Noise Pollution and Urban Birds Breeding in the Center of the Iberian Peninsula: Effects on Diversity and Abundance
Abstract
1. Introduction
2. Materials and Methods
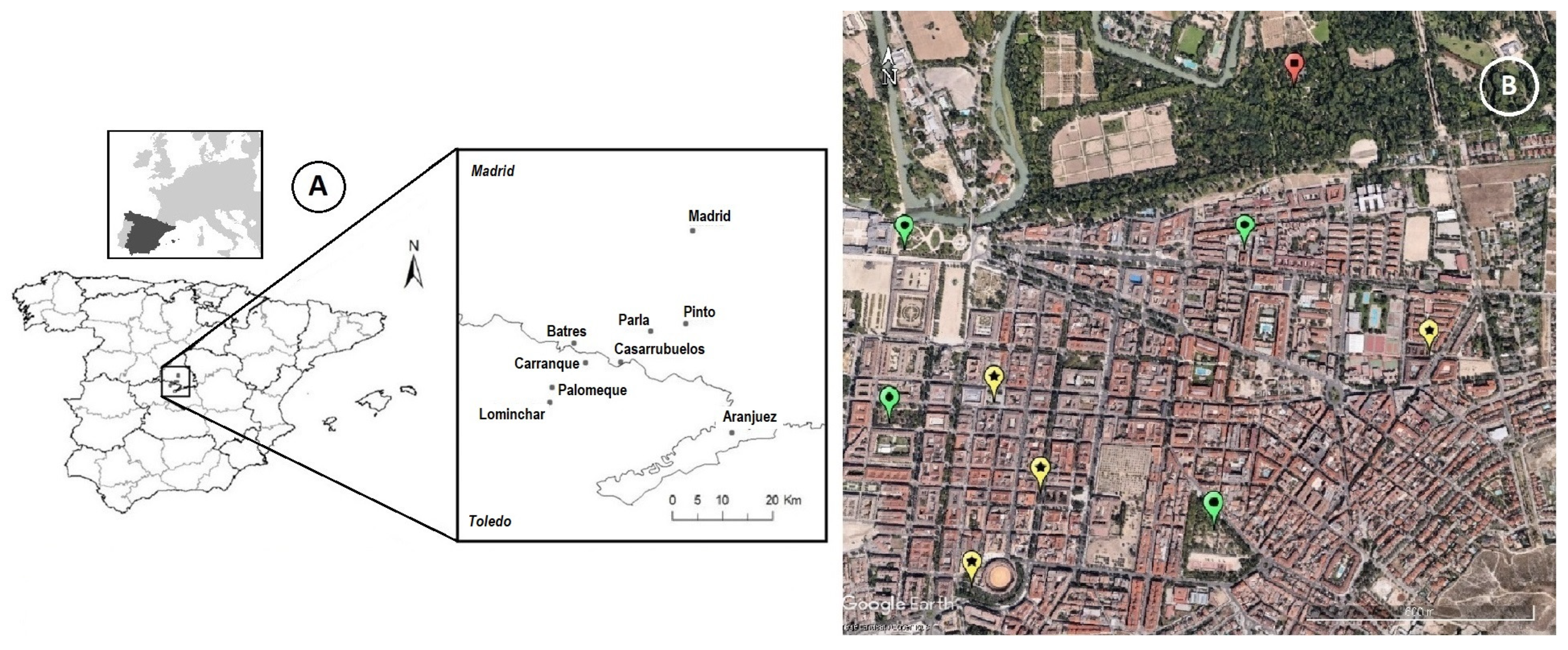
2.1. Study Area
- High level of acoustic pollution, where noise pollution levels were high and continuous: mean 56.1 dB (range: 49–66 dB).
- Medium level of acoustic pollution, where noise pollution levels were medium to low, with peaks of high noise pollution corresponding to the start and end of work, schools, rush hours, etc.: mean 49.6 dB (range: 45–59 dB).
- Low level of acoustic pollution, where noise pollution remained low: mean 43.3 dB (range: 39–48 dB).
2.2. Bird Sampling
2.3. Statistical Analysis
3. Results
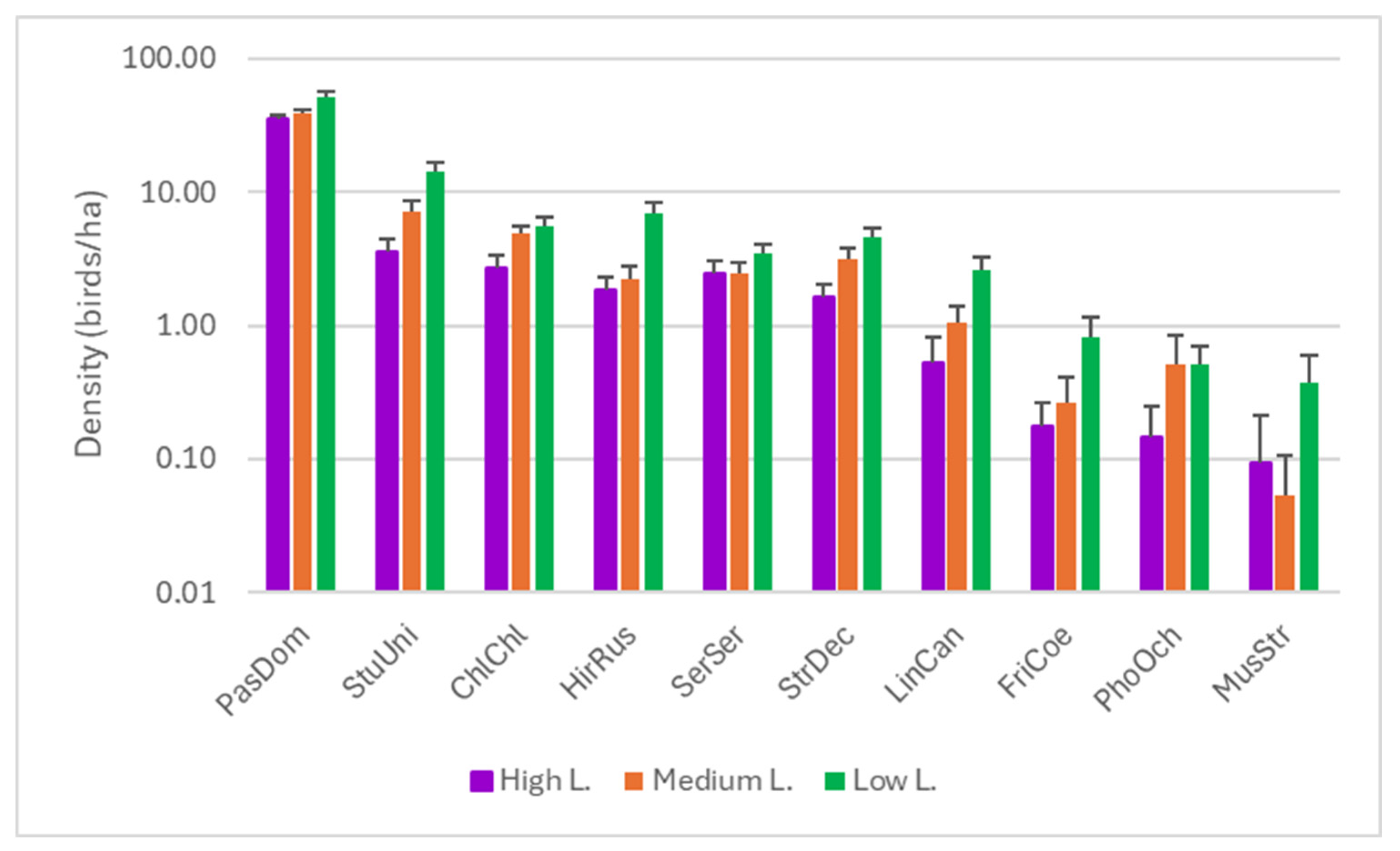
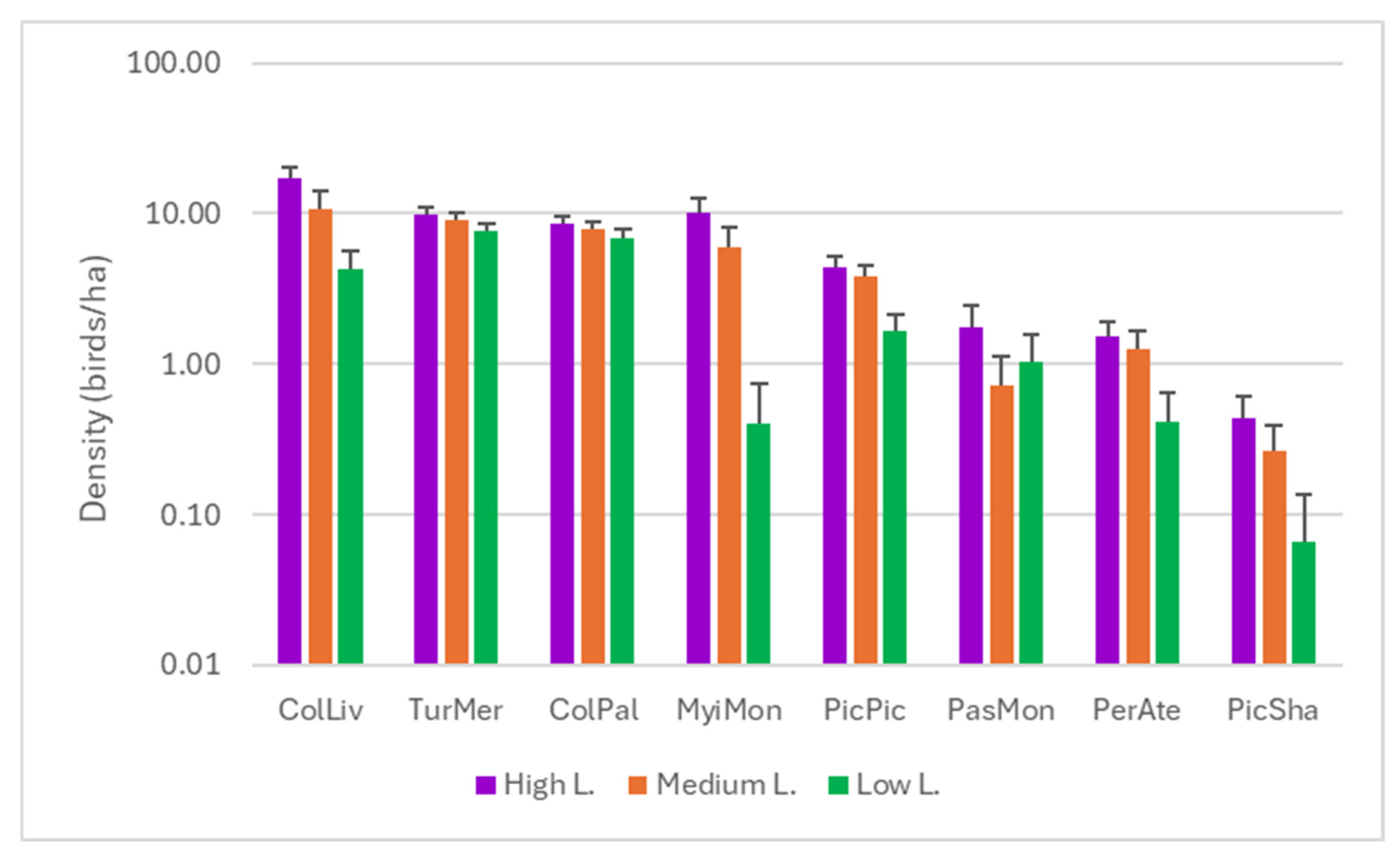
3.1. Species Diversity
3.2. Response to Noise
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| English Name | Scientific Name | C. Status | High Level | Medium Level | Low Level | Total |
|---|---|---|---|---|---|---|
| White Stork | Ciconia ciconia | LC | 4 | 6 | 3 | 13 |
| Western Bonelli’s Warbler | Phylloscopus bonelli | LC | 4 | 5 | 4 | 13 |
| Iberian Chiffchaff | Phylloscopus ibericus | LC | 6 | 4 | 1 | 11 |
| Mallard | Anas platyrhynchos | LC * | 4 | 4 | 2 | 10 |
| European Bee-eater | Merops apiaster | LC | 2 | 1 | 7 | 10 |
| Eurasian Hoopoe | Upupa epops | LC * | 2 | 1 | 7 | 10 |
| Black Kite | Milvus migrans | LC | 2 | 2 | 4 | 8 |
| Iberian Chiffchaff | Phylloscopus collybita | NT | 2 | 2 | 4 | 8 |
| Great Spotted Woodpecker | Dendrocopos major | LC | 2 | 2 | 3 | 7 |
| Common Nightingale | Luscinia megarhynchos | LC | 0 | 1 | 5 | 6 |
| Eurasian Nuthatch | Sitta europaea | LC | 3 | 1 | 2 | 6 |
| Red-rumped Swallow | Cecropis daurica | LC | 0 | 1 | 4 | 5 |
| Zitting Cisticola | Cisticola juncidis | NT | 0 | 2 | 3 | 5 |
| Red Kite | Milvus milvus | EN | 0 | 1 | 4 | 5 |
| Hawfinch | Coccothraustes coccothraustes | LC | 0 | 1 | 3 | 4 |
| Eurasian Wren | Troglodytes troglodytes | LC | 0 | 2 | 2 | 4 |
| Common Reed Warbler | Acrocephalus scirpaceus | LC | 0 | 1 | 2 | 3 |
| Carrion Crow | Corvus corone | LC | 1 | 1 | 1 | 3 |
| Eurasian Golden Oriole | Oriolus oriolus | LC | 1 | 0 | 2 | 3 |
| Rose-ringed Parakeet | Psittacula krameri | ALLOC | 1 | 1 | 1 | 3 |
| European Stonechat | Saxicola rubicola | LC * | 0 | 0 | 3 | 3 |
| Garden Warbler | Sylvia borin | LC | 0 | 1 | 2 | 3 |
| Greylag Goose | Anser anser | LC | 0 | 1 | 1 | 2 |
| Montagu’s Harrier | Circus pygargus | VU * | 0 | 0 | 2 | 2 |
| Corn Bunting | Emberiza calandra | LC * | 0 | 1 | 1 | 2 |
| Peregrine Falcon | Falco peregrinus | NT | 1 | 1 | 0 | 2 |
| Common Kestrel | Falco tinnunculus | EN * | 0 | 0 | 2 | 2 |
| European Crested Tit | Lophophanes cristatus | LC | 0 | 0 | 2 | 2 |
| Spanish Sparrow | Passer hispaniolensis | LC | 1 | 1 | 0 | 2 |
| Common Redstart | Phoenicurus phoenicurus | LC | 1 | 1 | 0 | 2 |
| Common Firecrest | Regulus ignicapilla | LC | 0 | 1 | 1 | 2 |
| Song Thrush | Turdus philomelos | LC | 0 | 0 | 2 | 2 |
| Red-legged Partridge | Alectoris rufa | VU * | 0 | 1 | 0 | 1 |
| Little Owl | Athene noctua | NT * | 0 | 1 | 0 | 1 |
| Muscovy Duck | Cairina moschata | ALLOC | 0 | 0 | 1 | 1 |
| Cetti’s Warbler | Cettia cetti | LC | 0 | 1 | 0 | 1 |
| Lesser Kestrel | Falco naumanni | VU | 0 | 1 | 0 | 1 |
| Common Moorhen | Gallinula chloropus | LC | 0 | 0 | 1 | 1 |
| Griffon Vulture | Gyps fulvus | LC | 0 | 0 | 1 | 1 |
| Woodchat Shrike | Lanius senator | EN | 0 | 1 | 0 | 1 |
| Red Crossbill | Loxia curvirostra | LC | 0 | 1 | 0 | 1 |
| Great Cormorant | Phalacrocorax carbo | LC | 0 | 1 | 0 | 1 |
| Wood Warbler | Phylloscopus sibilatrix | DD | 0 | 1 | 0 | 1 |
| Willow Warbler | Phylloscopus trochilus | DD | 0 | 1 | 0 | 1 |
| Common Whitethroat | Sylvia communis | LC * | 0 | 0 | 1 | 1 |
| Dartford Warbler | Curruca undata | EN | 0 | 1 | 0 | 1 |
| Common Swift | Apus apus | VU * | E | E | E | E |
| Pallid Swift | Apus pallidus | LC | E | E | E | E |
References
- United Nations Department of Economic and Social Affairs. The World’s Cities in 2018—Data Booklet; United Nations: New York, NY, USA, 2018. [Google Scholar]
- Lepczyk, C.A.; La Sorte, F.A.; Aronson, M.F.J.; Goddard, M.A.; MacGregor-Fors, I.; Nilon, C.H.; Warren, P.S. Global Patterns and Drivers of Urban Bird Diversity. In Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 13–33. ISBN 9783319433141. [Google Scholar]
- Murgui, E.; Hedblom, M. Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-43312-7. [Google Scholar]
- Ditchkoff, S.; Saalfeld, S.; Gibson, C. Animal Behavior in Urban Ecosystems: Modifications Due to Human-Induced Stress. Urban Ecosyst. 2006, 9, 5–12. [Google Scholar] [CrossRef]
- Díaz, M.; Ramos, A.; Concepción, E.D. Changing Urban Bird Diversity: How to Manage Adaptively Our Closest Relation with Wildlife. Ecosistemas 2022, 31, 2354. [Google Scholar] [CrossRef]
- Soifer, L.G.; Donovan, S.K.; Brentjens, E.T.; Bratt, A.R. Piecing Together Cities to Support Bird Diversity: Development and Forest Edge Density Affect Bird Richness in Urban Environments. Landsc. Urban Plan. 2021, 213, 104122. [Google Scholar] [CrossRef]
- Chace, J.F.; Walsh, J.J. Urban Effects on Native Avifauna: A Review. Landsc. Urban Plan. 2006, 74, 46–69. [Google Scholar] [CrossRef]
- Balmori, A.; Hallberg, Ö. The Urban Decline of the House Sparrow (Passer Domesticus): A Possible Link with Electromagnetic Radiation. Electromagn. Biol. Med. 2007, 26, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Marzluff, J.M. A Decadal Review of Urban Ornithology and a Prospectus for the Future. Ibis 2016, 159, 1–13. [Google Scholar] [CrossRef]
- Bernat-Ponce, E.; Gil-Delgado, J.A.; López-Iborra, G.M. Efectos de Las Características de Las Ciudades Occidentales Contemporáneas Sobre La Avifauna Urbana. Ecosistemas 2022, 31, 2158. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Ripmeester, E.A.P. Birdsong and Anthropogenic Noise: Implications and Applications for Conservation. Mol. Ecol. 2008, 17, 72–83. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the City: Can Anyone Become an “Urban Exploiter”? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Kaisanlahti-Jokimäki, M.L. Urbanization and Species Occupancy Frequency Distribution Patterns in Core Zone Areas of European Towns. Eur. J. Ecol. 2016, 2, 23–43. [Google Scholar] [CrossRef]
- Nemeth, E.; Brumm, H. Birds and Anthropogenic Noise: Are Urban Songs Adaptive? Am. Nat. 2010, 176, 465–475. [Google Scholar] [CrossRef]
- Slabbekoorn, H. Singing in the Wild: The Ecology of Birdsong. In Nature’s Music; Marler, P., Slabbekoorn, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 178–205. ISBN 9780124730700. [Google Scholar]
- Warren, P.S.; Katti, M.; Ermann, M.; Brazel, A. Urban Bioacoustics: It’s Not Just Noise. Anim. Behav. 2006, 71, 491–502. [Google Scholar] [CrossRef]
- Parris, K.M.; Schneider, A. Impacts of Traffic Noise and Traffic Volume on Birds of Roadside Habitats. Ecol. Soc. 2008, 14, 29. [Google Scholar] [CrossRef]
- Slabbekoorn, H. Songs of the City: Noise-Dependent Spectral Plasticity in the Acoustic Phenotype of Urban Birds. Anim. Behav. 2013, 85, 1089–1099. [Google Scholar] [CrossRef]
- Patricelli, G.L.; Blickley, J.L. Avian Communication in Urban Noise: Causes and Consequences of Vocal Adjustment. Auk 2006, 123, 639–649. [Google Scholar] [CrossRef]
- Oden, A.I.; Brandle, J.R.; Burbach, M.E.; Brown, M.B.; Gerber, J.E.; Quinn, J.E. Soundscapes and Anthromes: A Review of Proximate Effects of Traffic Noise on Avian Vocalization and Communication. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 203–208. ISBN 9780128160978. [Google Scholar]
- Brumm, H. The Impact of Environmental Noise on Song Amplitude in a Territorial Bird. J. Anim. Ecol. 2004, 73, 434–440. [Google Scholar] [CrossRef]
- Eens, M.; Rivera-Gutierrez, H.F.; Pinxten, R. Are Low-Frequency Songs Sexually Selected, and Do They Lose Their Potency in Male-Female Interactions under Noisy Conditions? Proc. Natl. Acad. Sci. USA 2012, 109, 1. [Google Scholar] [CrossRef] [PubMed]
- Gil, D.; Honarmand, M.; Pascual, J.; Pérez-Mena, E.; Macías Garcia, C. Birds Living near Airports Advance Their Dawn Chorus and Reduce Overlap with Aircraft Noise. Behav. Ecol. 2014, 26, 435–443. [Google Scholar] [CrossRef]
- Sweet, K.A.; Sweet, B.P.; Gomes, D.G.E.; Francis, C.D.; Barber, J.R. Natural and Anthropogenic Noise Increase Vigilance and Decrease Foraging Behaviors in Song Sparrows. Behav. Ecol. 2022, 33, 288–297. [Google Scholar] [CrossRef]
- Wiacek, J.; Polak, M.; Kucharczyk, M.; Bohatkiewicz, J. The Influence of Road Traffic on Birds during Autumn Period: Implications for Planning and Management of Road Network. Landsc. Urban Plan. 2015, 134, 76–82. [Google Scholar] [CrossRef]
- Ghadiri Khanaposhtani, M.; Gasc, A.; Francomano, D.; Villanueva-Rivera, L.J.; Jung, J.; Mossman, M.J.; Pijanowski, B.C. Effects of Highways on Bird Distribution and Soundscape Diversity around Aldo Leopold’s Shack in Baraboo, Wisconsin, USA. Landsc. Urban Plan. 2019, 192, 13. [Google Scholar] [CrossRef]
- SEO/BirdLife. Tendencia de Las Aves En Primavera. SACRE Resultados 1998–2013. (Bird Trend in Spring. SACRE Results 1998–2013.); SEO/BirdLife: Madrid, Spain, 2013. [Google Scholar]
- SEO/BirdLife. Programas de Seguimiento de Avifauna y Grupos de Trabajo de SEO/BirdLife 2018; SEO/BirdLife: Madrid, Spain, 2019. [Google Scholar]
- Mendes, S.; Cavalcante, K.; Colino Rabanal, V.; Peris, S.J. Evaluación Del Impacto de La Contaminación Acústica En El Rango de Vocalización de Paseriformes Basado En El SIL-“Speech Interference Level”. Rev. Acúst. 2010, 41, 33–41. [Google Scholar]
- Francis, C.D.; Ortega, C.P.; Cruz, A. Noise Pollution Filters Bird Communities Based on Vocal Frequency. PLoS ONE 2011, 6, 8. [Google Scholar] [CrossRef]
- Fuller, R.A.; Warren, P.H.; Gaston, K.J. Daytime Noise Predicts Nocturnal Singing in Urban Robins. Biol. Lett. 2007, 3, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Mockford, E.J.; Marshall, R.C. Effects of Urban Noise on Song and Response Behaviour in Great Tits. Proc. R. Soc. B Biol. Sci. 2009, 276, 2979–2985. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A.; Mustoe, S.H. Birds Census Techniques, 2nd ed.; Elsevier: London, UK, 2000. [Google Scholar]
- Soberón, J.; Llorente, J. The Use of Species Accumulation Functions for the Prediction of Species Richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating Terrestrial Biodiversity through Extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Quantifying Biodiversity: Procedures and Pitfalls in the Measurement and Comparison of Species Richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Estimating Species Richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 39–54. [Google Scholar]
- Quesada, J.; MacGregor-Fors, I. Avian Community Responses to the Establishment of Small Garden Allotments within a Mediterranean Habitat Mosaic. Anim. Biodivers. Conserv. 2010, 33, 53–61. [Google Scholar] [CrossRef]
- Shiu, H.; Lee, P. Assessing Avian Point-Count Duration and Sample Size Using Species Accumulation Functions. Zool. Stud. 2003, 42, 357–367. [Google Scholar]
- Sokal, R.; Rohlf, F. Biometry. The Principles and Practice of Statistics in Biological Research; W.H. Freeman: New York, NY, USA, 1994. [Google Scholar]
- SEO/BirdLife. Programas de Seguimiento y Grupos de Trabajo de SEO/BirdLife 2021; SEO/Birdlife: Madrid, Spain, 2022. [Google Scholar]
- SEO/BirdLife. Libro Rojo de Las Aves de España 2021; López-Jiménez, N., Ed.; SEO/Birdlife: Madrid, Spain, 2021. [Google Scholar]
- Caula, S.A.; Sirami, C.; Marty, P.; Martin, J.-L. Value of an Urban Habitat for the Native Mediterranean Avifauna. Urban Ecosyst. 2010, 13, 73–89. [Google Scholar] [CrossRef]
- Patón, D.; Romero, F.; Cuenca, J.; Escudero, J.C. Tolerance to Noise in 91 Bird Species from 27 Urban Gardens of Iberian Peninsula. Landsc. Urban Plan. 2012, 104, 1–8. [Google Scholar] [CrossRef]
- Carral-Murrieta, C.O.; García-Arroyo, M.; Marín-Gómez, O.H.; Sosa-López, J.R.; Macgregor-Fors, I. Noisy Environments: Untangling the Role of Anthropogenic Noise on Bird Species Richness in a Neotropical City. Avian Res. 2020, 11, 32. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities Are Hotspots for Threatened Species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Sorace, A.; Gustin, M. Species Richness and Species of Conservation Concern in Parks of Italian Towns. In Ecology and Conservation of Birds in Urban Environments; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 425–448. ISBN 9783319433141. [Google Scholar]
- Jokimäki, J.; Jukka, S.; Marja-Liisa, K.J. Urban Core Areas Are Important for Species Conservation: A European-Level Analysis of Breeding Bird Species. Landsc. Urban Plan. 2018, 178, 73–81. [Google Scholar] [CrossRef]
- Villaseñor, N.R.; Chiang, L.A.; Hernández, H.J.; Escobar, M.A.H. Vacant Lands as Refuges for Native Birds: An Opportunity for Biodiversity Conservation in Cities. Urban For. Urban Green. 2020, 49, 10. [Google Scholar] [CrossRef]
- Fernández-Juricic, E. Avian Spatial Segregation at Edges and Interiors of Urban Parks in Madrid, Spain. Biodivers. Conserv. 2001, 10, 1303–1316. [Google Scholar] [CrossRef]
- Kontsiotis, V.J.; Valsamidis, E.; Liordos, V. Organization and Differentiation of Breeding Bird Communities across a Forested to Urban Landscape. Urban For. Urban Green. 2019, 38, 242–250. [Google Scholar] [CrossRef]
- Morelli, F.; Reif, J.; Díaz, M.; Tryjanowski, P.; Diego Ibáñez-Álamo, J.; Suhonen, J.; Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L.; Pape Møller, A.; Bussiere, R.; et al. Top Ten Birds Indicators of High Environmental Quality in European Cities. Ecol. Indic. 2021, 133, 108397. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Blair, R.B. Birds and Butterflies Along Urban Gradients in Two Ecoregions of the United States: Is Urbanization Creating a Homogeneous Fauna? In Biotic Homogenization; Lockwood, J.L., McKinney, M.L., Eds.; Springer: Boston, MA, USA, 2001; pp. 33–56. [Google Scholar]
- Molina, B.; Postigo, J.L.; Muñoz, A.R.; Del Moral, J.C. La Cotorra Argentina En España, Población Reproductora En 2015 y Método de Censo; SEO/Birdlife: Madrid, Spain, 2016. [Google Scholar]
- Herrera-Montes, M.I.; Aide, T.M. Impacts of Traffic Noise on Anuran and Bird Communities. Urban Ecosyst. 2011, 14, 415–427. [Google Scholar] [CrossRef]
- Peris, S.J.; Pescador, M. Effects of Traffic Noise on Paserine Populations in Mediterranean Wooded Pastures. Appl. Acoust. 2004, 65, 357–366. [Google Scholar] [CrossRef]
- Brumm, H.; Slabbekoorn, H. Acoustic Communication in Noise. Adv. Study Behav. 2005, 35, 151–209. [Google Scholar] [CrossRef]
- Marler, P.; Slabbekoorn, H. Nature’s Music: The Science of Birdsong; Marler, P., Slabbekoorn, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780124730700. [Google Scholar]
- Hu, Y.; Cardoso, G.C. Which Birds Adjust the Frequency of Vocalizations in Urban Noise? Anim. Behav. 2010, 79, 863–867. [Google Scholar] [CrossRef]
- Dominoni, D.; Smit, J.A.H.; Visser, M.E.; Halfwerk, W. Multisensory Pollution: Artificial Light at Night and Anthropogenic Noise Have Interactive Effects on Activity Patterns of Great Tits (Parus Major). Environ. Pollut. 2020, 256, 113314. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.; Colino-Rabanal, V.; Peris, S. Bird Song Variations along an Urban Gradient: The Case of the European Blackbird (Turdus Merula). Landsc. Urban Plan. 2011, 99, 51–57. [Google Scholar] [CrossRef]
- Sierro, J.; Schloesing, E.; Pavón, I.; Gil, D. European Blackbirds Exposed to Aircraft Noise Advance Their Chorus, Modify Their Song and Spend More Time Singing. Front. Ecol. Evol. 2017, 5, 68. [Google Scholar] [CrossRef]
- McMullen, H.; Schmidt, R.; Kunc, H.P. Anthropogenic Noise Affects Vocal Interactions. Behav. Process. 2014, 103, 125–128. [Google Scholar] [CrossRef]
- Polak, M. Relationship between Traffic Noise Levels and Song Perch Height in a Common Passerine Bird. Transp. Res. D Transp. Environ. 2014, 30, 72–75. [Google Scholar] [CrossRef]
- Arroyo-Solís, A.; Castillo, J.M.; Figueroa, E.; López-Sánchez, J.L.; Slabbekoorn, H. Experimental Evidence for an Impact of Anthropogenic Noise on Dawn Chorus Timing in Urban Birds. J. Avian Biol. 2013, 44, 288–296. [Google Scholar] [CrossRef]
- Sheldon, E.L.; Ironside, J.E.; de Vere, N.; Marshal, R.C. Singing under Glass: Rapid Effects of Anthropogenic Habitat Modification on Song and Response Behaviours in an Isolated House Sparrow Passer Domesticus Population. J. Avian Biol. 2020, 51, 1–8. [Google Scholar] [CrossRef]
- Derryberry, E.P.; Phillips, J.N.; Derryberry, G.E.; Blum, M.J.; Luther, D. Singing in a Silent Spring: Birds Respond to a Half-Century Soundscape Reversion during the COVID-19 Shutdown. Science 2020, 370, 575–579. [Google Scholar] [CrossRef]
- De Framond, L.; Brumm, H. Long-Term Effects of Noise Pollution on the Avian Dawn Chorus: A Natural Experiment Facilitated by the Closure of an International Airport. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220906. [Google Scholar] [CrossRef] [PubMed]
- Ripmeester, E.A.P.; Mulder, M.; Slabbekoorn, H. Habitat-Dependent Acoustic Divergence Affects Playback Response in Urban and Forest Populations of the European Blackbird. Behav. Ecol. 2010, 21, 876–883. [Google Scholar] [CrossRef]
- Moseley, D.L.; Phillips, J.N.; Derryberry, E.P.; Luther, D.A. Evidence for Differing Trajectories of Songs in Urban and Rural Populations. Behav. Ecol. 2019, 30, 1734–1742. [Google Scholar] [CrossRef]
- Raiter, K.G.; Possingham, H.P.; Prober, S.M.; Hobbs, R.J. Under the Radar: Mitigating Enigmatic Ecological Impacts. Trends Ecol. Evol. 2014, 29, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.; Marzluff, J.M.; Shulenberger, E.; Bradley, G.; Ryan, C.; Zumbrunnen, C. Integrating Humans into Ecology: Opportunities and Challenges for Studying Urban Ecosystems. Bioscience 2003, 53, 1169–1179. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for Conserving Urban Biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Savard, J.P.L.; Clergeau, P.; Mennechez, G. Biodiversity Concepts and Urban Ecosystems. Landsc. Urban Plan. 2000, 48, 131–142. [Google Scholar] [CrossRef]
- Ikin, K.; Le Roux, D.S.; Rayner, L.; Villaseñor, N.R.; Eyles, K.; Gibbons, P.; Manning, A.D.; Lindenmayer, D.B. Key Lessons for Achieving Biodiversity-Sensitive Cities and Towns. Ecol. Manag. Restor. 2015, 16, 206–214. [Google Scholar] [CrossRef]
- Fernández Calvo, I.C. 100 Medidas Para La Conservación de La Biodiversidad En Entornos Urbanos; SEO/BirdLife: Madrid, Spain, 2019. [Google Scholar]
- Fernández-Juricic, E.; Jokimäki, J. A Habitat Island Approach to Conserving Birds in Urban Landscapes: Case Studies from Southern and Northern Europe. Biodivers. Conserv. 2001, 10, 2023–2043. [Google Scholar] [CrossRef]
- White, J.G.; Antos, M.J.; Fitzsimons, J.A.; Palmer, G.C. Non-Uniform Bird Assemblages in Urban Environments: The Influence of Streetscape Vegetation. Landsc. Urban Plan. 2005, 71, 123–135. [Google Scholar] [CrossRef]
- World Health Organization. Urban Green Spaces and Health. A Review of Evidence; World Health Organization: Copenhagen, Denmark, 2016. [Google Scholar]
- Swartz, T.M.; Gleditsch, J.M.; Behm, J.E. A Functional Trait Approach Reveals the Effects of Landscape Context on Ecosystem Services Provided by Urban Birds. Landsc. Urban Plan. 2023, 234, 104724. [Google Scholar] [CrossRef]
- Pollack, L.; Ondrasek, N.R.; Calisi, R. Urban Health and Ecology: The Promise of an Avian Biomonitoring Tool. Curr. Zool. 2017, 63, 205–212. [Google Scholar] [CrossRef]

| English Name | Scientific Name | C. Status | High Level | Medium Level | Low Level | Total |
|---|---|---|---|---|---|---|
| Common Wood Pigeon | Columba palumbus | LC | 24 | 24 | 24 | 72 |
| Common Blackbird | Turdus merula | LC | 24 | 24 | 24 | 72 |
| House Sparrow | Passer domesticus | LC * | 24 | 24 | 24 | 72 |
| Spotless Starling | Sturnus unicolor | LC | 18 | 24 | 24 | 66 |
| European Greenfinch | Chloris chloris | LC | 21 | 23 | 22 | 66 |
| Eurasian Magpie | Pica pica | LC * | 23 | 23 | 18 | 64 |
| European Goldfinch | Carduelis carduelis | LC | 20 | 22 | 21 | 63 |
| European Serin | Serinus serinus | LC * | 19 | 20 | 22 | 61 |
| Barn Swallow | Hirundo rustica | VU * | 16 | 22 | 20 | 58 |
| Eurasian Collared Dove | Streptopelia decaocto | LC | 15 | 20 | 20 | 55 |
| Rock Dove | Columba livia | LC * | 21 | 19 | 13 | 53 |
| Common House Martin | Delichon urbicum | LC | 12 | 21 | 19 | 52 |
| Common Linnet | Linaria cannabina | LC * | 12 | 19 | 19 | 50 |
| Eurasian Blue Tit | Cyanistes caerueleus | LC | 19 | 16 | 8 | 43 |
| Great Tit | Parus major | LC | 16 | 14 | 10 | 40 |
| Coal Tit | Periparus ater | LC | 18 | 15 | 5 | 38 |
| White Wagtail | Motacilla alba | LC * | 12 | 15 | 7 | 34 |
| Monk Parakeet | Myiopsitta monachus | ALLOC | 19 | 11 | 3 | 33 |
| European Robin | Erithacus rubecula | LC | 12 | 12 | 8 | 32 |
| Black Redstart | Phoenicurus ochruros | LC | 6 | 11 | 12 | 29 |
| Common Chaffinch | Fringilla coelebs | LC | 8 | 12 | 8 | 28 |
| Eurasian Blackcap | Sylvia atricapilla | LC | 9 | 8 | 9 | 26 |
| Short-toed Treecreeper | Certhia brachydactyla | LC | 13 | 6 | 6 | 25 |
| Stock Dove | Columba oenas | LC | 9 | 6 | 7 | 22 |
| Sardinian Warbler | Sylvia melanocephala | LC | 7 | 8 | 7 | 22 |
| Eurasian Tree Sparrow | Passer montanus | NT * | 10 | 5 | 7 | 22 |
| Iberian Green Woodpecker | Picus sharpei | LC * | 9 | 5 | 2 | 16 |
| European Pied Flycatcher | Ficedula hypoleuca | LC | 4 | 7 | 5 | 16 |
| Long-tailed Tit | Aegithalos caudatus | LC | 3 | 4 | 4 | 11 |
| Spotted Flycatcher | Muscicapa striata | LC | 2 | 2 | 6 | 10 |
| Western Jackdaw | Corvus monedula | EN * | 2 | 3 | 3 | 8 |
| Crested Lark | Galerida cristata | LC * | 0 | 2 | 5 | 7 |
| Mistle Thrush | Turdus viscivorus | LC | 0 | 2 | 5 | 7 |
| Species | H | p | High–Low | High–Medium | Medium–Low | |||
|---|---|---|---|---|---|---|---|---|
| H | p | H | p | H | p | |||
| Chloris chloris | 41.242 | <0.001 | −116.04 | <0.001 | −121.268 | <0.001 | 5.228 | ns |
| Fringilla coelebs | 12.965 | 0.002 | −32.284 | <0.001 | −6.079 | ns | −26.204 | 0.006 |
| Hirundo rustica | 103.128 | <0.001 | −186.353 | <0.001 | −32.721 | ns | −153.632 | <0.001 |
| Linaria cannabina | 48.4 | <0.001 | −101.727 | <0.001 | −40.32 | 0.006 | −61.406 | <0.001 |
| Muscicapa striata | 9.351 | 0.009 | −15.057 | 0.008 | 0.034 | ns | −15.091 | 0.008 |
| Passer domesticus | 17.486 | <0.001 | −96.139 | <0.001 | −23.732 | ns | −72.408 | 0.003 |
| Phoenicurus ochruros | 15.315 | <0.001 | −37.286 | <0.001 | −18.221 | ns | −19.065 | 0.045 |
| Serinus serinus | 9.603 | 0.008 | −54.047 | 0.004 | −8.738 | ns | −45.039 | 0.016 |
| Streptopelia decaocto | 34.98 | <0.001 | −109.415 | <0.001 | −77.382 | <0.001 | −32.034 | ns |
| Sturnus unicolor | 114.837 | <0.001 | −230.449 | <0.001 | −122.383 | <0.001 | −108.066 | <0.001 |
| Species | H | p | High–Low | High–Medium | Medium–Low | |||
|---|---|---|---|---|---|---|---|---|
| H | p | H | p | H | p | |||
| Columba livia | 104.682 | <0.001 | 201.109 | <0.001 | 132.379 | <0.001 | 68.73 | <0.001 |
| Columba palumbus | 7.883 | 0.019 | 63.723 | 0.005 | 38.488 | ns | 25.234 | ns |
| Myiopsitta monachus | 155.597 | <0.001 | 207.849 | <0.001 | 110.104 | <0.001 | 97.745 | <0.001 |
| Passer montanus | 12.597 | 0.002 | 27.934 | 0.008 | 35.703 | <0.001 | −7.77 | ns |
| Periparus ater | 36.389 | <0.001 | 79.773 | <0.001 | 23.836 | ns | 55.938 | <0.001 |
| Pica pica | 65.316 | <0.001 | 154.809 | <0.001 | 37.082 | ns | 117.727 | <0.001 |
| Picus sharpei | 17.168 | <0.001 | 34.521 | <0.001 | 13.604 | ns | 20.917 | 0.013 |
| Turdus merula | 13.5 | 0.001 | 85.383 | <0.001 | 36.145 | ns | 49.238 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almarza-Batuecas, P.; Pescador, M. Noise Pollution and Urban Birds Breeding in the Center of the Iberian Peninsula: Effects on Diversity and Abundance. Diversity 2025, 17, 338. https://doi.org/10.3390/d17050338
Almarza-Batuecas P, Pescador M. Noise Pollution and Urban Birds Breeding in the Center of the Iberian Peninsula: Effects on Diversity and Abundance. Diversity. 2025; 17(5):338. https://doi.org/10.3390/d17050338
Chicago/Turabian StyleAlmarza-Batuecas, Paula, and Moisés Pescador. 2025. "Noise Pollution and Urban Birds Breeding in the Center of the Iberian Peninsula: Effects on Diversity and Abundance" Diversity 17, no. 5: 338. https://doi.org/10.3390/d17050338
APA StyleAlmarza-Batuecas, P., & Pescador, M. (2025). Noise Pollution and Urban Birds Breeding in the Center of the Iberian Peninsula: Effects on Diversity and Abundance. Diversity, 17(5), 338. https://doi.org/10.3390/d17050338






