Linking Riparian Forest to the Functional Diversity of Ephemeroptera, Plecoptera, and Trichoptera in First-Order Tropical Streams
Abstract
1. Introduction
2. Materials and Methods
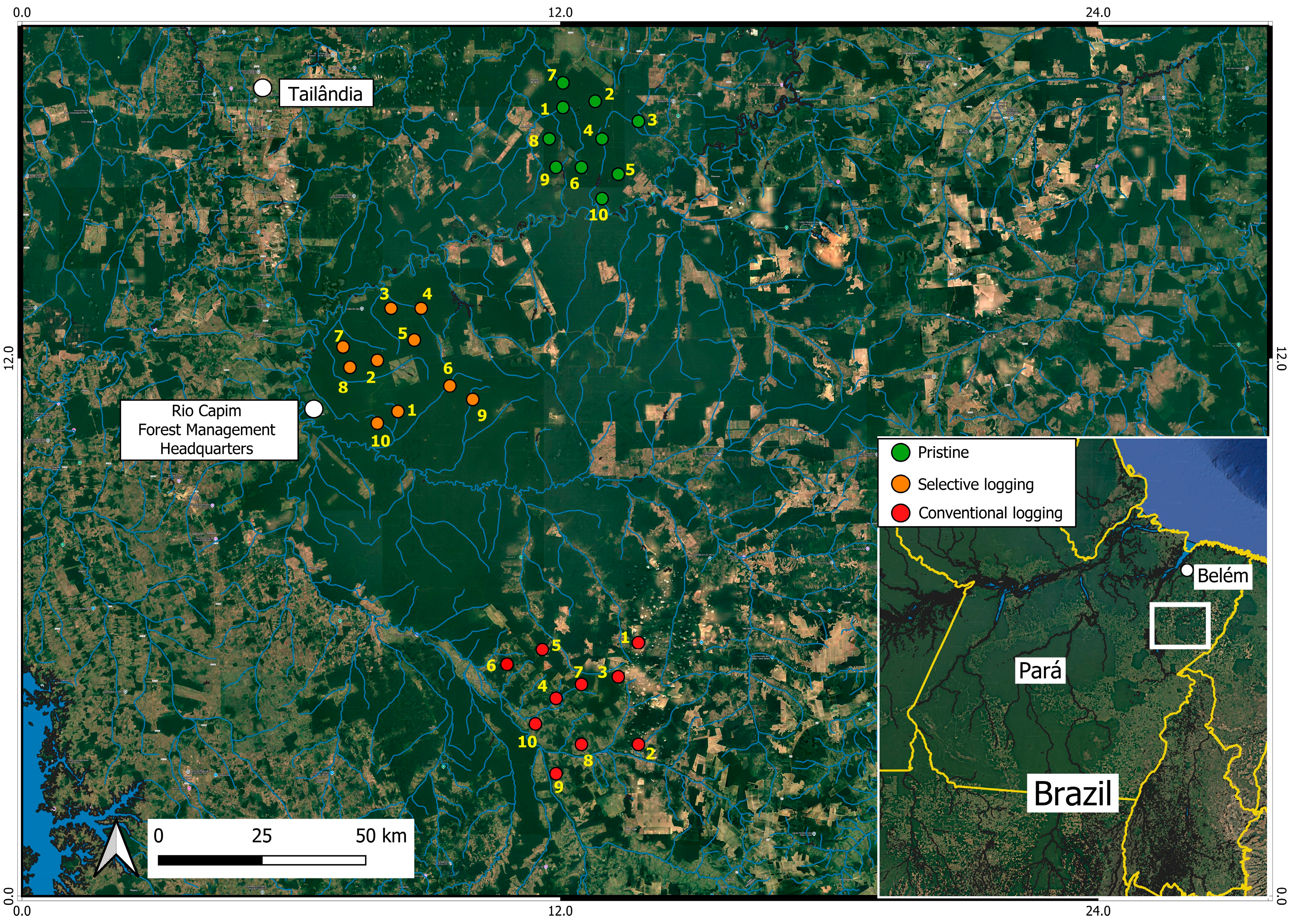
2.1. Study Area
2.2. Field Sampling Design
2.3. Measurement of Attributes Linked to Habitat Functioning
2.4. Sampling of EPT Assemblage
2.5. Functional Trait Selection
2.6. Functional Diversity Indices
2.7. Statistical Analysis
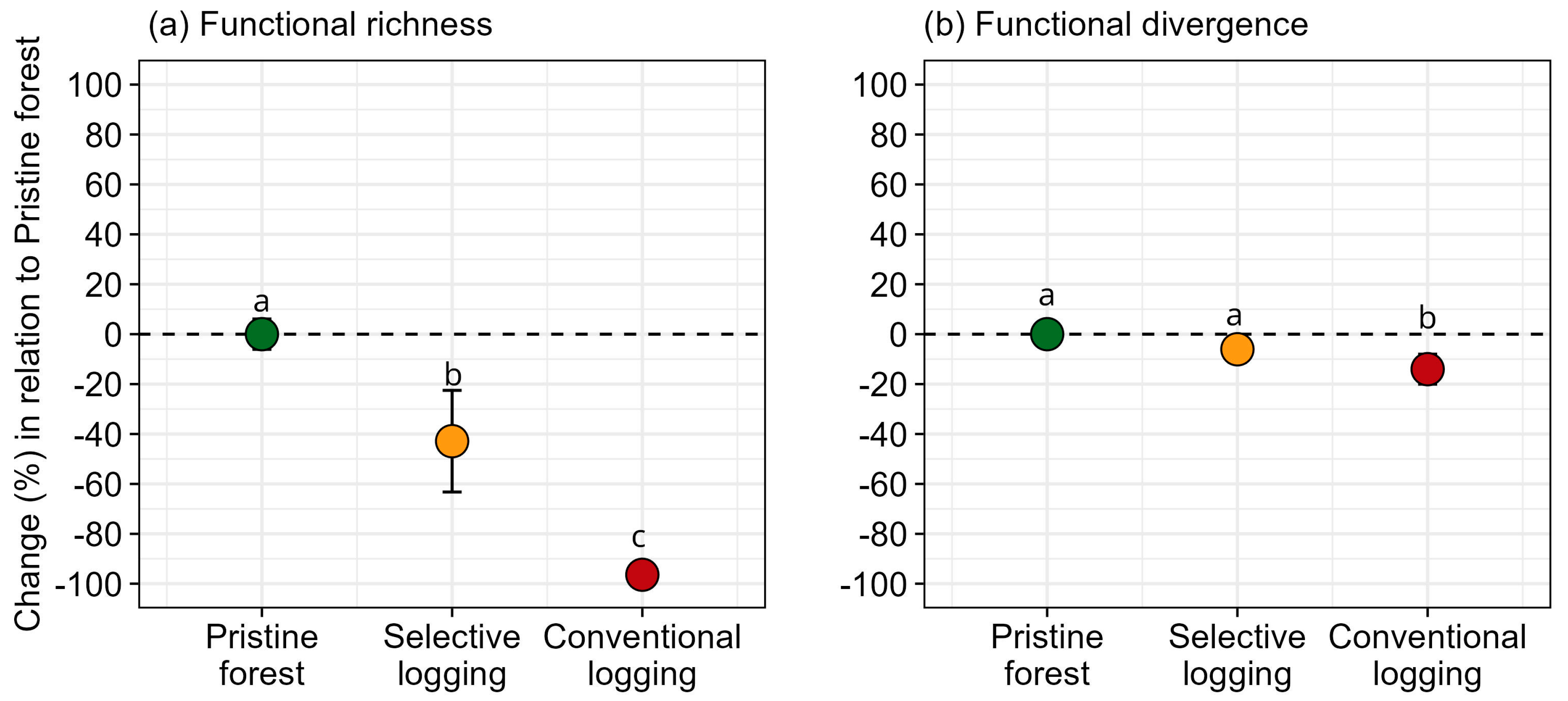
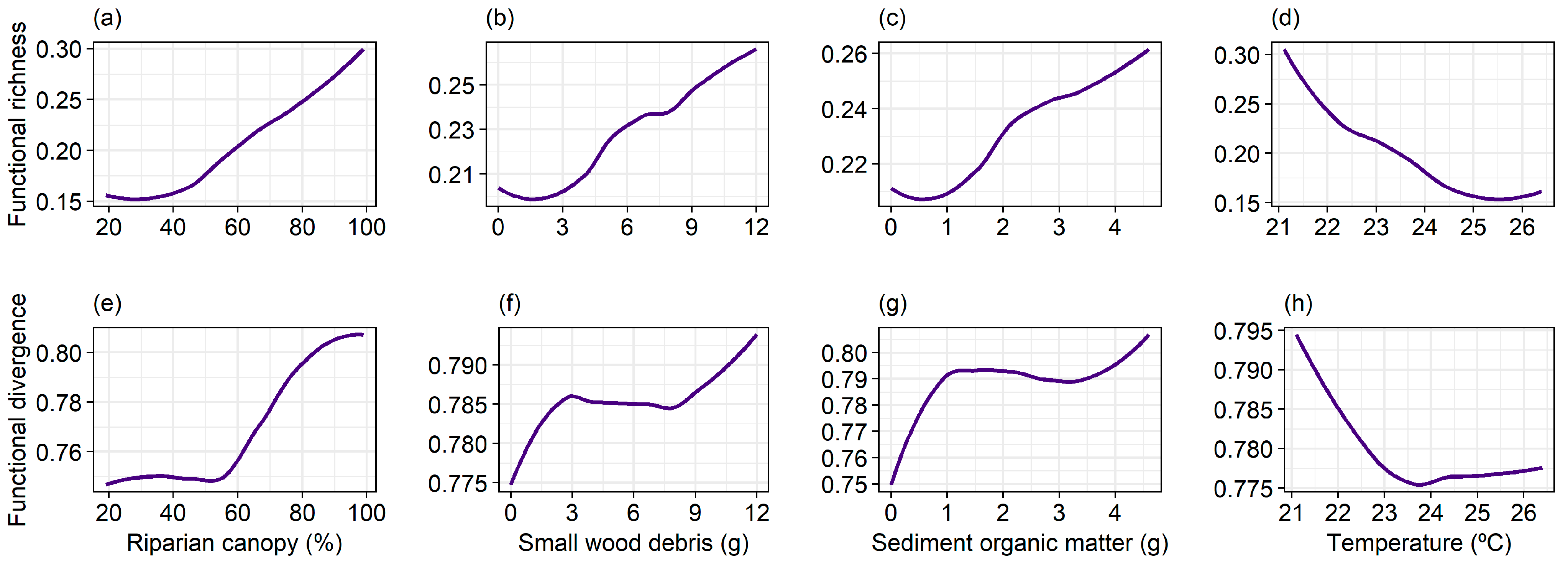
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Riparian Forest | Length (m) | Width (m) | Depth (m) | Canopy Cover (%) | Water Temperature (°C) |
|---|---|---|---|---|---|
| Pristine forest | |||||
| Stream 1 | 1095 | 4.0 ± 1.2 | 0.9 ± 1.05 | 96 ± 1.03 | 21.2 ± 1.41 |
| Stream 2 | 1071 | 4.3 ± 1.1 | 1.1 ± 1.04 | 96 ± 1.03 | 21.3 ± 1.31 |
| Stream 3 | 1126 | 5.1 ± 1.1 | 1.3 ± 1.05 | 95 ± 1.02 | 21.2 ± 1.15 |
| Stream 4 | 1065 | 4.5 ± 1.2 | 1.1 ± 1.02 | 97 ± 1.01 | 21.1 ± 1.22 |
| Stream 5 | 1192 | 5.8 ± 1.2 | 1.2 ± 1.03 | 97 ± 1.01 | 21.2 ± 1.23 |
| Stream 6 | 1064 | 5.2 ± 1.2 | 0.8 ± 1.03 | 97 ± 1.02 | 21.5 ± 1.21 |
| Stream 7 | 1073 | 4.5 ± 1.3 | 0.9 ± 1.02 | 98 ± 1.02 | 21.3 ± 1.25 |
| Stream 8 | 1051 | 4.7 ± 1.1 | 1.1 ± 1.02 | 98 ± 1.04 | 21.3 ± 1.32 |
| Stream 9 | 1072 | 4.3 ± 1.1 | 1.2 ± 1.04 | 96 ± 1.01 | 21.1 ± 1.35 |
| Stream 10 | 1057 | 4.3 ± 1.2 | 1.2 ± 1.02 | 98 ± 1.03 | 21.1 ± 1.42 |
| Selective logging | |||||
| Stream 1 | 1026 | 4.7 ± 1.2 | 1.1 ± 1.01 | 72 ± 1.01 | 21.9 ± 1.23 |
| Stream 2 | 1085 | 4.5 ± 1.2 | 1.3 ± 1.03 | 74 ± 1.01 | 22.1 ± 1.25 |
| Stream 3 | 1063 | 4.3 ± 1.1 | 1.3 ± 1.01 | 75 ± 1.03 | 21.8 ± 1.19 |
| Stream 4 | 1055 | 5.9 ± 1.3 | 0.8 ± 1.05 | 74 ± 1.02 | 22.1 ± 1.25 |
| Stream 5 | 1078 | 5.5 ± 1.1 | 0.8 ± 1.02 | 73 ± 1.02 | 22.5 ± 1.21 |
| Stream 6 | 1117 | 4.6 ± 1.1 | 0.9 ± 1.01 | 73 ± 1.01 | 22.6 ± 1.28 |
| Stream 7 | 1092 | 5.1 ± 1.4 | 1.1 ± 1.02 | 74 ± 1.03 | 22.3 ± 1.22 |
| Stream 8 | 1093 | 4.2 ± 1.3 | 0.9 ± 1.03 | 74 ± 1.03 | 21.9 ± 1.25 |
| Stream 9 | 1125 | 4.7 ± 1.3 | 1.3 ± 1.03 | 73 ± 1.01 | 22.4 ± 1.21 |
| Stream 10 | 1095 | 4.6 ± 1.2 | 1.1 ± 1.02 | 74 ± 1.01 | 22.5 ± 1.22 |
| Conventional logging | |||||
| Stream 1 | 1034 | 5.4 ± 1.1 | 1.2 ± 1.02 | 21 ± 1.02 | 25.5 ± 1.81 |
| Stream 2 | 1059 | 5.1 ± 1.1 | 1.1 ± 1.01 | 24 ± 1.02 | 25.4 ± 1.65 |
| Stream 3 | 1047 | 4.6 ± 1.2 | 1.2 ± 1.01 | 22 ± 1.01 | 26.1 ± 1.41 |
| Stream 4 | 1116 | 5.9 ± 1.2 | 1.2 ± 1.02 | 22 ± 1.01 | 25.4 ± 1.33 |
| Stream 5 | 1082 | 5.2 ± 1.2 | 0.8 ± 1.04 | 23 ± 1.01 | 25.3 ± 1.45 |
| Stream 6 | 1049 | 4.7 ± 1.3 | 0.8 ± 1.03 | 19 ± 1.02 | 26.1 ± 1.21 |
| Stream 7 | 1112 | 4.6 ± 1.3 | 0.9 ± 1.02 | 21 ± 1.02 | 25.2 ± 1.37 |
| Stream 8 | 1075 | 4.5 ± 1.2 | 1.1 ± 1.01 | 24 ± 1.03 | 26.1 ± 1.29 |
| Stream 9 | 1069 | 4.5 ± 1.1 | 1.1 ± 1.01 | 23 ± 1.02 | 25.7 ± 1.31 |
| Stream 10 | 1047 | 4.6 ± 1.1 | 1.2 ± 1.04 | 23 ± 1.02 | 24.9 ± 1.28 |
| Riparian Forest | Functional Richness | Functional Divergence |
|---|---|---|
| Pristine forest | ||
| Stream 1 | 0.43 | 0.83 |
| Stream 2 | 0.46 | 0.85 |
| Stream 3 | 0.43 | 0.84 |
| Stream 4 | 0.45 | 0.85 |
| Stream 5 | 0.34 | 0.80 |
| Stream 6 | 0.46 | 0.85 |
| Stream 7 | 0.46 | 0.85 |
| Stream 8 | 0.43 | 0.84 |
| Stream 9 | 0.46 | 0.85 |
| Stream 10 | 0.46 | 0.83 |
| Selective logging | ||
| Stream 1 | 0.29 | 0.82 |
| Stream 2 | 0.37 | 0.85 |
| Stream 3 | 0.08 | 0.76 |
| Stream 4 | 0.40 | 0.84 |
| Stream 5 | 0.40 | 0.85 |
| Stream 6 | 0.26 | 0.81 |
| Stream 7 | 0.09 | 0.70 |
| Stream 8 | 0.29 | 0.73 |
| Stream 9 | 0.17 | 0.82 |
| Stream 10 | 0.12 | 0.70 |
| Conventional logging | ||
| Stream 1 | 0.009 | 0.67 |
| Stream 2 | 0.03 | 0.62 |
| Stream 3 | 0.009 | 0.67 |
| Stream 4 | 0.009 | 0.67 |
| Stream 5 | 0.009 | 0.67 |
| Stream 6 | 0.03 | 0.62 |
| Stream 7 | 0.03 | 0.62 |
| Stream 8 | 0.009 | 0.63 |
| Stream 9 | 0.008 | 0.63 |
| Stream 10 | 0.009 | 0.63 |
References
- Oester, R.; Altermatt, F.; Bruder, A. Riparian forests shape trophic interactions in detrital stream food webs. Funct. Ecol. 2024, 38, 2196–2206. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Manning, D.W.P. Aquatic-terrestrial linkages as complex systems: Insights and advances from network models. Freshw. Sci. 2019, 38, 936–945. [Google Scholar] [CrossRef]
- Simeone, D. Effect of logged forests on diet of small characids from Neotropical streams. Ecol. Freshw. Fish 2023, 33, e12743. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Lin, A.-C.; Tsai, H.-N.; Yen, Y.-T.; Tzeng, C.-S.; Yang, M.-M.; Lin, H.-J. The significance of riparian communities in the energy flow of subtropical stream ecosystems. Aquat. Sci. 2022, 84, 20. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Tapia, J.; Hauenstein, E.; Peña-Cortés, F.; Vergara, C.; Cerna, C.; Vargas-Chacoff, L. Effects of local land-use on riparian vegetation, water quality, and the functional organization of macroinvertebrate assemblages. Sci. Total Environ. 2017, 609, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Firmino, V.C.; Sasahara de Paiva, C.K.; Juen, L.; Brasil, L.S. Land use changes disrupt streams and affect the functional feeding groups of aquatic insects in the Amazon. J. Insect Conserv. 2022, 26, 137–148. [Google Scholar] [CrossRef]
- Rojas-Castillo, O.A.; Kepfer-Rojas, S.; Vargas, N.; Jacobsen, D. Forest buffer-strips mitigate the negative impact of oil palm plantations on stream communities. Sci. Total Environ. 2023, 873, 162259. [Google Scholar] [CrossRef] [PubMed]
- Calvão, L.B.; Nogueira, D.S.; de Assis Montag, L.F.; Lopes, M.A.; Juen, L. Are Odonata communities impacted by conventional or reduced impact logging? For. Ecol. Manag. 2016, 382, 143–150. [Google Scholar] [CrossRef]
- Rojas-Castillo, O.A.; Kepfer Rojas, S.; Juen, L.; Montag, L.F.d.A.; Carvalho, F.G.; Mendes, T.P.; Chua, K.W.J.; Wilkinson, C.L.; Amal, M.N.A.; Fahmi-Ahmad, M.; et al. Meta-analysis contrasting freshwater biodiversity in forests and oil palm plantations with and without riparian buffers. Conserv. Biol. 2023, 38, e14172. [Google Scholar] [CrossRef]
- Turunen, J.; Elbrecht, V.; Steinke, D.; Aroviita, J. Riparian forests can mitigate warming and ecological degradation of agricultural headwater streams. Freshw. Biol. 2021, 66, 785–798. [Google Scholar] [CrossRef]
- Silva, L.F.R.; Castro, D.M.P.; Juen, L.; Callisto, M.; Hughes, R.M.; Hermes, M.G. Functional responses of Odonata larvae to human disturbances in neotropical savanna headwater streams. Ecol. Indic. 2021, 133, 108367. [Google Scholar] [CrossRef]
- Marengo, J.A.; Souza, C.M.; Thonicke, K.; Burton, C.; Halladay, K.; Betts, R.A.; Alves, L.M.; Soares, W.R. Changes in climate and land use over the Amazon region: Current and future variability and trends. Front. Earth Sci. 2018, 6, 228. [Google Scholar] [CrossRef]
- Zeni, J.O.; Perez-Mayorga, M.A.; Roa-Fuentes, C.A.; Brejao, G.L.; Casatti, L. How Deforestation drives stream habitat changes and the functional structure of fish assemblages in different tropical regions. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1238–1252. [Google Scholar] [CrossRef]
- Espinosa, A.C.E.; Cunha, E.J.; Shimano, Y.; Rolim, S.; Mioli, L.; Juen, L.; Dunck, B. Functional diversity of mayflies (Ephemeroptera, Insecta) in streams in mining areas located in the Eastern Amazon. Hydrobiologia 2023, 850, 929–945. [Google Scholar] [CrossRef]
- Espinoza-Toledo, A.; Mendoza-Carranza, M.; Castillo, M.M.; Barba-Macias, E.; Capps, K.A. Taxonomic and functional responses of macroinvertebrates to riparian forest conversion in tropical streams. Sci. Total Environ. 2021, 757, 143972. [Google Scholar] [CrossRef]
- Leiva, M.; Marchese, M.; Lorenz, G.; Diodato, L. Functional diversity of benthic macroinvertebrates regarding hydrological and land use disturbances in a heavily impaired lowland river. Limnologica 2022, 92, 125940. [Google Scholar] [CrossRef]
- Mangadze, T.; Wasserman, R.J.; Froneman, P.W.; Dalu, T. Macroinvertebrate functional feeding group alterations in response to habitat degradation of headwater Austral streams. Sci. Total Environ. 2019, 695, 133910. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A Distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Graeber, D.; Jensen, T.M.; Rasmussen, J.J.; Riis, T.; Wiberg-Larsen, P.; Baattrup-Pedersen, A. Multiple stress response of lowland stream benthic macroinvertebrates Depends on Habitat Type. Sci. Total Environ. 2017, 599–600, 1517–1523. [Google Scholar] [CrossRef]
- Silva, B.L.; Guterres, A.P.M.; Santana, S.S.; Cunha, E.J.; Juen, L. The loss of riparian vegetation along streams causes morphological divergences in functional traits of semiaquatic insects (Heteropteran: Gerromorpha) in the eastern Amazon. Environ. Monit. Assess. 2024, 196, 914. [Google Scholar] [CrossRef]
- Sargac, J.; Johnson, R.K.; Burdon, F.J.; Truchy, A.; Risnoveanu, G.; Goethals, P.; McKie, B.G. Forested riparian buffers change the taxonomic and functional composition of stream invertebrate communities in agricultural catchments. Water 2021, 13, 1028. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Tichý, L. Field test of canopy cover estimation by hemispherical photographs taken with a smartphone. J. Veg. Sci. 2016, 27, 427–435. [Google Scholar] [CrossRef]
- Hamada, N.; Nessimian, J.L.; Querino, R.B. Insetos Aquáticos na Amazônia Brasileira: Taxonomia, Biologia e Ecologia; Editora INPA: Manaus, Brazil, 2014. [Google Scholar]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biomonitoring through biological traits of benthic macroinvertebrates: How to use species trait databases? Hydrobiologia 2000, 153–162. [Google Scholar] [CrossRef]
- Tomanova, S.; Usseglio-Polatera, P. Patterns of benthic community traits in Neotropical streams: Relationship to mesoscale spatial variability. Fundam. Appl. Limnol. 2007, 170, 243–255. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G. Species traits in relation to a habitat templet for river systems. Freshw. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. 2014. Available online: https://search.r-project.org/CRAN/refmans/FD/html/FD-package.html (accessed on 18 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Bocard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Calhoun, P.; Levine, R.A.; Fan, J. Repeated measures random forests (RMRF): Identifying factors associated with nocturnal hypoglycemia. Biometrics 2020, 77, 343–351. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sitati, A.; Raburu, P.O.; Yegon, M.J.; Masese, F.O. Land-use influence on the functional organization of Afrotropical macroinvertebrate assemblages. Limnologica 2021, 88, 125875. [Google Scholar] [CrossRef]
- Calvão, L.B.; Brito, J.d.S.; Ferreira, D.; Cunha, E.J.; Oliveira-Junior, J.M.B.; Juen, L. Effects of the loss of forest cover on Odonate communities in eastern Amazonia. J. Insect Conserv. 2022, 27, 205–218. [Google Scholar] [CrossRef]
- Palt, M.; Hering, D.; Kail, J. Context-specific positive effects of woody riparian vegetation on aquatic invertebrates in rural and urban landscapes. J. Appl. Ecol. 2023, 60, 1010–1021. [Google Scholar] [CrossRef]
- Desrosiers, M.; Usseglio-Polatera, P.; Archaimbault, V.; Larras, F.; Méthot, G.; Pinel-Alloul, B. Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Sci. Total Environ. 2019, 649, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Monteles, J.S.; Gerhard, P.; Ferreira, A.; Sonoda, K.C. Agriculture impacts benthic insects on multiple scales in the Eastern Amazon. Biol. Conserv. 2021, 255, 108998. [Google Scholar] [CrossRef]
- Williams, M.F.; Houghton, D.C. Influence of a protected riparian corridor on the benthic aquatic macroinvertebrate assemblages of a small northern Michigan (USA) agricultural stream. J. Freshw. Ecol. 2024, 39, 2312807. [Google Scholar] [CrossRef]
| Order | Genus | Presence and Absence | Functional Traits | ||||
|---|---|---|---|---|---|---|---|
| Pristine Forest | Selective Logging | Conventional Logging | Main Food Type a | Feeding Habit b | Respiration c | ||
| Ephemeroptera | Baetodes | 1 | 1 | 0 | Fd | CG | GT |
| Apobaetis | 1 | 1 | 0 | FdMi | CGSC | GT | |
| Farrodes | 1 | 1 | 0 | FdCd | SH | G | |
| Campylocia | 1 | 0 | 0 | FdCd | SH | G | |
| Plecoptera | Anacroneuria | 1 | 0 | 0 | Ma | PR | G |
| Trichoptera | Marilia | 1 | 1 | 1 | FdMi | CGSC | GT |
| Leptonema | 1 | 1 | 1 | FdCdMa | CFSHPR | GT | |
| Nectopsyche | 1 | 1 | 1 | FdCd | CGSH | GT | |
| Cernotina | 1 | 1 | 0 | Ma | PR | T | |
| Chimarra | 1 | 1 | 0 | Fd | CF | T | |
| Hydroptila | 1 | 1 | 0 | Mi | PI | T | |
| Helicopsyche | 1 | 0 | 0 | Fd | CG | T | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeone, D.; Fernandes, M.E.B. Linking Riparian Forest to the Functional Diversity of Ephemeroptera, Plecoptera, and Trichoptera in First-Order Tropical Streams. Diversity 2025, 17, 438. https://doi.org/10.3390/d17070438
Simeone D, Fernandes MEB. Linking Riparian Forest to the Functional Diversity of Ephemeroptera, Plecoptera, and Trichoptera in First-Order Tropical Streams. Diversity. 2025; 17(7):438. https://doi.org/10.3390/d17070438
Chicago/Turabian StyleSimeone, Diego, and Marcus E. B. Fernandes. 2025. "Linking Riparian Forest to the Functional Diversity of Ephemeroptera, Plecoptera, and Trichoptera in First-Order Tropical Streams" Diversity 17, no. 7: 438. https://doi.org/10.3390/d17070438
APA StyleSimeone, D., & Fernandes, M. E. B. (2025). Linking Riparian Forest to the Functional Diversity of Ephemeroptera, Plecoptera, and Trichoptera in First-Order Tropical Streams. Diversity, 17(7), 438. https://doi.org/10.3390/d17070438







