Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior
Abstract
1. Introduction
2. Materials and Methods
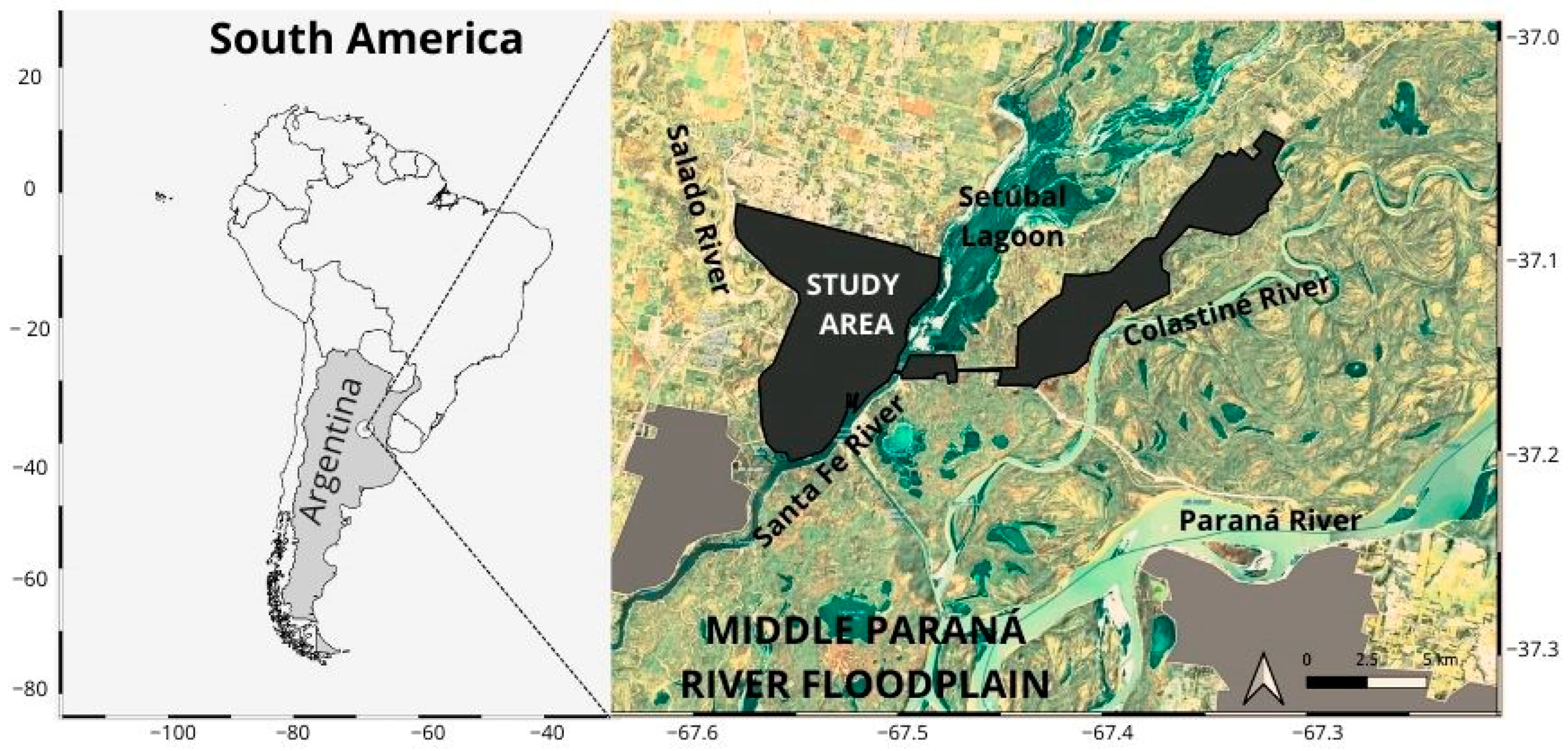
2.1. Study Area
2.2. Sampling Methods
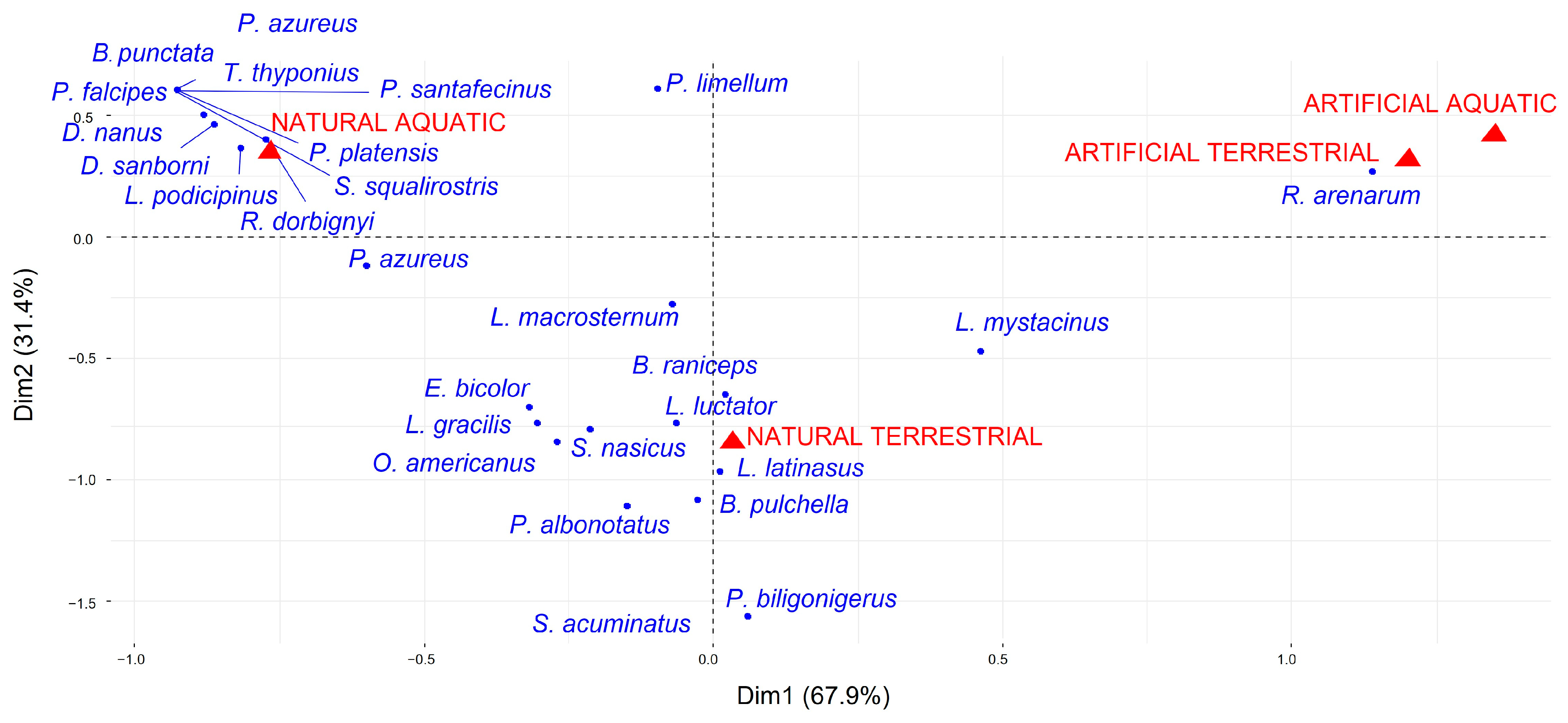
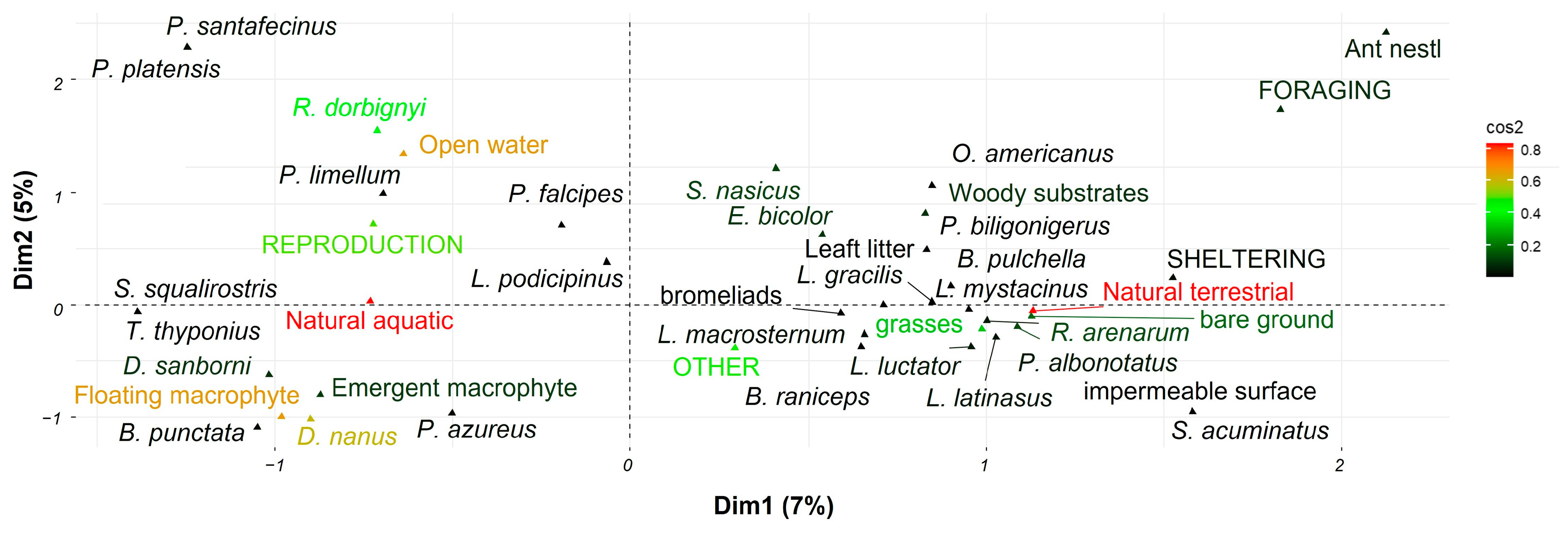
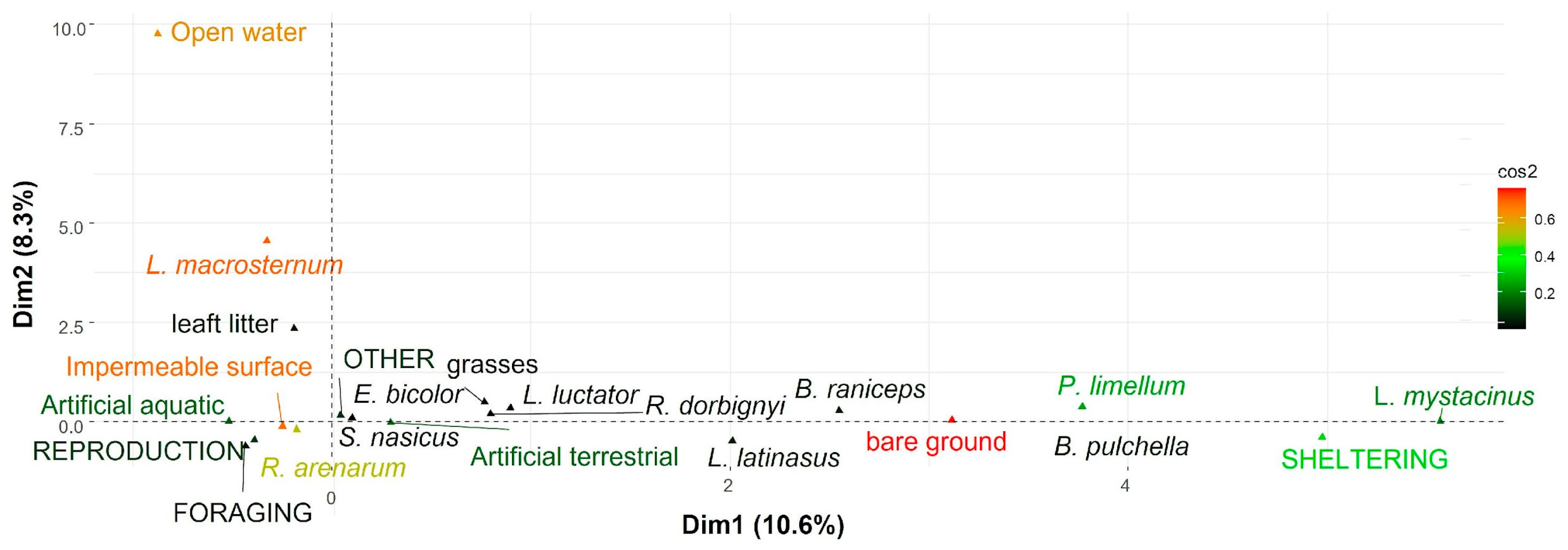
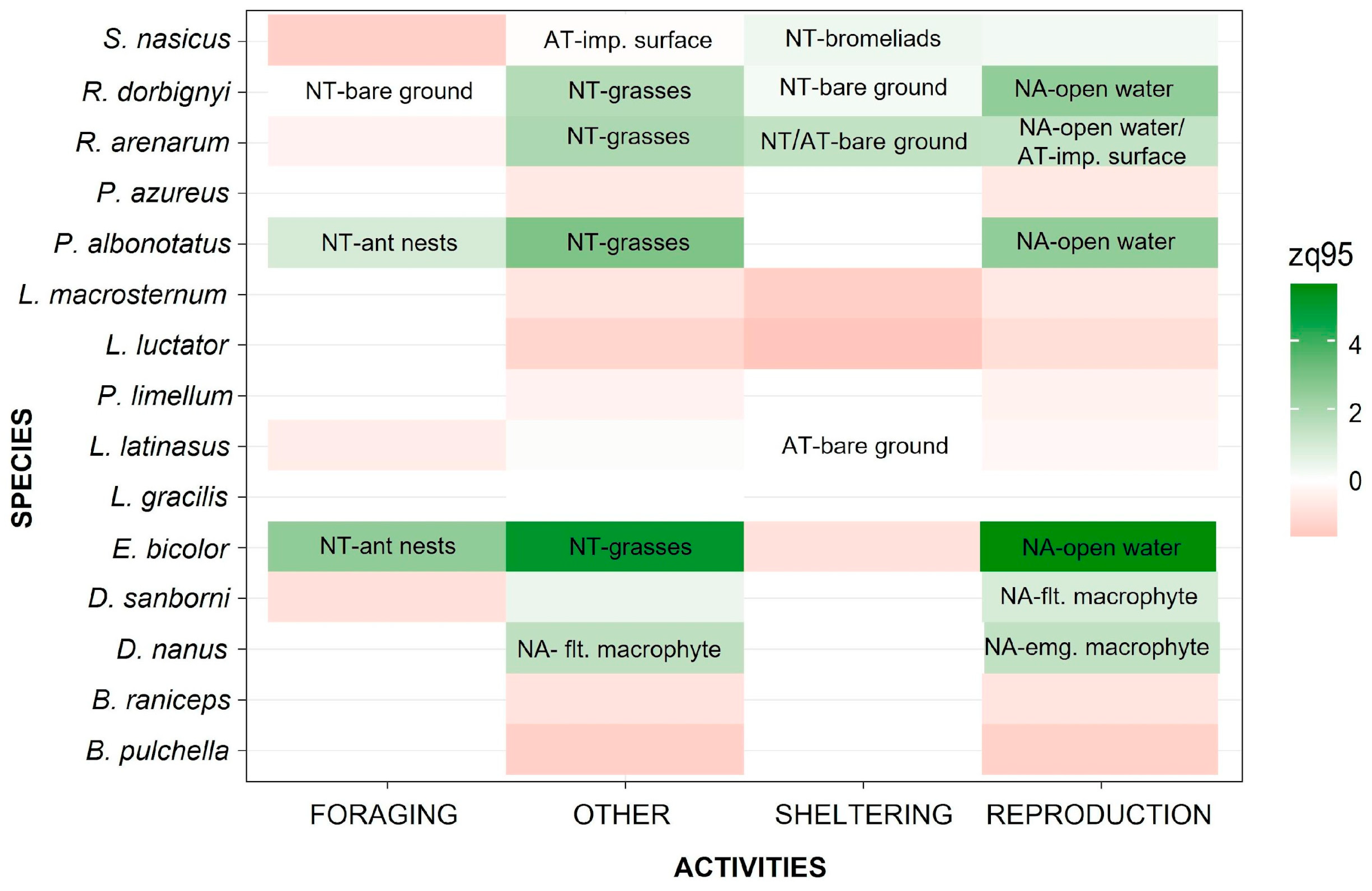
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOAJ | Directory of Open Access Journals |
| TLA | three-letter acronym |
| LD | linear dichroism |
Appendix A
| Species | Habitat | Microhabitat |
|---|---|---|
| Scinax nasicus | Natural terrestrial | Natural aquatic open water |
| Natural terrestrial bromeliad | ||
| Natural terrestrial woody substrates | ||
| Rhinella dorbignyi | Natural aquatic | Natural aquatic open water |
| Natural aquatic grasses | ||
| Natural aquatic bare ground | ||
| Rhinella arenarum | Artificial aquatic | Artificial aquatic impermeable surface |
| Artificial terrestrial | Artificial terrestrial impermeable surface | |
| Pithecopus azureus | Natural aquatic emergent macrophyte | |
| Natural terrestrial emergent macrophyte | ||
| Physalaemus albonotatus | Natural terrestrial | Natural terrestrial grasses |
| Leptodactylus macrosternum | Natural terrestrial | Artificial aquatic water |
| Natural aquatic grasses | ||
| Natural terrestrial grasses | ||
| Natural terrestrial bare ground | ||
| Leptodactylus luctator | Natural terrestrial | Natural aquatic bare ground |
| Natural terrestrial grass | ||
| Natural terrestrial leaf litter | ||
| Natural terrestrial bare ground | ||
| Lysapsus limellum | Artificial terrestrial | Artificial terrestrial bare ground |
| Natural aquatic open water | ||
| Leptodactylus latinasus | Natural terrestrial | Natural aquatic bare ground |
| Natural terrestrial grasses | ||
| Natural terrestrial impermeable surface | ||
| Leptodactylus gracilis | Natural terrestrial | Natural aquatic open water |
| Natural terrestrial bare ground | ||
| Elachistocleis bicolor | Natural terrestrial | Natural aquatic open water |
| Natural terrestrial grasses | ||
| Natural terrestrial ant nest | ||
| Natural terrestrial bare ground | ||
| Dendropsophus sanborni | Natural aquatic | Natural aquatic bromeliad |
| Natural aquatic floating macrophyte | ||
| Natural aquatic emergent macrophyte | ||
| Dendropsophus nanus | Natural aquatic | Natural aquatic woody vegetation |
| Natural aquatic floating macrophyte | ||
| Natural aquatic emergent macrophyte | ||
| Boana raniceps | Natural terrestrial | Artificial terrestrial bare ground |
| Natural terrestrial woody vegetation | ||
| Boana pulchella | Natural terrestrial | Natural terrestrial woody vegetation |
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Prats, J.M.C.; Vicente-Serrano, S.M.; Sánchez, M.A.S. Los efectos de la urbanización en el clima de Zaragoza (España): La Isla de Calor y sus factores condicionantes. Boletín Asoc. Geógrafos Españoles 2005, 40, 311–327. [Google Scholar]
- Alonzo, L.A.A.; Vera, M.A.G. Pérdida de cobertura vegetal como efecto de la urbanización en Chetumal, Quintana Roo. Quivera Rev. Estud. Territ. 2010, 12, 1–19. Available online: https://www.redalyc.org/articulo.oa?id=40115676001 (accessed on 2 February 2025).
- Tuff, K.T.; Tuff, T.; Davies, K.F. A framework for integrating thermal biology into fragmentation research. Ecol. Lett. 2016, 19, 361–374. [Google Scholar] [CrossRef]
- Rojas, C.; de la Barrera, F.; Aranguíz, T.; Munizaga, J.; Pino, J. Efectos de la urbanización sobre la conectividad ecológica de paisajes metropolitanos. Rev. Univ. Geogr. 2017, 26, 155–182. Available online: https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1852-42652017000200007&lng=es&nrm=iso (accessed on 30 January 2025).
- Okamiya, H.; Kusano, T. Lower genetic diversity and hatchability in amphibian populations isolated by urbanization. Popul. Ecol. 2018, 60, 347–360. [Google Scholar] [CrossRef]
- Schmidt, C.; Garroway, C.J. The population genetics of urban and rural amphibians in North America. Mol. Ecol. 2021, 30, 3918–3929. [Google Scholar] [CrossRef]
- Sievers, M.; Parris, K.M.; Swearer, S.E.; Hale, R. Stormwater wetlands can function as ecological traps for urban frogs. Ecol. Appl. 2018, 28, 1106–1115. [Google Scholar] [CrossRef]
- Clevenot, L.; Carré, C.; Pech, P. A review of the factors that determine whether stormwater ponds are ecological traps and/or high-quality breeding sites for amphibians. Front. Ecol. Evol. 2018, 6, 40. [Google Scholar] [CrossRef]
- Bounas, A.; Keroglidou, M.; Toli, E.A.; Chousidis, I.; Tsaparis, D.; Leonardos, I.; Sotiropoulos, K. Constrained by aliens, shifting landscape, or poor water quality? Factors affecting the persistence of amphibians in an urban pond network. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1037–1049. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, Y.; Gao, S.; Wang, S. The impact of anthropogenic noise, artificial light at night and road kills on amphibians. Biodivers. Sci. 2023, 31, 22427. [Google Scholar] [CrossRef]
- Hutto, D., Jr.; Barrett, K. Do urban open spaces provide refugia for frogs in urban environments? PLoS ONE 2021, 16, e0244932. [Google Scholar] [CrossRef]
- Gagné, S.A.; Fahrig, L. Effect of landscape context on anuran communities in breeding ponds in the National Capital Region, Canada. Landsc. Ecol. 2007, 22, 205–215. [Google Scholar] [CrossRef]
- López, J.A.; Scarabotti, P.A.; Ghirardi, R. Amphibian trophic ecology in increasingly human-altered wetlands, US Geological Survey. Herpetol. Conserv. Bio. 2015, 10, 819–832. [Google Scholar]
- Pereyra, L.C.; Akmentins, M.S.; Salica, M.J.; Quiroga, M.F.; Moreno, C.E.; Vaira, M. Tolerant and avoiders in an urban landscape: Anuran species richness and functional groups responses in the Yungas’ forest of NW Argentina. Urban Ecosyst. 2021, 24, 141–152. [Google Scholar] [CrossRef]
- Buckley, L.B.; Jetz, W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B. 2007, 274, 1167–1173. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Kallimanis, A.S. Trait selection matters! A study on European amphibian functional diversity patterns. Ecol. Res. 2019, 34, 225–234. [Google Scholar] [CrossRef]
- Dalmolin, D.A.; Tozetti, A.M.; Ramos Pereira, M.J. Taxonomic and functional anuran beta diversity of a subtropical metacommunity respond differentially to environmental and spatial predictors. PLoS ONE 2019, 14, e0214902. [Google Scholar] [CrossRef]
- Nordberg, E.J.; Schwarzkopf, L. Reduced competition may allow generalist species to benefit from habitat homogenization. J. App. Ecol. 2019, 56, 305–318. [Google Scholar] [CrossRef]
- Gainsbury, A.M.; Santos, E.G.; Wiederhecker, H. Does urbanization impact terrestrial vertebrate ectotherms across a biodiversity hotspot? Sci. Total Environ. 2022, 835, 155446. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; Ganci, C.C.; Rivas, M.L. Thermoregulation and hydric balance in amphibians. In Evolutionary Ecology of Amphibians, 1st ed.; Moreno-Rueda, G., Comas, M., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 103–119. [Google Scholar]
- Sinervo, B.; Mendez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Walsh, R.P.; Ferreira, A.J. Degradation in urban areas. Curr. Opin. Environ. Sci. Health 2018, 5, 19–25. [Google Scholar] [CrossRef]
- Fangzheng, L.W.; Wang, Y.; Liang, J.; Xie, S.; Guo, S.; Li, X.; Yu, C. Urban green space fragmentation and urbanization: A spatiotemporal perspective. Forests 2019, 10, 333. [Google Scholar] [CrossRef]
- Zipperer, W.C.; Northrop, R.; Andreu, M. Urban development and environmental degradation. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: London, UK, 2020. [Google Scholar]
- Basu, T.; Das, A. Urbanization induced degradation of urban green space and its association to the land surface temperature in a medium-class city in India. Sustain. Cities Soc. 2023, 90, 104373. [Google Scholar] [CrossRef]
- Bozkurt, S.G.; Basaraner, M. Spatio-temporal investigation of urbanization and its impact on habitat fragmentation in natural ecosystems of Istanbul using Shannon’s entropy and landscape metrics in GIS. Environ. Dev. Sustain. 2024, 26, 26879–26907. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology & Behavior of Amphibians, 1st ed.; University of Chicago Press: Chicago, IL, USA, 2007; 1400p. [Google Scholar]
- Burrow, A.; Maerz, J. How plants affect amphibian populations. Biol. Rev. 2022, 97, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.L.; Stathopoulos, T.; Marey, A.M. Urban microclimate and its impact on built environment—A review. Build. Environ. 2023, 238, 110334. [Google Scholar] [CrossRef]
- Grimmond, S.U.E. Urbanization and global environmental change: Local effects of urban warming. Geogr. J. 2007, 173, 83–88. Available online: http://www.jstor.org/stable/30113496 (accessed on 22 January 2025). [CrossRef]
- Belasen, A.; Brock, K.; Li, B.; Chremou, D.; Valakos, E.; Pafilis, P.; Sinervo, B.; Foufopoulos, J. Fine with heat, problems with water: Microclimate alters water loss in a thermally adapted insular lizard. Oikos 2017, 126, 447–457. [Google Scholar] [CrossRef]
- Seebacher, F.; Alford, R.A. Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J. Herpetol. 2002, 36, 69–75. [Google Scholar] [CrossRef]
- Rittenhouse, T.A.; Harper, E.B.; Rehard, L.R.; Semlitsch, R.D. The role of microhabitats in the desiccation and survival of anurans in recently harvested oak–hickory forest. Copeia 2008, 4, 807–814. [Google Scholar] [CrossRef]
- Hutto, D.; Barrett, K. Do open spaces within an urban matrix increase anuran abundance. Herpetol. Conserv. Biol. 2022, 17, 582–592. [Google Scholar]
- Mueller, T.; Fagan, W.F. Search and navigation in dynamic environments from individual behaviors to population distributions. Oikos 2008, 117, 654–664. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.M.; Mejía-Domínguez, N.R.; Velasco, J.A.; Marske, K.A.; Rahbek, C. Amphibian functional diversity is related to high annual precipitation and low precipitation seasonality in the New World. Global Ecol. Biogeogr. 2019, 28, 1219–1229. [Google Scholar] [CrossRef]
- INDEC (Instituto Nacional de Estadística y Censos). Censo Nacional de Población, Hogares y Viviendas 2022; INDEC (Instituto Nacional de Estadística y Censos): Buenos Aires, Argentina, 2022. Available online: https://www.indec.gob.ar (accessed on 18 February 2025).
- Iriondo, M.H.; Paggi, J.C.; Parma, M.J. The Middle Paraná River; Limnology of a Subtropical Wetland; Springer: Berlin/Heidelberg, Germany, 2007; 352p. [Google Scholar]
- Demartín, R.P.; Ghirardi, R.; López, J.A. Amphibian diversity across an urban gradient in southern South America. Front. Ecol. Evol. 2024, 12, 1461147. [Google Scholar] [CrossRef]
- Abdi, H.; Valentin, D. Multiple correspondence analysis. Encycl. Meas. Stat. 2007, 2, 651–657. [Google Scholar]
- Clark, D.B.; Palmer, M.W.; Clark, D.A. Edaphic factors and the landscape-scale distributions of tropical rain forest trees. Ecology 1999, 80, 2662–2675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, version 4.4.1; R Core Team: Vienna, Austria, 2024.
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 January 2025).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 43, 1686. [Google Scholar] [CrossRef]
- Douglas, B.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. (B) 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Lista Roja de Especies Amenazadas de la UICN. Versión 2025-1. Available online: https://www.iucnredlist.org (accessed on 11 April 2025).
- Figueiredo, G.D.T.; Storti, L.F.; Lourenço-De-Moraes, R.; Shibatta, O.A.; Anjos, L.D. Influence of microhabitat on the richness of anuran species: A case study of different landscapes in the Atlantic Forest of southern Brazil. An. Acad. Bras. Cienc. 2019, 9, e20171023. [Google Scholar] [CrossRef]
- Hackett, T.D.; Sauve, A.M.C.; Maia, K.P.; Montoya, D.; Davies, N.; Archer, R.; Potts, S.G.; Tylianakis, J.M.; Vaughan, I.P.; Memmott, J. Multi-habitat landscapes are more diverse and stable with improved function. Nature 2024, 633, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Babini, M.S.; de Lourdes Bionda, C.; Salinas, Z.A.; Salas, N.E.; Martino, A.L. Reproductive endpoints of Rhinella arenarum (Anura, Bufonidae): Populations that persist in agroecosystems and their use for the environmental health assessment. Ecotoxicol. Environ. Saf. 2018, 154, 294–301. [Google Scholar] [CrossRef]
- Pollo, F.; Bionda, C.; Baraquet, M.; Otero, M.; Martino, A.; Grenat, P. Anuran responses to urbanization: Evaluating life history traits of Rhinella arenarum in urban wetlands. Curr. Zool. 2024, 43, 1–10. [Google Scholar]
- Entiauspe-Neto, O.M.; Perleberg, T.D.; de Freitas, M.A. Herpetofauna from an urban Pampa fragment in southern Brazil: Composition, structure and conservation. Check List 2016, 12, 1964. [Google Scholar] [CrossRef][Green Version]
- Vaca, A.D.; Kassor, R.G.; Curi, L.M.; Sandoval, M.T. Anomalías de los miembros de larvas de Scinax nasicus (Cope, 1862) (Anura: Hyladae), de Corrientes, Argentina. FACENA 2023, 33, 1–8. [Google Scholar] [CrossRef]
- Demartín, R.P.; Ghirardi, R.; López, J.A. High amphibian diversity throughout urban environmental heterogeneity. Urban Ecosyst. 2024, 27, 2061–2072. [Google Scholar] [CrossRef]
- Holzer, K.A.; Bayers, R.P.; Nguyen, T.T.; Lawler, S.P. Habitat value of cities and rice paddies for amphibians in rapidly urbanizing Vietnam. J. Urban Ecol. 2017, 3, juw007. [Google Scholar] [CrossRef]
- Komine, H.; Koike, S.; Schwarzkopf, L. Impacts of artificial light on food intake in invasive toads. Sci. Rep. 2020, 10, 6527. [Google Scholar] [CrossRef]
- Beranek, C.T.; Sanders, S.; Clulow, J.; Mahony, M. Predator-free short-hydroperiod wetlands enhance metamorph output in a threatened amphibian: Insights into frog breeding behaviour evolution and conservation management. Wildl. Res. 2021, 49, 360–371. [Google Scholar] [CrossRef]
- Vargová, V.; Balogová, M.; Pristašová, P.; Kaňuch, P.; Uhrin, M. Spatiotemporal dynamics in the roosting ecology of the green toad: Implications for urban planning and nature conservation. J. Nat. Conserv. 2024, 77, 126543. [Google Scholar] [CrossRef]
- Ghirardi, R.; López, J.A. Anfibios de Santa Fe, 2nd ed.; Ediciones UNL: Santa Fe, Argentina, 2020. [Google Scholar]
- Murphy, M.; Boone, M. Evaluating the role of body size and habitat type in movement behavior in human-dominated systems: A frog’s eye view. Ecol. Evol. 2022, 12, e9022. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Habitat selection and population interactions: The search for mechanism. Am. Nat. 1991, 137, S5–S28. [Google Scholar] [CrossRef]
- Torres, P.J.; Boeris, J.M.; Insaurralde, J.A.; Baldo, J.D.; Brunetti, A.E. Potential effects of dams in the geographic range expansion of hylid frogs associated with aquatic macrophytes. Herpetol. Conserv. Biol. 2021, 16, 259–270. [Google Scholar]
- Grant, B.W.; Middendorf, G.; Colgan, M.J.; Ahmad, H.; Vogel, M.B. Ecology of urban amphibians and reptiles: Urbanophiles, urbanophobes, and the urbanoblivious. In Urban Ecology: Patterns, Processes and Applications, 1st ed.; Niemelä, J., Breuste, J.H., Elmqvist, T., Guntenspergen, G., James, P., McIntyre, N.E., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 167–178. [Google Scholar]
- Baxter-Gilbert, J.; Riley, J.L.; Measey, J. Fortune favors the bold toad: Urban-derived behavioral traits may provide advantages for invasive amphibian populations. Behav. Ecol. Socio. Biol. 2021, 75, 130. [Google Scholar] [CrossRef]
- de Oliveira, F.F.R.; Eterovick, P.C. Patterns of spatial distribution and microhabitat use by syntopic anuran species along permanent lotic ecosystems in the Cerrado of southeastern Brazil. Herpetologica 2010, 66, 159–171. [Google Scholar] [CrossRef]
- Neves, M.O.; Yves, A.; Pereira Silva, E.A.; Alves, L.; Vasques, J.B.; Coelho, J.F.T.; Silva, P.S. Herpetofauna in a highly endangered area: The Triângulo Mineiro region, in Minas Gerais State, Brazil. Herpetozoa 2019, 32, 113–123. [Google Scholar] [CrossRef]
- Jiménez-Ortega, O.J.; Tílvez, K.L.; Castro-Palacios, J.; García, A.; Navas, G.R.; Ferrer-Sotelo, J.A.; Naranjo-Calderón, D.; Díaz-Castellar, J.G.; Buelvas-Meléndez, V. Diversity of anurans and use of microhabitats in three vegetation coverages of the Santuario de Flora y Fauna Los Colorados, Colombian Caribbean. Rev. Mex. Biodiv. 2024, 95, e955385. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Bodie, J.R. Are small, isolated wetlands expendable? Conserv. Biol. 1998, 12, 1129–1133. [Google Scholar] [CrossRef]
- Crnobrnja-Isailović, J.; Adrović, A.; Bego, F.; Čađenović, N.; Jurida, E.H.; Jablonski, D.; Sterijovski, B.; Glavaš, O.J. The Importance of Small Water Bodies’ Conservation for Maintaining Local Amphibian Diversity in the Western Balkans. In Small Water Bodies of the Western Balkans, 1st ed.; Pešić, V., Milošević, D., Miliša, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 351–387. [Google Scholar]
- Biggs, L.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Schwarzkopf, L.; Alford, R.A. Desiccation and Shelter-Site Use in a Tropical Amphibian: Comparing Toads with Physical Models. Func. Ecol. 1996, 10, 193–200. [Google Scholar] [CrossRef]
- Tong, Q.; Dong, W.J.; Long, X.Z.; Hu, Z.F.; Luo, Z.W.; Guo, P.; Cui, L.Y. Effects of fine-scale habitat quality on activity, dormancy, habitat use, and survival after reproduction in Rana dybowskii (Chordata, Amphibia). BMC Zool. 2023, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abukenova, V.S.; Bobrovskaya, Z.A. Invertebrate animals of landscape gardening lawn cenoses of the city of Karaganda (area of the South-East). Bull. Karaganda Univ. Ser. Biol. Med. Geogr. 2020, 99, 6–13. [Google Scholar] [CrossRef]
- Watson, C.J.; Carignan-Guillemette, L.; Turcotte, C.; Maire, V.; Proulx, R. Ecological and economic benefits of low-intensity urban lawn management. J. App. Ecol. 2020, 57, 436–446. [Google Scholar] [CrossRef]
- López, J.A.; Ghirardi, R.; Scarabotti, P.A.; Medrano, M.C. Feeding ecology of Elachistocleis bicolor (Anura, Microhylidae) in a riparian locality of Middle Paraná river. Herpetol. J. 2007, 17, 48–53. [Google Scholar]
- López, J.A.; Antoniazzi, C.E.; Lorenzón, R.; Ghirardi, R. Spatio-temporal patterns of foraging and feeding behavior of Elachistocleis bicolor (Anura: Microhylidae). Caldasia 2017, 39, 345–353. [Google Scholar] [CrossRef]
- Isacch, J.; Barg, M. Are bufonid toads specialized ant-feeders? A case test from the Argentinian flooding pampa. J. Nat. Hist. 2005, 36, 2005–2012. [Google Scholar] [CrossRef]
- Lopez, J.A.; Peltzer, P.; Lajmanovich, R.C. Dieta y solapamiento del subnicho trófico de nueve especies de leptodactílidos en el Parque General San Martín (Argentina). Rev. Esp. Herp. 2005, 19, 19–31. [Google Scholar]
- Falico, D.A.; López, J.A.; Antoniazzi, C.E.; Beltzer, A.H. Variación interpoblacional y ontogenética en la dieta de la rana llorona Physalaemus albonotatus (Anura: Leiuperidae). Rev. Mex. Biodiver. 2012, 83, 1187–1193. [Google Scholar] [CrossRef]
- Duré, M.I.; Kehr, A.I.; Schaefer, E.F. Superposición de nichos y partición de recursos entre cinco bufónidos simpátricos (Anura, Bufonidae) del noreste de Argentina. Phyllomedusa Rev. Herpetol. 2009, 8, 27–39. [Google Scholar] [CrossRef]
- Cossovich, S.; Aun, L.; Martori, R. Análisis trófico de la herpetofauna de la localidad de Alto Alegre (Depto. Unión, Córdoba, Argentina). Cuad. Herpet. 2011, 25, 11–19. Available online: https://www.scielo.org.ar/scielo.php?pid=S1852-57682011000100002&script=sci_arttext&tlng=pt (accessed on 10 February 2025).
- López, J.A.; Scarabotti, P.A.; Medrano, M.C.; Ghirardi, R. Is the red spotted green frog Hypsiboas punctatus (Anura: Hylidae) selecting its preys?: The importance of prey availability. Rev. Biol. Trop. 2009, 57, 847–857. Available online: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0034-77442009000300031&lng=en&nrm=iso (accessed on 4 February 2025). [CrossRef]
- Sinsch, U.; Hecht, K.; Kost, S.; Grenat, P.R.; Martino, A.L. Asymmetric Male Mating Success in Lek-Breeding Rhinella arenarum. Animals 2022, 12, 3268. [Google Scholar] [CrossRef] [PubMed]
- González-Bernal, E.; Greenlees, M.J.; Brown, G.P.; Shine, R. Toads in the backyard: Why do invasive cane toads (Rhinella marina) prefer buildings to bushland? Popul. Ecol. 2016, 58, 293–302. [Google Scholar] [CrossRef]
- Montezol, M.; Cassel, M.; Silva, D.; Ferreira, A.; Mehanna, M. Gametogenesis and reproductive dynamics of Rhinella schneideri (Anura: Bufonidae): Influence of environmental and anthropogenic factors. Acta Zool. 2018, 99, 93–104. [Google Scholar] [CrossRef]
- Jacinto-Maldonado, M.; García-Peña, G.E.; Lesbarrères, D.; Meza-Figueroa, D.; Robles-Morúa, A.; Salgado-Maldonado, G.; Suzán, G. Urbanization impacts parasite diversity in the cane toad Rhinella horribilis (Anura: Bufonidae). Glob. Ecol. Conserv. 2022, 38, e02275. [Google Scholar] [CrossRef]
- Mackenzie, C.; Vladimirova, V. Food in the city: The urbanized diets of Rhinella diptycha (Anura: Bufonidae), Hemidactylus mabouia (Squamata: Gekkonidae), and Tropidurus torquatus (Squamata: Tropiduridae) in Pilar, Paraguay. Herpetol. Notes 2022, 15, 523–532. [Google Scholar]
- Oliveira-Souza, A.E.; Santana, M.M.S.; Martins, M.J.L.; Anaissi, J.S.C.; Sanches, P.R.S.; Costa-Campos, C.E. Diversity of ants in the diet of Rhinella major (Anura: Bufonidae) in an urban area in North Brazil. Herpetol. Notes 2022, 15, 663–670. [Google Scholar]
- Bettencourt-Amarante, S.; Abensur, R.; Furet, R.; Ragon, C.; Herrel, A. Do human-induced habitat changes impact the morphology of a common amphibian, Bufo bufo? Urban Ecosyst. 2025, 28, 87. [Google Scholar] [CrossRef]
- Liedtke, H.C.; Müller, H.; Rödel, M.O.; Menegon, M.; Gonwouo, L.N.; Barej, M.F.; Gvoždík, V.; Schmitz, A.; Channing, A.; Nagel, P.; et al. No ecological opportunity signal on a continental scale? Diversification and life-history evolution of African true toads (Anura: Bufonidae). Evolution 2016, 70, 1717–1733. [Google Scholar] [CrossRef]
- Regis-Alves, E.; Jared, S.G.S.; Maurício, B.; Mailho-Fontana, P.L.; Antoniazzi, M.M.; Fleury-Curado, M.C.; Brodie, E.D.; Jared, C., Jr. Structural cutaneous adaptations for defense in toad (Rhinella icterica) parotoid macroglands. Toxicon 2017, 137, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bókony, V.; Üveges, B.; Verebélyi, V.; Ujhegyi, N.; Móricz, Á.M. Toads phenotypically adjust their chemical defences to anthropogenic habitat change. Sci. Rep. 2019, 9, 3163. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Vega, R.; Galván-Hernández, A.R.; Salazar-Monge, H.; Zataraín-Palacios, R.; García-Villalvazo, P.E.; Zavalza-Galvez, D.I.; Valdez-Velazquez, L.L.; Jiménez-Vargas, J.M. Antimicrobial compounds from skin secretions of species that belong to the Bufonidae Family. Toxins 2023, 15, 145. [Google Scholar] [CrossRef]
- Hamer, A.J.; Parris, K.M. Predation modifies larval amphibian communities in urban wetlands. Wetlands 2013, 33, 641–652. [Google Scholar] [CrossRef]
- Plaza, P.I.; Speziale, K.L.; Zamora-Nasca, L.B.; Lambertucci, S.A. Dogs and cats put wildlife at risk. J. Wildl. Manag. 2019, 83, 767–768. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, W.; He, K.; Zhao, K.; Zhou, C.; Wright, J.A.; Li, F. Evaluating the urban-rural differences in the environmental factors affecting amphibian roadkill. Sustainability 2023, 15, 6051. [Google Scholar] [CrossRef]
- Perry, G.; Buchanan, B.W.; Fisher, R.N.; Salmon, M.; Wise, S.E. Effects of artificial night lighting on amphibians and reptiles in urban environments. Urban Herpetol. 2008, 3, 239–256. [Google Scholar]
- Tennessen, J.B.; Parks, S.E.; Langkilde, T. Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs. Conserv. Physio. 2014, 2, cou032. [Google Scholar] [CrossRef]
- Zaffaroni-Caorsi, V.; Both, C.; Márquez, R.; Llusia, D.; Narins, P.; Debon, M.; Borges-Martins, M. Effects of anthropogenic noise on anuran amphibians. Bioacoustics 2023, 32, 90–120. [Google Scholar] [CrossRef]
- Croteau, M.C.; Hogan, N.; Gibson, J.C.; Lean, D.; Trudeau, V.L. Toxicological threats to amphibians and reptiles in urban environments. Urban Herpetol. 2008, 3, 197–209. [Google Scholar]
- Battaglin, W.A.; Smalling, K.L.; Anderson, C.; Calhoun, D.; Chestnut, T.; Muths, E. Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Sci. Total Environ. 2016, 566, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Zamudio, K.R. Genetic structure and habitat fragmentation in a salamander species with limited vagility. Conserv. Genet. 2002, 3, 265–274. [Google Scholar]

| Families (6) | Genera (12) | Species (26) | Common Name | Local Common Name |
|---|---|---|---|---|
| Bufonidae | Rhinella | R. arenarum | Argentine toad | Sapo común |
| R. dorbingyi | D’Orbigny’s toad | Sapo de panza amarilla | ||
| Hylidae | Boana | B. pulchella | Montevideo tree frog | Rana del zarzal |
| B. punctata | Polka-dot tree frog | Rana punteada | ||
| B. raniceps | Chaco tree frog | Rana trepadora chaqueña | ||
| Dendropsophus | D. nanus | Dwarf tree frog | Rana enana | |
| D. sanborni | Sanborn’s tree frog | Rana enana de Sanborni | ||
| Pseudis | P. limellum | Uruguay harlequin frog | Rana nadadora chica | |
| P. platensis | Paradoxical frog | Rana paradoxa | ||
| Scinax | S. acuminatus | Mato Grosso snouted tree frog | Rana hocicuda chaqueña | |
| S. nasicus | Lesser snouted tree frog | Rana de los baños | ||
| S. squalirostris | Striped snouted tree frog | Rana trepadora rayada | ||
| Trachycephalus | T. typhonius | Rana Lechera Comun | Rana lechosa | |
| Leptodactylidae | Leptodactylus | L. gracilis | Dumeril’s striped frog | Rana rayada |
| L. latinasus | Oven frog | Rana piadora | ||
| L. luctator | Wrestler frog | Rana criolla | ||
| L. macrosternum | Miranda’s white-lipped frog | Rana chaqueña | ||
| L. mystacinus | Mustached frog | Rana de bigotes | ||
| L. podicipinus | Pointedbelly frog | Rana puntiaguda espumera | ||
| Physalaemus | P. albonotatus | Menwig frog | Rana llorona | |
| P. biligonigerus | Weeping frog | Rana llorona | ||
| P. santafecinus | Helvetia dwarf frog | Rana llorona | ||
| Pseudopaludicola | P. falcipes | Hensel’s swamp frog | Rana enana de hensel | |
| Microhylidae | Elachistocleis | E. bicolor | Two-colored oval frog | Rana aceituna |
| Odontophrynidae | Odontophrynus | O. americanus | Common lesser escuerzo | Escuerzo común |
| Phyllomedusidae | Pithecopus | P. azureus | Earless monkey leaf frog | Rana mono |
| Species | Artificial | Natural | ||||
|---|---|---|---|---|---|---|
| Aquatic | Terrestrial | Total | Aquatic | Terrestrial | Total | |
| R. arenarum | 157 (30) | 245 (47) | 402 (77) | 25 (5) | 98 (18) | 123 (23) |
| R. dorbignyi | 1 (0.6) | 3 (2) | 4 (2.6) | 140 (88) | 15 (9.4) | 155 (97.4) |
| B. pulchella | 0 | 1 (6) | 1 (6) | 3 (17) | 14 (77) | 17 (94) |
| B. punctata | 0 | 0 | 0 | 9 (100) | 0 | 9 (100) |
| B. raniceps | 0 | 3 (16) | 3 (16) | 5 (26) | 11 (58) | 16 (84) |
| D. nanus | 0 | 0 | 0 | 300 (95) | 15 (5) | 315 (100) |
| D. sanborni | 0 | 0 | 0 | 70 (93) | 5 (7) | 75 (100) |
| P. limellum | 0 | 8 (35) | 8 (35) | 15 (65) | 0 | 15 (65) |
| P. platensis | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) |
| S. acuminatus | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) |
| S. nasicus | 0 | 3 (3) | 3 (3) | 29 (32) | 58 (65) | 87 (97) |
| S. squalirostris | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) |
| T. thyponius | 0 | 0 | 0 | 2 (100) | 0 | 2 (100) |
| L. gracilis | 0 | 0 | 0 | 14 (36) | 24 (64) | 38 (100) |
| L. latinasus | 1 (2.3) | 3 (7) | 4 (9.3) | 8 (18) | 32 (72.7) | 40 (90.7) |
| L. luctator | 0 | 6 (10) | 6 (10) | 16 (27) | 38 (63) | 54 (90) |
| L. macrosternum | 5 (6) | 10 (12) | 15 (18) | 33 (40) | 34 (42) | 67 (82) |
| L. mystacinus | 0 | 3 (37) | 3 (37) | 1 (13) | 4 (50) | 5 (63) |
| L. podicipinus | 0 | 0 | 0 | 8 (89) | 1 (11) | 9 (100) |
| P. albonotatus | 0 | 0 | 0 | 12 (21) | 45 (79) | 57 (100) |
| P. biligonigerus | 0 | 0 | 0 | 0 | 2 (100) | 2 (100) |
| P. santafecinus | 0 | 0 | 0 | 5 (100) | 0 | 5 (100) |
| P. falcipes | 0 | 0 | 0 | 1 (100) | 0 | 1 (100) |
| E. bicolor | 0 | 1 (0.7) | 1 (0.7) | 60 (39.3) | 92 (60) | 152 (99.3) |
| O. americanus | 0 | 0 | 0 | 1 (33) | 2 (67) | 3 (100) |
| P. azureus | 0 | 0 | 0 | 12 (67) | 6 (33) | 18 (100) |
| Species | Flt. Macro. | Emg. Macro. | Bare Ground | Open Water | Grasses | Leaf Litter | Woody Subs. | Bromeliads | Imp. Surf. | Ant Nests |
|---|---|---|---|---|---|---|---|---|---|---|
| R. arenarum | 0 | 0 | 24 (20) | 16 (13) | 78 (63) | 0 | 5 (4) | 0 | 0 | 0 |
| R. dorbignyi | 0 | 0 | 10 (6) | 122 (79) | 23 (15) | 0 | 0 | 0 | 0 | 0 |
| B. pulchella | 2 (12) | 1 (6) | 1 (6) | 0 | 0 | 0 | 13 (76) | 0 | 0 | 0 |
| B. punctata | 9 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B raniceps | 2 (13) | 3(19) | 4 (25) | 0 | 1 (6) | 1 (6) | 5 (31) | 0 | 0 | 0 |
| D. nanus | 243 (77) | 51 (16) | 1 (0.3) | 1 (0.3) | 11 (3) | 1 (0.3) | 6 (2) | 1 (0.4) | 0 | 0 |
| D. sanborni | 58 (77) | 11(15) | 0 | 0 | 3 (4) | 0 | 2 (3) | 1 (1) | 0 | 0 |
| P. limellum | 1 (7) | 0 | 0 | 14 (93) | 0 | 0 | 0 | 0 | 0 | 0 |
| P. platensis | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| S. acuminatus | 0 | 0 | 1 (50) | 0 | 0 | 0 | 0 | 0 | 1 (50) | 0 |
| S. nasicus | 0 | 0 | 6 (7) | 23 (26) | 9 (10) | 0 | 47 (54) | 2 (2) | 0 | 0 |
| S. squalirostris | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T. thyponius | 0 | 2 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L. gracilis | 0 | 0 | 18 (47) | 14 (37) | 6 (16) | 0 | 0 | 0 | 0 | 0 |
| L. latinasus | 0 | 0 | 9 (23) | 5 (13) | 25 (62) | 0 | 0 | 0 | 1 (2) | 0 |
| L. luctator | 3 (6) | 0 | 20 (37) | 8 (15) | 21 (39) | 2 (4) | 0 | 0 | 0 | 0 |
| L. macrosternum | 2 (3) | 0 | 12 (18) | 13 (19) | 40 (60) | 0 | 0 | 0 | 0 | 0 |
| L. mystacinus | 0 | 0 | 3 (60) | 1 (20) | 1 (20) | 0 | 0 | 0 | 0 | 0 |
| L. podicipinus | 0 | 0 | 3 (33) | 5 (56) | 0 | 1 (11) | 0 | 0 | 0 | 0 |
| P. albonotatus | 0 | 0 | 8 (14) | 8 (14) | 40 (70) | 0 | 0 | 0 | 0 | 1 (2) |
| P. biligonigerus | 0 | 0 | 0 | 0 | 2 (100) | 0 | 0 | 0 | 0 | 0 |
| P. santafecinus | 0 | 0 | 0 | 5 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| P. falcipes | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. bicolor | 0 | 0 | 36 (24) | 57 (37) | 50 (33) | 2 (1) | 1 (1) | 0 | 0 | 6 (4) |
| O. americanus | 0 | 0 | 0 | 1 (33) | 2 (67) | 0 | 0 | 0 | 0 | 0 |
| P. azureus | 0 | 17 (94) | 0 | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Species | Grasses | Leaf Litter | Bare Ground | Impermeable Surface | Open Water |
|---|---|---|---|---|---|
| R. arenarum | 1 (0.2) | 2 (0.5) | 16 (4) | 383 (95.3) | 0 |
| R. dorbignyi | 0 | 0 | 1 (25) | 3 (75) | 0 |
| B. pulchella | 0 | 0 | 1 (100) | 0 | 0 |
| B. raniceps | 0 | 0 | 2 (67) | 1 (33) | 0 |
| P. limellum | 0 | 0 | 8 (100) | 0 | 0 |
| S. nasicus | 0 | 0 | 0 | 3 (100) | 0 |
| L. latinasus | 0 | 0 | 1 (25) | 3 (75) | 0 |
| L. luctator | 1 (17) | 0 | 1 (17) | 4 (67) | 0 |
| L. macrosternum | 0 | 1 (7) | 0 | 11 (73) | 3 (20) |
| L. mystacinus | 0 | 0 | 3 (100) | 0 | 0 |
| E. bicolor | 0 | 0 | 0 | 1 (100) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demartín, R.P.; Ghirardi, R.; López, J.A. Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior. Diversity 2025, 17, 292. https://doi.org/10.3390/d17040292
Demartín RP, Ghirardi R, López JA. Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior. Diversity. 2025; 17(4):292. https://doi.org/10.3390/d17040292
Chicago/Turabian StyleDemartín, Rocio Pamela, Romina Ghirardi, and Javier Alejandro López. 2025. "Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior" Diversity 17, no. 4: 292. https://doi.org/10.3390/d17040292
APA StyleDemartín, R. P., Ghirardi, R., & López, J. A. (2025). Influence of Natural and Artificial Habitats and Microhabitats on Urban Amphibian Diversity and Behavior. Diversity, 17(4), 292. https://doi.org/10.3390/d17040292






