Hidden Microbial Diversity in Mangrove Depths: New Cyanobacterial Species of Picosynechococcus and Two New Records of Sirenicapillaria and Allocoleopsis from the Andaman Coast of Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Culture
2.3. Morphological Observation
2.4. Herbarium Voucher Preparation
2.5. Molecular and Phylogenetic Analyses
2.6. Salinity Tolerance and Growth
3. Results
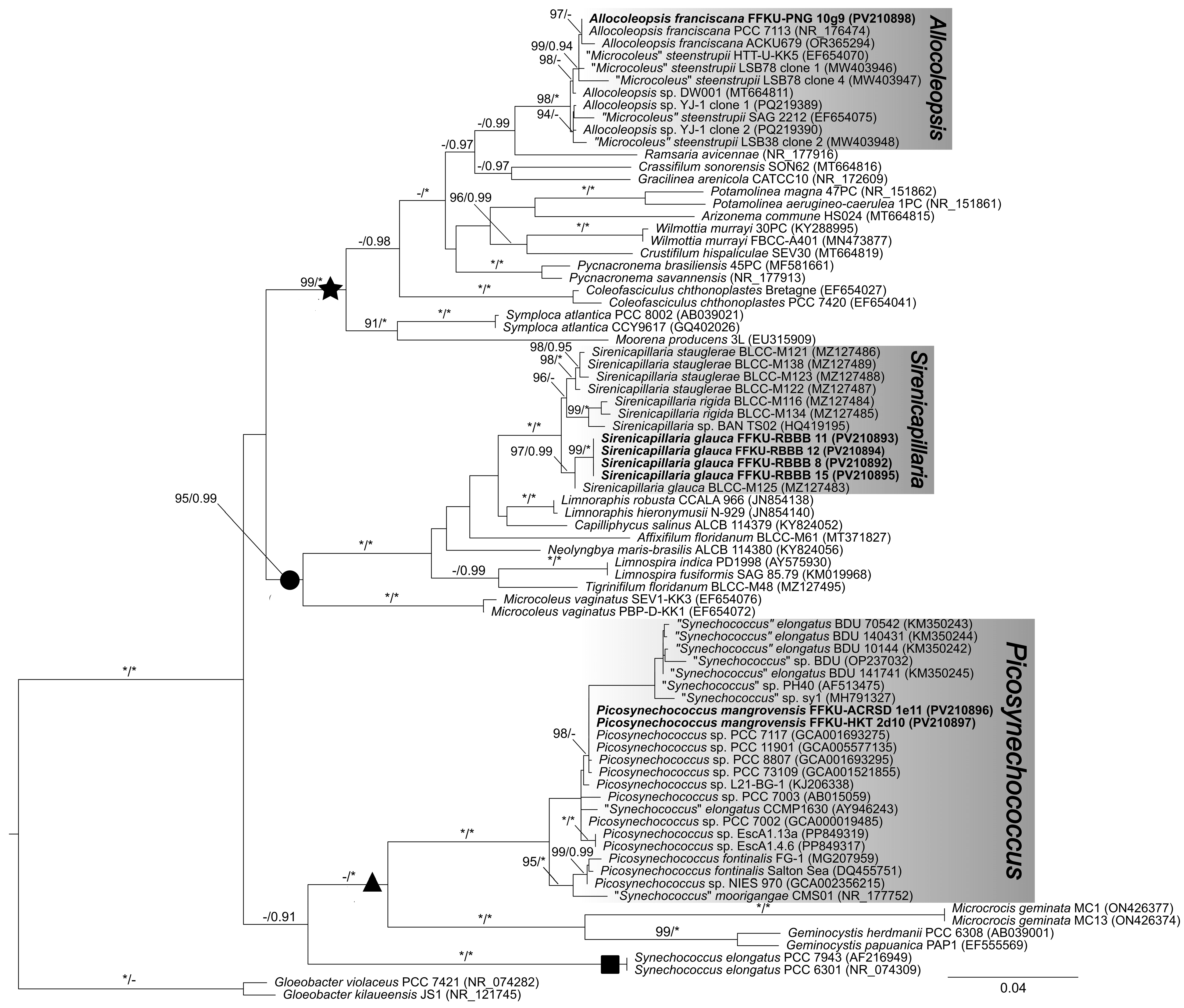
3.1. 16S rRNA Gene Phylogenetic and p-Distance Analyses
3.2. 16S–23S rRNA ITS Secondary Structure and p-Distance Analyses
3.3. Taxonomic Description
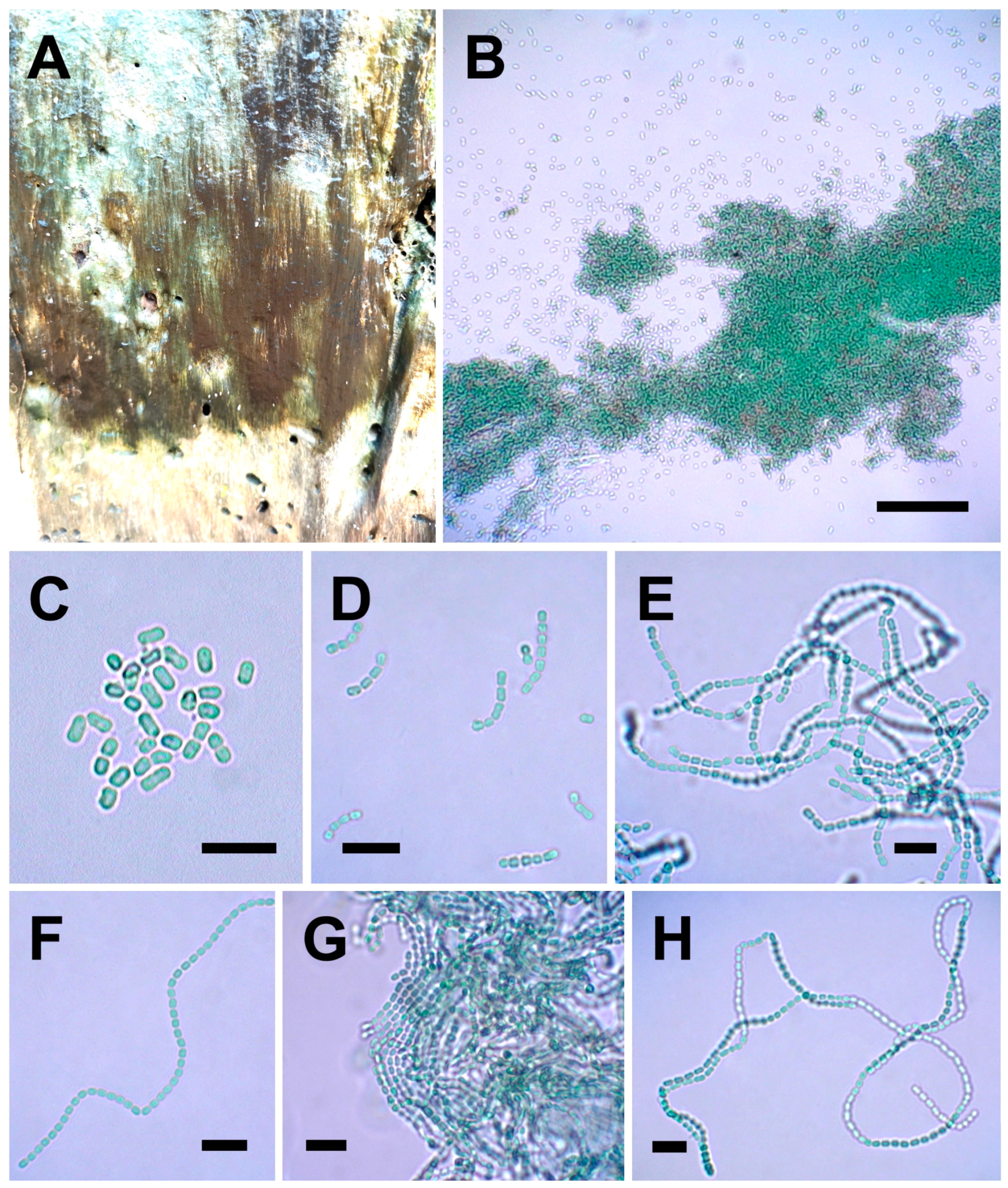
3.3.1. Picosynechococcus mangrovensis sp. nov. Lim and Muangmai (Figure 5A–H)
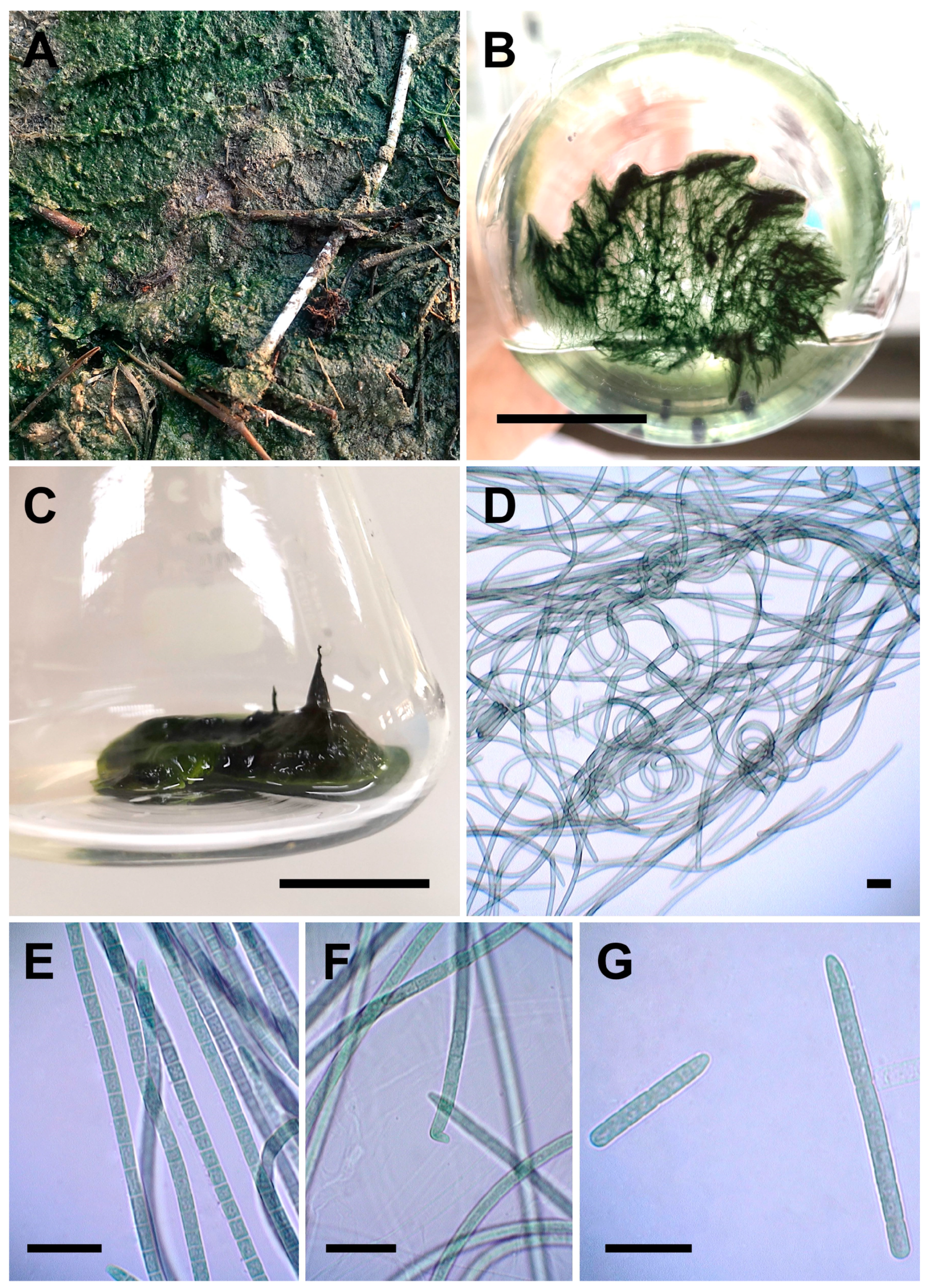
3.3.2. Allocoleopsis franciscana Moreira-Fernandes, Giraldo-Silva, D. Roush, and Garcia-Pichel (Figure 6A–G)
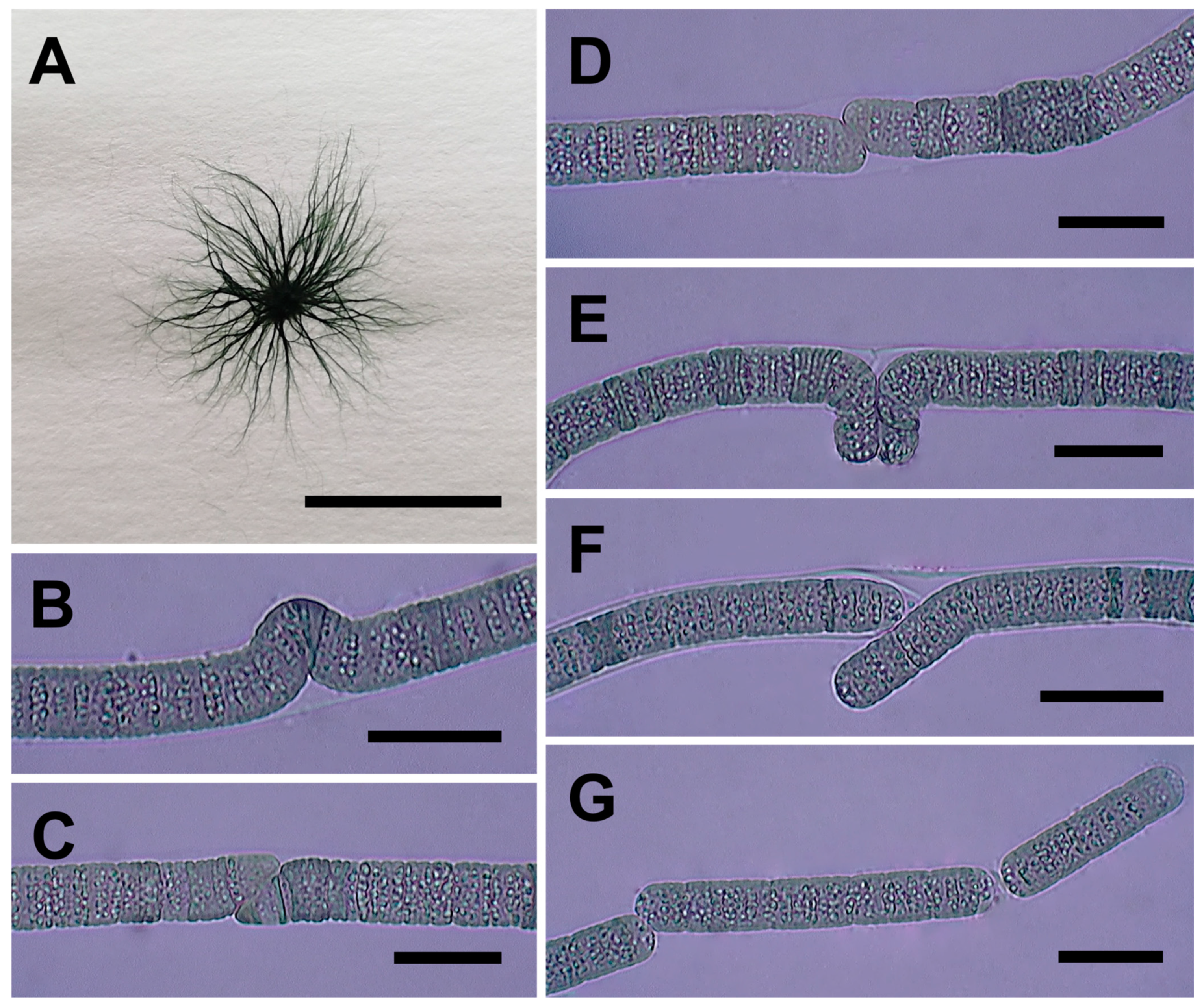
3.3.3. Sirenicapillaria glauca Berthold, Leftler, and Laughinghouse (Figure 7A–G)
3.4. Growth at Different Salinities
4. Discussion
4.1. Picosynechococcus mangrovensis sp. nov.
4.1.1. 16S rRNA Phylogeny and ITS Region Analyses
4.1.2. Morphological Characterization
4.1.3. Ecological Adaptation and Geographical Distribution
4.2. Allocoleopsis franciscana
4.2.1. 16S rRNA Phylogeny and ITS Region Analyses
4.2.2. Morphological Characterization
4.2.3. Ecological Adaptation and Geographical Distribution
4.3. Sirenicapillaria glauca
4.3.1. 16S rRNA Phylogeny and ITS Region Analyses
4.3.2. Morphological Characterization
4.3.3. Ecological Adaptation and Geographical Distribution
4.4. Current Status and Future Surveys of Cyanobacterial Diversity in Thailand Mangroves
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarenga, D.O.; Rigonato, J.; Branco, L.H.Z.; Fiore, M.F. Cyanobacteria in mangrove ecosystems. Biodivers. Conserv. 2015, 24, 799–817. [Google Scholar] [CrossRef]
- Dvořák, P.; Hašler, P.; Casamatta, D.A.; Poulíčková, A. Underestimated cyanobacterial diversity: Trends and perspectives of research in tropical environments. Fottea 2021, 21, 110–127. [Google Scholar] [CrossRef]
- Dvořák, P.; Jahodářová, E.; Stanojković, A.; Skoupý, S.; Casamatta, D.A. Population genomics meets the taxonomy of cyanobacteria. Algal Res. 2023, 72, 103128. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Rigonato, J.; Branco, L.H.Z.; Melo, I.S.; Fiore, M.F. Phyllonema aviceniicola gen. nov., sp. nov. and Foliisarcina bertiogensis gen. nov., sp. nov., epiphyllic cyanobacteria associated with Avicennia schaueriana leaves. Int. J. Syst. Evol. Microbiol. 2016, 66, 689–700. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Andreote, A.P.D.; Branco, L.H.Z.; Fiore, M.F. Kryptousia macronema gen. nov., sp. nov. and Kryptousia microlepis sp. nov., nostocalean cyanobacteria isolated from phyllospheres. Int. J. Syst. Evol. Microbiol. 2017, 67, 3301–3309. [Google Scholar] [CrossRef]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Chakraborty, S.; Maruthanayagam, V.; Achari, A.; Mahansaria, R.; Pramanik, A.; Jaisankar, P.; Mukherjee, J. Oxynema aestuarii sp. nov. (Microcoleaceae) isolated from an Indian mangrove forest. Phytotaxa 2018, 374, 24–40. [Google Scholar] [CrossRef]
- Chakraborty, S.; Maruthanayagam, V.; Achari, A.; Pramanik, A.; Jaisankar, P.; Mukherjee, J. Aerofilum fasciculatum gen. nov., sp. nov. (Oculatellaceae) and Euryhalinema pallustris sp. nov. (Prochlorotrichaceae) isolated from an Indian mangrove forest. Phytotaxa 2021, 522, 165–186. [Google Scholar] [CrossRef]
- Genuário, D.B.; Vaz, M.G.M.V.; Hentschke, G.S.; Sant’Anna, C.L.; Fiore, M.F. Halotia gen. nov., a phylogenetically and physiologically coherent cyanobacterial genus isolated from marine coastal environments. Int. J. Syst. Evol. Microbiol. 2015, 65, 663–675. [Google Scholar] [CrossRef]
- Mangrove Forest Conservation Division. Mangrove Knowledge Guide, Department of Marine and Coastal Resources (DMCR); Ploy Media Co., Ltd.: Bangkok, Thailand, 2020; pp. 1–68. Available online: https://www.dmcr.go.th/detailLib/5720 (accessed on 2 April 2025).
- Muangham, S.; Suksaard, P.; Mingma, R.; Matsumoto, A.; Takahashi, Y.; Duangmal, K. Nocardiopsis sediminis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 3835–3840. [Google Scholar] [CrossRef]
- Nimnoi, P.; Pongsilp, N. Insights into bacterial communities and diversity of mangrove forest soils along the Upper Gulf of Thailand in response to environmental factors. Biology 2022, 11, 1787. [Google Scholar] [CrossRef] [PubMed]
- Am-In, S.; Limtong, S.; Yongmanitchai, W.; Jindamorakot, S. Candida andamanensis sp. nov., Candida laemsonensis sp. nov. and Candida ranongensis sp. nov., anamorphic yeast species isolated from estuarine waters in a Thai mangrove forest. Int. J. Syst. Evol. Microbiol. 2011, 61, 454–461. [Google Scholar] [CrossRef]
- Limtong, S.; Yongmanitchai, W.; Kawasaki, H.; Seki, T. Candida phangngensis sp. nov., an anamorphic yeast species in the Yarrowia clade, isolated from water in mangrove forests in Phang-Nga Province, Thailand. Int. J. Syst. Evol. Microbiol. 2008, 58, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Kunthiphun, S.; Endoh, R.; Takashima, M.; Ohkuma, M.; Tanasupawat, S.; Savarajara, A. Prototheca paracutis sp. nov., a novel oleaginous achlorophyllous microalga isolated from a mangrove forest. Mycoscience 2019, 60, 165–169. [Google Scholar] [CrossRef]
- Komárek, J.; Johansen, J.R.; Šmarda, J.; Strunecký, O. Phylogeny and taxonomy of Synechococcus-like cyanobacteria. Fottea 2020, 20, 171–191. [Google Scholar] [CrossRef]
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Berthold, D.E.; Lefler, F.W.; Laughinghouse, H.D., IV. Recognizing novel cyanobacterial diversity in marine benthic mats, with the description of Sirenicapillariaceae fam. nov., two genera Sirenicapillaria gen. nov. and Tigrinifilum gen. nov., and seven new species. Phycologia 2022, 61, 146–165. [Google Scholar] [CrossRef]
- Fernandes, V.M.C.; Giraldo-Silva, A.; Roush, D.; Garcia-Pichel, F. Coleofasciculaceae, a monophyletic home for the Microcoleus steenstrupii complex and other desiccation-tolerant filamentous cyanobacteria. J. Phycol. 2021, 57, 1563–1579. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Waterbury, J.B. The cyanobacteria—Isolation, purification and identification. Prokaryotes 2006, 4, 10531073. [Google Scholar]
- Neilan, B.A.; Jacobs, D.; Del Dot, T.; Blackall, L.L.; Hawkins, P.R.; Cox, P.T.; Goodman, A.E. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Bacteriol. 1997, 47, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Lèpere, C.; Wilmotte, A.; Meyer, B. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Syst. Geogr. Plants 2000, 70, 275–283. [Google Scholar] [CrossRef]
- Boyer, S.L.; Flechtner, V.R.; Johansen, J.R. Is the 16S-23S rRNA internal transcribed spacer region a good tool for use in molecular systematics and population genetics? A case study in cyanobacteria. Mol. Biol. Evol. 2001, 18, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Tanabe, A.S. Kakusan4 and Aminosan: Two programs for comparing nonpartitioned proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Mol. Ecol. Resour 2011, 11, 914–921. [Google Scholar] [CrossRef]
- Tracer v1.6. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 27 February 2025).
- FigTree v1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 27 February 2025).
- Iteman, I.; Rippka, R.; de Marsac, T.N.; Herdman, M. Comparison of conserved structural and regulatory domains within divergent 16S rRNA-23S rRNA spacer sequences of cyanobacteria. Microbiology 2000, 146, 1275–1286. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nuc. Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glockner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzeby, J.; Amann, R.; Rossello-Mora, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Erwin, P.M.; Thacker, R.W. Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge hosts. Mol. Ecol. 2008, 17, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- González-Resendiz, L.; Johansen, J.R.; Alba-Lois, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Jimenez Garcia, L.F.; Hauer, T.; León-Tejera, H. Nunduva, a new marine genus of Rivulariaceae (Nostocales, Cyanobacteria) from marine rocky shores. Fottea 2018, 18, 86–105. [Google Scholar] [CrossRef]
- Johansen, J.R.; Casamatta, D.A. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algol. Stud. 2005, 116, 71–93. [Google Scholar] [CrossRef]
- Johansen, J.; Kovácik, L.; Casamatta, D.A.; Fucikova, K.; Kaštovský, J. Utility of 16S-23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: Leptolyngbya corticola sp. nov. (Pseudanabaenaceae, Cyanobacteria). Nova Hedwig. 2011, 92, 283–302. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- Perkerson III, R.B.; Johansen, J.R.; Kovácik, L.; Brand, J.; Kaštovský, J.; Casamatta, D.A. A unique Pseudanabaenalean (Cyanobacteria) genus Nodosilinea gen. nov. based on morphological and molecular data. J. Phycol. 2011, 47, 1397–1412. [Google Scholar] [CrossRef]
- Pietrasiak, N.; Mühlsteinová, R.; Siegesmund, M.A.; Johansen, J.R. Phylogenetic placement of Symplocastrum (Phormidiaceae, Cyanophyceae) with a new combination S. californicum and two new species: S. flechtnerae and S. torsivum. Phycologia 2014, 53, 529–541. [Google Scholar] [CrossRef]
- Řeháková, K.; Johansen, J.R.; Casamatta, D.A.; Li, X.; Vincent, J. Morphological and molecular characterization of selected desert soil cyanobacteria: Three species new to science including Mojavia pulchra gen. et sp. nov. Phycologia 2007, 46, 481–502. [Google Scholar] [CrossRef]
- Siegesmund, M.A.; Johansen, J.R.; Karsten, U.; Friedl, T. Coleofasciculus gen. nov. (Cyanobacteria): Morphological and molecular criteria for revision of the genus Microcoleus Gomont. J. Phycol. 2008, 44, 1572–1585. [Google Scholar] [CrossRef]
- Vázquez-Martínez, J.; Gutierrez-Villagomez, J.M.; Fonseca-Garc, C.; Ramírez-Chávez, E.; Mondragón-Sánchez, M.L.; Partida-Martínez, L.; Johansen, J.R.; Molina-Torres, J. Nodosilinea chupicuarensis sp. nov. (Leptolyngbyaceae, Synechococcales) a subaerial cyanobacterium isolated from a stone monument in central Mexico. Phytotaxa 2018, 334, 167–182. [Google Scholar] [CrossRef]
- Singh, T.; Bhadury, P. Description of a new marine planktonic cyanobacterial species Synechococcus moorigangaii (Order Chroococcales) from Sundarbans mangrove ecosystem. Phytotaxa 2019, 393, 263–277. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Selão, T.T.; Norling, B.; Nixon, P.J. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun. Biol. 2020, 3, 215. [Google Scholar] [CrossRef]
- Wang, H.R.; Song, J.H.; Lee, N.J.; Kim, D.H.; Kim, S.W.; Lee, O.M. Six newly recorded species of epilithic cyanobacteria isolated in Korea. J. Species Res. 2024, 13, 10–31. [Google Scholar] [CrossRef]
- Chen, J.X.; Cai, F.F. Allocoleopsis (Coleofasciculales, Cyanobacteria), a new record genus of terrestrial cyanobacteria in China. Acta Hydrobiol. Sin. 2024, 49, 1–9. [Google Scholar] [CrossRef]
- Mehda, S.; Muñoz-Martín, M.A.; Oustani, M.; Hamdi-Aïssa, B.; Perona, E.; Mateo, P. Microenvironmental conditions drive the differential cyanobacterial community composition of biocrusts from the Sahara Desert. Microorganisms 2021, 9, 487. [Google Scholar] [CrossRef]
- Tang, S.; Pichugin, Y.; Hammerschmidt, K. An environmentally induced multicellular life cycle of a unicellular cyanobacterium. Curr. Biol. 2023, 33, 764–769. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jiao, M.; Zhao, Z.; Wang, Y.H.; Li, T.; Wei, Y.; Li, R.; Yang, F. Insight into the role of niche concept in deciphering the ecological drivers of MPs-associated bacterial communities in mangrove forest. Water Res. 2024, 249, 120995. [Google Scholar] [CrossRef]
- Hasan, J.; Shaha, D.C.; Kundu, S.R.; Yusoff, F.M.; Cho, Y.; Haque, F.; Salam, M.A.; Ahmed, S.; Wahab, M.A.; Ahmed, M.; et al. Phytoplankton community in relation to environmental variables in the tidal mangrove creeks of the Pasur River Estuary, Bangladesh. Conservation 2022, 2, 587–612. [Google Scholar] [CrossRef]
- Hentschke, G.S.; Sant’Anna, C.L. Current trends and prospects for cyanobacterial taxonomy—Are only cultured populations enough? Algol. Stud. 2015, 147, 3–6. [Google Scholar] [CrossRef]
- Cordeiro, R.; Luz, R.; Lage, S.; Menezes, C.; Dias, E.; Flores, C.; Fonseca, A.; Gonçalves, V. Metabolomic and taxonomic characterization of Haloleptolyngbya lsitanica sp. nov. (Cyanobacteria, Synechococcales). Phycologia 2024, 63, 225–234. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Garcia-Pichel, F.; Hernández-Mariné, M. Polyphasic characterization of benthic, moderately halophilic, moderately thermophilic cyanobacteria with very thin trichomes and the proposal of Halomicronema excentricum gen. nov., sp. nov. Arch. Microbiol. 2002, 177, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, C.D.; Bohunická, M.; Johansen, J.R. We are doing it wrong: Putting homology before phylogeny in cyanobacterial taxonomy. J. Phycol. 2024, 60, 1071–1089. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kaštovský, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef]
- Komárek, J. Cyanoprokaryota. 3. Teil/3rd Part: Heterocytous Genera. In Süßwasserflora von Mitteleuropa, Bd. 19/3; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Heidelberg, Germany, 2013; pp. 67–1131. [Google Scholar]

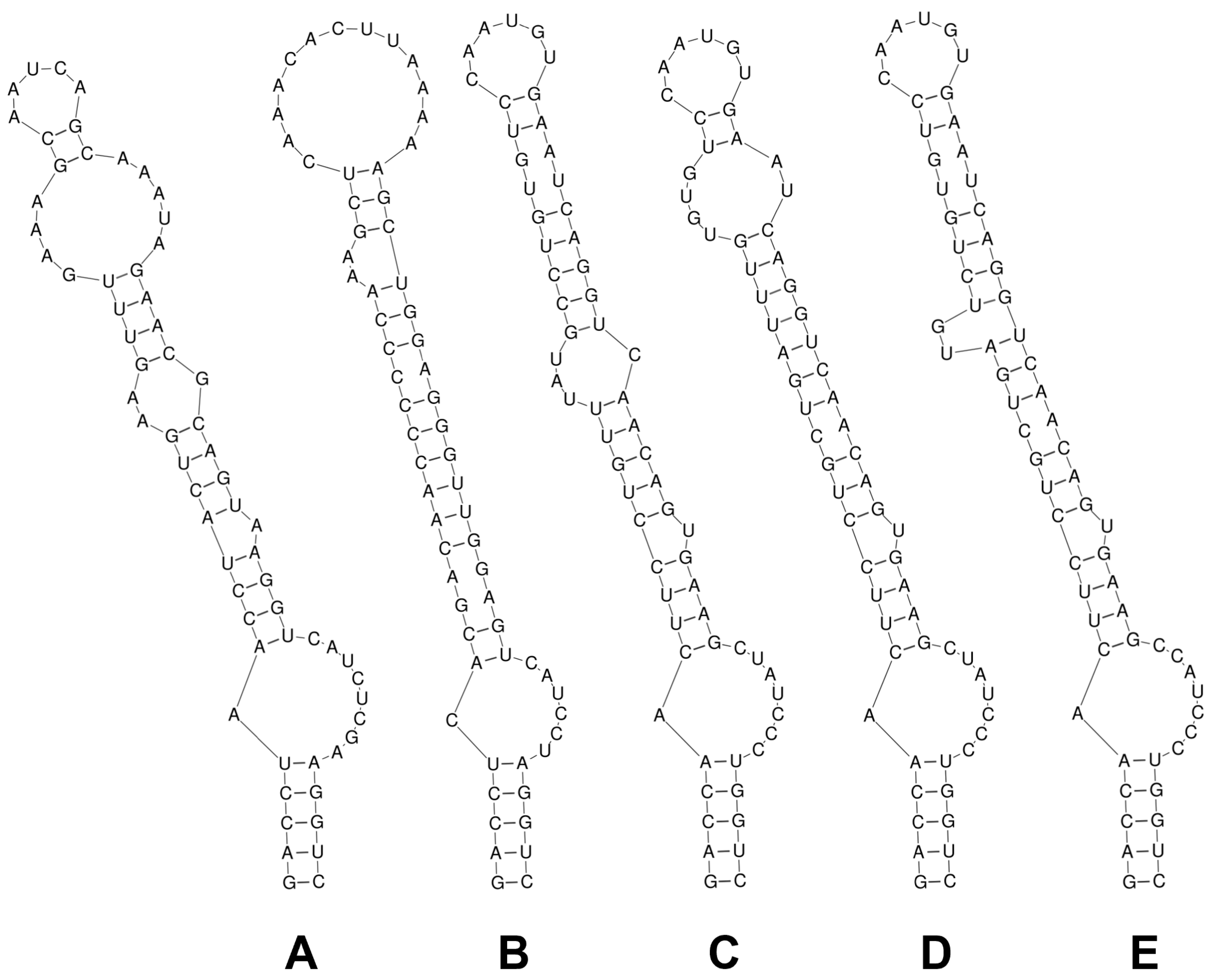
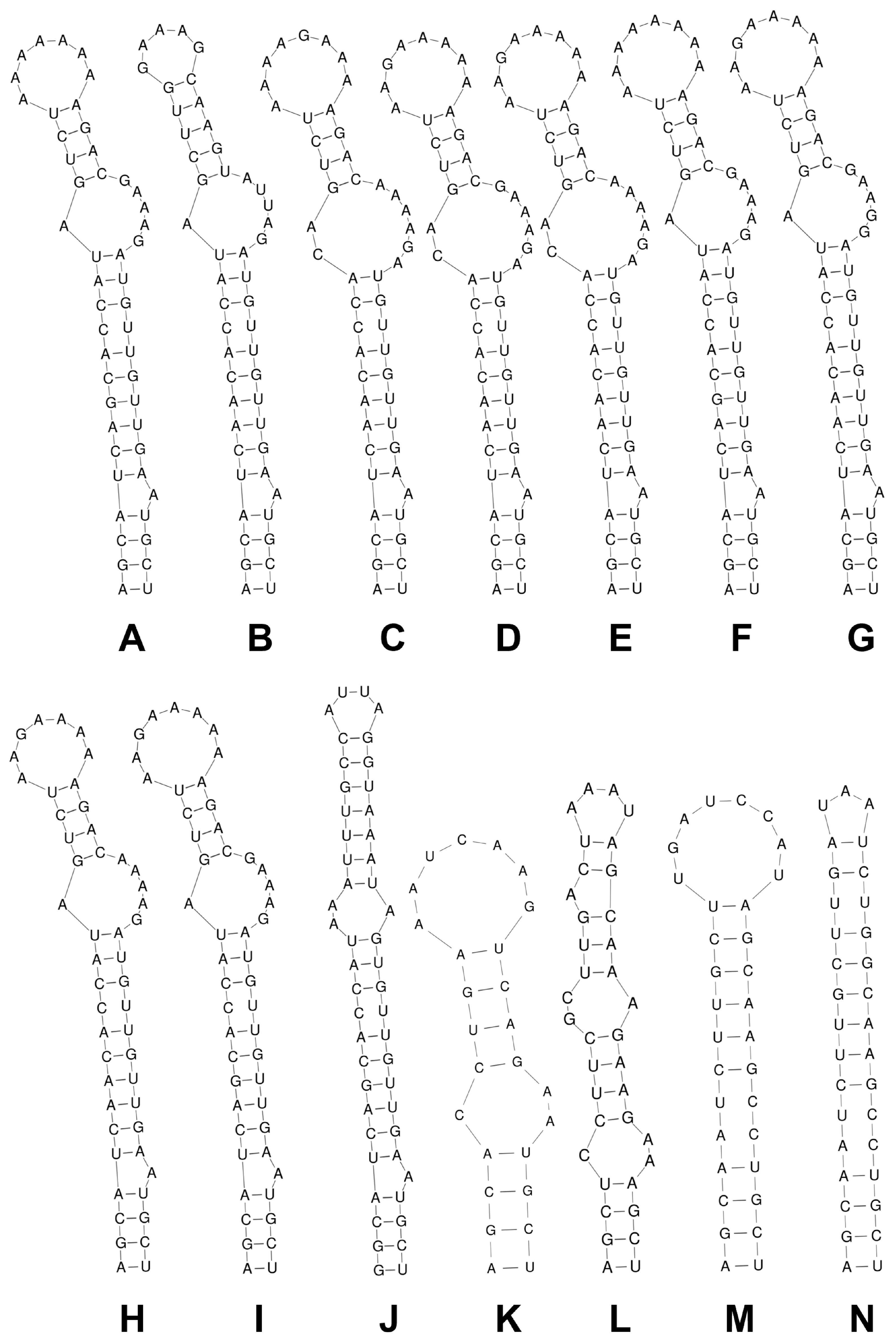
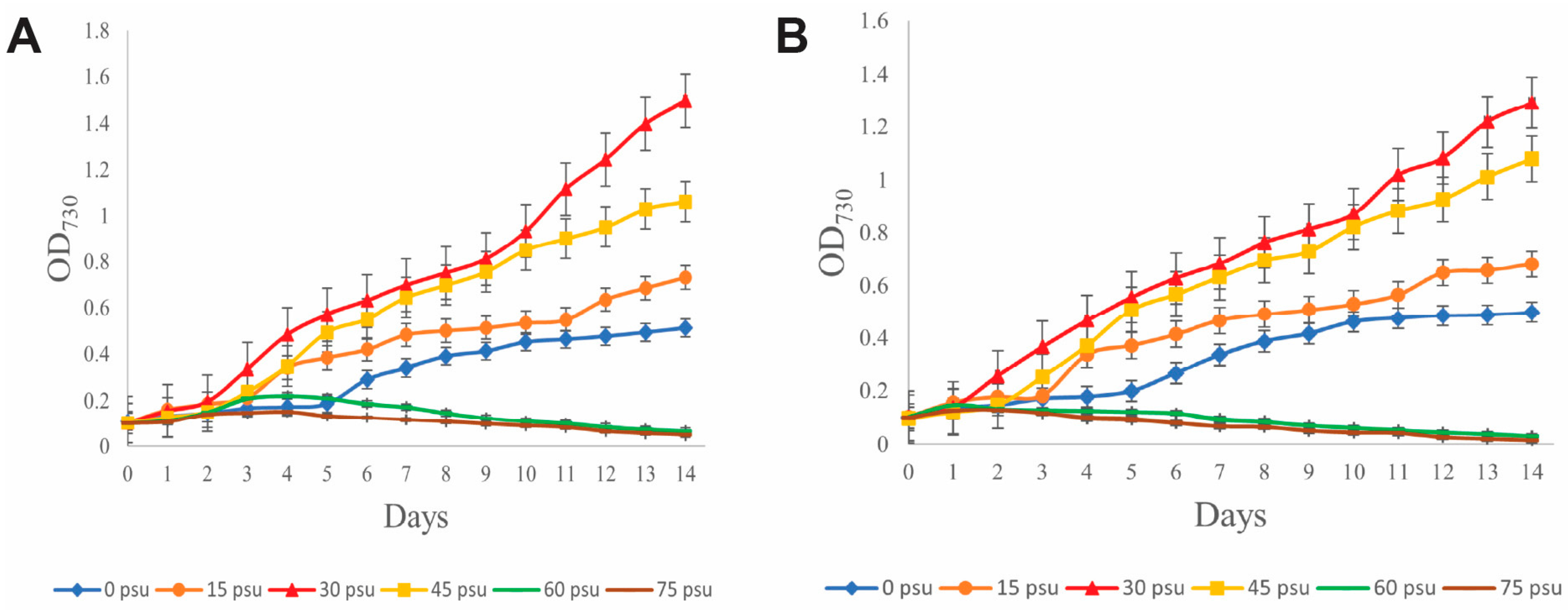
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P. mangrovensis FFKU-ACRSD 1e11 | ||||||||||||
| 2 | P. mangrovensis FFKU-HKT 2d10 | 0 | |||||||||||
| 3 | “Synechococcus” elongatus BDU 141741 | 0.9 | 0.9 | ||||||||||
| 4 | “Synechococcus” elongatus BDU 10144 | 0.9 | 0.9 | 1.1 | |||||||||
| 5 | “Synechococcus” elongatus BDU 70542 | 1.1 | 1.1 | 1.3 | 0.9 | ||||||||
| 6 | “Synechococcus” elongatus BDU 140431 | 1.3 | 1.3 | 1.7 | 1.5 | 0.9 | |||||||
| 7 | Picosynechococcus sp. NIES 970 | 14.5 | 14.5 | 5.2 | 5.4 | 4.9 | 5.4 | ||||||
| 8 | Picosynechococcus sp. PCC 73109 | 9.4 | 9.4 | 0.9 | 0.9 | 1.1 | 1.3 | 5.1 | |||||
| 9 | Picosynechococcus sp. PCC 11901 | 10.4 | 10.4 | 1.5 | 1.1 | 0.2 | 1.1 | 4.9 | 1.2 | ||||
| 10 | Picosynechococcus sp. PCC 7117 | 2.0 | 2.0 | 1.5 | 1.5 | 1.3 | 1.5 | 0.6 | 1.6 | 1.5 | |||
| 11 | Picosynechococcus sp. PCC 7002 | 10.8 | 10.8 | 1.7 | 1.7 | 1.1 | 1.3 | 5.5 | 1.3 | 1.0 | 1.3 | ||
| 12 | Picosynechococcus sp. PCC 7003 | 10.9 | 10.9 | 5.4 | 5.4 | 5.4 | 5.7 | 5.7 | 5.1 | 5.1 | 6.1 | 5.3 | |
| 13 | Picosynechococcus sp. PCC 8807 | 9.2 | 9.2 | 0.9 | 0.9 | 1.1 | 1.3 | 5.6 | 0.2 | 1.1 | 1.6 | 1.3 | 5.1 |
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Sirenicapillaria glauca FFKU-RBBB 15 | ||||||||
| S. glauca FFKU-RBBB 8 | 0 | |||||||
| S. glauca FFKU-RBBB 12 ** | 29.9 | 34.0 | ||||||
| S. glauca FFKU-RBBB 11 ** | 29.9 | 34.0 | 0 | |||||
| S. glauca BLCC-M125 clone B | 1.1 | 1.1 | 27.0 | 27.0 | ||||
| S. rigida BLCC-M116 clone A | 8.6 | 8.6 | 27.4 | 27.4 | 8.8 | |||
| S. rigida BLCC-M116 clone B ** | 34.7 | 39.0 | 5.9 | 5.9 | 28.4 | 24.4 | ||
| S. stauglerae BLCC-M121 clone C | 19.1 | 19.1 | 38.4 | 38.4 | 18.9 | 18.9 | 39.2 | |
| S. stauglerae BLCC-M121 clone A ** | 44.8 | 49.6 | 9.6 | 9.6 | 37.9 | 38.6 | 9.7 | 23.9 |
| Taxa | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1 | Allocoleopsis franciscana FFKU-PNG 10g9 * | ||||||
| 2 | A. franciscana PCC 7113 * | 0.4 | |||||
| 3 | Allocoleopsis sp. YJ-1 clone 1 | 14.0 | 14.1 | ||||
| 4 | Allocoleopsis sp. YJ-1 clone 2 | 14.3 | 14.4 | 0.2 | |||
| 5 | “Microcoleus” steenstrupii LSB38 clone 2 | 14.2 | 14.4 | 2.7 | 3.0 | ||
| 6 | “Microcoleus” steenstrupii LSB78 clone 4 | 17.3 | 17.5 | 7.2 | 7.5 | 5.7 | |
| 7 | “Microcoleus” steenstrupii LSB78 clone 1 | 17.6 | 17.9 | 7.5 | 7.8 | 5.8 | 0.2 |
| Taxon | P. mangrovensis | P. fontinalis | “Synechococcus” moorigangae | Picosynechococcus sp. |
|---|---|---|---|---|
| Strains | FFKU-ACRSD 1e11, FFKU-HKT 2d10 | FG-1 | CMS01 | PCC 11901 |
| Cell morphology | solitary, oval to cylindrical, connected in short to very long filamentous formations up to 160 cells | solitary, slightly oval | solitary, oval to cylindrical, form short chain filaments of 4 to 16 cells | solitary, elongated, form short filaments of 2 to 6 cells regardless of growth phase |
| Cell length (μm) | 1.7–4.0 | 1.2–3.0 | 1.2–3.0 | 1.5–3.5 |
| Cell width (μm) | 1.3–2.5 | 0.8–2.0 | 0.8–2.0 | 1–1.5 |
| Habitat/locality | on tree bark, Ranong, Thailand; on stump, Phuket, Thailand | planktic, periphytic, or metaphytic in freshwater pool, Bulgaria | Sundurban mangrove, India | Planktonic, Johor Strait, Singapore |
| Reference | This study | [16] | [46] | [47] |
| Strains | FFKU-PNG 10g9 | PCC 7113 | ACKU 679 | YJ-1 | LSB78 | LSB38 |
|---|---|---|---|---|---|---|
| Filament morphology in culture | erect or fasciculate, solitary or forming rope-like bundles | solitary, forming rope-like bundles | fasciculate | solitary, forming rope-like bundles | forming rope-like bundles | |
| Cell length (μm) | 3.4–8.7 | 6–7.5 | 4.1–6.8 | 4–7 | 3.4–6.7 | 4–9.4 |
| Cell width (μm) | 3.4–5.0 | 5–6 | 4.6–5.4 | 5–7 | 5.3–7.7 | 4.4–8.4 |
| Trichome | heteropolar, cross-walls slightly constricted | heteropolar, cross-walls slightly constricted | heteropolar, cross-walls slightly constricted | heteropolar, cross-walls slightly constricted | - | - |
| Apical cell | rounded, elongated, conical or bent | rounded, slightly conical | rounded to conical | rounded to conical | - | - |
| Reproduction | hormogonia, necridia | - | hormogonia, necridia | hormogonia | - | - |
| Habitat/locality | mangrove soil, Phang-Nga, Thailand | orchid greenhouse soil and biocrust, USA | freshwater gravel, South Korea | moist soil nearby an IKEA building, Wuhan, China | desert soil crust, Taleb Larbi, Algeria | |
| References | This study | [19] | [48] | [49] | [50] | |
| Strains | FFKU-RBBB 8, 11, 12, 15 | BLCC-M125 |
|---|---|---|
| Thallus morphology in culture | pale to dark green, free-floating globose colony with filaments radiating from a common center | light to dark blue-green, erect, loose |
| Filament width (µm) | 10.9–18.0 | 18.4–25.2(–28) |
| Cell length (μm) | 0.9–2.2 | 1.3–2.8 |
| Cell width (μm) | 8.9–11.8 (12.8) | (14–)15–20(–21) |
| Trichome | cylindrical, rarely attenuated towards the ends, forming knot-like arrangement or two trichomes in the same sheath | cylindrical, attenuated towards the ends, cross-walls slightly constricted |
| Apical cell | rounded, slightly conical, no calyptra | rounded, conical, occasional calyptra |
| Reproduction | single cell release, straight or diagonal trichome fragmentation, hormogonia, necridia, false branching | single cell release, straight or diagonal trichome fragmentation, hormogonia, necridia |
| Habitat/locality | epilithic, mangrove forest, Ban Bang Ben, Ranong | benthic, growing on seagrass, Florida, USA |
| Reference | This study | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim Chun Ginn, B.; Merican, F.; Praiboon, J.; Maneekat, S.; Muangmai, N. Hidden Microbial Diversity in Mangrove Depths: New Cyanobacterial Species of Picosynechococcus and Two New Records of Sirenicapillaria and Allocoleopsis from the Andaman Coast of Thailand. Diversity 2025, 17, 319. https://doi.org/10.3390/d17050319
Lim Chun Ginn B, Merican F, Praiboon J, Maneekat S, Muangmai N. Hidden Microbial Diversity in Mangrove Depths: New Cyanobacterial Species of Picosynechococcus and Two New Records of Sirenicapillaria and Allocoleopsis from the Andaman Coast of Thailand. Diversity. 2025; 17(5):319. https://doi.org/10.3390/d17050319
Chicago/Turabian StyleLim Chun Ginn, Billy, Faradina Merican, Jantana Praiboon, Sinchai Maneekat, and Narongrit Muangmai. 2025. "Hidden Microbial Diversity in Mangrove Depths: New Cyanobacterial Species of Picosynechococcus and Two New Records of Sirenicapillaria and Allocoleopsis from the Andaman Coast of Thailand" Diversity 17, no. 5: 319. https://doi.org/10.3390/d17050319
APA StyleLim Chun Ginn, B., Merican, F., Praiboon, J., Maneekat, S., & Muangmai, N. (2025). Hidden Microbial Diversity in Mangrove Depths: New Cyanobacterial Species of Picosynechococcus and Two New Records of Sirenicapillaria and Allocoleopsis from the Andaman Coast of Thailand. Diversity, 17(5), 319. https://doi.org/10.3390/d17050319







