuzzaci, G.; de Lillo, E. Detection, extraction, and collection of eriophyoid mites. Zootaxa 2007, 1662, 35–43. [Google Scholar] [CrossRef]
- Amrine, J.W., Jr.; Manson, D.C.M. Preparation, mounting and descriptive study of eriophyoid mites. In Eriophyoid Mites. Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1996; Volume 6, pp. 383–396. [Google Scholar]
- de Lillo, E.; Craemer, C.; Amrine, J.W., Jr.; Nuzzaci, G. Recommended procedures and techniques for morphological studies of Eriophyoidea (Acari: Prostigmata). Exp. Appl. Acarol. 2010, 51, 283–307. [Google Scholar] [CrossRef]
- Lindquist, E.E. External anatomy and notation of structures. In Eriophyoid Mites: Their Biology, Natural Enemies and Control. World Crop Pests; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 3–31. [Google Scholar]
- Kamali, H.; Majidi, M.; Akrami, M.A. Two new eriophyid mite species (Acari, Prostigmata, Eriophyidae) on date trees from Iran. Persian J. Acarol. 2015, 3, 321–329. [Google Scholar]
- Flechtmann, C.H. Four new species of Eriophyoid mites from Brazil (Acarina, Eriophyoidea). Rev. Bras. Zool. 1996, 13, 109–119. [Google Scholar] [CrossRef]
- Mohanasundaram, M.; Jagadish, P.S.; Venkatesh, N.; Ranganath, H.R. New species and records of eriophyid mites (Acari: Eriophyoidea) from Karnataka, India. Indian J. Acarol. 1985, 9, 1–10. [Google Scholar]
- Kuang, H.Y.; Hong, X.Y. Three new species of the subfamily Phyllocoptinae from China (Acariformes: Eriophyidae). Acta Entomol. Sin. 1991, 34, 376–379. [Google Scholar]
- Wei, S.G.; Li, D.W.; Wang, G.Q. Description of five new species of eriophyid mites (Acari: Eriophyidae) from Guangxi, China. Int. J. Acarol. 2007, 33, 115–122. [Google Scholar] [CrossRef]
- Boczek, J.; Petanovic, R.U. Studies on eriophyoid mites (Acari: Eriophyoidea), XVI. Bull. Pol. Acad. Sci. Biol. Sci. 1995, 43, 69–75. [Google Scholar]
- Chakrabarti, S.; Sarkar, S. Three new species of eriophyoid mites (Acari: Eriophyoidea) infesting fruit yielding plants from India. Zootaxa 2011, 2988, 28–36. [Google Scholar] [CrossRef]
- Ghosh, B.; Chakrabarti, S. An account of the genus Tegonotus nalepa (Acarina: Eriophyoidea): Five new species and a key to the Indian species. Proc. Indian Sci. Congr. 1983, 70, 126. [Google Scholar]
- Xu, X.Q.; Chen, J.; Xue, X.F. A new eriophyid mite species (Acari, Eriophyidae) in the genus Tegonotus nalepa infesting Chinese white olive, Canarium album, in Fujian Province, southeastern China. Acarologia 2017, 57, 901–911. [Google Scholar] [CrossRef]
- Li, H.S.; Xue, X.F.; Hong, X.Y. Homoplastic evolution and host association of Eriophyoidea (Acari, Prostigmata) conflict with the morphological-based taxonomic system. Mol. Phylogenet. Evol. 2014, 78, 185–198. [Google Scholar] [CrossRef]
- Farkas, H.K. On the Eriophyids of Hungary IV. The descriptions of new species (Acari: Eriophyidae). Acta Zool. Acad. Sci. Hung. 1963, 9, 237–270. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, E.M.; Mirza, J.H.; Kamran, M.; Alatawi, F.J. The Genus Tegonotus Nalepa (Acariformes: Eriophyidae: Phyllocoptinae): Description of a New Species and Key to Valid Species. Diversity 2025, 17, 465. https://doi.org/10.3390/d17070465
Khan EM, Mirza JH, Kamran M, Alatawi FJ. The Genus Tegonotus Nalepa (Acariformes: Eriophyidae: Phyllocoptinae): Description of a New Species and Key to Valid Species. Diversity. 2025; 17(7):465. https://doi.org/10.3390/d17070465
Chicago/Turabian StyleKhan, Eid Muhammad, Jawwad Hassan Mirza, Muhammad Kamran, and Fahad Jaber Alatawi. 2025. "The Genus Tegonotus Nalepa (Acariformes: Eriophyidae: Phyllocoptinae): Description of a New Species and Key to Valid Species" Diversity 17, no. 7: 465. https://doi.org/10.3390/d17070465
APA StyleKhan, E. M., Mirza, J. H., Kamran, M., & Alatawi, F. J. (2025). The Genus Tegonotus Nalepa (Acariformes: Eriophyidae: Phyllocoptinae): Description of a New Species and Key to Valid Species. Diversity, 17(7), 465. https://doi.org/10.3390/d17070465






