Spartina alterniflora-Derived Biochar Alters Biomass Allocation and Root Traits of Native Scirpus mariqueter
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sediment Collection
2.2. Biochar Preparation
2.3. Experimental Design
2.4. Plant and Soil Measurements
2.5. Statistical Analysis
3. Results
3.1. Effects of Spartina alterniflora-Derived Biochar on the Plant Traits of Scirpus mariqueter
3.2. Effects of Spartina alterniflora-Derived Biochar on Soil Physicochemical Properties
3.3. Effects of Spartina alterniflora-Derived Biochar on Root Morphology of Scirpus mariqueter
3.4. Correlation Analysis Among Plant Traits, Root Morphology, and Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Murtaza, G.; Deng, G.; Usman, M.; Jamil, A.; Qasim, M.; Iqbal, J.; Ercisli, S.; Akram, M.I.; Rizwan, M.; Elshikh, M.S. Impact of Acacia-derived biochar to mitigate salinity stress in Zea mays L. by morpho-physiological and biochemical indices. Sci. Rep. 2024, 14, 31883. [Google Scholar] [CrossRef] [PubMed]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, B.; Yuan, L.; Li, X.; Huang, Y.; Shi, R.; Jiang, X.; Wang, L.; Su, C. Mapping coastal salt marshes in China using time series of Sentinel-1 SAR. ISPRS J. Photogramm. Remote Sens. 2021, 173, 122–134. [Google Scholar] [CrossRef]
- Nan, L.; Longwei, L.; Yinlong, Z.; Ming, W. Monitoring of the invasion of Spartina alterniflora from 1985 to 2015 in Zhejiang Province, China. BMC Ecol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Wang, S.; Martin, P.A.; Hao, Y.; Sutherland, W.J.; Shackelford, G.E.; Wu, J.; Ruiting, J.; Zhou, W.; Li, B. A global synthesis of the effectiveness and ecological impacts of management interventions for Spartina species. Front. Environ. Sci. Eng. 2023, 17, 141. [Google Scholar] [CrossRef]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Change 2013, 3, 961–968. [Google Scholar] [CrossRef]
- Feng, L.J.; Wei, L.Q.; Qin, G.J.; Ge, F.J.; Ya, Z.X.; Jing, H.Y.; Hai, Y.F. Biochar-compost addition benefits Phragmites australis growth and soil property in coastal wetlands. Sci. Total Environ. 2021, 769, 145166. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, X. Role of biochar in raising blue carbon stock capacity of salt marshes. Pedosphere 2024, 34, 19–22. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Liao, X.; Ramus, A.P.; Angelini, C.; Liu, L.; Silliman, B.R.; Bertness, M.D.; He, Q. Shorebirds-driven trophic cascade helps restore coastal wetland multifunctionality. Nat. Commun. 2023, 14, 8076. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, D.; Tian, B.; Yuan, X.; Bo, S.; Ma, Q.; Wu, W.; Zhao, Z.; Zhang, L.; Keesing, J.K. A solution for restoration of critical wetlands and waterbird habitats in coastal deltaic systems. J. Environ. Manag. 2022, 302, 113996. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Gu, J.; Yun, P.; Gan, J.; Fan, W.; Chen, Z.; Shao, J.; Zhang, G.; Zeng, J. Spatiotemporal dynamics and influencing factors of native and invasive saltmarshes in a rapidly silting bay during 1985–2023. Front. Mar. Sci. 2025, 11, 1459935. [Google Scholar] [CrossRef]
- Zheng, X.; Javed, Z.; Liu, B.; Zhong, S.; Cheng, Z.; Rehman, A.; Du, D.; Li, J. Impact ofSpartina alternifloraInvasion in Coastal Wetlands of China: Boon or Bane? Biology 2023, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liao, C.; Zhang, X.; Chen, H.; Wang, Q.; Chen, Z.; Gan, X.; Wu, J.; Zhao, B.; Ma, Z. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Kong, R.; Ren, Z.; Liu, L.; Zhu, Y.; Sun, Y.; Peng, N.; He, J.; Ji, Y. Strategies in growth and reproduction of the native endangered plant species Scripus mariqueter and the driving factors in a coastal salt marsh wetland, eastern China. Acta Oecologica 2024, 122, 103979. [Google Scholar] [CrossRef]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.Z.; Xue, L.M.; Yan, Z.Z.; Liang, X.; Chen, X.C. Pioneer salt marsh species Scirpus mariqueter disperses quicker in summer with seed contribution from current and last year. Estuar. Coast. Shelf Sci. 2022, 264, 107682. [Google Scholar] [CrossRef]
- Qun, Z.; Bo, L. Field practice of Scirpus mariqueter restoration in the bird habitats of Chongming Dongtan Wetland, China. Chin. J. Appl. Ecol./Yingyong Shengtai Xuebao 2023, 34, 2663–2671. [Google Scholar] [CrossRef]
- Antonangelo, J.A.; Sun, X.; Eufrade-Junior, H.D. Biochar impact on soil health and tree-based crops: A review. Biochar 2025, 7, 51. [Google Scholar] [CrossRef]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. Nature-based solutions to address global societal challenges. IUCN Gland Switz. 2016, 97, 2036. [Google Scholar] [CrossRef]
- Han, L.; Lu, C.; Chen, L.; Wang, F.; Gao, K.; Yu, Y.; Xu, C. Carbon sequestration potential of biochar in soil from the perspective of organic carbon structural modification. Appl. Soil Ecol. 2024, 198, 105389. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Effect of invasive weed biochar amendment on soil enzymatic activity and respiration of coal mine spoil: A laboratory experiment study. Biochar 2021, 3, 519–533. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Kaushik, A.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Impact of Broussonetia papyrifera Biochar on the Biological Attributes of Cajanus cajan and Soil Enzymatic Activities. J. Soil Sci. Plant Nutr. 2024, 24, 1990–2007. [Google Scholar] [CrossRef]
- Cai, J.F.; Jiang, F.; Liu, X.S.; Sun, K.; Wang, W.; Zhang, M.X.; Li, H.L.; Xu, H.F.; Kong, W.J.; Yu, F.H. Biochar-amended coastal wetland soil enhances growth of Suaeda salsa and alters rhizosphere soil nutrients and microbial communities. Sci. Total Environ. 2021, 788, 147707. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Chen, F.; Zhang, T.; Kong, W. Preparation of two types plant biochars and application in soil quality improvement. Sci. Total Environ. 2024, 906, 167334. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Gao, Z.; Jia, H.; Du, D.; Eltawab, R.; Abdelfattah, A. Innovative solutions for coastal wetlands pollution: Application of Spartina alterniflora Loisel.-biochar for cadmium removal and carbon sequestration. J. Environ. Manag. 2025, 373, 123444. [Google Scholar] [CrossRef]
- Ghulam, M.; Zeeshan, A.; Eldin, S.M.; Basharat, A.; Sami, B.; Muhammad, U.; Rashid, I.; Dhurba, N.; Abd, U.; Ahmad, K.; et al. Biochar-Soil-Plant interactions: A cross talk for sustainable agriculture under changing climate. Front. Environ. Sci. 2023, 11, 1059449. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, L.; He, C.; Wang, F.; Liu, J.; Li, Y.; Chen, X.; Liu, X.; Lv, G.; Wang, D. Spartina alterniflora Invaded Coastal Wetlands by Raising Soil Sulfur Contents: A Meta-Analysis. Water 2022, 14, 1633. [Google Scholar] [CrossRef]
- Hussein, F.; Hasan, F.A. Biochar Enhanced Chemical and Biological Properties of Contaminated Soils with Lead. IOP Conf. Ser. Earth Environ. Sci. 2023, 1259, 012024. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yang, Q.; Xu, H.; Shen, G.; Chen, Q. Optimizing Biochar Application Rates to Improve Soil Properties and Crop Growth in Saline–Alkali Soil. Sustainability 2024, 16, 2523. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Becagli, M.; Lauria, G.; Cantini, V.; Ceccanti, C.; Cardelli, R.; Massai, R.; Remorini, D.; Guidi, L.; Landi, M. Biochar as a soil amendment in the tree establishment phase: What are the consequences for tree physiology, soil quality and carbon sequestration? Sci. Total Environ. 2022, 844, 157175. [Google Scholar] [CrossRef]
- Jikai, L.; Yina, L.; Junlin, H.; Bingyan, H.; Bing, W.; Kenji, O.; Jian, Z.; Hongyu, S. The effect of biochar on the migration theory of nutrient ions. Sci. Total Environ. 2022, 845, 157262. [Google Scholar] [CrossRef]
- Rasse, D.P.; Weldon, S.; Joner, E.J.; Joseph, S.; Kammann, C.I.; Liu, X.; O’Toole, A.; Pan, G.; Kocatürk Schumacher, N.P. Enhancing plant N uptake with biochar-based fertilizers: Limitation of sorption and prospects. Plant Soil 2022, 475, 213–236. [Google Scholar] [CrossRef]
- Wang, X.; Riaz, M.; Babar, S.; Eldesouki, Z.; Liu, B.; Xia, H.; Li, Y.; Wang, J.; Xia, X.; Jiang, C. Alterations in the composition and metabolite profiles of the saline-alkali soil microbial community through biochar application. J. Environ. Manag. 2024, 352, 120033. [Google Scholar] [CrossRef]
- Ahmed, M.; Hasanuzzaman, M.; Raza, M.A.; Malik, A.; Ahmad, S. Plant nutrients for crop growth, development and stress tolerance. Sustain. Agric. Era Clim. Change 2020, 43–92. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Subedi, R.; Bertora, C.; Zavattaro, L.; Grignani, C. Crop response to soils amended with biochar: Expected benefits and unintended risks. Ital. J. Agron. 2017, 12, 794. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Tao, W.; Zhang, X.; Xu, Z.; Xu, C. The Biological Effects of Biochar on Soil’s Physical and Chemical Characteristics: A Review. Sustainability 2025, 17, 2214. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef]
- Woo, K.M.; Mesenbet, Y.; Hyun, K.Y.; Jin, O.S.; Cheol, L.J.; Kwon, E.E.; Soo, L.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef]
- Jin, F.; Piao, J.; Miao, S.; Che, W.; Li, X.; Li, X.; Shiraiwa, T.; Tanaka, T.; Taniyoshi, K.; Hua, S.; et al. Long-term effects of biochar one-off application on soil physicochemical properties, salt concentration, nutrient availability, enzyme activity, and rice yield of highly saline-alkali paddy soils: Based on a 6-year field experiment. Biochar 2024, 6, 40. [Google Scholar] [CrossRef]
- Liu, Q.; Meki, K.; Zheng, H.; Yuan, Y.; Shao, M.; Luo, X.; Li, X.; Jiang, Z.; Li, F.; Xing, B. Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 2023, 5, 45. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Ecotoxicological responses of weed biochar on seed germination and seedling growth in acidic soil. Environ. Technol. Innov. 2020, 20, 101074. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Dziurka, K.; Dziurka, M.; Rodrigues, A.F.; Latawiec, A.E. Biochars as culture medium additives influence organogenic potential of plant explants through changes in endogenous phytohormone and carbohydrate contents in Daphne species. Plant Cell Tissue Organ Cult. 2022, 152, 45–66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M. Modeling hydrodynamic and hydrological processes in tidal wetlands. Wetlands 2022, 42, 1. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Patel, M.; Pittman, C.U., Jr. Harnessing biochar in contaminated soil for heavy metal immobilization, soil health enhancement, and carbon sequestration. Ind. Eng. Chem. Res. 2024, 63, 10380–10396. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Hu, J.; Tang, H.; Wang, Y.Z.; Yang, C.; Gao, M.-T.; Tsang, Y.F.; Li, J. Effect of dissolved solids released from biochar on soil microbial metabolism. Environ. Sci. Process. Impacts 2022, 24, 598–608. [Google Scholar] [CrossRef]
- Sadia, A.; Shabana, B.; Mehedi, H.M.; Partha, B.; Ishtiaq, A.M.; Muhammad, B.; Hitesh, C.; Nobendu, M.; Swastika, M. A review on influence of biochar amendment on soil processes and environmental remediation. Biotechnol. Genet. Eng. Rev. 2023, 40, 31–35. [Google Scholar] [CrossRef]
- Ding, J.; Yu, S. Impact of Biochar on Nitrogen-Cycling Functional Genes: A Comparative Study in Mollisol and Alkaline Soils. Life 2024, 14, 1631. [Google Scholar] [CrossRef]
- Kassa, Y.; Amare, A.; Nega, T.; Alem, T.; Gedefaw, M.; Chala, B.; Freyer, B.; Waldmann, B.; Fentie, T.; Mulu, T.; et al. Water hyacinth conversion to biochar for soil nutrient enhancement in improving agricultural product. Sci. Rep. 2025, 15, 1820. [Google Scholar] [CrossRef]
- Yang, F.; Wang, C.; Sun, H. A comprehensive review of biochar-derived dissolved matters in biochar application: Production, characteristics, and potential environmental effects and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105258. [Google Scholar] [CrossRef]
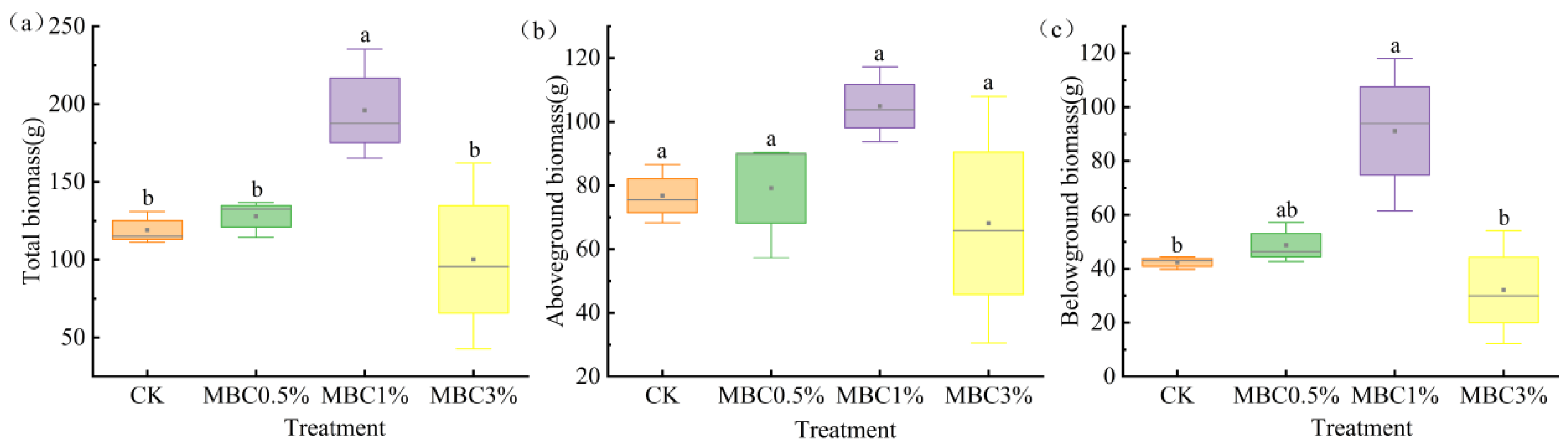
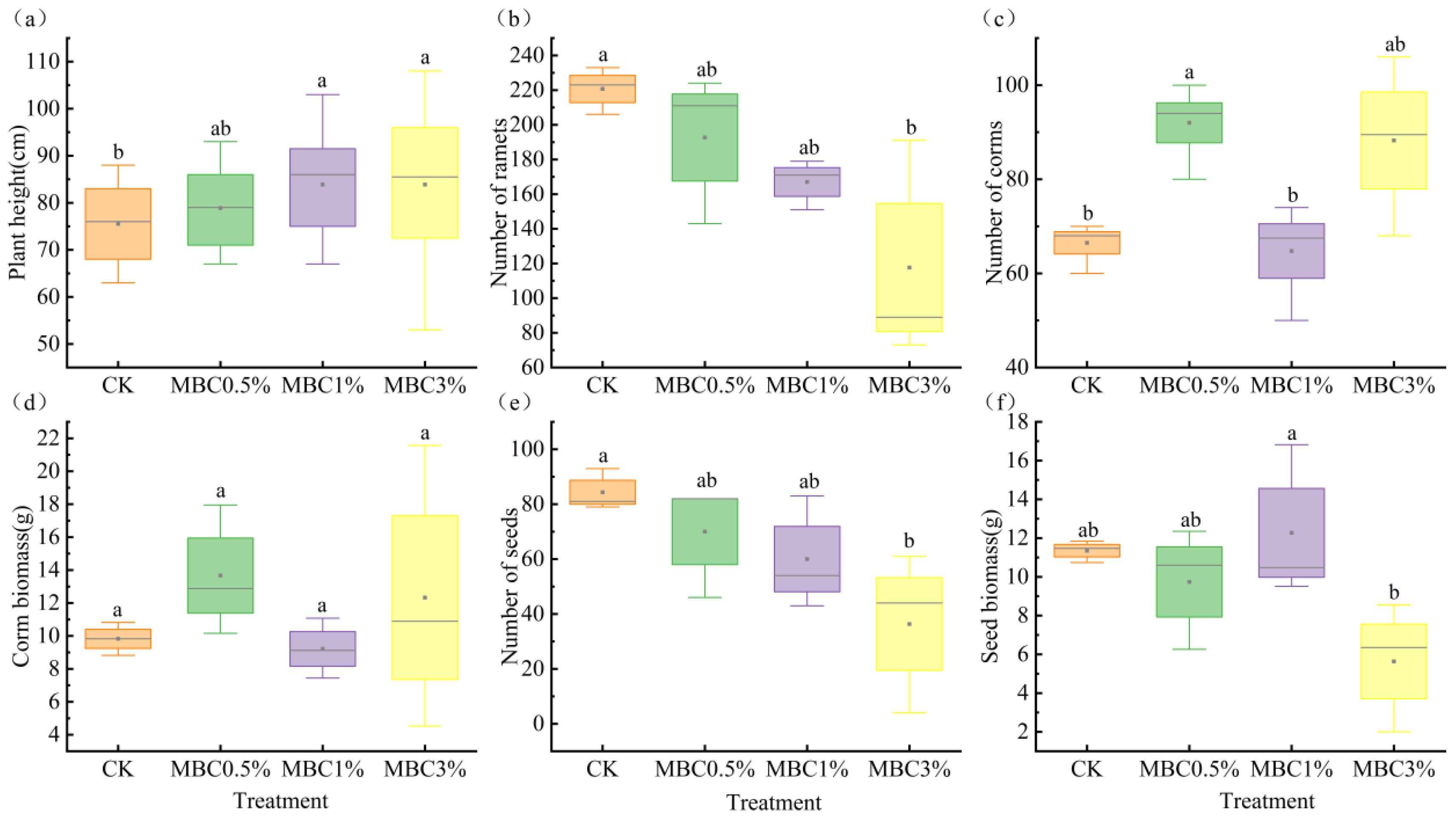

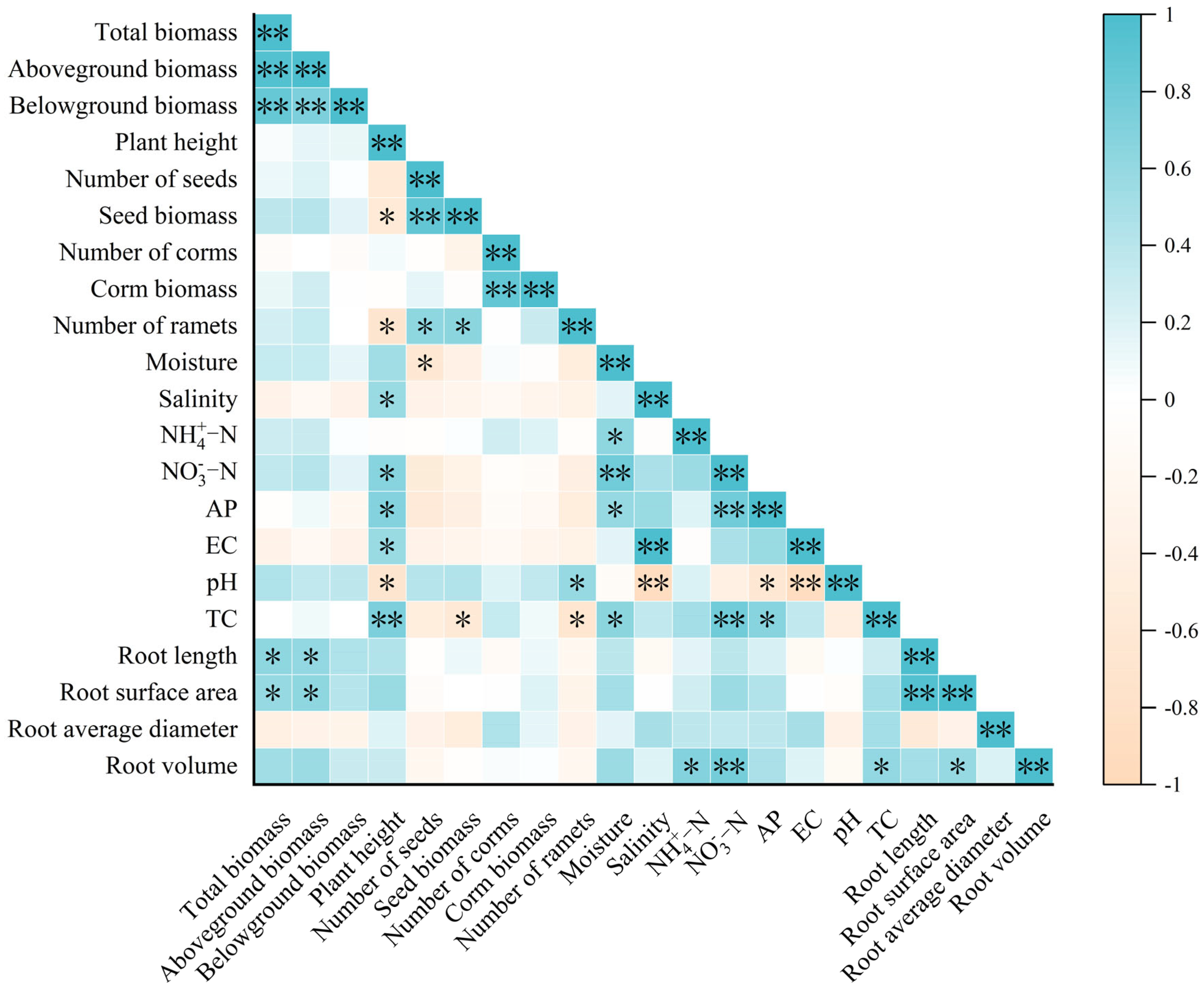
| Treatment | CK | MBC0.5% | MBC1% | MBC3% |
|---|---|---|---|---|
| pH | 7.67 ± 0.08 a | 7.82 ± 0.03 a | 7.75 ± 0.04 a | 7.49 ± 0.01 b |
| Moisture (%) | 46.48 ± 0.89 b | 48.81 ± 0.87 ab | 51.96 ± 0.98 a | 52.31 ± 2.09 a |
| EC (μS/cm) | 136.57 ± 9.03 b | 121.20 ± 2.60 b | 133.03 ± 7.79 b | 174.87 ± 3.06 a |
| Salinity (g/kg) | 0.27 ± 0.02 b | 0.24 ± 0.01 b | 0.27 ± 0.02 b | 0.35 ± 0.01 a |
| AP (mg/kg) | 84.40 ± 13.49 ab | 83.88 ± 2.74 b | 101.40 ± 3.02 ab | 119.30 ± 1.95 a |
| NH4+-N (mg/kg) | 26.16 ± 0.84 b | 33.18 ± 3.13 a | 30.39 ± 0.81 ab | 30.86 ± 0.73 ab |
| NO3−-N (mg/kg) | 15.02 ± 0.57 c | 15.69 ± 0.66 bc | 17.17 ± 0.17 ab | 17.81 ± 0.36 a |
| TC (g/kg) | 53.89 ± 0.87 c | 59.78 ± 1.60 b | 61.56 ± 1.07 b | 76.62 ± 2.17 a |
| Treatments | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Root Avgrage Diameter (mm) |
|---|---|---|---|---|
| CK | 101.02 ± 20.47 b | 18.25 ± 3.14 b | 0.26 ± 0.04 b | 0.58 ± 0.02 b |
| MBC0.5% | 108.13 ± 21.42 b | 21.62 ± 3.20 b | 0.35 ± 0.03 ab | 0.65 ± 0.04 ab |
| MBC1% | 238.25 ± 34.52 a | 41.67 ± 3.68 a | 0.48 ± 0.09 a | 0.56 ± 0.03 b |
| MBC3% | 133.40 ± 26.18 b | 29.84 ± 5.09 ab | 0.42 ± 0.06 ab | 0.72 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Gao, J.; Jiang, P.; Li, J.; Wu, M.; Jiao, S.; Zhang, L.; Li, N.; Shao, X. Spartina alterniflora-Derived Biochar Alters Biomass Allocation and Root Traits of Native Scirpus mariqueter. Diversity 2025, 17, 357. https://doi.org/10.3390/d17050357
Tang Y, Gao J, Jiang P, Li J, Wu M, Jiao S, Zhang L, Li N, Shao X. Spartina alterniflora-Derived Biochar Alters Biomass Allocation and Root Traits of Native Scirpus mariqueter. Diversity. 2025; 17(5):357. https://doi.org/10.3390/d17050357
Chicago/Turabian StyleTang, Yaoyao, Jingwen Gao, Pengcheng Jiang, Junzhen Li, Ming Wu, Shengwu Jiao, Long Zhang, Niu Li, and Xuexin Shao. 2025. "Spartina alterniflora-Derived Biochar Alters Biomass Allocation and Root Traits of Native Scirpus mariqueter" Diversity 17, no. 5: 357. https://doi.org/10.3390/d17050357
APA StyleTang, Y., Gao, J., Jiang, P., Li, J., Wu, M., Jiao, S., Zhang, L., Li, N., & Shao, X. (2025). Spartina alterniflora-Derived Biochar Alters Biomass Allocation and Root Traits of Native Scirpus mariqueter. Diversity, 17(5), 357. https://doi.org/10.3390/d17050357







