Local Variation in Ground Arthropod Diversity Rises as Distance to Residential Areas Decreases in a Mature Evergreen Forest
Abstract
1. Introduction
2. Materials and Methods
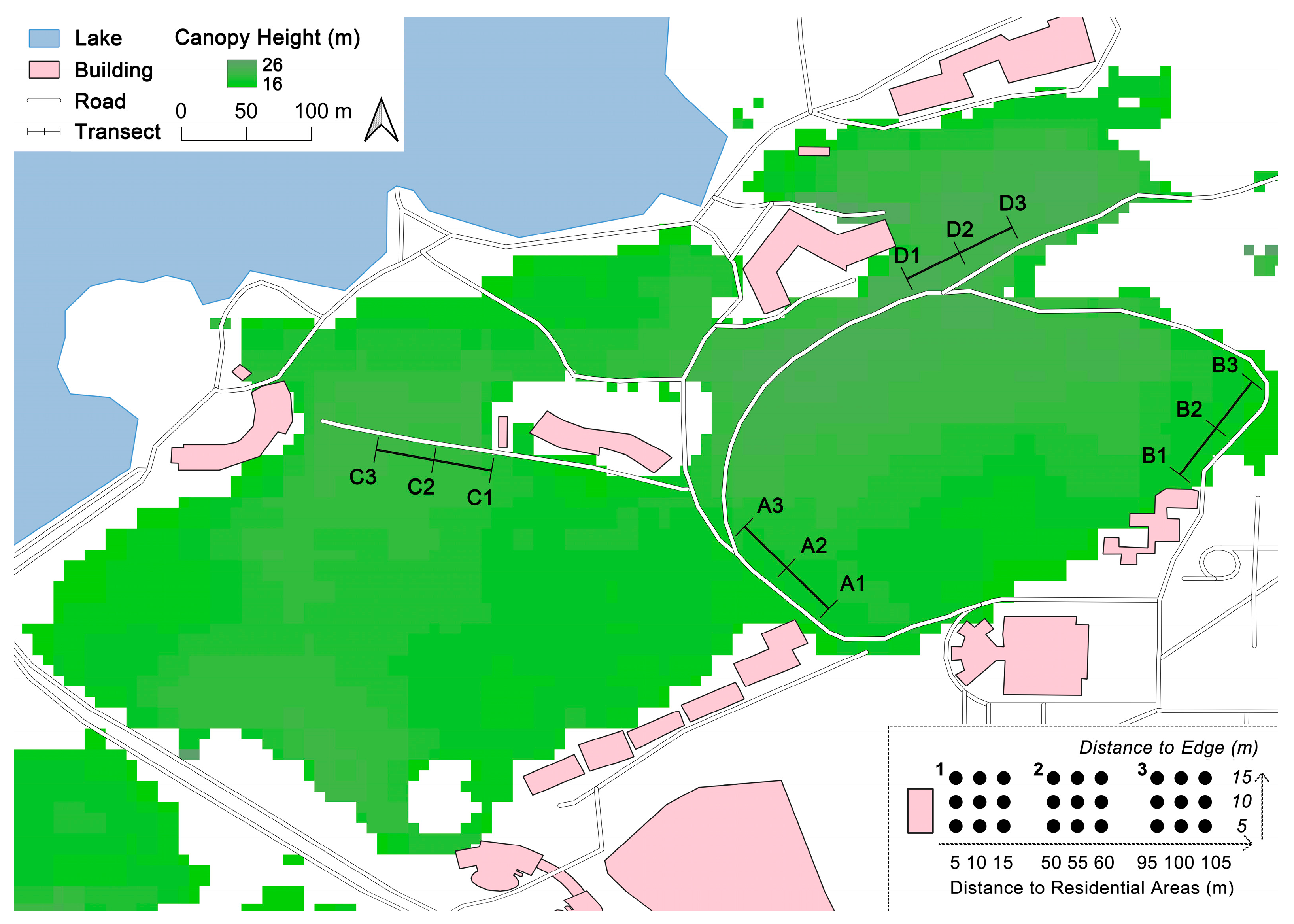
2.1. Study Area
2.2. Pitfall Trapping
2.3. Diversity Indices
2.4. Analysis of Variance
3. Results
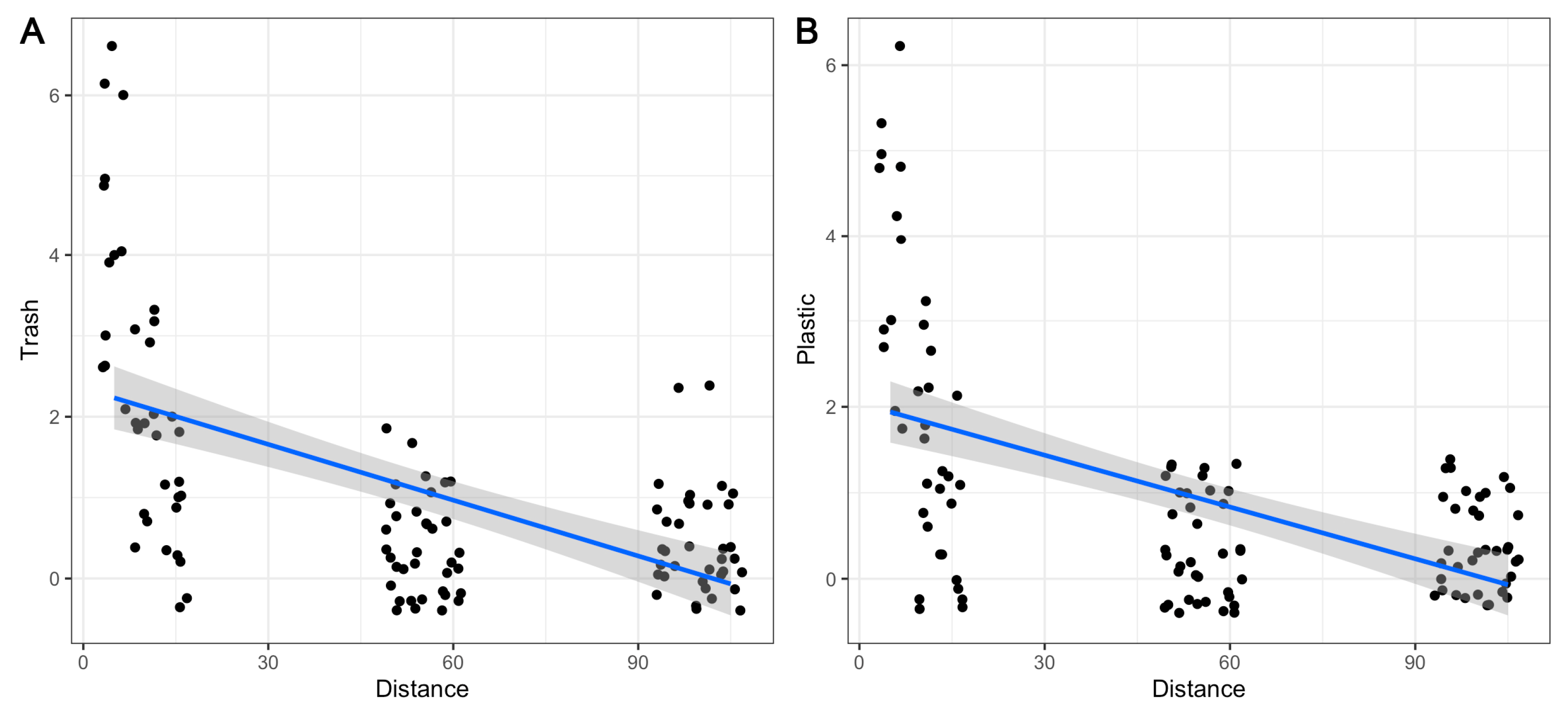
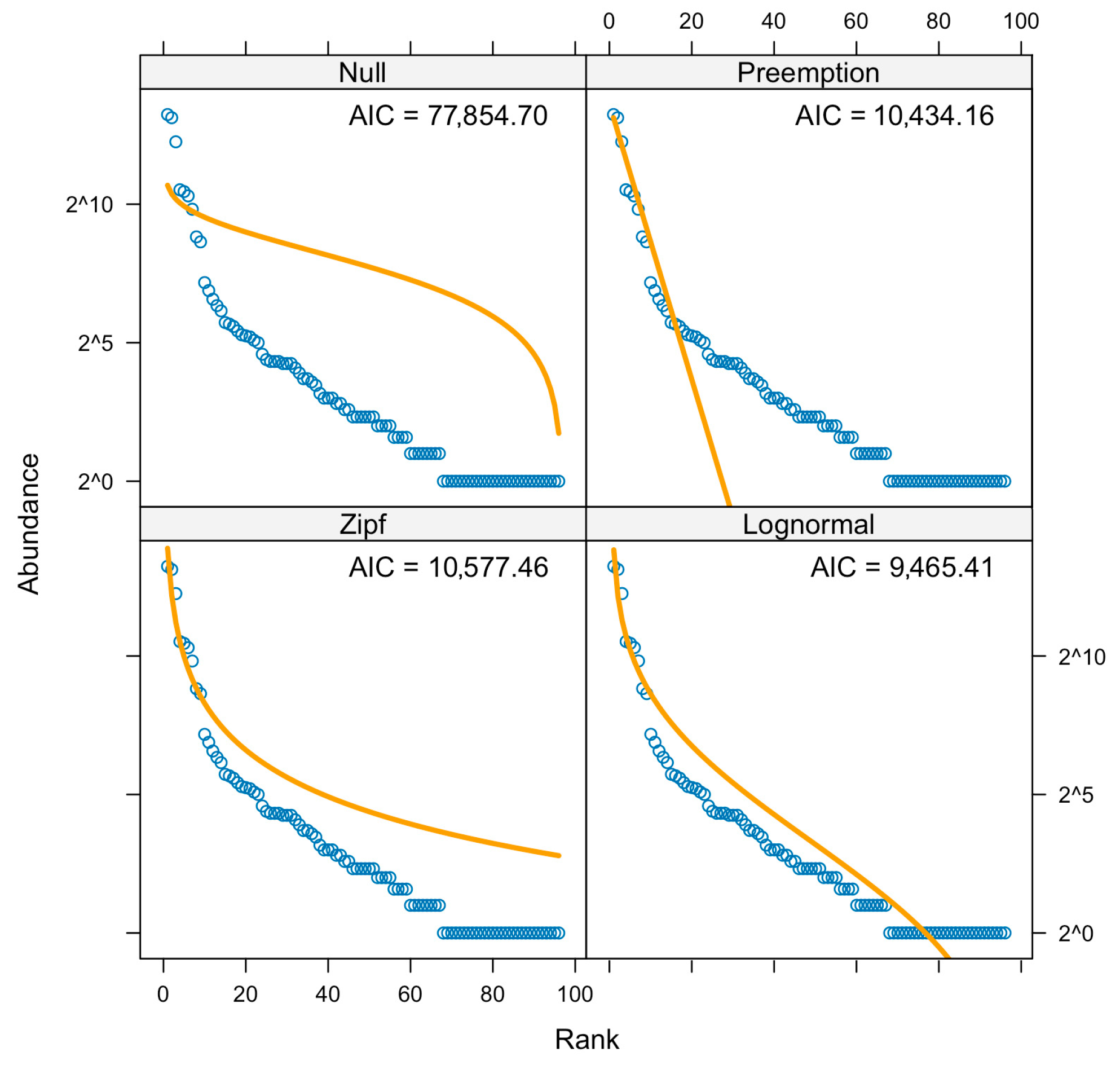
3.1. Community Composition
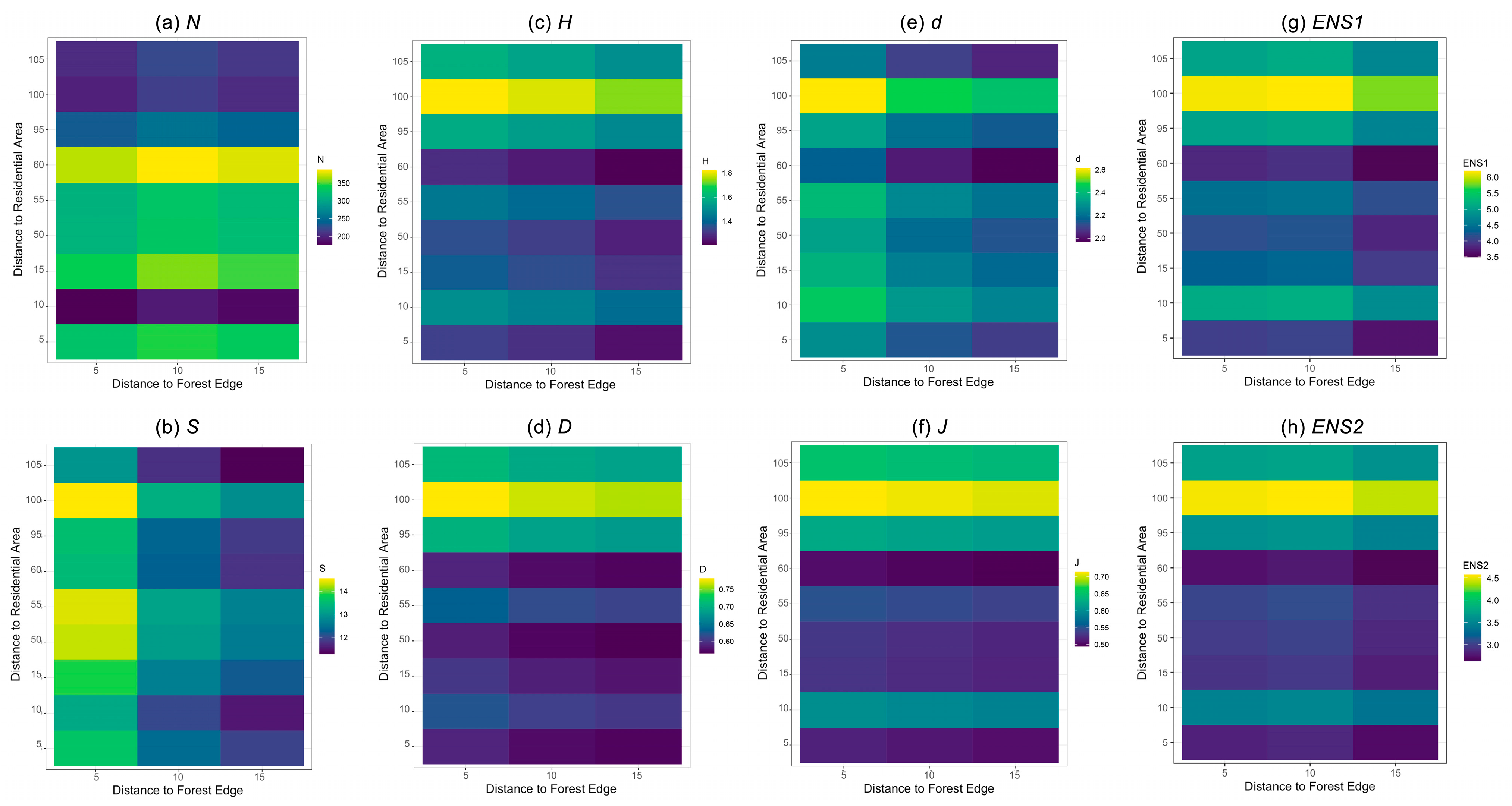
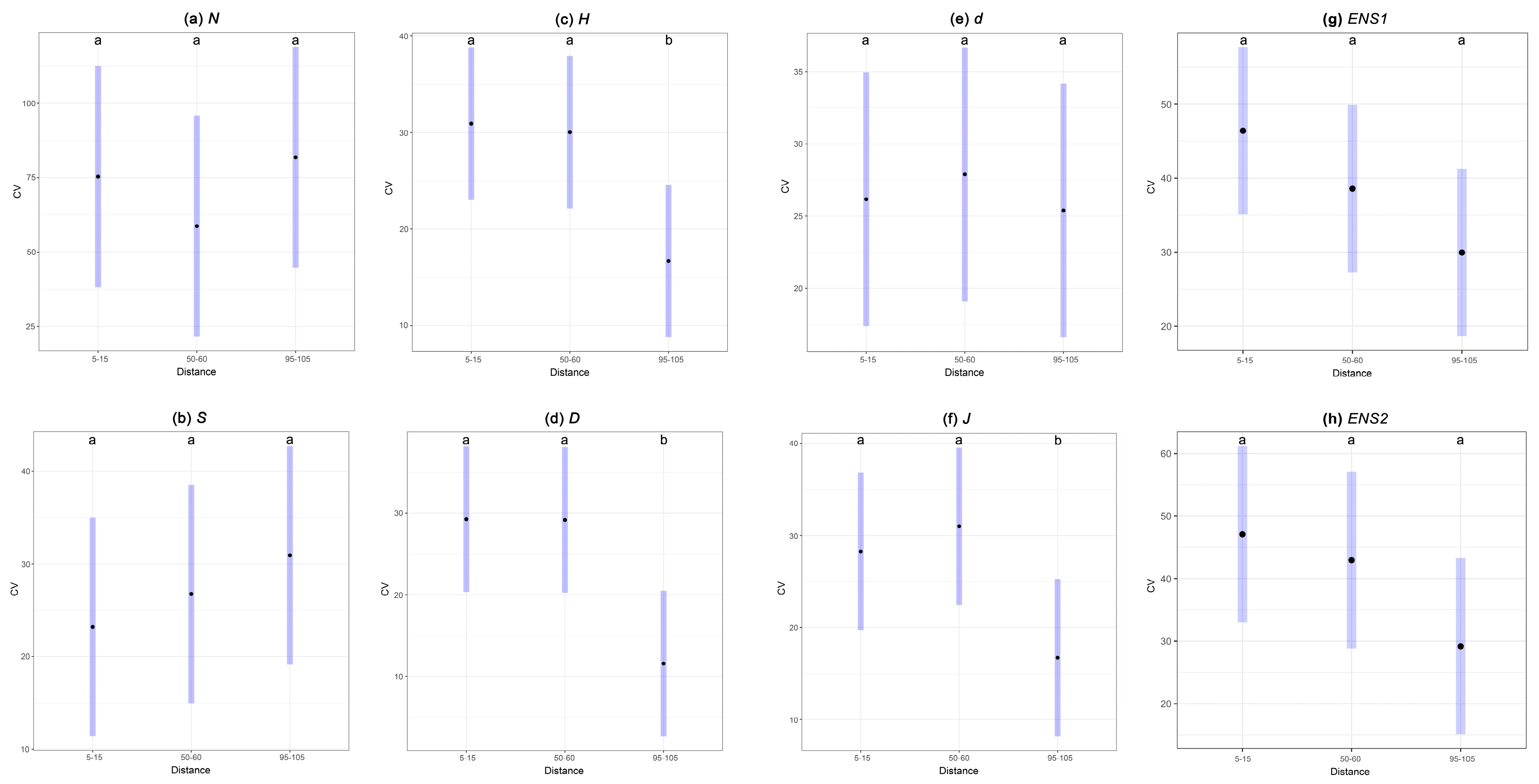
3.2. Variation in Diversity Indices
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Order | Richness | Abundance | Relative Abundance (%) |
|---|---|---|---|
| Scolopendromorpha | 2 | 974 | 3.181 |
| Scutigeromorpha | 1 | 1 | 0.003 |
| Araneae | 14 | 71 | 0.232 |
| Sarcoptiformes | 8 | 86 | 0.281 |
| Isopoda | 1 | 39 | 0.127 |
| Entomobryomorpha | 1 | 452 | 1.476 |
| Dermaptera | 3 | 38 | 0.124 |
| Orthoptera | 2 | 40 | 0.131 |
| Blattodea | 2 | 58 | 0.189 |
| Thysanoptera | 1 | 3 | 0.010 |
| Hemiptera | 7 | 1280 | 4.181 |
| Hymenoptera | 20 | 25,008 | 81.683 |
| Coleoptera | 11 | 107 | 0.349 |
| Diptera | 20 | 2055 | 6.712 |
| Siphonaptera | 1 | 399 | 1.303 |
| Mecoptera | 2 | 5 | 0.016 |
References
- Maleque, M.A.; Ishii, H.T.; Maeto, K. The use of arthropods as indicators of ecosystem integrity in forest management. J. For. 2006, 104, 113–117. [Google Scholar] [CrossRef]
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Huang, J.; Miao, X.; Wang, Q.; Menzel, F.; Tang, P.; Yang, D.; Wu, H.; Vogler, A.P. Metabarcoding reveals massive species diversity of Diptera in a subtropical ecosystem. Ecol. Evol. 2022, 12, e8535. [Google Scholar] [CrossRef]
- Lattin, J.D. Arthropod diversity and conservation in old-growth northwest forests. Am. Zool. 1993, 33, 578–587. [Google Scholar] [CrossRef]
- Yekwayo, I.; Pryke, J.S.; Roets, F.; Samways, M.J. Surrounding vegetation matters for arthropods of small, natural patches of indigenous forest. Insect Conserv. Divers. 2016, 9, 224–235. [Google Scholar] [CrossRef]
- Lichtenberg, E.M.; Kennedy, C.M.; Kremen, C.; Batary, P.; Berendse, F.; Bommarco, R.; Bosque-Pérez, N.A.; Carvalheiro, L.G.; Snyder, W.E.; Williams, N.M.; et al. A global synthesis of the effects of diversified farming systems on arthropod diversity within fields and across agricultural landscapes. Glob. Change Biol. 2017, 23, 4946–4957. [Google Scholar] [CrossRef]
- Kotze, D.J.; Lowe, E.C.; MacIvor, J.S.; Ossola, A.; Norton, B.A.; Hochuli, D.F.; Mata, L.; Moretti, M.; Gagné, S.A.; Handa, I.T.; et al. Urban forest invertebrates: How they shape and respond to the urban environment. Urban Ecosyst. 2022, 25, 1589–1609. [Google Scholar] [CrossRef]
- Maleque, M.A.; Maeto, K.; Ishii, H.T. Arthropods as bioindicators of sustainable forest management, with a focus on plantation forests. Appl. Entomol. Zool. 2009, 44, 1–11. [Google Scholar] [CrossRef]
- Zommers, Z. “Follow the Spiders”: Ecosystems as Early Warnings. In Reducing Disaster: Early Warning Systems for Climate Change; Singh, A., Zommers, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Lamarre, G.P.; Hérault, B.; Fine, P.V.; Vedel, V.; Lupoli, R.; Mesones, I.; Baraloto, C. Taxonomic and functional composition of arthropod assemblages across contrasting Amazonian forests. J. Anim. Ecol. 2016, 85, 227–239. [Google Scholar] [CrossRef]
- Swart, R.C.; Pryke, J.S.; Roets, F. Arthropod assemblages deep in natural forests show different responses to surrounding land use. Biodivers. Conserv. 2018, 27, 583–606. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Cours, J.; Bouget, C.; Barsoum, N.; Horák, J.; Le Souchu, E.; Leverkus, A.B.; Pincebourde, S.; Thorn, S.; Sallé, A. Surviving in changing forests: Abiotic disturbance legacy effects on arthropod communities of temperate forests. Cur. For. Rep. 2023, 9, 189–218. [Google Scholar] [CrossRef]
- Kung’u, G.N.; Cousseau, L.; Githiru, M.; Habel, J.C.; Kinyanjui, M.; Matheka, K.; Schmitt, C.B.; Seifert, T.; Teucher, M.; Lens, L.; et al. Anthropogenic activities affect forest structure and arthropod abundance in a Kenyan biodiversity hotspot. Biodivers. Conserv. 2023, 32, 3255–3282. [Google Scholar] [CrossRef]
- Li, Y.; Schmid, B.; Schuldt, A.; Li, S.; Wang, M.Q.; Fornoff, F.; Staab, M.; Guo, P.F.; Anttonen, P.; Chesters, D.; et al. Multitrophic arthropod diversity mediates tree diversity effects on primary productivity. Nat. Ecol. Evol. 2023, 7, 832–840. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Hawes, C.; Peace, A.J.; Ferris-Kaan, R.; Jukes, M.R. Relationships between insect diversity and habitat characteristics in plantation forests. For. Ecol. Manag. 1999, 113, 11–21. [Google Scholar] [CrossRef]
- Lange, M.; Weisser, W.W.; Gossner, M.M.; Kowalski, E.; Türke, M.; Joner, F.; Fonseca, C.R. The impact of forest management on litter-dwelling invertebrates: A subtropical–temperate contrast. Biodivers. Conserv. 2011, 20, 2133–2147. [Google Scholar] [CrossRef]
- Lingbeek, B.J.; Higgins, C.L.; Muir, J.P.; Kattes, D.H.; Schwertner, T.W. Arthropod diversity and assemblage structure response to deforestation and desertification in the Sahel of western Senegal. Glob. Ecol. Conserv. 2017, 11, 165–176. [Google Scholar] [CrossRef]
- Estay, S.A.; Navarrete, S.A.; Rothmann Toro, S. Effects of human mediated disturbances on exotic forest insect diversity in a Chilean Mediterranean ecosystem. Biodivers. Conserv. 2012, 21, 3699–3710. [Google Scholar] [CrossRef]
- Agra, J.; Ligeiro, R.; Heino, J.; Macedo, D.R.; Castro, D.M.; Linares, M.S.; Callisto, M. Anthropogenic disturbances alter the relationships between environmental heterogeneity and biodiversity of stream insects. Ecol. Indic. 2021, 121, 107079. [Google Scholar] [CrossRef]
- Canelles, Q.; Aquilué, N.; James, P.M.; Lawler, J.; Brotons, L. Global review on interactions between insect pests and other forest disturbances. Landsc. Ecol. 2021, 36, 945–972. [Google Scholar] [CrossRef]
- Crossley, M.S.; Meier, A.R.; Baldwin, E.M.; Berry, L.L.; Crenshaw, L.C.; Hartman, G.L.; Lagos-Kutz, D.; Nichols, D.H.; Patel, K.; Varriano, S.; et al. No net insect abundance and diversity declines across US Long Term Ecological Research sites. Nat. Ecol. Evol. 2020, 4, 1368–1376. [Google Scholar] [CrossRef]
- Brandon-Mong, G.J.; Littlefair, J.E.; Sing, K.W.; Lee, Y.P.; Gan, H.M.; Clare, E.L.; Wilson, J.J. Temporal changes in arthropod activity in tropical anthropogenic forests. Bull. Entomol. Res. 2018, 108, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Baidya, P.; Bagchi, S. Influence of human land use and invasive species on beta diversity of tropical ant assemblages. Insect Conserv. Divers. 2022, 15, 73–85. [Google Scholar] [CrossRef]
- Lu, Z.; Hoffmann, B.D.; Chen, Y. Can reforested and plantation habitats effectively conserve SW China’s ant biodiversity? Biodivers. Conserv. 2016, 25, 753–770. [Google Scholar] [CrossRef]
- Cerda, X.; Arnan, X.; Retana, J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol. News 2013, 18, 131–147. [Google Scholar]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Horgan, F.G. Effects of deforestation on diversity, biomass and function of dung beetles on the eastern slopes of the Peruvian Andes. For. Ecol. Manag. 2005, 216, 117–133. [Google Scholar] [CrossRef]
- Pearce, J.L.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecol. Indic. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- de Castro Solar, R.R.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity consequences of land-use change and forest disturbance in the Amazon: A multi-scale assessment using ant communities. Biol. Conserv. 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Potapov, A.M.; Dupérré, N.; Jochum, M.; Dreczko, K.; Klarner, B.; Barnes, A.D.; Krashevska, V.; Rembold, K.; Kreft, H.; Brose, U.; et al. Functional losses in ground spider communities due to habitat structure degradation under tropical land-use change. Ecology 2020, 101, e02957. [Google Scholar] [CrossRef]
- Lira, A.F.; Foerster, S.I.; Salomão, R.P.; Porto, T.J.; Albuquerque, C.M.; Moura, G.J. Understanding the effects of human disturbance on scorpion diversity in Brazilian tropical forests. J. Insect. Conserv. 2021, 25, 147–158. [Google Scholar] [CrossRef]
- Košulič, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced oak forests. PLoS ONE 2016, 11, e0148585. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Tso, I.M.; Lin, H.C.; Lin, L.K.; Lin, C.P. Effects of thinning on spider diversity of an East Asian subtropical plantation forest. Zool. Stud. 2011, 50, 705–717. [Google Scholar]
- Lee, R.H.; Kwong, I.H.; Tsang, T.P.; Wong, M.K.; Guénard, B. Remotely sensed environmental data as ecological proxies for ground-dwelling ant diversity along a subtropical forest succession gradient. J. Ecol. 2023, 111, 1428–1442. [Google Scholar] [CrossRef]
- Uehara-Prado, M.; de Oliveira Fernandes, J.; de Moura Bello, A.; Machado, G.; Santos, A.J.; Vaz-de-Mello, F.Z.; Freitas, A.V.L. Selecting terrestrial arthropods as indicators of small-scale disturbance: A first approach in the Brazilian Atlantic Forest. Biol. Conserv. 2009, 142, 1220–1228. [Google Scholar] [CrossRef]
- Dennis, P.; Thomas, M.B.; Sotherton, N.W. Structural features of field boundaries which influence the overwintering densities of beneficial arthropod predators. J. Appl. Ecol. 1994, 31, 361–370. [Google Scholar] [CrossRef]
- Knapp, M.; Štrobl, M.; Venturo, A.; Seidl, M.; Jakubíková, L.; Tajovský, K.; Kadlec, T.; González, E. Importance of grassy and forest non-crop habitat islands for overwintering of ground-dwelling arthropods in agricultural landscapes: A multi-taxa approach. Biol. Conserv. 2022, 275, 109757. [Google Scholar] [CrossRef]
- Allen, J.C.; Barnes, D.F. The causes of deforestation in developing countries. Ann. Am. Assoc. Geogr. 1985, 75, 163–184. [Google Scholar] [CrossRef]
- Leblois, A.; Damette, O.; Wolfersberger, J. What has driven deforestation in developing countries since the 2000s? Evidence from new remote-sensing data. World Dev. 2017, 92, 82–102. [Google Scholar] [CrossRef]
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Purswani, E.; Pathak, B.; Kumar, D.; Verma, S. Land-use Change as a Disturbance Regime. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; pp. 127–144. [Google Scholar] [CrossRef]
- Yang, H.; Zhong, X.; Deng, S.; Nie, S. Impact of LUCC on landscape pattern in the Yangtze River Basin during 2001–2019. Ecol. Infom. 2022, 69, 101631. [Google Scholar] [CrossRef]
- Zhou, Y.; Ning, L.; Bai, X. Spatial and temporal changes of human disturbances and their effects on landscape patterns in the Jiangsu coastal zone, China. Ecol. Indic. 2018, 93, 111–122. [Google Scholar] [CrossRef]
- Jacobson, A.P.; Riggio, J.; Tait, A.M.; Baillie, J.E.M. Global areas of low human impact (‘Low Impact Areas’) and fragmentation of the natural world. Sci. Rep. 2019, 9, 14179. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, P.; Xia, J.; Wang, W.; Cai, W.; Chen, N.; Hu, S.; Luo, X.; Li, J.; Zhan, C. Land use/land cover prediction and analysis of the middle reaches of the Yangtze River under different scenarios. Sci. Total Environ. 2022, 833, 155238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qu, X.; Huang, Y.; Tan, M.; Xu, K. Assessing the potential of species loss caused by deforestation in a mature subtropical broadleaf forest in Central China. Trees For. People 2024, 18, 100673. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Kong, R.; Chang, J.; Tian, J.; Zhu, B.; Jiang, S.; Chen, X.; Xu, C.Y. Changes in forest net primary productivity in the Yangtze River Basin and its relationship with climate change and human activities. Remote Sens. 2019, 11, 1451. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Rao, S.; Xu, K. Sleet damage to branches and crowns of street camphor trees (Cinnamomum camphora) in a central China mega-city: Damage statistics, modelling, and implications. Front. For Glob. Change 2024, 7, 1391645. [Google Scholar] [CrossRef]
- Lu, S.; Tang, X.; Guan, X.; Qin, F.; Liu, X.; Zhang, D. The assessment of forest ecological security and its determining indicators: A case study of the Yangtze River Economic Belt in China. J. Environ. Manag. 2020, 258, 110048. [Google Scholar] [CrossRef]
- Brown, B.V. Sampling methods for adult flies (Diptera). In Measuring Arthropod Biodiversity; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 187–204. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Bassoki, H.; Dassou, A.G. Chronic anthropogenic disturbance mediates the bottom-up influence of plant diversity on arthropods in tropical forests. Ecol. Evol. 2024, 14, e11055. [Google Scholar] [CrossRef]
- Greenberg, C.H.; McGrane, A. A comparison of relative abundance and biomass of ground-dwelling arthropods under different forest management practices. For. Ecol. Manag. 1996, 89, 31–41. [Google Scholar] [CrossRef]
- Kwon, T.S.; Lee, C.M.; Sung, J.H. Diversity decrease of ant (Formicidae, Hymenoptera) after a forest disturbance: Different responses among functional guilds. Zool. Stud. 2014, 53, 37. [Google Scholar] [CrossRef]
- Szinwelski, N.; Rosa, C.S.; Schoereder, J.H.; Mews, C.M.; Sperber, C.F. Effects of forest regeneration on crickets: Evaluating environmental drivers in a 300-year chronosequence. Int. J. Zool. 2012, 2012, 793419. [Google Scholar] [CrossRef]
- Frouz, J. Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: A review of ecological requirements and response to disturbance. Agric. Ecosyst. Environ. 1999, 74, 167–186. [Google Scholar] [CrossRef]
- Hassall, M.; Jones, D.T.; Taiti, S.; Latipi, Z.; Sutton, S.L.; Mohammed, M. Biodiversity and abundance of terrestrial isopods along a gradient of disturbance in Sabah, East Malaysia. Eur. J. Soil Biol. 2006, 42 (Suppl. S1), S197–S207. [Google Scholar]
- Wilson, L.T.; Room, P.M. The relative efficiency and reliability of three methods for sampling arthropods in Australian cotton fields. Aust. J. Entomol. 1982, 21, 175–181. [Google Scholar] [CrossRef]
- Woodcock, B.A. Pitfall trapping in ecological studies. In Insect Sampling in Forest Ecosystems; Leather, S.R., Ed.; Blackwell Science: West Sussex, UK, 2005; pp. 37–57. [Google Scholar] [CrossRef]
- Hertz, M. Huomioita petokuoriaisten olinpaikoista. Luonnon Ystävä 1927, 31, 218–222. [Google Scholar]
- Barber, H.S. Traps for cave-inhabiting insects. J. Elisha Mitchell Sci. Soc. 1931, 46, 259–266. [Google Scholar]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef]
- Jokimäki, J.; Huhta, E.; Itämies, J.; Rahko, P. Distribution of arthropods in relation to forest patch size, edge, and stand characteristics. Can. J. For. Res. 1998, 28, 1068–1072. [Google Scholar] [CrossRef]
- De Smedt, P.; Baeten, L.; Proesmans, W.; Van de Poel, S.; Van Keer, J.; Giffard, B.; Martin, L.; Vanhulle, R.; Brunet, J.; Cousins, S.A.; et al. Strength of forest edge effects on litter-dwelling macro-arthropods across Europe is influenced by forest age and edge properties. Divers. Distrib. 2019, 25, 963–974. [Google Scholar] [CrossRef]
- Hohbein, R.R.; Conway, C.J. Pitfall traps: A review of methods for estimating arthropod abundance. Wildl. Soc. Bull. 2018, 42, 597–606. [Google Scholar] [CrossRef]
- Hartshorn, J. A review of forest management effects on terrestrial leaf litter inhabiting arthropods. Forests 2020, 12, 23. [Google Scholar] [CrossRef]
- Perry, K.I.; Herms, D.A. Dynamic responses of ground-dwelling invertebrate communities to disturbance in forest ecosystems. Insects 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, S. Identifying and zoning key areas of ecological restoration for territory in resource-based cities: A case study of Huangshi city, China. Sustainability 2021, 13, 3931. [Google Scholar] [CrossRef]
- Lin, X.; Wu, X.; Chao, J.; Ge, X.; Tan, L.; Liu, W.; Sun, Z.; Hou, J. Effects of combined ecological restoration measures on water quality and underwater light environment of Qingshan Lake, an urban eutrophic lake in China. Ecol. Indic. 2024, 163, 112107. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Chen, Z. Development of soil classification in China. In Soil Classification; Eswaran, H., Ahrens, R., Rice, T.J., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 101–126. [Google Scholar] [CrossRef]
- Zhu, Q.; De Vries, W.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmos. Environ. 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Koivula, M.; Kotze, J.; Hiisivuori, L.; Rita, H. Pitfall trap efficiency: Do trap size, collecting fluid and vegetation structure matter? Entomol. Fenn. 2003, 14, 1–14. [Google Scholar] [CrossRef]
- Ji, L. China Checklist of Animals, In Catalogue of Life China: 2024 Annual Checklist; The Biodiversity Committee of Chinese Academy of Sciences, Ed.; Chinese Academy of Sciences: Beijing, China, 2024. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘Vegan’. Community Ecology Package, version 2.9.; CRAN: Windhoek, Namibia, 2013. [Google Scholar]
- Matthews, T.J.; Whittaker, R.J. On the species abundance distribution in applied ecology and biodiversity management. J. Appl. Ecol. 2015, 52, 443–454. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Chapman, M.G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Sherwin, W.B. Bray-Curtis (AFD) differentiation in molecular ecology: Forecasting, an adjustment (AA), and comparative performance in selection detection. Ecol. Evol. 2022, 12, e9176. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Rempala, G.A.; Seweryn, M. Methods for diversity and overlap analysis in T-cell receptor populations. J. Math. Biol. 2013, 67, 1339–1368. [Google Scholar] [CrossRef]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring biological diversity. Curr. Biol. 2021, 31, R1174–R1177. [Google Scholar] [CrossRef]
- Peet, R.K. The measurement of species diversity. Annu. Rev. Ecol. Syst. 1974, 5, 285–307. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A conceptual guide to measuring species diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.10.7.; CRAN: Windhoek, Namibia, 2019. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons, Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Coddington, J.A.; Agnarsson, I.; Miller, J.A.; Kuntner, M.; Hormiga, G. Undersampling bias: The null hypothesis for singleton species in tropical arthropod surveys. J. Anim. Ecol. 2009, 78, 573–584. [Google Scholar] [CrossRef]
- Anu, A.; Sabu, T.K.; PJ, V. Seasonality of litter insects and relationship with rainfall in a wet evergreen forest in south Western Ghats. J. Insect Sci. 2009, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Elek, Z.; Lövei, G.L. Patterns in ground beetle (Coleoptera: Carabidae) assemblages along an urbanisation gradient in Denmark. Acta Oecol. 2007, 32, 104–111. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, Y.T.; Wu, Q.J.; Wang, S.L.; Xie, W.; Zhang, Y.J. Genome-wide analysis of odorant-binding proteins and chemosensory proteins in the sweet potato whitefly, Bemisia tabaci. Insect Sci. 2019, 26, 620–634. [Google Scholar] [CrossRef]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef]

| Chilopoda | Arachnida | Malacostraca | Collembola | Insecta | Arthropoda | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | N | S | N | S | N | S | N | S | N | S | N | S |
| A1 | 9 | 2 | 3 | 2 | 1 | 1 | 23 | 1 | 1988 | 26 | 2024 | 32 |
| A2 | 10 | 2 | 13 | 10 | 6 | 1 | 7 | 1 | 3927 | 30 | 3963 | 44 |
| A3 | 30 | 2 | 1 | 1 | 5 | 1 | 9 | 1 | 2541 | 25 | 2586 | 30 |
| B1 | 14 | 1 | 5 | 3 | 1 | 1 | 20 | 1 | 1452 | 26 | 1492 | 32 |
| B2 | 179 | 2 | 12 | 9 | 3 | 1 | 31 | 1 | 2124 | 26 | 2349 | 39 |
| B3 | 395 | 1 | 22 | 8 | 3 | 1 | 103 | 1 | 1518 | 40 | 2041 | 51 |
| C1 | 14 | 2 | 6 | 5 | 1 | 1 | 15 | 1 | 1484 | 34 | 1520 | 43 |
| C2 | 13 | 2 | 7 | 5 | 6 | 1 | 8 | 1 | 1867 | 24 | 1901 | 33 |
| C3 | 5 | 2 | 9 | 3 | 0 | 0 | 25 | 1 | 1142 | 27 | 1181 | 33 |
| D1 | 51 | 2 | 18 | 4 | 2 | 1 | 18 | 1 | 5298 | 20 | 5387 | 30 |
| D2 | 107 | 1 | 8 | 5 | 9 | 1 | 58 | 1 | 3840 | 23 | 4022 | 31 |
| D3 | 148 | 1 | 5 | 5 | 2 | 1 | 135 | 1 | 1860 | 21 | 2150 | 29 |
| Total | 975 | 3 | 109 | 22 | 39 | 1 | 452 | 1 | 29,041 | 69 | 30,616 | 96 |
| Distance (m) | N | S | H | D | d | J | ENS1 | ENS2 |
|---|---|---|---|---|---|---|---|---|
| 5 | 336 a | 13 a | 1.285 a | 0.579 a | 2.169 a | 0.512 a | 3.907 a | 2.800 a |
| 10 | 185 a | 12 a | 1.466 ab | 0.611 ab | 2.338 a | 0.596 ab | 5.022 ab | 3.450 ab |
| 15 | 349 a | 13 a | 1.344 a | 0.590 a | 2.268 a | 0.524 a | 4.222 ab | 2.903 a |
| 50 | 321 a | 13 a | 1.314 a | 0.575 a | 2.221 a | 0.526 a | 4.056 ab | 2.954 a |
| 55 | 320 a | 13 a | 1.407 ab | 0.619 ab | 2.291 a | 0.544 ab | 4.374 ab | 3.010 ab |
| 60 | 378 a | 13 a | 1.251 a | 0.579 a | 2.050 a | 0.500 a | 3.733 a | 2.719 a |
| 95 | 244 a | 13 a | 1.536 a | 0.691 ab | 2.230 a | 0.620 ab | 4.932 ab | 3.558 ab |
| 100 | 206 a | 14 a | 1.782 b | 0.770 b | 2.505 a | 0.709 a | 6.053 b | 4.502 b |
| 105 | 213 a | 12 a | 1.553 ab | 0.696 ab | 2.124 a | 0.645 ab | 4.964 ab | 3.681 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Wang, M.; Liu, H.; Shang, W.; Zhou, F.; Cao, H.; Pan, J.; Zeng, Y.; Xu, K.; Li, G. Local Variation in Ground Arthropod Diversity Rises as Distance to Residential Areas Decreases in a Mature Evergreen Forest. Diversity 2025, 17, 344. https://doi.org/10.3390/d17050344
Su J, Wang M, Liu H, Shang W, Zhou F, Cao H, Pan J, Zeng Y, Xu K, Li G. Local Variation in Ground Arthropod Diversity Rises as Distance to Residential Areas Decreases in a Mature Evergreen Forest. Diversity. 2025; 17(5):344. https://doi.org/10.3390/d17050344
Chicago/Turabian StyleSu, Jing, Meili Wang, Hui Liu, Wenqi Shang, Fanfang Zhou, Haochen Cao, Jinwen Pan, Yang Zeng, Kun Xu, and Ganghua Li. 2025. "Local Variation in Ground Arthropod Diversity Rises as Distance to Residential Areas Decreases in a Mature Evergreen Forest" Diversity 17, no. 5: 344. https://doi.org/10.3390/d17050344
APA StyleSu, J., Wang, M., Liu, H., Shang, W., Zhou, F., Cao, H., Pan, J., Zeng, Y., Xu, K., & Li, G. (2025). Local Variation in Ground Arthropod Diversity Rises as Distance to Residential Areas Decreases in a Mature Evergreen Forest. Diversity, 17(5), 344. https://doi.org/10.3390/d17050344






