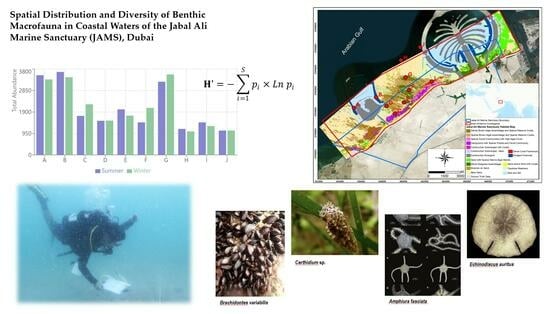
Spatial Distribution and Diversity of Benthic Macrofauna in Coastal Waters of the Jabal Ali Marine Sanctuary (JAMS), Dubai
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Analysis
2.3. Univariate Indices
3. Results
3.1. Environmental Parameters
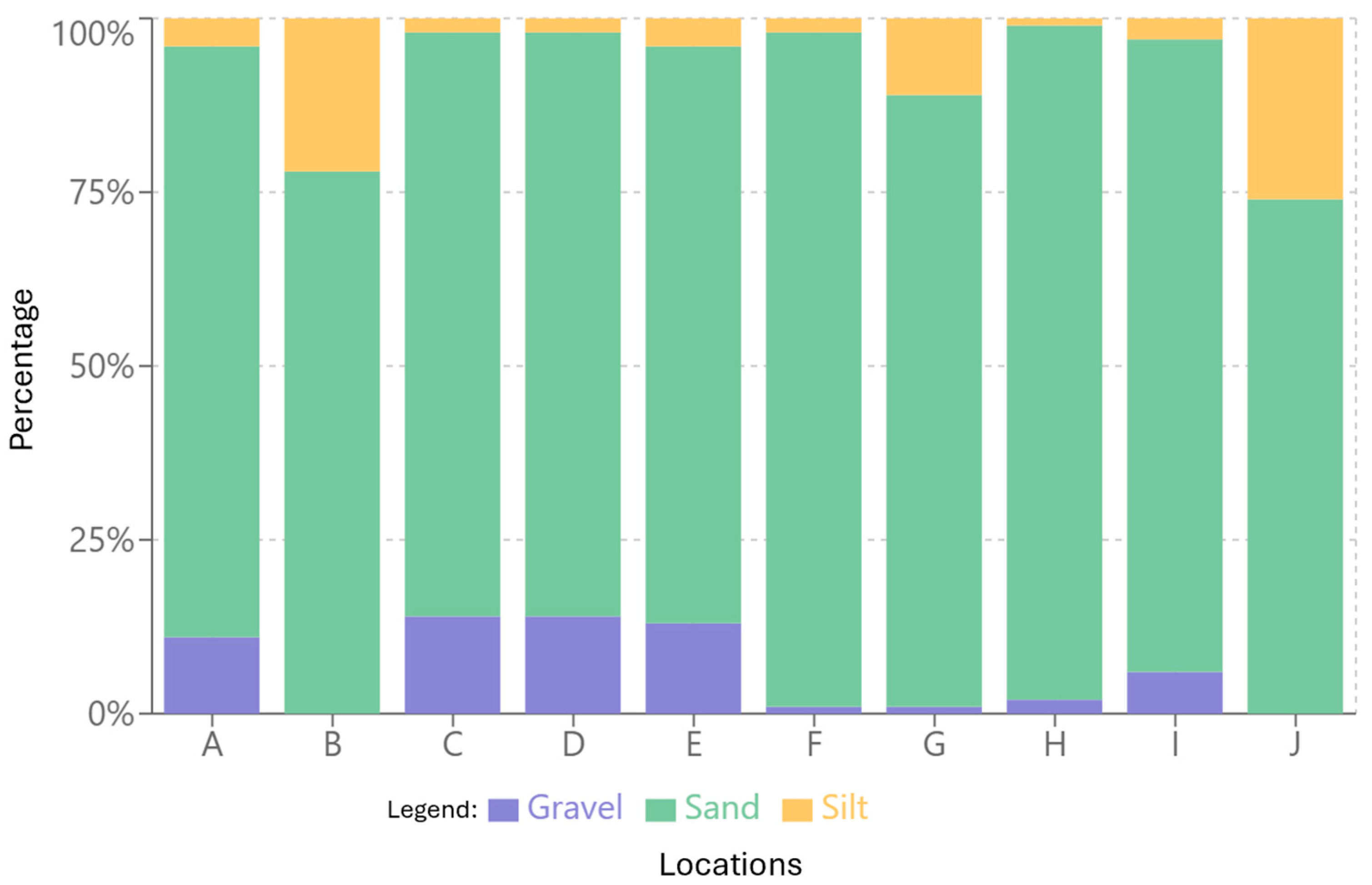
3.2. Sediment Characterization
3.3. Sediment Quality Assessment
3.4. Species Richness and Diversity
3.4.1. Species Richness
3.4.2. Diversity
3.5. Spatial Distribution
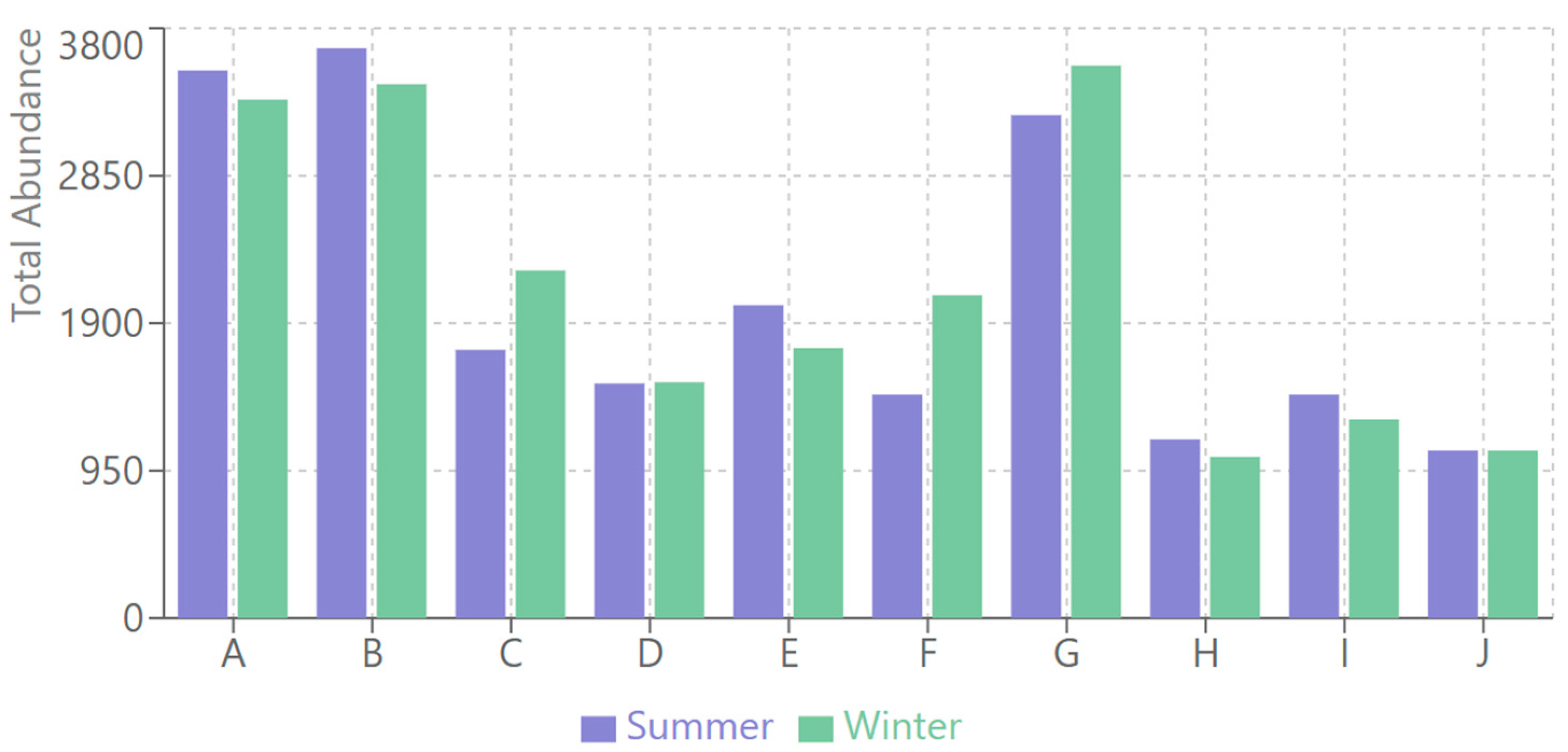
3.5.1. Abundance
3.5.2. Cluster Analysis
3.6. Relationship Between Environmental Parameters and Benthic Communities
4. Discussion
5. Conclusions
- Expanding sampling to include additional seasons to better understand annual cycles;
- Implementing continuous monitoring of key environmental parameters;
- Conducting targeted studies on dominant species’ physiological adaptations to extreme conditions;
- Establishing permanent monitoring stations at the identified diversity hotspots (stations E and F);
- Developing region-specific biotic indices calibrated to the extreme conditions of the Arabian Gulf.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Hamza, W.; Munawar, M. Protecting and managing the Arabian Gulf: Past, present and future. Aquat. Ecosyst. Health Manag. 2009, 12, 429–439. [Google Scholar] [CrossRef]
- Borja, Á.; Marín, S.L.; Muxika, I.; Pino, L.; Rodríguez, J.G. Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar. Pollut. Bull. 2015, 97, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.P.; Bernard, A.T.F.; Lombard, A.T.; Sink, K.J. Assessing marine ecosystem condition: A review to support indicator choice and framework development. Ecol. Indic. 2021, 121, 107148. [Google Scholar] [CrossRef]
- McCall, P. Community patterns and adaptive strategies of the infaunal benthos of Long Island Sound. J. Mar. Res. 1977, 35, 221–265. [Google Scholar]
- Rosenberg, R. Effects of dredging operation on estuarine benthic macrofauna. Mar. Pollut. Bull. 1977, 8, 102–104. [Google Scholar] [CrossRef]
- De Groot, S.J. The physical impact of marine aggregate extraction in the North Sea. ICES J. Mar. Sci. 1996, 53, 1051–1053. [Google Scholar] [CrossRef]
- Newell, R.C.; Seiderer, L.J.; Hitchcock, D.R. The impact of dredging works in coastal waters: A review of the sensitivity to disturbance and subsequent recovery of biological resources on the sea bed. Oceanogr. Mar. Biol. Annu. Rev. 1998, 36, 127–178. [Google Scholar]
- Desprez, M. Physical and biological impact of marine aggregate extraction along the French coast of the eastern English Channel: Short- and long-term post-dredging restoration. ICES J. Mar. Sci. 2000, 57, 1428–1438. [Google Scholar] [CrossRef]
- Lopez-Jamar, E.; Mejuto, J. Infaunal benthic recolonization after dredging operations in La Coruna Bay, NW Spain. Cah. Biol. Mar. 1988, 29, 37–49. [Google Scholar]
- Bonsdorff, E. Recovery potential of macrozoobenthos from dredging in shallow brackish waters. In Fluctuation and Succession in Marine Ecosystems, Proceedings of the 17th European Marine Biology Symposium, Brest, France, 27 September–1 October 1982; Cabioch, L., Glemarec, M., Samains, J.F., Eds.; Oceanologica Acta; Gauthier-Villars: Paris, France, 1983; pp. 27–32. [Google Scholar]
- Vandalfsen, J.; Essink, K.; Toxvigmadsen, H.; Birklund, J.; Romero, J.; Manzanera, M. Differential response of macrozoobenthos to marine sand extraction in the North Sea and the Western Mediterranean. ICES J. Mar. Sci. 2000, 57, 1439–1445. [Google Scholar] [CrossRef]
- Lowe, R.L.; LaLiberte, G.D. Benthic Stream Algae. Methods Stream Ecol. 2017, 1, 193–221. [Google Scholar]
- Tong, R.; Yesson, C.; Yu, J.; Luo, Y.; Zhang, L. Key factors for species distribution modeling in benthic marine environments. Front. Mar. Sci. 2023, 10, 1222382. [Google Scholar] [CrossRef]
- Muñoz, P.T.; Rodrıguez-Rojas, F.; Celis-Pla, P.S.M.; Lopez-Marras, A.; Blanco-Murillo, F.; Sola, I.; Lavergne, C.; Valenzuela, F.; Orrego, R.; Sanchez-Lizaso, J.L.; et al. Desalination effects on macroalgae (part A): Laboratory-controlled experiments with Dictyota spp. from the Pacific Ocean and Mediterranean Sea. Front. Mar. Sci. 2023, 10, 1042782. [Google Scholar] [CrossRef]
- Burger, J. Bioindicators: Types, Development, and Use in Ecological Assessment and Research. Environ. Bioindic. 2006, 1, 22–39. [Google Scholar] [CrossRef]
- Holt, E.; Miller, S. Bioindicators: Using Organisms to Measure Environmental Impacts. Nat. Educ. Knowl. 2010, 3, 8–13. [Google Scholar]
- Parmar, T.K.; Rawtani, D.; Agrawal, Y.K. Bioindicators: The natural indicator of environmental pollution. Front. Life Sci. 2016, 9, 110–118. [Google Scholar] [CrossRef]
- Lu, L. The relationship between soft-bottom macrobenthic communities and environmental variables in Singaporean waters. Mar. Pollut. Bull. 2005, 51, 1034–1040. [Google Scholar] [CrossRef]
- Gudencio, M.J.; Cabral, H.N. Trophic structure of macrobenthos in the Tagus estuary and adjacent coastal shelf. Hydrobiologia 2007, 587, 241–251. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.; Wang, H.; Close, P.G. Macroinvertebrates in the bed sediment of the Yellow River. Int. J. Sediment Res. 2011, 26, 255–268. [Google Scholar] [CrossRef]
- Flanagan, A.M.; Flood, R.D.; Maher, N.P.; Cerrato, R.M. Quantitatively characterizing benthic community-habitat relationships in soft-sediment, nearshore environments to yield useful results for management. J. Environ. Manag. 2019, 249, 109361. [Google Scholar] [CrossRef] [PubMed]
- Aguhob, J.; Hamza, W.; Reul, A.; Musabih, M.; Mustafa, S.; Muñoz, M. Baseline Habitat Setting for Future Evaluation of Environmental Status Quality of Jabal Ali Marine Sanctuary, Dubai, UAE. Sustainability 2024, 16, 2374. [Google Scholar] [CrossRef]
- McCain, J.C. Benthic Communities of the Coastal Waters of Dubai, United Arab Emirates; Technical Report; Dubai Municipality: Dubai, United Arab Emirates, 1999; 78p. [Google Scholar]
- John, D.M.; George, J.D.; Al-Thani, R.F. The macrobenthos of the Jabal Ali Bay and Abu Dhabi coastal waters, United Arab Emirates. Qatar Univ. Sci. J. 2004, 24, 115–128. [Google Scholar]
- Hamza, W.; Al-Hassini, M.; Al-Ansari, E.M.A.S. Monitoring Program for Dubai Coastal Waters: Analysis of Long-Term Variations in Physicochemical and Biological Parameters (2006–2015); Technical Report; Dubai Municipality Environmental Department: Dubai, United Arab Emirates, 2016; 142p. [Google Scholar]
- Al-Zaidan, A.S.Y.; Al-Mohanna, S.Y.; George, P. Status of macrobenthos in Arabian Gulf marine protected areas: Spatial and temporal patterns. Reg. Stud. Mar. Sci. 2018, 24, 78–89. [Google Scholar]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment; Springer Nature: Dordrecht, GX, The Netherlands, 1981. [Google Scholar] [CrossRef]
- Gray, J.S.; Clarke, K.R.; Warwick, R.M.; Hobbs, G. Detection of initial effects of pollution on marine benthos: An example from the Ekofisk and Eldfisk oilfields, North Sea. Mar. Ecol. Prog. Ser. 1990, 66, 285–299. [Google Scholar] [CrossRef]
- Carter, J.L.; Resh, V.H.; Hannaford, M.J. Macroinvertebrates as Biotic Indicators of Environmental Quality. In Methods in Stream Ecology; Academic Press: Cambridge, MA, USA, 2017; pp. 293–318. [Google Scholar]
- Clinton, M.E.; Snelgrove, P.V.R.; Bates, A.E. Macrofaunal diversity patterns in coastal marine sediments: Re-examining common metrics and methods. Mar. Ecol. Prog. Ser. 2024, 735, 1–26. [Google Scholar] [CrossRef]
- Al-Yamani, F.Y.; Polikarpov, I.; Saburova, M. Marine life mortalities and Harmful Algal Blooms in the Northern Arabian Gulf. Aquat. Ecosyst. Health Manag. 2020, 23, 196–209. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; USEPA: Washington, DC, USA, 1996. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-3050b.pdf (accessed on 19 February 2025).
- Yip, Y.C.; Sham, W.C. Applications of collision/reaction-cell technology in isotope dilution mass spectrometry. TrAC Trends Anal. Chem. 2007, 26, 727–743. [Google Scholar] [CrossRef]
- Agilent Technologies. Agilent 7700 Series ICP-MS: Hardware Maintenance Manual; Publication No. G3280-90001; Agilent Technologies Inc.: Santa Clara, CA, USA, 2022. [Google Scholar]
- Ministry of Infrastructure and Environment. Soil Remediation Circular; Government Gazette No. 16675; Ministry of Infrastructure and Environment: The Hague, The Netherlands, 2013. [Google Scholar]
- USEPA Method 1631. Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry; EPA-821-R-02-019; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Company: Austin, TX, USA, 1974; 182p. [Google Scholar]
- Al-Omari NHA(2016) Guide to Polychaetes Annelida in Qatar Marine Sediments; Qatar University Environmental Studies Center: Doha, Qatar, 2011; ISBN 99921-786-1-2.
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 990p. [Google Scholar]
- Van Hoey, G.; Degraer, S.; Vincx, M. Long-term patterns in the temporal variation of macrobenthic communities in coastal waters. Mar. Environ. Res. 2018, 129, 1–12. [Google Scholar]
- Al-Yamani, F.Y.; Bishop, J.M.; Al-Rifaie, K. Diversity and distribution of polychaetes in the Arabian Gulf: A comprehensive review. Oceanogr. Mar. Biol. Annu. Rev. 2020, 58, 177–219. [Google Scholar]
- Al-Zaidan, A.S.Y.; Jones, D.A.; Al-Mohanna, S.Y. Physical and chemical characteristics of marine sediments in the Arabian Gulf: A review of recent studies. Environ. Monit. Assess. 2019, 191, 1–18. [Google Scholar]
- Basson, P.W.; Burchard, J.E.; Hardy, J.T.; Price, A.R.G. Biotopes and biotas of the Arabian Gulf: With particular reference to the impacts of extreme environmental stressors. Environ. Sci. Pollut. Res. 2021, 28, 31046–31068. [Google Scholar]
- Sheppard, C.; Al-Husiani, M.; Al-Jamali, F.; Al-Yamani, F.; Baldwin, R.; Bishop, J.; Zainal, K. The Gulf: A young sea in decline. Mar. Pollut. Bull. 2010, 60, 13–38. [Google Scholar] [CrossRef]
- Al-Kandari, M.; Al-Yamani, F.; Al-Rifaie, K. Benthic community structure and diversity in Kuwait Bay, Arabian Gulf. Estuar. Coast. Shelf Sci. 2021, 248, 106754. [Google Scholar]
- Price, A.R.G.; Donlan, M.C.; Sheppard, C.R.C.; Munawar, M. Environmental problems in the Gulf: A holistic approach to management. Aquat. Ecosyst. Health Manag. 2012, 15, 8–15. [Google Scholar]
- Morrisey, D.J.; Swales, A.; Dittmann, S.; Morrison, M.A.; Lovelock, C.E.; Beard, C.M. The Ecology and Management of Temperate Mangroves. Oceanogr. Mar. Biol. Annu. Rev. 2018, 48, 43–160. [Google Scholar]
- Burt, J.A.; Paparella, F.; Al-Mansoori, N.; Al-Mansoori, A.; Al-Jailani, H. Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 2019, 38, 567–589. [Google Scholar] [CrossRef]
- Reiss, H.; Kröncke, I. Seasonal variability of infaunal community structures in three areas of the North Sea under different environmental conditions. Estuar. Coast. Shelf Sci. 2019, 65, 253–274. [Google Scholar] [CrossRef]
- Ellis, J.I.; Norkko, A.; Thrush, S.F. Temporal variability in infaunal communities: Influence of environmental drivers and biological interactions. J. Exp. Mar. Biol. Ecol. 2019, 534, 151–172. [Google Scholar]
- Al-Khayat, J.A.; Al-Ansi, M.A.; Al-Khater, A.A.R. Macrobenthic community structure and environmental parameters in Saudi Arabian waters of the Arabian Gulf. Reg. Stud. Mar. Sci. 2022, 47, 101945. [Google Scholar]
- Al-Ansari, I.M.A.S.; Rowe, G.T.; Abdel-Moati, M.A.R.; Yigiterhan, O. Benthic community structure and response to environmental variables in the coastal waters of Qatar, Arabian Gulf. J. Mar. Syst. 2021, 168, 73–81. [Google Scholar]
- Al-Hashmi, K.A.; Al-Muzaini, S.; Al-Yamani, F. Spatial and temporal variations in the abundance and biomass of benthic communities in Kuwait’s waters, Arabian Gulf. Mar. Pollut. Bull. 2019, 142, 108–121. [Google Scholar]
- Price, A.R.G.; Al-Yamani, F.; Sheppard, C.R.C. Environmental challenges in a complex marine ecosystem: A review of scientific research in the Arabian Gulf. Mar. Pollut. Bull. 2021, 163, 111927. [Google Scholar]
- Al-Rumaidh, M.J.; Al-Sofyani, A.A.; Manikandan, B. Seasonal variations in benthic communities along the coast of Bahrain, Arabian Gulf. J. King Abdulaziz Univ. Mar. Sci. 2019, 30, 47–60. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Lohrer, A.M. Interaction networks in coastal soft-sediment communities: Implications for pattern and process. Mar. Ecol. Progress Ser. 2020, 642, 17–32. [Google Scholar]
- Gray, J.S.; Elliott, M. Ecology of Marine Sediments: From Science to Management; Oxford University Press: Oxford, UK, 2018; pp. 225–256. [Google Scholar]
- Price, A.R.G.; Coles, S.L. Forty years of change in coral communities: What have we learned in the Arabian Gulf? Mar. Pollut. Bull. 2019, 141, 40–52. [Google Scholar]
- Al-Wedaei, K.; Naser, H.; Al-Sayed, H. Diversity and distribution of benthic molluscs in the coastal waters of Bahrain, Arabian Gulf. Reg. Stud. Mar. Sci. 2018, 24, 23–31. [Google Scholar]
- Erftemeijer, P.L.A.; Shuail, D.A. Seagrass habitats in the Arabian Gulf: Distribution, tolerance thresholds and threats. Aquat. Bot. 2019, 155, 73–83. [Google Scholar] [CrossRef]
- Cosentino, A.; Giacobbe, S. Biodiversity patterns of soft-bottom marine benthic communities: A temporal and spatial analysis. J. Sea Res. 2019, 145, 32–44. [Google Scholar]
- Vaughan, G.O.; Al-Mansoori, N.; Burt, J.A. The Arabian Gulf. In World Seas: An Environmental Evaluation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–23. [Google Scholar]
- Hewitt, J.E.; Thrush, S.F.; Dayton, P.K. The effect of spatial and temporal heterogeneity on the design and analysis of empirical studies of scale-dependent systems. Am. Nat. 2021, 178, 113–130. [Google Scholar] [CrossRef]
- Snelgrove, P.V.R.; Butman, C.A. Animal-sediment relationships revisited: Cause versus effect. Oceanogr. Mar. Biol. Annu. Rev. 2017, 55, 201–233. [Google Scholar]
- Naser, H.A. Environmental Impacts of Dredging Activities in the Arabian Gulf: A Review. Mar. Pollut. Bull. 2020, 159, 111498. [Google Scholar]
- Al-Zaidan, A.S.Y.; Kennedy, H.; Jones, D.A.; Al-Mohanna, S.Y. Role of microbial mats in Sulaibikhat Bay (Kuwait) mudflat food webs: Evidence from δ13C analysis. Mar. Ecol. Progress Ser. 2019, 308, 27–36. [Google Scholar] [CrossRef]
- Bu-Olayan, A.H.; Thomas, B.V. Trace metals toxicity and bioaccumulation in mudskipper Periophthalmus waltoni Koumans 1941 (Gobiidae: Perciformes). Turk. J. Fish. Aquat. Sci. 2014, 14, 517–525. [Google Scholar]
- Catsiki, V.A.; Papathanassiou, E.; Bei, F. Heavy metal levels in characteristic benthic flora and fauna in the Central Aegean Sea. Mar. Pollut. Bull. 1991, 22, 566–569. [Google Scholar] [CrossRef]
- Jewett, S.C.; Feder, H.M.; Blanchard, A. Assessment of the benthic environment following offshore placer gold mining in the northeastern Bering Sea. Mar. Environ. Res. 1999, 48, 91–122. [Google Scholar] [CrossRef]
- Al-Husaini, M.; Al-Baz, A.; Al-Ayoub, S. Trace metals in sediments and their relationship to benthic communities along the Omani coast. Mar. Environ. Res. 2022, 173, 105539. [Google Scholar]
- De Mora, S.; Fowler, S.W.; Readman, J.W. Distribution of heavy metals in marine bivalves, fish and coastal sediments in the Gulf and Gulf of Oman. Mar. Pollut. Bull. 2020, 157, 111102. [Google Scholar] [CrossRef]
- Al-Majed, N.; Al-Muzaini, S. Metals in surficial sediments of Kuwait’s marine areas: An assessment of contamination and ecological risk. Mar. Pollut. Bull. 2019, 145, 23–34. [Google Scholar]
- Al-Abdali, F.; Massoud, M.S.; Al-Ghadban, A.N. Metals in sediments and benthic organisms from Kuwait’s marine environment. Environ. Monit. Assess. 2019, 191, 1–15. [Google Scholar]
- Al-Sarawi, M.A.; Massoud, M.S.; Wahba, S.A. Recent trace metals in coastal waters of Kuwait: Influences on benthic communities. Mar. Pollut. Bull. 2021, 169, 112534. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 2019, 7, 497–520. [Google Scholar] [CrossRef]
- Al-Mutairi, N.; Abahussain, A.; El-Battay, A. Spatial and temporal characterizations of water quality parameters in Kuwait Bay. Mar. Pollut. Bull. 2018, 127, 53–64. [Google Scholar]
- Naser, H.A. Heavy metals contamination in sediments and their effect on benthic biodiversity in Bahrain coastal waters. Environ. Monit. Assess. 2018, 190, 620. [Google Scholar]
- Al-Naimi, H.A.; Al-Ghouti, M.A.; Al-Shaikh, I. Environmental assessment of trace metal concentrations and their ecological risk in Qatari coastal sediments. J. Mar. Syst. 2020, 201, 103245. [Google Scholar]
- Al-Darwish, H.A.; Abd El-Gawad, E.A.; Lotfy, M.M. Assessment of trace metals in coastal sediments and benthic fauna of the UAE coast. Mar. Pollut. Bull. 2018, 127, 311–324. [Google Scholar]
- Dauer, D.M.; Ranasinghe, J.A. Seasonal dynamics of benthic communities in coastal marine ecosystems: A review. Oceanogr. Mar. Biol. Annu. Rev. 2020, 58, 127–168. [Google Scholar]
- Al-Madfa, H.; Abdel-Moati, M.A.R.; Al-Naama, A. Heavy metals in coastal sediments and their influence on benthic communities of Qatar. Mar. Pollut. Bull. 2021, 168, 112419. [Google Scholar]
- Al-Farawati, R.; Al-Mahtaseb, M.A.; El Sayed, M.A. Environmental quality assessment of Saudi Arabian coastal waters: Heavy metal enrichment factors in surficial sediments. Mar. Pollut. Bull. 2019, 142, 595–604. [Google Scholar]
- Al-Sayed, H.; Al-Wedaei, K.; Naser, H.A. Trace metals distribution and their relationship to benthic assemblages in Bahrain coastal waters. Environ. Monit. Assess. 2022, 194, 440. [Google Scholar]
- Dehghan-Madiseh, S.; Nabavi, S.M.B.; Ghofleh Marammazi, J. Heavy metals concentration in sediment and benthic communities from the Iranian coast of the Persian Gulf. Environ. Sci. Pollut. Res. 2021, 28, 18921–18934. [Google Scholar]
- Al-Yamani, F.Y.; Skryabin, V.; Durvasula, S.R.K. Benthic infaunal composition and abundance in the Northwestern Arabian Gulf. Reg. Stud. Mar. Sci. 2020, 35, 101152. [Google Scholar]
- Al-Khayat, J.A.; Al-Ansi, M.A. Ecological assessment of bivalves in the marine sediments of the Qatari waters, Arabian Gulf. Int. J. Environ. Res. 2018, 12, 367–376. [Google Scholar]
- Rainbow, P.S.; Luoma, S.N. Metal toxicity, uptake and bioaccumulation in aquatic invertebrates—Modelling zinc in crustaceans. Aquat. Toxicol. 2011, 105, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 2018, 56, 229–311. [Google Scholar]
- Naser, H.A. Assessment of heavy metal pollution in sediments and related impacts on benthic macrofauna communities in the Kingdom of Bahrain, Arabian Gulf. Environ. Monit. Assess. 2021, 193, 1–16. [Google Scholar]
- Bu-Olayan, A.H.; Thomas, B.V. Monitoring bioaccumulation of trace metals in mullet fish from Kuwait Bay. Int. J. Environ. Stud. 2018, 75, 65–77. [Google Scholar]
- Al-Yamani, F.Y.; Skryabin, V.; Boltachova, N.; Revkov, N.; Makarov, M.; Grintsov, V.; Kolesnikova, E. Illustrated Atlas on the Zoobenthos of Kuwait; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2020; 385p. [Google Scholar]
- Thrush, S.F.; Hewitt, J.E.; Norkko, A.; Nicholls, P.E.; Funnell, G.A.; Ellis, J.I. Habitat change in estuaries: Predicting broad-scale responses of intertidal macrofauna to sediment mud content. Mar. Ecol. Progress. Ser. 2017, 263, 101–112. [Google Scholar] [CrossRef]
- Fowler, S.W.; Villeneuve, J.-P.; Wyse, E.; Jupp, B.; de Mora, S. Temporal survey of petroleum hydrocarbons, organochlorinated compounds and heavy metals in benthic marine organisms from Dhofar, southern Oman. Mar. Pollut. Bull. 2007, 54, 357–367. [Google Scholar] [CrossRef]
- Saunders, J.E.; Al Zahed, K.M.; Paterson, D.M. The impact of organic pollution on the macrobenthic fauna of Dubai Creek (UAE). Mar. Pollut. Bull. 2007, 54, 1715–1723. [Google Scholar] [CrossRef]
- Alongi, D.M. Temporal patterns in benthic infaunal communities of tropical estuaries. Mar. Ecol. Progress Ser. 2018, 126, 235–249. [Google Scholar]


 —environmental vectors;
—environmental vectors;  —dominant species;
—dominant species;  —stations.
—stations.
 —environmental vectors;
—environmental vectors;  —dominant species;
—dominant species;  —stations.
—stations.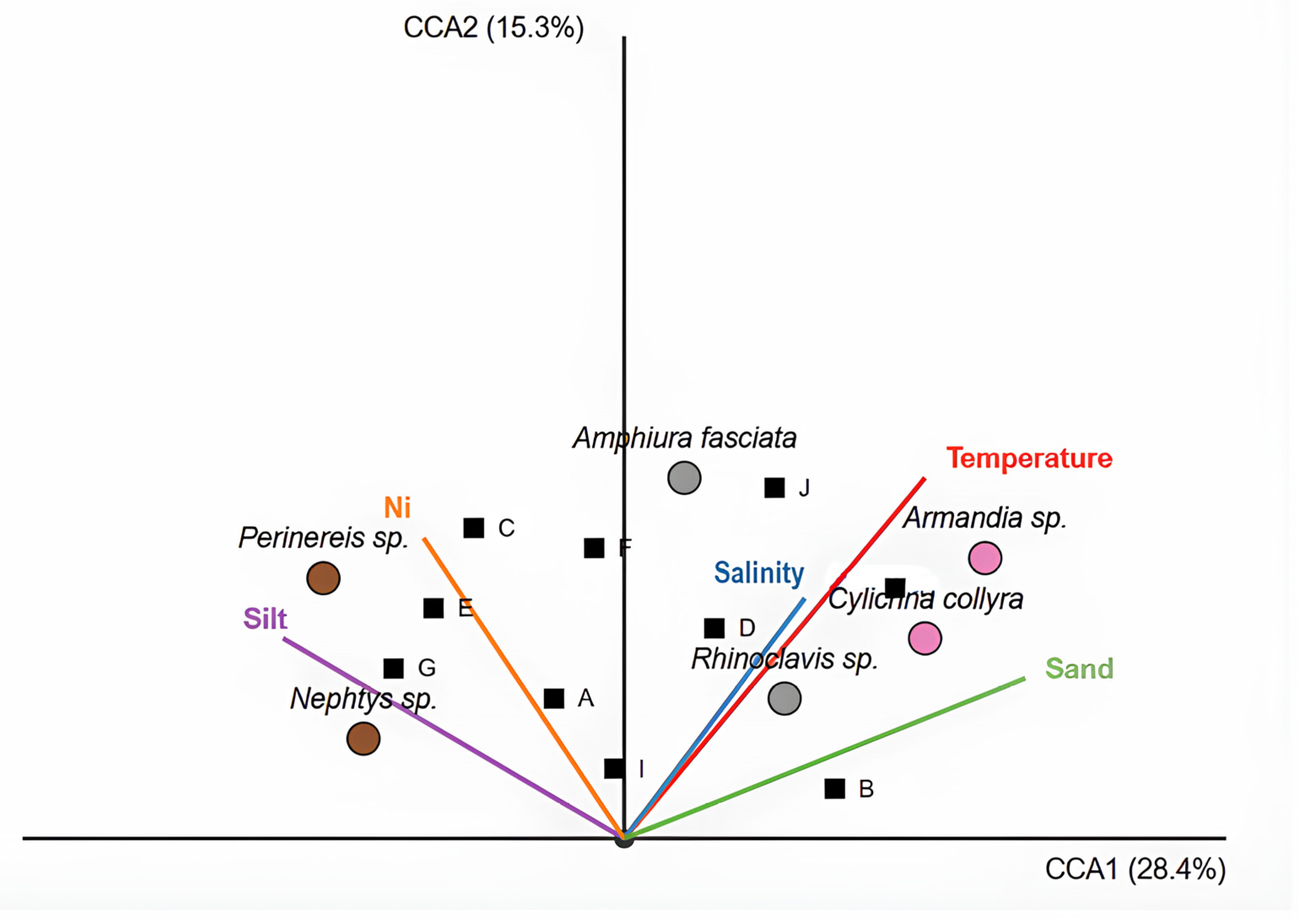
| Station | Summer | Winter | ||
|---|---|---|---|---|
| Temperature (°C) | Salinity (ppt) | Temperature (°C) | Salinity (ppt) | |
| A | 35.8 ± 0.3 | 43.5 ± 0.2 | 24.7 ± 0.2 | 42.6 ± 0.1 |
| B | 35.3 ± 0.4 | 42.8 ± 0.3 | 24.2 ± 0.3 | 42.1 ± 0.2 |
| C | 34.7 ± 0.2 | 42.4 ± 0.2 | 23.8 ± 0.2 | 41.7 ± 0.2 |
| D | 34.1 ± 0.3 | 41.9 ± 0.2 | 23.4 ± 0.1 | 41.2 ± 0.1 |
| E | 33.8 ± 0.4 | 41.5 ± 0.3 | 23.2 ± 0.2 | 40.9 ± 0.2 |
| F | 33.5 ± 0.3 | 41.2 ± 0.2 | 23.1 ± 0.3 | 40.5 ± 0.2 |
| G | 33.2 ± 0.2 | 40.8 ± 0.3 | 23.0 ± 0.2 | 40.3 ± 0.3 |
| H | 32.8 ± 0.5 | 40.5 ± 0.4 | 22.8 ± 0.4 | 40.1 ± 0.2 |
| I | 32.5 ± 0.3 | 40.3 ± 0.3 | 22.6 ± 0.3 | 40.0 ± 0.3 |
| J | 32.1 ± 0.4 | 40.2 ± 0.2 | 22.4 ± 0.2 | 39.8 ± 0.2 |
| Mean | 34.2 ± 1.1 | 41.8 ± 1.2 | 23.5 ± 0.7 | 41.1 ± 0.9 |
| Parameters | Results/Range | Dutch Target Values | Ads 19/2017 Adqcc— Marine Protected Area Use |
|---|---|---|---|
| Arsenic, As | <0.05 | 29 | 7 |
| Boron, B | 4.48–16.13 | - | - |
| Cadmium, Cd | <0.05–0.62 | <1 | 0.2 |
| Chromium, Cr | 4.73–95.51 | 100 | 11 |
| Copper, Cu | 0.66–0.68 | 36 | 20 |
| Lead, Pb | <0.05 | 85 | 5 |
| Manganese, Mn | 26.75–164.90 | - | - |
| Nickel, Ni | 8.38–228.10 | - | 7 |
| Zinc, Zn | 6.06–25.46 | 140 | 70 |
| Aluminum, Al | 396.8–3142.0 | - | - |
| Tin, Sn | <0.05 | - | - |
| Mercury, Hg | 0.01–0.02 | 0.3 | - |
| Vanadium, V | 3.34–22.61 | 42 | - |
| Selenium, Si (mg/Kg) | <0.050 | 0.7 | - |
| Barium (mg/Kg) | 7.83–76.75 | 160 | - |
| Cobalt (mg/Kg) | <0.05 | 9 | - |
| Iron (mg/Kg) | 718.5–7848 | - | - |
| Total Organic Carbon | 0.05–1.10 | - | - |
| Species | Summer | Summer Abundance | Winter | Winter Abundance | Seasonality |
|---|---|---|---|---|---|
| Amphiura fasciata | ✓ | 576 | ✓ | 440 | Year-round presence |
| Amplescia sp. | ✓ | 648 | ✓ | 540 | Year-round presence |
| Ancilla sp. | ✓ | 144 | ✓ | 240 | Year-round presence |
| Apanthura sp. | ✓ | 144 | ✓ | 120 | Year-round presence |
| Apseudes sp. | ✓ | 288 | ✓ | 160 | Year-round presence |
| Aricidea sp. | ✓ | 144 | ✓ | 320 | Year-round presence |
| Armandia sp. | ✓ | 72 | - | - | Summer only |
| Bassina sp. | ✓ | 3096 | ✓ | 3080 | Year-round presence |
| Bittium sp. | ✓ | 288 | ✓ | 240 | Year-round presence |
| Bivalve spp. | ✓ | 72 | ✓ | 80 | Year-round presence |
| Brachidontes variabilis | ✓ | 144 | ✓ | 80 | Year-round presence |
| Bulla ampulla | ✓ | 144 | ✓ | 240 | Year-round presence |
| Capetiella capitella | ✓ | 360 | - | - | Summer only |
| Cardites sp. | ✓ | 144 | ✓ | 240 | Year-round presence |
| Certhidium cerithinum | ✓ | 72 | ✓ | 120 | Year-round presence |
| Circe sp. | ✓ | 72 | - | - | Summer only |
| Cronia konkanensis | ✓ | 72 | - | - | Summer only |
| Cylichna collyra | ✓ | 72 | - | - | Summer only |
| Cypris sp. larva | ✓ | 72 | - | - | Summer only |
| Echinodiscus auritus | ✓ | 72 | - | - | Summer only |
| Euclymene insecta | ✓ | 216 | ✓ | 280 | Year-round presence |
| Eunice sp. | ✓ | 144 | ✓ | 160 | Year-round presence |
| Glycinde sp. | ✓ | 576 | ✓ | 640 | Year-round presence |
| Maldane sp. | ✓ | 288 | ✓ | 240 | Year-round presence |
| Mitrella blanda | ✓ | 288 | ✓ | 280 | Year-round presence |
| Nephtys sp. | ✓ | 1152 | ✓ | 1400 | Year-round presence |
| Onuphis sp. | ✓ | 432 | ✓ | 560 | Year-round presence |
| Paphia sp. | ✓ | 72 | ✓ | 120 | Year-round presence |
| Perinereis sp. | ✓ | 1440 | ✓ | 1880 | Year-round presence |
| Phascolion sp. | ✓ | 144 | ✓ | 160 | Year-round presence |
| Polinices sp. | ✓ | 144 | ✓ | 240 | Year-round presence |
| Polychaete spp. | ✓ | 72 | ✓ | 80 | Year-round presence |
| Rapana sp. | ✓ | 144 | - | - | Summer only |
| Rhinoclavis sp. | ✓ | 6912 | ✓ | 6600 | Year-round presence |
| Sabella sp. | ✓ | 648 | ✓ | 400 | Year-round presence |
| Scabricola destangsii | ✓ | 144 | - | - | Summer only |
| Scoloplos sp. | ✓ | 720 | ✓ | 1580 | Year-round presence |
| Tellina vernalis | ✓ | 72 | ✓ | 120 | Year-round presence |
| Terebellides sp. | ✓ | 144 | ✓ | 200 | Year-round presence |
| Umbonium vestiarium | ✓ | 216 | ✓ | 320 | Year-round presence |
| Veneridae (gen) sp. | ✓ | 144 | ✓ | 160 | Year-round presence |
| Station | Season | Margalef Index (d) | Shannon–Wiener (H’) | Pielou Evenness (J) |
|---|---|---|---|---|
| A | Summer | 1.102 | 2.056 | 0.893 |
| Winter | 1.109 | 1.719 | 0.747 | |
| B | Summer | 0.122 | 0.362 | 0.523 |
| Winter | 0.86 | 1.023 | 0.492 | |
| C | Summer | 0.671 | 1.135 | 0.633 |
| Winter | 0.519 | 0.759 | 0.472 | |
| D | Summer | 1.229 | 2.066 | 0.897 |
| Winter | 1.228 | 1.909 | 0.829 | |
| E | Summer | 1.051 | 2.02 | 0.919 |
| Winter | 1.206 | 2.025 | 0.879 | |
| F | Summer | 1.375 | 2.221 | 0.926 |
| Winter | 1.309 | 2.162 | 0.901 | |
| G | Summer | 1.361 | 1.948 | 0.784 |
| Winter | 1.223 | 1.94 | 0.809 | |
| H | Summer | 0.993 | 1.836 | 0.883 |
| Winter | 0.72 | 1.651 | 0.922 | |
| I | Summer | 0.688 | 1.466 | 0.818 |
| Winter | 0.699 | 1.581 | 0.882 | |
| J | Summer | 0.43 | 1.245 | 0.898 |
| Winter | 0.43 | 1.049 | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguhob, J.; Hamza, W.; Reul, A.; Musabih, M.; Villegas, J.P.; Muñoz, M. Spatial Distribution and Diversity of Benthic Macrofauna in Coastal Waters of the Jabal Ali Marine Sanctuary (JAMS), Dubai. Diversity 2025, 17, 332. https://doi.org/10.3390/d17050332
Aguhob J, Hamza W, Reul A, Musabih M, Villegas JP, Muñoz M. Spatial Distribution and Diversity of Benthic Macrofauna in Coastal Waters of the Jabal Ali Marine Sanctuary (JAMS), Dubai. Diversity. 2025; 17(5):332. https://doi.org/10.3390/d17050332
Chicago/Turabian StyleAguhob, Jeruel, Waleed Hamza, Andreas Reul, Muna Musabih, Jhonnel P. Villegas, and Maria Muñoz. 2025. "Spatial Distribution and Diversity of Benthic Macrofauna in Coastal Waters of the Jabal Ali Marine Sanctuary (JAMS), Dubai" Diversity 17, no. 5: 332. https://doi.org/10.3390/d17050332
APA StyleAguhob, J., Hamza, W., Reul, A., Musabih, M., Villegas, J. P., & Muñoz, M. (2025). Spatial Distribution and Diversity of Benthic Macrofauna in Coastal Waters of the Jabal Ali Marine Sanctuary (JAMS), Dubai. Diversity, 17(5), 332. https://doi.org/10.3390/d17050332