Marine Bryozoans from the Northern Pacific Coast of Costa Rica
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Live Colonies Processing
2.3. Scanning Electron Microscope Images
2.4. DNA Tissue Extraction
3. Results
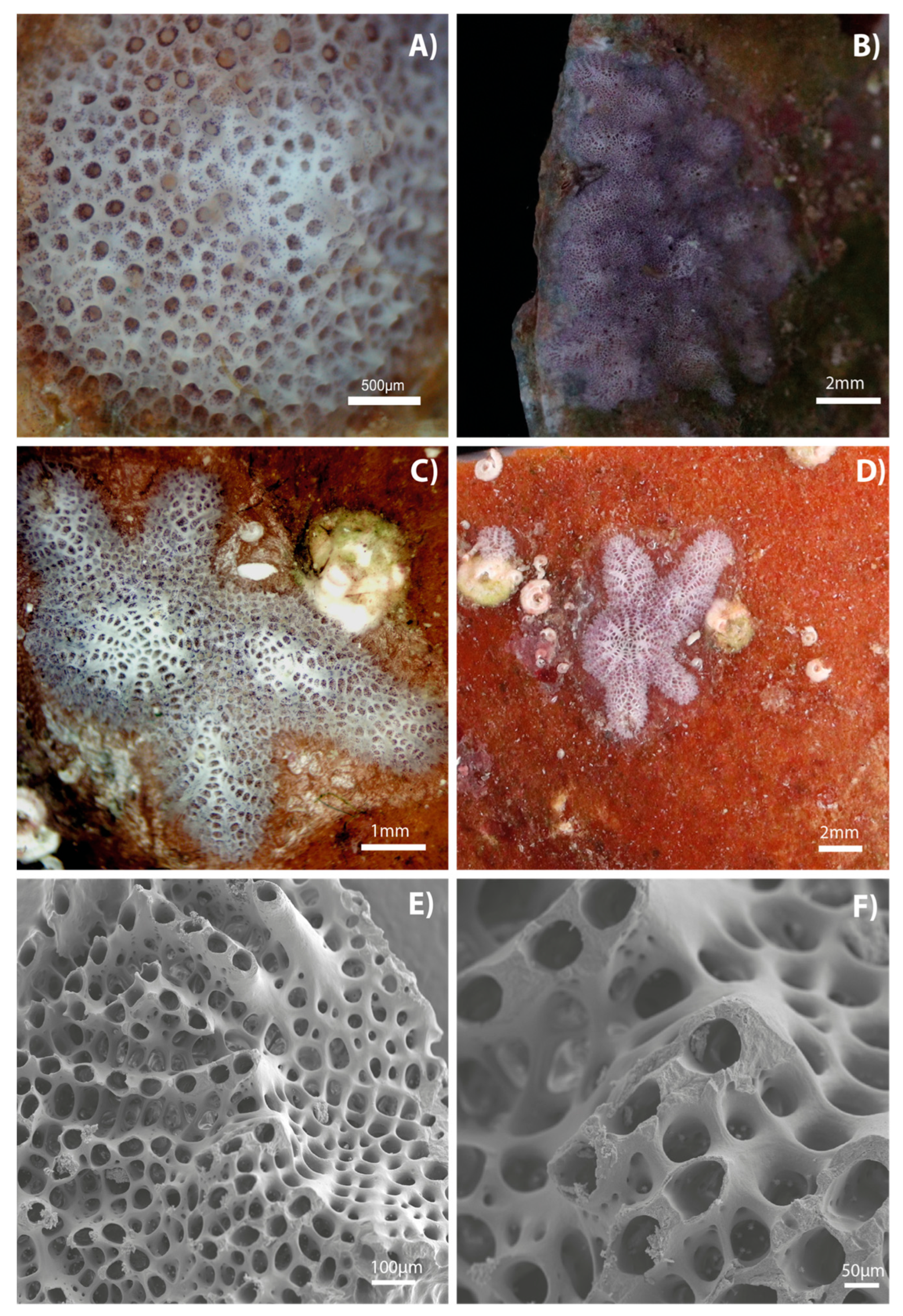
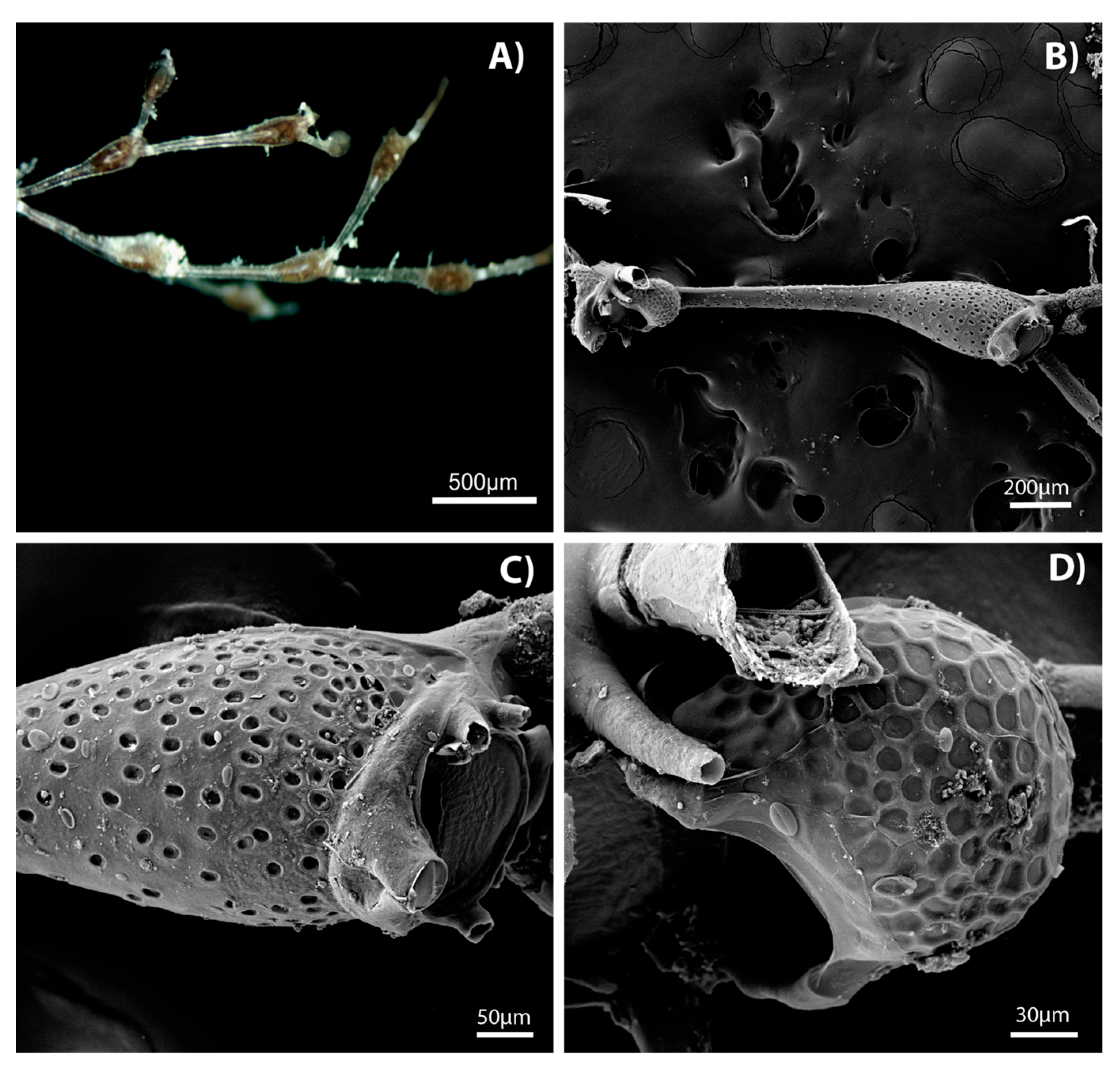
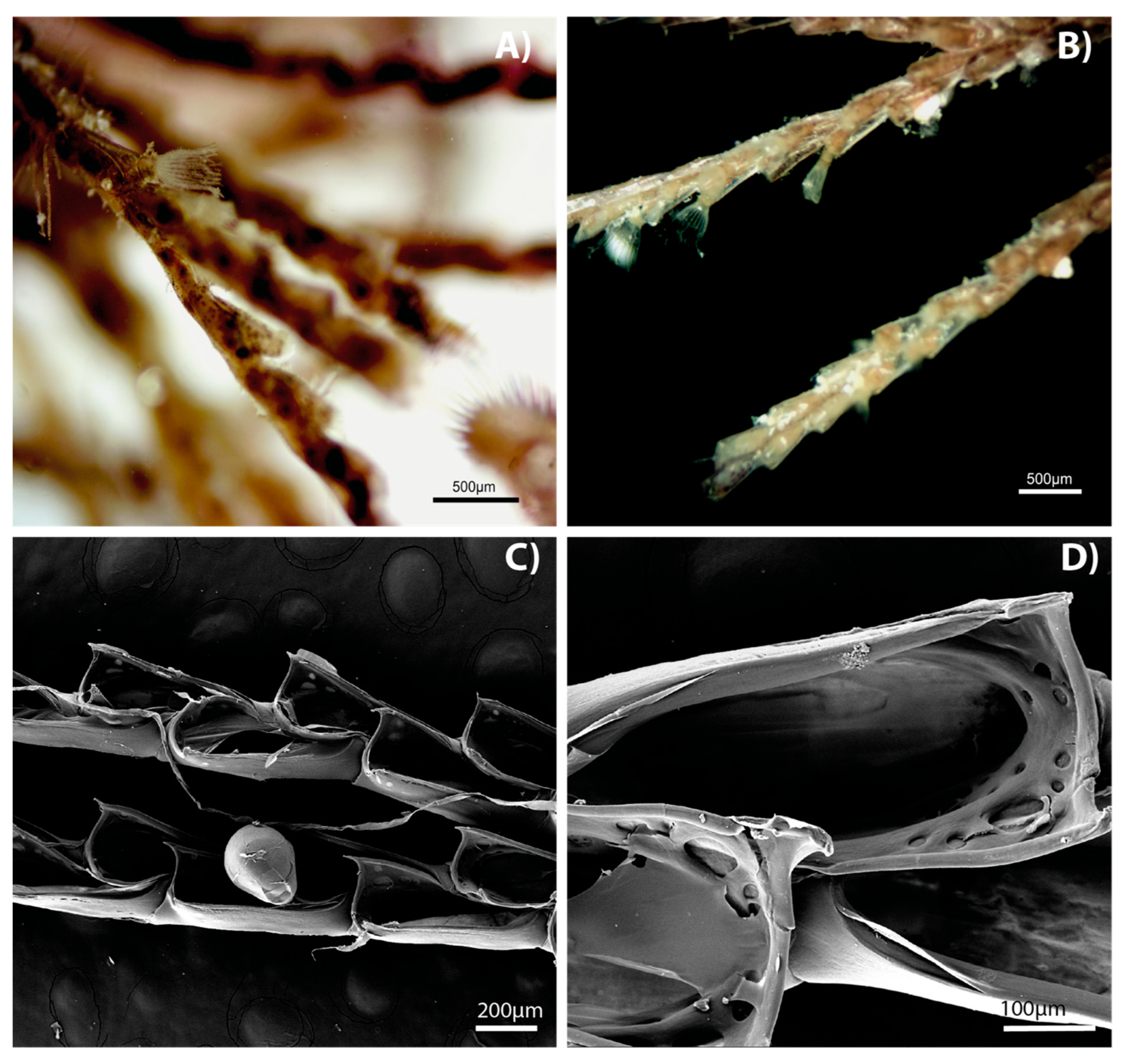
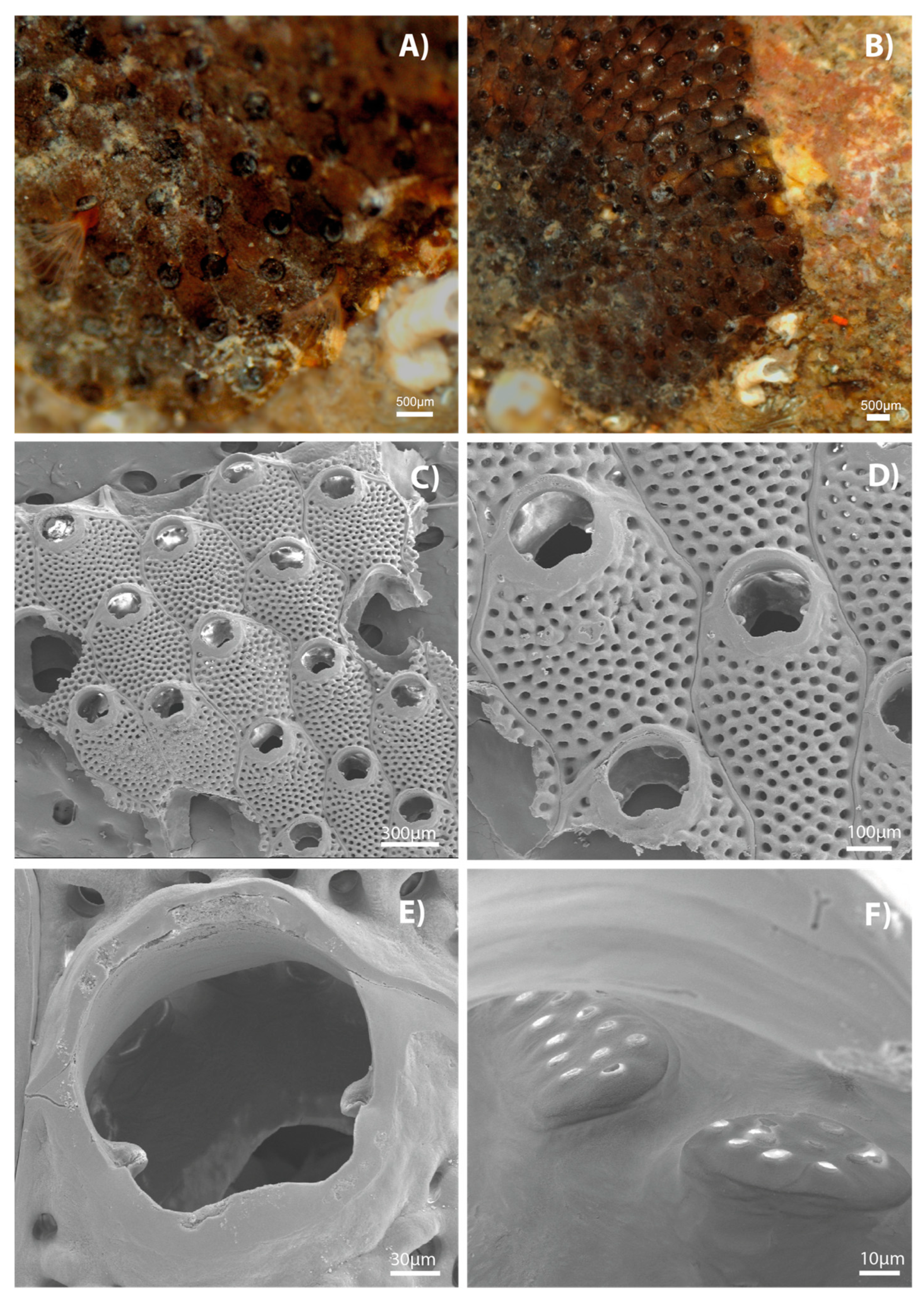
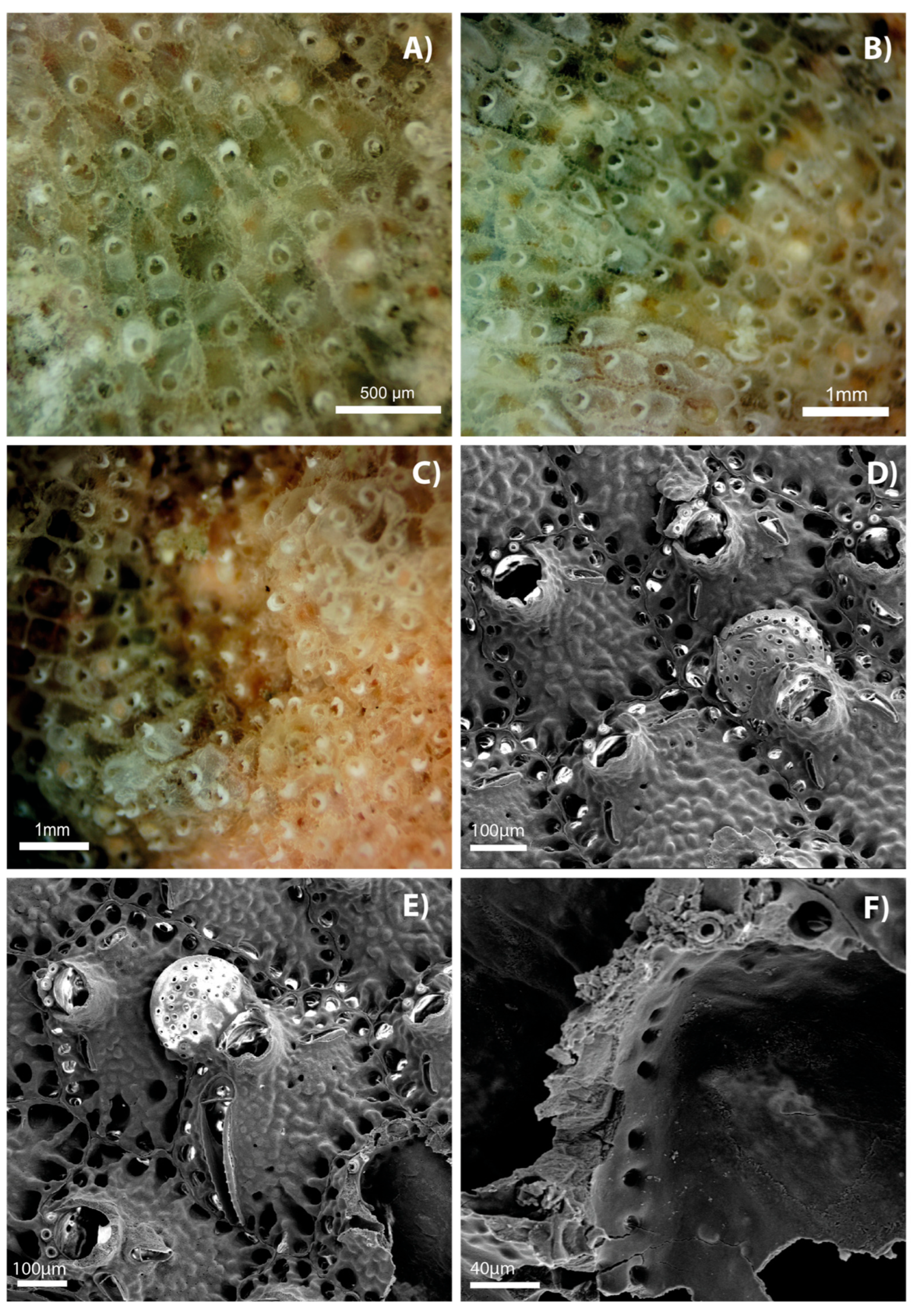
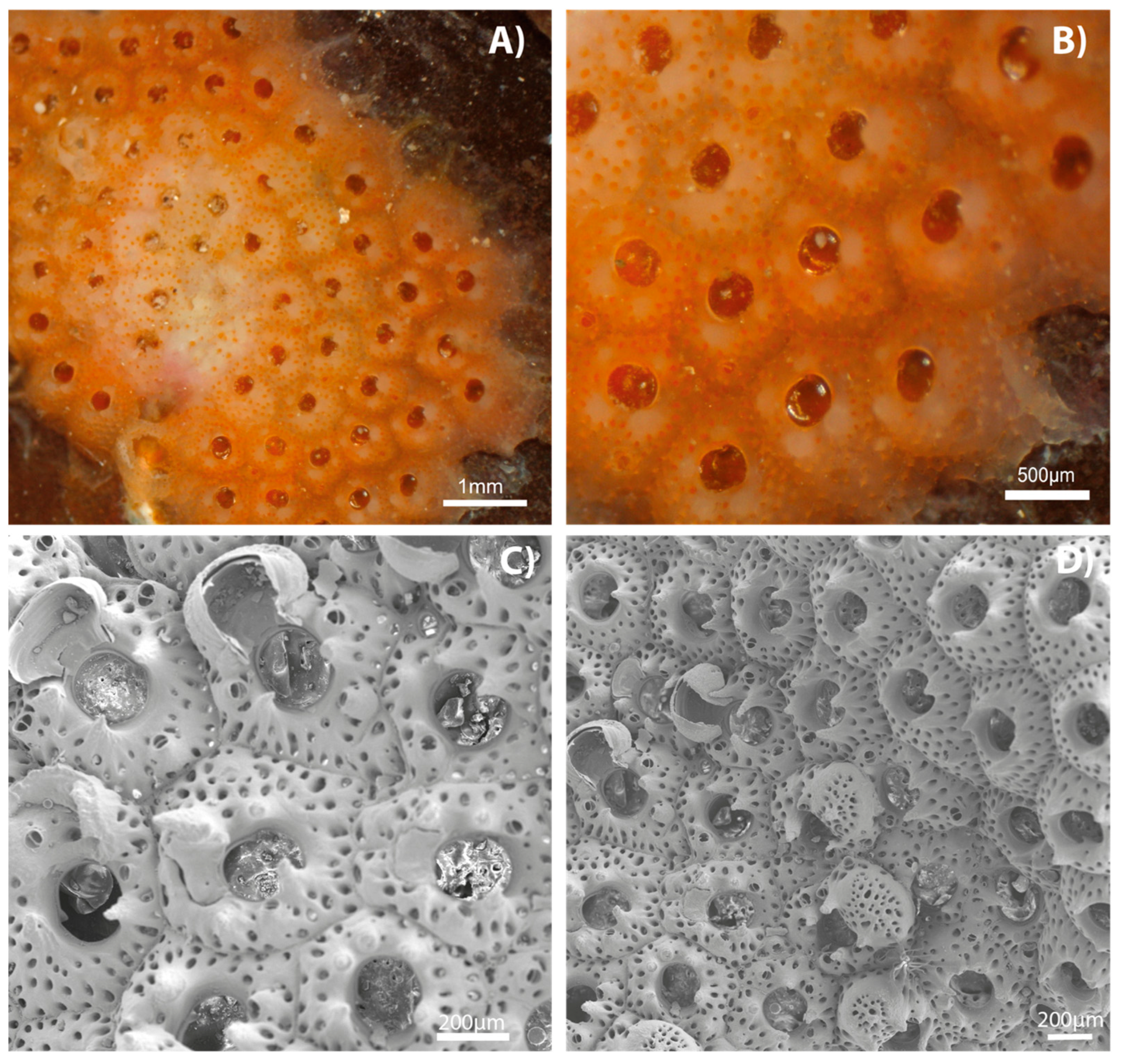

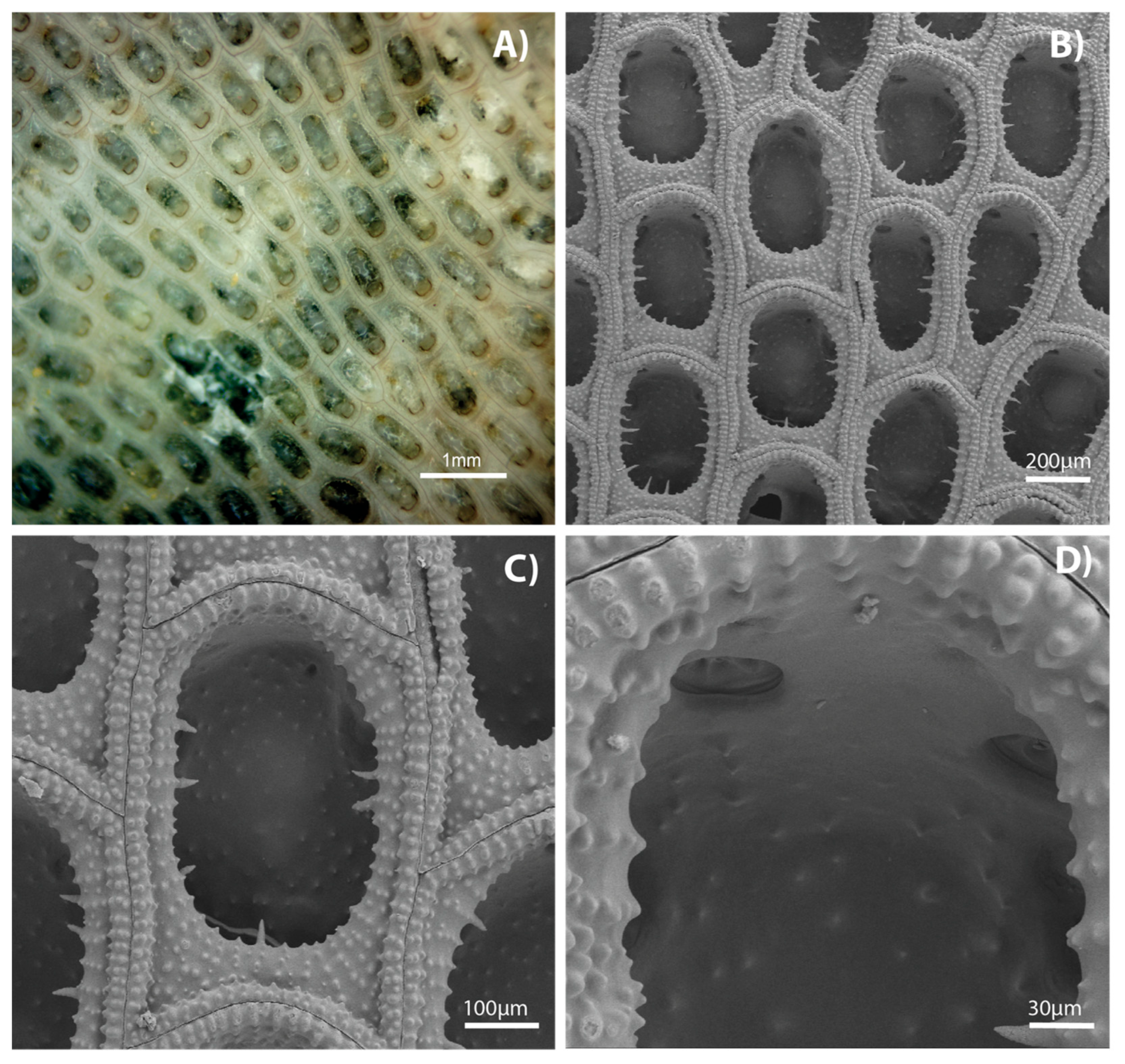
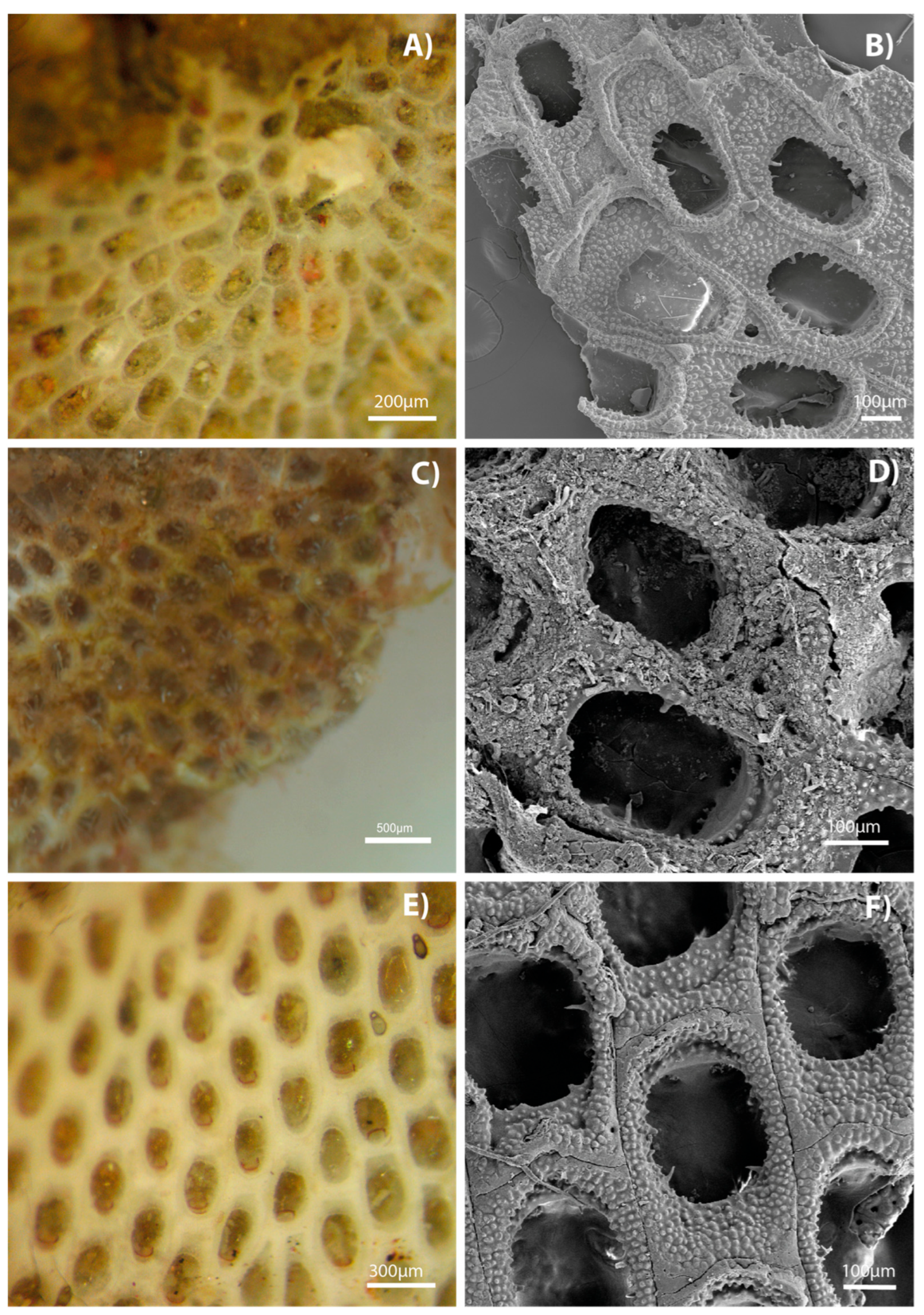
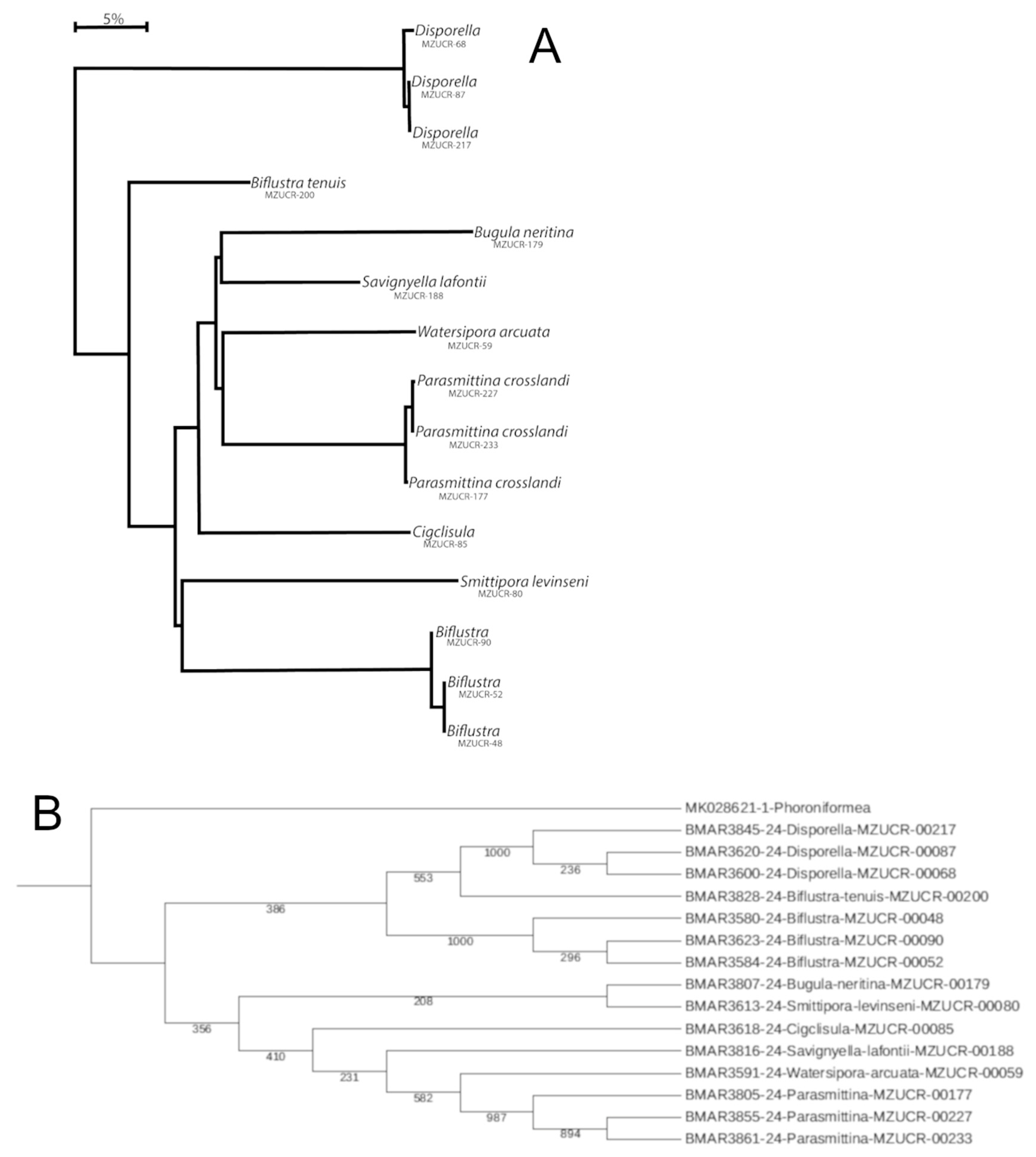
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscope |
| ACG | Área de Conservación Guanacaste |
Appendix A. Sequences Generated
References
- Cook, P.L.; Bock, P.E.; Gordon, D.P.; Weaver, H.J. Australian BRYOZOA: Volume 1 Biology, Ecology and Natural History; CSIRO Publishing: Melbourne, Australia, 2018. [Google Scholar]
- Cortés, J.; Nielsen, V.; Herrera-Cubilla, A. Bryozoans. In Marine Biodiversity of Costa Rica, Central America; Wehrtmann, I.S., Cortés, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 413–416. [Google Scholar]
- Flórez-Romero, P.; Montoya-Cadavid, E.; Reyes-Forero, J.; Santodomingo, N. Briozoos cheilostomados del Caribe colombiano. Boletín De Investig. Mar. Y Costeras—INVEMAR 2007, 36, 229–250. [Google Scholar] [CrossRef]
- Bock, P.E. Bryozoans (Phylum Bryozoa). In Marine Invertebrates of Southern Australia Part 1; Shepherd, S.A., Thomas, I.M., Eds.; South Australian Government: Adelaide, Australia, 1982; pp. 319–394. [Google Scholar]
- Key, M.M.; Jeffries, W.B.; Voris, H.K. Epizoic Bryozoans, Sea Snakes, and Other Nektonic Substrates. Bull. Mar. Sci. 1995, 56, 462–474. [Google Scholar]
- Ryland, J.S. Physiology and ecology of marine Bryozoans. Adv. Mar. Biol. 1976, 14, 285–443. [Google Scholar]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The phylum Bryozoa: From biology to biomedical potential. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, S. Predation on marine bryozoan colonies: Taxa, traits and trophic groups. Mar. Ecol. Prog. Ser. 2008, 359, 117–131. [Google Scholar] [CrossRef]
- Smith, A.M. Growth and Calcification of Marine Bryozoans in a Changing Ocean. Biol. Bull. 2014, 226, 203–210. [Google Scholar] [CrossRef]
- Winston, J.E.; Jackson, J.B.C. Ecology of cryptic coral reef communities. IV. Community development and life histories of encrusting Cheilostome Bryozoa. J. Exp. Mar. Biol. Ecol. 1984, 76, 1–21. [Google Scholar] [CrossRef]
- Labarbera, M. Mechanisms of spatial competition of Discinisca strigata (Inarticulata: Brachiopoda) in the intertidal of Panama. Biol. Bull. 1985, 168, 91–105. [Google Scholar] [CrossRef]
- Banta, W.C. The Bryozoa of the Galápagos. In Galapagos Marine Invertebrates; Springer: Berlin/Heidelberg, Germany, 1991; pp. 371–389. [Google Scholar]
- Banta, W.C.; Carson, R.J.M. Bryozoa from Costa Rica. Pac. Sci. 1977, 31, 381–424. [Google Scholar]
- Liuzzi, M.G.; López-Gappa, J.; Salgado, L. Bryozoa from the continental shelf off Tierra del Fuego (Argentina): Species richness, colonial growth-forms, and their relationship with water depth. Estuar. Coast. Shelf Sci. 2018, 214, 48–56. [Google Scholar] [CrossRef]
- Miranda, A.A.; Almeida, A.C.S.; Vieira, L.M. Non-native marine bryozoans (Bryozoa: Gymnolaemata) in Brazilian waters: Assessment, dispersal and impacts. Mar. Pollut. Bull. 2018, 130, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Huang, Z. Study of fouling organisms in daya bay, China. Biofouling 1990, 2, 229–237. [Google Scholar] [CrossRef]
- Osburn, R.C. Bryozoa of the Pacific Coast of America. Part 1, Cheilostomata-Anasca; University of Southern California Press: Los Angeles, CA, USA, 1950. [Google Scholar]
- Osburn, R.C. Bryozoa of the Pacific Coast of America. Part 2, Cheilostomata-Ascophora; University of Southern California Press: Los Angeles, CA, USA, 1952. [Google Scholar]
- Osburn, R.C. Bryozoa of the Pacific Coast of America. Part 3, Cyclostomata, Ctenostomata, Entoprocta, and Addenda; University of Southern California Press: Los Angeles, CA, USA, 1953. [Google Scholar]
- Sibaja-Cordero, J.A.; Troncoso, J.S.; Cortés, J.; Moreira, J.; Vargas, J.A.; Benavides-Varela, C. Biodiversity and density of subtidal benthos of an oceanic tropical island (a comparison within the Pacific Ocean). J. Sea Res. 2016, 115, 47–58. [Google Scholar] [CrossRef]
- Cortés, J. Marine biodiversity baseline for Área de conservación Guanacaste, Costa Rica: Published records. Zookeys 2017, 2017, 129–179. [Google Scholar] [CrossRef]
- Cortés, J.; Joyce, F. BioMar-ACG: A successful partnership to inventory and promulgate marine biodiversity. Biotropica 2020, 52, 1103–1106. [Google Scholar] [CrossRef]
- Blanco, R. ACG Sitio Patrimonio Mundial. 2012. Available online: https://www.acguanacaste.ac.cr/acg/designaciones-internacionales/acg-sitio-patrimonio-mundial (accessed on 7 May 2024).
- ACG. Área de Conservación Guanacaste: Sector Marino. Available online: https://www.acguanacaste.ac.cr/biodesarrollo/sector-marino2022 (accessed on 7 May 2024).
- Alfaro, E.J.; Cortés, J.; Alvarado, J.J.; Jiménez, C.; León, A.; Sánchez-Noguera, C.; Nivia-Ruiz, J.; Ruiz, E. Clima y temperatura sub-superficial del mar en Bahía Culebra, Golfo de Papagayo, Costa Rica. Rev. Biol. Trop. 2012, 60, 159–171. [Google Scholar] [CrossRef]
- Alfaro, E.J.; Cortés, J. Atmospheric forcing of cool subsurface water events in Bahía Culebra, Gulf of Papagayo, Costa Rica. Rev. Biol. Trop. 2012, 60, 173–186. [Google Scholar] [CrossRef]
- Cortés, J.; Samper-Villarreal, J.; Bernecker, A. Seasonal phenology of Sargassum liebmannii J. Agardh (Fucales, Heterokontophyta) in an upwelling area of the Eastern Tropical Pacific. Aquat. Bot. 2014, 119, 105–110. [Google Scholar] [CrossRef]
- Fernández-García, C.; Salas-Moya, C.; Mena, S.; Azofeifa-Solano, J.C.; Alvarado, J.J. Subtidal habitats diversity of Santa Elena Peninsula and Murciélago Islands, North Pacific, Costa Rica. Rev. Biol. Trop. 2021, 69, S160–S179. [Google Scholar] [CrossRef]
- Banta, W.C.; Soule, J.D.; Soule, D.F. Preparing Whole Mounts of Hard Parts of Recent Bryozoa. Source Chesap. Sci. 1973, 14, 62–64. [Google Scholar] [CrossRef]
- Breedy, O.; Guzmán, H. A revision of the genus Pacifigorgia (Coelenterata: Octocorallia: Gorgoniidae). Proc. Biol. Soc. Wash. 2002, 115, 782–839. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Ivanova, N.V.; Dewaard, J.R.; Hebert, P.D.N. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol. Ecol. Notes 2006, 6, 998–1002. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Wei, C.; Chan, D.; Agda, J.; Agda, J.; Ballesteros-Mejia, L.; Boutou, H.A.; El Bastami, Z.M.; Ma, E.; Manjunath, R.; et al. BOLD v4: A Centralized Bioinformatics Platform for DNA-Based Biodiversity Data. In DNA Barcoding: Methods and Protocols; Humana: New York, NY, USA, 2024; pp. 403–441. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29–W33. [Google Scholar] [CrossRef]
- Taylor, P.D.; Grischenko, A.V. Two new species of heavily calcified cyclostome bryozoans from the intertidal of Akkeshi Bay, Hokkaido, Japan. J. Nat. Hist. 2015, 49, 1763–1775. [Google Scholar] [CrossRef]
- Dick, M.H.; Tilbrook, K.J.; Mawatari, S.F. Diversity and taxonomy of rocky-intertidal Bryozoa on the Island of Hawaii, USA. J. Nat. Hist. 2006, 40, 2197–2257. [Google Scholar] [CrossRef]
- Cook, P.L.; Bock, P.E.; Gordon, D.P.; Weaver, H.J. Australian BRYOZOA Volume 2: Taxonomy of Australian Families; CSIRO Publishing: Melbourne, Australia, 2018. [Google Scholar]
- Banta, W.C. Watersipora arcuata, A new species in the Subovoidea-Cucullata-nigra complex (Bryozoa, Cheilostomata). Bull. South. Calif. Acad. Sci. 1969, 68, 96–102. [Google Scholar]
- Fransen, C. Caribbean Bryozoa: Anasca and Ascopora Imperfecta of the inner bays of Curaçao and Bonaire. Stud. Fauna Curaçao Other Caribb. Islands 1986, 68, 1–102. [Google Scholar]
- Badve, R.M.; Sonar, M.A. Bryozoa Cheilostomata from Holocene, West Coast of Maharashtra, India. Geobios 1995, 28, 317–335. [Google Scholar] [CrossRef]
- Herrera Cubilla, A. Bryozoos del Pacifico. Available online: https://biogeodb.stri.si.edu/briozoos/pages/acerca/2017 (accessed on 20 May 2024).
- Soule, D.F.; Soule, J.D.; Morris, P.A.; Chaney, H.W. Bryozoa. In The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon; Carlton, J.T., Ed.; University of California Press: Berkeley, CA, USA, 2007; pp. 866–904. [Google Scholar]
- Duncan, M.; Chow, B.; Myron, K.; Stone, J.; Hubbell, M.; Schriock, E.; Hunt, C.; Khtikian, W.K.; Cohen, C.S. First report of genetic data from two invasive Watersipora (Bryozoa) species in the central California coast rocky intertidal. Aquat. Invasions 2022, 17, 136–152. [Google Scholar] [CrossRef]
- Key, M.M.J.R.; Hendrickx, M.E. Biflustra irregulata (Cheilostomata: Membraniporidae): A tsunami debris rafted Indo-Pacific bryozoan found in the eastern Pacific Ocean. Zootaxa 2022, 5128, 340–354. [Google Scholar] [CrossRef] [PubMed]
| Collection Code | Best Match BOLD | Overlap (bp) | %Match | Best Match GenBank | %Match | Nearest Neighbor ID | Final Species ID |
|---|---|---|---|---|---|---|---|
| MZUCR-00068 | BOLD:AEI5412 | 387 | 87.67 | MW284801 | 82.85 | Disporella sp. | Disporella sp. |
| MZUCR-00087 | BOLD:AEI5412 | 397 | 87.91 | MW284801 | 82.64 | Disporella sp. | Disporella sp. |
| MZUCR-00217 | BOLD:AEI5412 | 405 | 87.91 | MW284801 | 83.21 | Disporella sp. | Disporella sp. |
| MZUCR-00048 | BOLD:ADQ2487 | 287 | 93.92 | MW124839 | 85.75 | Membranipora sp. * | Biflustra sp. |
| MZUCR-00052 | BOLD:ADQ2487 | 292 | 95.43 | MW124844 | 84.68 | Membranipora sp. * | Biflustra sp. |
| MZUCR-00090 | BOLD:ADQ2487 | 462 | 96.12 | MW124839 | 86.42 | Membranipora sp. * | Biflustra sp. |
| MZUCR-00177 | BOLD:AAI2619 | 465 | 82.55 | HM425581 | 83.96 | Parasmittina sp. * | Parasmittina crosslandi |
| MZUCR-00227 | BOLD:AAI2619 | 478 | 84.07 | HM425581 | 84.25 | Parasmittina sp. * | Parasmittina crosslandi |
| MZUCR-00233 | BOLD:AAI2619 | 501 | 84.11 | HM425581 | 83.93 | Parasmittina sp. * | Parasmittina crosslandi |
| MZUCR-00085 | BOLD:AFE9081 | 444 | 81.76 | AFU9282 | Cigclisula sp. | Cigclisula sp. | |
| MZUCR-00080 | BOLD:AFE4573 | 366 | 84.33 | NA | Onychocella marioni * | Smittipora levinseni | |
| MZUCR-00059 | BOLD:AAF4822 | 523 | 98.02 | DQ417459 | 98.02 | Watersipora arcuata | Watersipora arcuata |
| MZUCR-00179 | BOLD:AAD2483 | 423 | 93.61 | MN064606 | 93.68 | Bugula neritina | Bugula neritina |
| MZUCR-00188 | BOLD:ADJ3487 | 447 | 99.8 | ADJ3487 | Savignyella lafontii | Savignyella lafontii | |
| MZUCR-00200 | BOLD:ACH2215 | 351 | 82.66 | NA | Biflustra tenuis | Biflustra tenuis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antillón-Obando, B.; Cortés, J.; Sibaja-Cordero, J.A. Marine Bryozoans from the Northern Pacific Coast of Costa Rica. Diversity 2025, 17, 451. https://doi.org/10.3390/d17070451
Antillón-Obando B, Cortés J, Sibaja-Cordero JA. Marine Bryozoans from the Northern Pacific Coast of Costa Rica. Diversity. 2025; 17(7):451. https://doi.org/10.3390/d17070451
Chicago/Turabian StyleAntillón-Obando, Beatriz, Jorge Cortés, and Jeffrey A. Sibaja-Cordero. 2025. "Marine Bryozoans from the Northern Pacific Coast of Costa Rica" Diversity 17, no. 7: 451. https://doi.org/10.3390/d17070451
APA StyleAntillón-Obando, B., Cortés, J., & Sibaja-Cordero, J. A. (2025). Marine Bryozoans from the Northern Pacific Coast of Costa Rica. Diversity, 17(7), 451. https://doi.org/10.3390/d17070451






