Alien Mammals in the Afrotropical Region and Their Impact on Vertebrate Biodiversity: A Review
Abstract
1. Introduction
2. Material and Methods
- Indigenous species (native, autochthonous): a species living within its natural range.
- Alien species (introduced, non-native, non-indigenous, foreign, exotic): a species introduced by human intentionally or accidentally.
- Invasive alien species (invader): a species which has been introduced, to areas not previously occupied, established a viable breeding population, spread and become a pest affecting ecosystem, local biodiversity, economy and society (including human health).
- Non-invasive alien species: introduced species with a developed viable population of low dispersal abilities, and not adversely affecting ecosystems, economy and society in the conquered areas.
- Translocated species: accidental escapee from an enclosure; it may reproduce in the wild but has not developed a viable population.
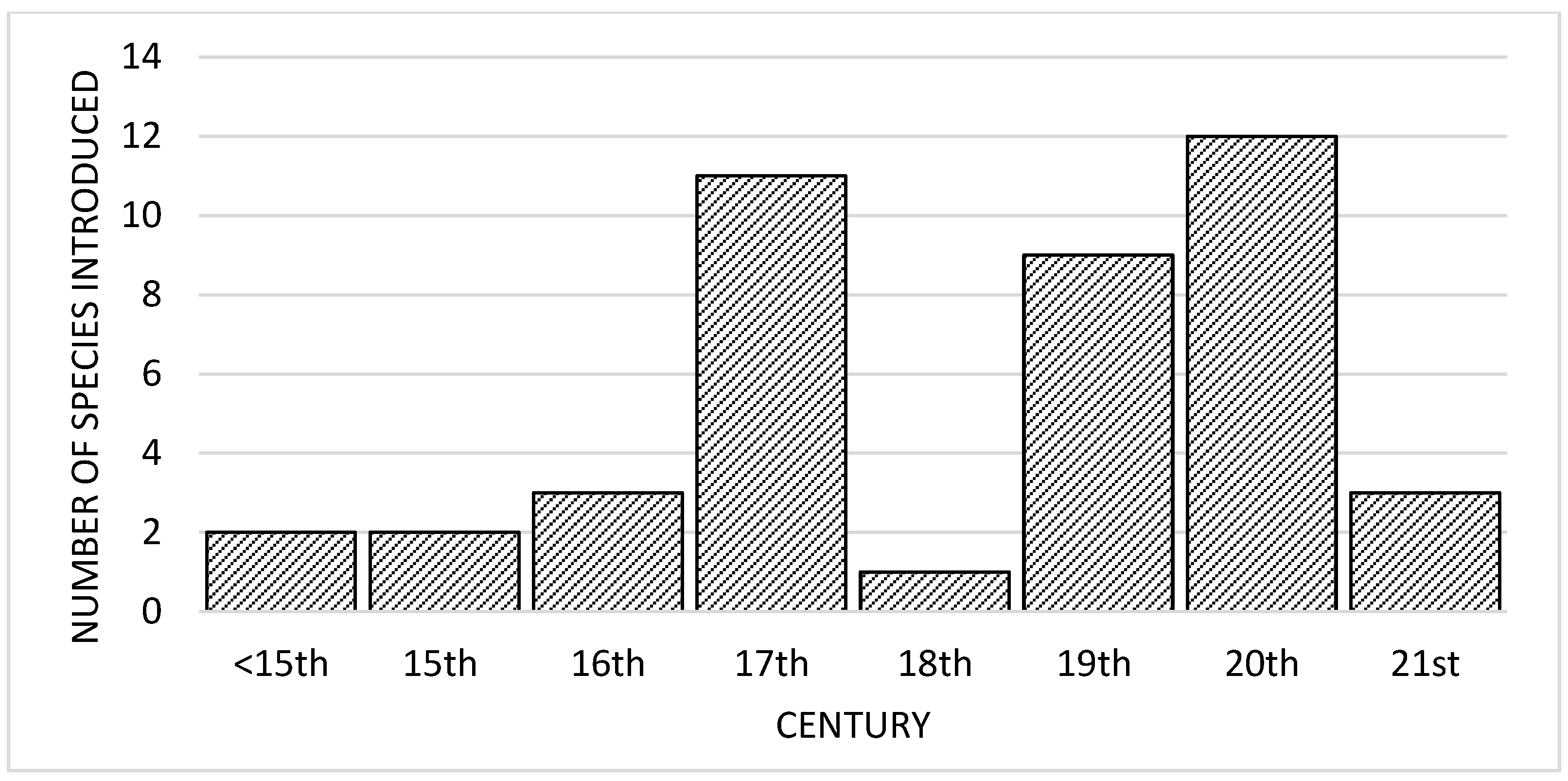
3. The Introduced Species
3.1. Feral/Domestic Species
3.1.1. Feral Cat Felis catus
3.1.2. Feral Dog Canis familiaris
3.1.3. Feral Donkey Equus asinus
3.1.4. Feral Horse Equus ferus caballus
3.1.5. Wild Boar/Feral Pig Sus scrofa
3.1.6. Feral Goats Capra hircus
3.1.7. Other Feral Mammals
3.2. Ungulates Introduced for Ranching and Hunting
3.2.1. Fallow Deer Cervus dama
3.2.2. Red Deer Cervus elaphus elaphus/Wapiti C. e. canadensis
3.2.3. Sambar Deer Rusa unicolor
3.2.4. Himalayan Tahr Hemitragus jemlahicus
3.2.5. Other Species
3.3. Leporids
3.3.1. European Rabbit Oryctolagus cuniculus
3.3.2. Black-Footed Hare Lepus nigricollis
3.4. Rodents
3.4.1. House Mouse Mus musculus
3.4.2. Black Rat (House Rat) Rattus rattus
3.4.3. Brown Rat Rattus norvegicus
3.4.4. Asian House Rat Rattus tanezumi
3.4.5. Grey Squirrel Sciurus carolinensis
3.4.6. Coypu Myocaster coypus
3.5. Carnivores
3.6. Primates
3.7. Insectivores
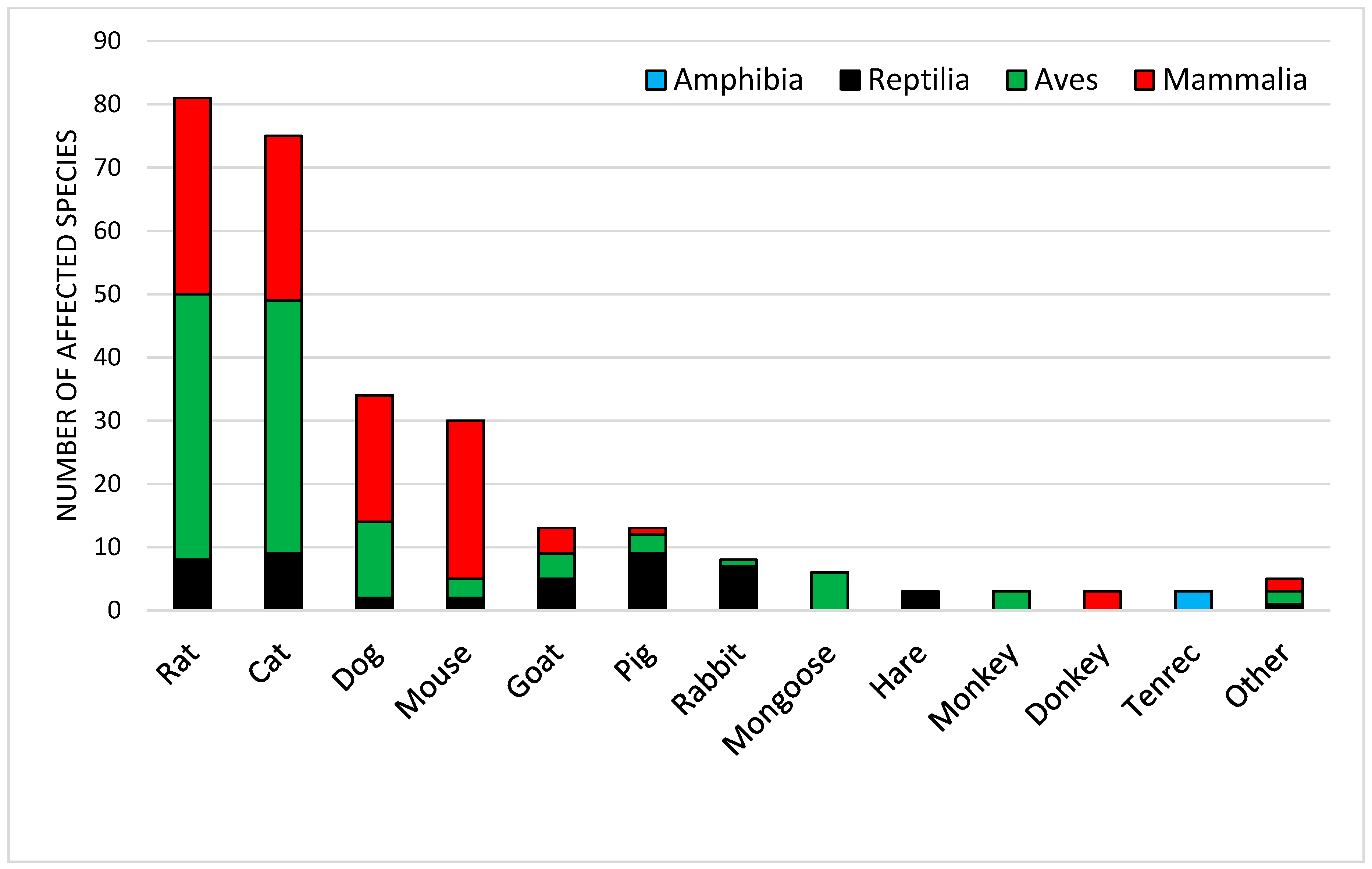
3.8. General Characteristics of the Mammal Introductions in Sub-Saharan Africa
4. Relocated Mammal Species in Sub-Saharan Africa
| Species Scientific Name | Species Common Name | Family | Original Range | Translocated to |
|---|---|---|---|---|
| Kobus ellipsiprymnus ellipsiprymnus | Waterbuck | Bovidae | South Africa | Namibia, Senegal |
| Kobus vardonii | Puku | Bovidae | South Africa | South Africa |
| Kobus leche | Lechwe | Bovidae | South Africa | South Africa |
| Syncerus caffer | African Savanna Buffalo | Bovidae | Africa | South Africa, Namibia |
| Aepyceros melampus petersi | Black-faced Impala | Bovidae | Namibia, Angola | Angola, Namibia |
| Antidorcas marsupialis | Springbok | Bovidae | South Africa | South Africa, Namibia |
| Redunca arundineum | Common Reedbuck | Bovidae | South Africa | Namibia |
| Redunca fulvorufula | Mountain Reedbuck | Bovidae | South Africa | Namibia |
| Pelea capreolus | Grey rhebok | Bovidae | South Africa | South Africa |
| Oryx dammah | Scimitar-horned Oryx | Bovidae | N Africa | South Africa |
| Oryx gazella gazella | Southern Oryx | Bovidae | South Africa | Senegal |
| Tragelaphus derbianus | Derby’s Eland | Bovidae | C and W Africa | South Africa |
| Tragelaphus euryceros | Bongo | Bovidae | Central Africa | South Africa |
| Tragelaphus imberbis | Lesser Kudu | Bovidae | NE Africa | South Africa |
| Tragelaphus scriptus | Greater Kudu | Bovidae | South Africa | South Africa; Tanzania: Rubondo (240 km2), Lake Victoria island |
| Tragelaphus angasi | Nyala | Bovidae | South Africa | Botswana, Namibia, Angola |
| Tragelaphus spekii | Sitatunga | Bovidae | Southern Africa | South Africa; Tanzania: Rubondo (240 km2), Lake Victoria island |
| Taurotragus oryx oryx | Common Eland | Bovidae | Southern Africa | Senegal |
| Cephalophus natalensis | Natal Duiker | Bovidae | South Africa | South Africa |
| Neotragus moschatus | Suni | Bovidae | South Africa | South Africa, Tanzania: Rubondo (240 km2), Lake Victoria island |
| Madoqua kirkii | Kirk’s Dik-dik | Bovidae | E Africa | South Africa |
| Capra ibex | Nubian Ibex | Bovidae | NE Africa | Namibia |
| Hippotragus niger | Sable Antelope | Bovidae | Botswana, Namibia, Angola | South Africa |
| Hippotragus equinus koba | Roan Antelope | Bovidae | West Africa | South Africa; Tanzania: Rubondo (240 km2), Lake Victoria island |
| Damaliscus pygargus dorcas | Bontebok | Bovidae | South Africa | South Africa |
| Damaliscus pygargus phillipsi | Blesbok | Bovidae | NE South Africa | SW South Africa, Botswana, Mozam-bique, Namibia, Zimbabwe, Angola |
| Beatragus hunteri | Hirola | Bovidae | SE Kenya | W Kenya (Tsavo) |
| Oreotragus oreotragus | Klipspringer | Bovidae | South Africa | South Africa |
| Connochaetes gnou | Black Wildebeest | Bovidae | Africa | Namibia |
| Connochaetes taurinus | Blue Wildebeest | Bovidae | Africa | Gabon: W.WP.R., <1986, now? |
| Giraffa camelopardalis | Giraffa | Giraffidae | South Africa | South Africa; Tanzania: Rubondo (240 km2), Lake Victoria island |
| Equus zebra hartmannae | Hartmann’s Mountain Zebra | Equidae | Namibia | South Africa: Western cape, Eastern Cape |
| Diceros bicornis michaeli | Black Rhinoceros | Rhinocerotidae | Kenya | South Africa; Tanzania: Rubondo (240 km2), Lake Victoria island |
| Ceratotherium simum simum | White Rhinoceros | Rhinocerotidae | South Africa, Kenya | Uganda, Kenya, Zambia |
| Loxodonta africana | African Elephant | Loxodontidae | Africa | Tanzania: Rubondo (240 km2), Lake Victoria island |
| Panthera leo melanochaita | African Lion | Felidae | South Africa | Rwanda |
| Colobus abyssinicus | Angola Pied Colobus | Cercopithecidae | Central Africa | Tanzania: Rubondo (240 km2), Lake Victoria island |
| Cercopithecus aethiops | Vervet Monkey | Cercopithecidae | Africa | Tanzania: Rubondo (240 km2), Lake Victoria island |
| Pan troglodytes | Chimpanzee | Hominidae | Cental Africa | Tanzania: Rubondo (240 km2), Lake Victoria island |
5. Impacts of Introduced Mammals on Vertebrate Biodiversity
6. Impact of Alien Mammals on Islands
6.1. Cape Verde
6.2. São Tomé e Principe and Other Islands in the Gulf of Guinea
6.3. Madagascar
6.4. Mascarenes
6.5. Comoros
6.6. Seychelles
6.7. Socotra
6.8. Prince Edward Islands
7. Conclusions
Funding
Conflicts of Interest
References
- Wroe, S.; Field, J.; Fullagar, R.; Jermin, L.S. Megafaunal extinction in the late Quaternary and the global overkill hypothesis. Alcheringa 2004, 28, 291–331. [Google Scholar] [CrossRef]
- Stuart, A.J. Late Quaternary megafaunal extinctions on the continents: A short review. Geol. J. 2015, 50, 338–363. [Google Scholar] [CrossRef]
- Svenning, J.C.; Lemoine, R.T.; Bergman, J.; Buitenwerf, R.; Le Roux, E.; Lundgren, E.; Mungi, N.; Pedersen, R.Ø. The late-Quaternary megafauna extinctions: Patterns, causes, ecological consequences and implications for ecosystem management in the Anthropocene. Camb. Prism. Extinction 2024, 2, e5. [Google Scholar] [CrossRef] [PubMed]
- Beinart, W. African history and environmental history. Afr. Aff. 2000, 99, 269–302. [Google Scholar] [CrossRef]
- Beinart, W. The Rise of Conservation in South Africa: Settlers, Livestock, and the Environment 1770–1950; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Southey, N. Islands in a Forgotten Sea: A History of the Seychelles, Mauritius, Réunion and Madagascar; University of South Africa: Pretoria, South Africa, 2010. [Google Scholar]
- Mitchell, P. Settling Madagascar: When Did People First Colonize the World’s Largest Island? J. Isl. Coast. Archaeol. 2020, 15, 576–595. [Google Scholar] [CrossRef]
- Neumann, R.P. The postwar conservation boom in British colonial Africa. Environ. Hist. 2002, 7, 22–47. [Google Scholar] [CrossRef]
- Munro, P. Colonial Wildlife Conservation and National Parks in Sub-Saharan Africa. In Oxford Research Encyclopedia of African History; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Groom, M.J.; Meffe, G.K.; Carroll, C.R. Principals of Conservation Biology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- King, B. Conservation geographies in Sub-Saharan Africa: The politics of national parks, community conservation and peace parks. Geogr. Compass 2010, 4, 14–27. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12, p. 12. [Google Scholar]
- Bigalke, R.C.; Pepler, D. Mammals Introduced to the Mediterranean Region of South Africa. Biogeography of Mediterranean Invasions; Cambridge University Press: Cambridge, UK, 1991; pp. 285–292. [Google Scholar]
- Measey, J.; Hui, C.; Somers, M.J. Terrestrial vertebrate invasions in South Africa. In Biological Invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Cham, Switzerland, 2020; Volume 14, pp. 787–830. [Google Scholar] [CrossRef]
- Russell, J.C.; Cole, N.C.; Zuël, N. Gérard Rocamora Introduced mammals on Western Indian Ocean islands. Glob. Ecol. Conserv. 2016, 6, 132–144. [Google Scholar]
- Genovesi, P.; Bacher, S.; Kobelt, M.; Pascal, M.; Scalera, R. Alien mammals of Europe. In Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherland, 2009. [Google Scholar]
- Ballari, S.A.; Anderson, C.B.; Valenzuela, A.E. Understanding trends in biological invasions by introduced mammals in southern South America: A review of research and management. Mammal Rev. 2016, 46, 229–240. [Google Scholar] [CrossRef]
- Rodríguez, P.J. Exotic species introductions into South America: An underestimated threat? Biodivers. Conserv. 2001, 10, 1983–1996. [Google Scholar] [CrossRef]
- Toomes, A.; García-Díaz, P.; Wittmann, T.A.; Virtue, J.; Cassey, P. New aliens in Australia: 18 years of vertebrate interceptions. Wildl. Res. 2019, 47, 55–67. [Google Scholar] [CrossRef]
- Kopij, G. Alien Birds in Sub-Saharan Africa: An Overview. Conservation 2025, 5, 16. [Google Scholar] [CrossRef]
- Llobet, T.; Velikov, I.; Sogorb, L.; Peacock, F.; Jutglar, F.; Mascarell, A.; Martí, B.; Rodríguez-Osorio, J. Mammals of the World; Lynx Nature Books: Barcelona, Spain, 2023. [Google Scholar]
- Clements, J.F.; Schulenberg, T.S.; Iliff, M.J.; Roberson, D.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Sullivan, B.L.; Smith, M.; et al. The eBird/Clements Checklist of Birds of the World. 2018. Available online: https://www.birds.cornell.edu/clementschecklist/introduction/updateindex/october-2024/2024-citation-checklist-downloads/ (accessed on 1 March 2015).
- Midtgaard, R. RepFocus—A Survey of the Reptiles of the World. 2025. Available online: https://www.repfocus.dk/ (accessed on 3 March 2025).
- ANMH (American Museum of Natural History). Amphibian Species of the World 6.2, an Online Reference. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 3 March 2025).
- Kurdila, J. The introduction of exotic species into the United States: There goes the neighborhood. Boston Coll. Environ. Aff. Law Rev. 1988, 16, 95. [Google Scholar]
- Campbell, K.; Donlan, C.J. Feral goat eradications on islands. Conserv. Biol. 2005, 19, 1362–1374. [Google Scholar] [CrossRef]
- IUNC. 100 of the World’s Worst Invasive Alien Species. Global Invasive Species Database. 2025. Available online: https://www.iucngisd.org/gisd/100_worst.php (accessed on 1 March 2025).
- Fitzgerald, B.M.; Veitch, C.R. The cats of Herekopare Island, New Zealand; their history, ecology and effects on birdlife. N. Z. J. Zool. 1985, 12, 319–330. [Google Scholar] [CrossRef]
- Winter, L.; Wallace, G.E. Impacts of feral and free-ranging cats on bird species of conservation concern. Other Publications in Wildlife Management; University of Nebraska: Lincoln, NE, USA, 2006; p. 28. [Google Scholar]
- Bonnington, C.; Gaston, K.J.; Evans, K.L. Fearing the feline: Domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J. Appl. Ecol. 2013, 50, 15–24. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Foxcroft, L.C.; Herbst, M.; MacFadyen, S. Genetic analysis shows low levels of hybridization between African wildcats (Felis silvestris lybica) and domestic cats (F. s. catus) in South Africa. Ecol. Evol. 2015, 5, 288–299. [Google Scholar] [CrossRef]
- Bester, M.N.; Bloomer, J.P.; Van Aarde, R.J.; Erasmus, B.H.; Van Rensburg, P.J.J.; Skinner, J.D.; Howell, P.G.; Naude, T.W. A review of the successful eradication of feral cats from sub-Antarctic Marion Island, Southern Indian Ocean. S. Afr. J. Wildl. Res. 2002, 32, 65–73. [Google Scholar]
- Davies, S.J.; Jordaan, M.; Karsten, M.; Terblanche, J.S.; Turner, A.A.; van Wilgen, N.J.; Veldtman, R.; Zengeya, T.A.; Measey, J. Experience and lessons from alien and invasive animal control projects in South Africa. In Biological Invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 625–660. [Google Scholar] [CrossRef]
- Doherty, T.S.; Dickman, C.R.; Glen, A.S.; Newsome, T.M.; Nimmo, D.G.; Ritchie, E.G.; Vanak, A.T.; Wirsing, A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Conserv. 2017, 210, 56–59. [Google Scholar] [CrossRef]
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Wandeler, A.I.; Matter, H.C.; Kappeler, A.; Budde, A. The ecology of dogs and canine rabies: A selective review. Rev. Sci. Tech. 1993, 12, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Merz, L.; Kshirsagar, A.R.; Rafaliarison, R.R.; Rajaonarivelo, T.; Farris, Z.J.; Randriana, Z.; Valenta, K. Wildlife predation by dogs in Madagascar. Biotropica 2022, 54, 181–190. [Google Scholar] [CrossRef]
- Macpherson, C.N.L.; Karstad, L.; Stevenson, P.; Arundel, J.H. Hydatid disease in the Turkana District of Kenya. 3. The significance of wild animals in the transmission of Echinococcus granulosus, with particular reference to Turkana and Masailand in Kenya. Ann. Trop. Med. Parasitol. 1983, 77, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Blench, R. The history and spread of donkeys in Africa. In Donkeys, People and Development. A Resource Book of the Animal Traction Network for Eastern and Southern Africa (ATNESA); ACP-EU Technical Center for Agriculture and Rural Cooperation: Wageningen, The Netherlands, 2004. [Google Scholar]
- Muller, H.; Bourne, A. Minimum population size and potential impact of feral and semi-feral donkeys and horses in an arid rangeland. Afr. Zool. 2018, 53, 139–144. [Google Scholar] [CrossRef]
- Mwenya, E.; Keib, G. History and utilization of donkeys in Namibia. In Donkeys, People and Development: A Resource Book for Eastern and Southern Africa; ATNESA: Wageningen, The Netherlands, 2004; pp. 172–174. [Google Scholar]
- Brooke, R.K.; Lloyd, P.H.; de Villiers, A.L. Alien and translocated terrestrial vertebrates in South Africa. In The Ecology and Management of Biological Invasions in Southern Africa; Macdonald, I.A.W., Kruger, F.J., Ferrar, A.A., Eds.; Oxford University Press: Cape Town, South Africa, 1986; pp. 63–74. [Google Scholar]
- Hoffmann, K.L.; Wood, A.K.; Mccarthy, P.H.; Griffiths, K.A.; Evans, D.L.; Gill, R.W. Sonographic observations of the peripheral vasculature of the equine thoracic limb. Anat. Histol. Embryol. 1999, 28, 281–289. [Google Scholar] [CrossRef]
- Greyling, T. Factors Affecting Possible Management Strategies for the Namib Feral Horses. Ph.D. Thesis, North-West University, Pochefstroom, South Africa, 2005. [Google Scholar]
- Goldbeck, G.; Greyling; Swilling, R. Wild Horses in the Namib Desert: An Equine Biography; Namibia Scientific Society: Windhoek, Namibia, 2011. [Google Scholar]
- Kefena, E.; Dessie, T. The Kundido Feral Horses: Fugitives of the Abyssinian Domestic Horses; Ethiopian Society of Animal Production (ESAP): Addis Abeba, Ethiopia, 2011; Volume 25. [Google Scholar]
- Picker, M.D.; Griffiths, C.L. Alien and Invasive Animals: A South African Perspective; Penguin Random House South Africa: Cape Town, South Africa, 2011. [Google Scholar]
- Skead, C.J.; Boshoff, A.; Kerley, G.I.H.; Lloyd, P. Introduced (non-indigenous) species: A growing threat to biodiversity. In Historical Incidence of the Larger Land Mammals in the Broader Northern and Western Cape; Skead, C.J., Boshoff, A., Gih, K., Lloyd, P., Eds.; Centre for African Conservation Ecology, Nelson Mandela Metropolitan University: Port Elizabeth, South Africa, 2011. [Google Scholar]
- van Wilgen, B.W.; Wilson, J.R. (Eds.) The Status of Biological Invasions and Their Management in South Africa in 2017; South African National Biodiversity Institute, Kirstenbosch and DST-NRF Centre of Excellence for Invasion Biology: Stellenbosch, South Africa, 2018. [Google Scholar]
- Oliver, W.L.R. (Ed.) Pigs, Peccaries and Hippos; IUCN: Gland, Switzerland, 1993. [Google Scholar]
- Greve, M.; von der Meden, C.E.O.; Janion-Scheepers, C. Biological invasions in South Africa’s offshore sub-Antarctic territories. In Biological Invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–226. [Google Scholar] [CrossRef]
- Moolman, H.J.; Cowling, R.M. The impact of elephant and goat grazing on the endemic flora of South African succulent thicket. Biol. Conserv. 1994, 68, 53–61. [Google Scholar] [CrossRef]
- Long, J.L. Introduced Mammals of the World: Their History, Distribution and Influence; CSIRO Publishing: Clayton, Australia, 2003. [Google Scholar]
- Crawford, A.B.; Serventy, D.L. The birds of Marion Island, South Indian Ocean. Emu-Austral. Ornithol. 1952, 52, 73–85. [Google Scholar] [CrossRef]
- De Villiers, M.S.; Mecenero, S.; Sherley, R.B.; Heinze, E.; Kieser, J.; Leshoro, T.M.; Merbold, L.; Nordt, A.; Parsons, N.J.; Peter, H.U. Introduced European rabbits (Oryctolagus cuniculus) and domestic cats (Felis catus) on Robben Island: Population trends and management recommendations. S. Afr. J. Wildl. Res. 2010, 40, 139–148. [Google Scholar] [CrossRef]
- Dobigny, G.; Dalecky, A. Native and Invasive Small Mammals in Urban Habitats along the Commercial Axis Connecting Benin and Niger, West Africa. Diversity 2019, 11, 238. [Google Scholar] [CrossRef]
- Dutton, J. Introduced mammals in São Tomé and Príncipe: Possible threats to biodiversity. Biodivers. Conserv. 1994, 3, 927–938. [Google Scholar] [CrossRef]
- Feiler, A. Uber die Saugetiere der Insel São Tome. Zool. Abh. Mus. Tierk. 1984, 40, 75–78. [Google Scholar]
- Feiler, A. Die Saugetiere der Inseln im Golf von Guinea und ihre Beziehungen zur Saugetierfauna der westafrikanischen Festlandes. Zool. Abh. Mus. Tierk. 1988, 44, 83–88. [Google Scholar]
- Feiler, A.; Haft, J.; Widmann, P. Beobachtungen und Untersuchungen an Saugetieren der Insel São Tome (Golf von Guinea) (Mammalia). Faun. Abh. Mus. Tierkd. Dresden 1993, 19, 21–35. [Google Scholar]
- Figueiredo, E.; Gascoigne, A. Conservation of pteridophytes in São Tomé e Príncipe (Gulf of Guinea). Biodivers. Conserv. 2001, 10, 45–68. [Google Scholar] [CrossRef]
- Frade, F. Aves e mammiferos das Ilhas de Sao Tome e do Principe—Notas de sistematica e de proteccao a fauna. Conf. Int. Dos Afr. Occidendais 1958, 4, 137–150. [Google Scholar]
- Goodman, S.M. Rattus on Madagascar and the Dilemma of Protecting the Endemic Rodent Fauna. Conserv. Biol. 1995, 9, 450–453. Available online: https://www.jstor.org/stable/2386788 (accessed on 2 April 2025). [CrossRef]
- Goodman, S.M. The New Natural History of Madagascar; Princeton University Press: Princeton, NJ, USA, 2022. [Google Scholar]
- Hinton, H.E.; Dunn, A.M.S. Mongooses, Their Natural History and Behaviour, Oliver & Boyd, 42s; Cambridge University Press: Cambridge, UK, 1967. [Google Scholar]
- Hixon, S.W.; Douglass, K.G.; Godfrey, L.R.; Eccles, L.; Crowley, B.E.; Rakotozafy, L.M.A.; Clark, G.; Haberle, S.; Anderson, A.; Wright, H.T.; et al. Ecological Consequences of a Millennium of Introduced Dogs on Madagascar. Front. Ecol. Evol. 2021, 9, 689559. [Google Scholar] [CrossRef]
- Jassat, W.; Naicker, N.; Naidoo, S.; Mathee, A. Rodent control in urban communities in Johannesburg, South Africa: From research to action. Int. J. Environ. Health Res. 2013, 23, 474–483. [Google Scholar] [CrossRef]
- Kerley, G.; Boshoff, A. The Introduction of Alien Mammals into the Broader Western and Northern Cape Provinces of South Africa; Centre for African Conservation Ecology Nelson Mandela Metropolitan University: Port Elizabeth, South Africa, 2015. [Google Scholar]
- van Wilgen, B.; Measey, J.; Richardson, D.; Wilson, J.; Zengeya, T. (Eds.) Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lever, Naturalized Animals: The Ecology of Successfully Introduced Species; Poyser Natural History: London, UK, 1994.
- Nameer, P.O.; Smith, A.T. Lepus nigricollis. In IUCN Red List of Threatened Species; UNCN: Gland, Switzerland, 2019; p. e.T41282A45188041. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World; T. 1. Johns Hopkins University Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Picker, M.D.; Griffiths, C.L. Alien animals in South Africa-composition, introduction history, origins and distribution patterns. Bothalia-Afr. Biodivers. Conserv. 2017, 47, 1–19. [Google Scholar] [CrossRef]
- Rocamora, G.; Henriette, E. Invasive Alien Species in Seychelles: Why and How to Eliminate Them. Identification and Management of Priority Species; Collection Inventaires et Biodiversité. Biotope Éditions, Mèze; Muséum National d’Histoire Naturelle: Paris, France, 2015; p. 384. [Google Scholar]
- Russell, J.C.; Towns, D.R.; Clout, M.N. Review of Rat Invasion Biology: Implications for Island Biosecurity; Science for Conservation 286; Department of Conservation: Wellington, New Zealand, 2018. [Google Scholar]
- Ryan, P. Alein and islands. Quest 2015, 11, 26–30. [Google Scholar]
- Sow, A.; Duplantier, J.M. Sharing space between native and invasive small mammals: Study of commensal communities in Senegal. Ecol. Evol. 2023, 13, e10539. [Google Scholar] [CrossRef]
- Sussman, R.W.; Tattersall, I. A Preliminary Study of the Crab-eating Macaque Macaca fascicularis in Mauritius. Mauritius Inst. Bull. 1980, 9, 31–51. [Google Scholar]
- Sussman, R.W.; Tattersall, I. Distribution, Abundance, and Putative Ecological Strategy of Macaca fascicularis on the Island of Mauritius, Southwestern Indian Ocean. Folia Primatol. 1986, 46, 28–43. [Google Scholar] [CrossRef]
- Spear, D.; Chown, S.L. Taxonomic homogenization in ungulates: Patterns and mechanisms at local and global scales. J. Biogeogr. 2008, 35, 1962–1975. [Google Scholar] [CrossRef]
- Waker, S. Introducing a “New Species”: The South African Himalayan Tahr. Zoos Print 2001, 16, 21–22. [Google Scholar]
- Cooper, R.K.; Brooke, R.K. Past and present distribution of the feral European rabbit, Oryctolagus cuniculus, on southern African offshore islands. S. Afr. J. Wildl. Res. 1982, 12, 71–75. [Google Scholar]
- Happold, D.C.D. (Ed.) Oryctolagus cuniculus European Rabbit. In Mammals of Africa; Bloomsbury: London, UK; Volume 3, pp. 708–710.
- Steadman, D.W. Prehistoric extinctions of Pacific island birds: Biodiversity meets zooarchaeology. Science 1995, 267, 1123–1131. [Google Scholar] [CrossRef]
- Courchamp, F.; Chapuis, J.L.; Pascal, M. Mammal invaders on islands: Impact, control and control impact. Biol. Rev. 2003, 78, 347–383. [Google Scholar] [CrossRef]
- Atkinson, I.A.E. The spread of commensal species of Rattus to oceanic islands and their effect on island avifaunas. In Conservation of Island Birds; Moors, P.J., Ed.; ICBP Technical Publication no.3; ICBP: Cambridge, UK, 1985. [Google Scholar]
- Towns, D.R.; Atkinson, I.A.E.; Daugherty, C.H. Have the harmful effects of introduced rats on islands been exaggerated? Biol. Invasions 2006, 8, 863–891. [Google Scholar]
- Jones, H.P.; Tershy, B.R.; Zavaleta, E.S.; Croll, D.A.; Keitt, B.S.; Finkelstein, M.E.; Howald, G.R. Severity of the effects of invasive rats on seabirds: A global review. Conserv. Biol. 2008, 22, 16–26. [Google Scholar] [CrossRef]
- Hagern, B.L.; Kumschick, S. The relevance of using various scoring schemes revealed by an impact assessment of feral mammals. NeoBiota 2018, 38, 37. [Google Scholar] [CrossRef]
- Watkins, B.P.; Cooper, J. Introduction, present status and control of alien species at the Prince Edward Islands, Sub-Antarctic. S. Afr. J. Antarct. Res. 1986, 16, 86–94. [Google Scholar]
- Jones, M.G.W.; Ryan, P.G. Evidence of mouse attacks on albatross chicks on sub-Antarctic Marion Island. Antarct. Sci. 2010, 22, 39–42. [Google Scholar] [CrossRef]
- Dilley, B.J.; Schoombie, S.; Schoombie, J.; Ryan, P.G. ‘Scalping’ of albatross fledglings by introduced mice spreads rapidly at Marion Island. Antarct. Sci. 2016, 28, 73–80. [Google Scholar] [CrossRef]
- Treasure, A.M.; Chown, S.L. Antagonistic effects of biological invasion and temperature change on body size of island ectotherms. Divers. Distrib. 2014, 20, 202–213. [Google Scholar] [CrossRef]
- Deacon, J. Human settlement in South Africa and archaeological evidence for alien plants and animals. In The Ecology and Management of Biological Invasions in Southern Africa; Macdonald, I.A.W., Kruger, F.J., Ferrar, A.A., Eds.; Oxford University Press: Cape Town, South Africa, 1986; pp. 3–19. [Google Scholar]
- Crawford, R.J.M.; Dyer, B.M. Wildlife of Robben Island. Bright Continent Guide 1; Avian Demography Unit: Cape Town, South Africa, 2000. [Google Scholar]
- Monadjem, A.; Taylor, P.J.; Denys, C.; Cotterill, F.P.D. Rodents of Sub-Saharan Africa: A Biogeographic and Taxonomic Synthesis; Walter de Gruyter: Berlin, Germany, 2015. [Google Scholar] [CrossRef]
- Happold, D.C.D. (Ed.) Rattus norvegicus Brown Rat. In Mammals of Africa; Bloomsbury: London, UK, 2013; Volume 3, pp. 540–541. [Google Scholar]
- Dobigny, G.; Poirier, P.; Hima, K.; Cabaret, O.; Gauthier, P.; Tatard, C.; Costa, J.M.; Bretagne, S. Molecular survey of rodent-borne Trypanosoma in Niger with special emphasis on T. lewisi imported by invasive black rats. Acta Trop. 2011, 117, 183–188. [Google Scholar]
- Happold, D.C.D. (Ed.) Rattus rattus Black Rat. In Mammals of Africa; Bloomsbury: London, UK, 2013; Volume 3, pp. 541–543. [Google Scholar]
- De Graaff, G. The Rodents of Southern Africa: Notes on Their Identification, Distribution, Ecology, and Taxonomy; Butterworths: Durban, South Africa, 1981. [Google Scholar]
- Julius, R.S.; Schwan, E.V.; Chimimba, C.T. Helminth composition and prevalence of indigenous and invasive synanthropic murid rodents in urban areas of Gauteng Province, South Africa. J. Helminthol. 2018, 92, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, L.; Douwes, E.; Gaertner, M.; Measey, J.; Paap, T.; Richardson, D.M. Biological invasions in South Africa’s urban ecosystems: Patterns, processes, impacts and management. In Biological invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 273–310. [Google Scholar] [CrossRef]
- Taylor, P.J.; Arntzen, L.; Hayter, M.; Iles, M.; Frean, J.; Belmain, S. Understanding and managing sanitary risks due to rodent zoonoses in an African city: Beyond the Boston Model. Integr. Zool. 2008, 3, 38–50. [Google Scholar] [CrossRef]
- van Helden, L.; van Helden, P.D.; Meiring, C. Pathogens of vertebrate animals as invasive species: Insights from South Africa. In Biological Invasions in South Africa; van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 247–272. [Google Scholar] [CrossRef]
- Bastos, A.D.; Nair, D.; Taylor, P.J.; Brettschneider, H.; Kirsten, F.; Mostert, E.; von Maltitz, E.; Lamb, J.M.; van Hooft, P.; Belmain, S.R.; et al. Genetic monitoring detects an overlooked cryptic species and reveals the diversity and distribution of three invasive Rattus congeners in South Africa. BMC Genet. 2011, 12, 26. [Google Scholar] [CrossRef]
- Smithers, R.H.N. The Mammals of the Southern African Subregion; University of Pretoria: Pretoria, South Africa, 1983. [Google Scholar]
- Happold, D.C.D. (Ed.) Sciurus carolinensis Grey Squirrel. In Mammals of Africa; Bloomsbury: London, UK, 2013; Volume 3, pp. 92–93. [Google Scholar]
- Happold, D.C.D. (Ed.) Myocaster coypus Coypu. In Mammals of Africa; Bloomsbury: London, UK, 2013; Volume 3, pp. 691–692. [Google Scholar]
- Bocage, J.V.B. Contribution a la faune des quatre isles du Golfe du Guinee. 2. lie de Prince. J. Acad. Sci. 1904, 7, 25–54. [Google Scholar]
- Bocage, J.V.B. Contribution a la faune des quatre isles du Golfe du Guinee. 4. Be de Sao Tome. J. Acad. Sci. 1904, 7, 65–96. [Google Scholar]
- Wyner, Y.; Absher, R.; Amato, G.; Sterling, E.; Stumpf, R.; Rumpler, Y.; Desalle, R. Species concepts and the determination of historic gene flow patterns in the Eulemur fulvus (brown lemur) complex. Biol. J. Linn. Soc. 1999, 66, 39–56. [Google Scholar] [CrossRef][Green Version]
- Harpet, C. Les Lémuriens de L’océan Indien: Du Temps de la Colonisation au Temps de la Cohabitation. Civilisations des Mondes Insulaires (Madagascar, Îles du Canal du Mozambique, Mascareignes, Polynésie, Guyanes)—Mélanges en L’honneur du Professeur Claude Allibert, sous la Direction de Chantal Radimilahy et Narivelo Rajaonarimanana; Karthala Editions: Paris, France, 2011; pp. 122–138. [Google Scholar]
- Havemann, C.P.; Retief, T.A.; Tosh, C.A.; de Bruyn, P.J.N. Roan antelope Hippotragus equinus in Africa: A review of abundance, threats and ecology. Mammal. Rev. 2016, 46, 144–158. [Google Scholar] [CrossRef]
- Kruger, J.; Koen, J.; Collins, K.; Nel, L. A conservation assessment of Hippotragus equinus. In The Red List of Mammals of South Africa, Swaziland and Lesotho; Child, M.F., Roxburgh, L., Do Linh San, E., Raimondo, D., Davies-Mostert, H.T., Eds.; South African National Biodiversity Institute and Endangered Wildlife Trust: Pretoria, South Africa, 2016. [Google Scholar]
- Matthee, C.A.; Robinson, T.J. Mitochondrial DNA population structure of roan and sable antelope: Implications for the translocation and conservation of the species. Mol. Ecol. 1999, 8, 227–238. [Google Scholar] [CrossRef]
- Castley, J.G.; Boshoff, A.F.; Kerley, G.I.H. Compromising South Africa’s natural biodiversity-inappropriate herbivore introductions. S. Afr. J. Sci. 2001, 97, 344–348. [Google Scholar]
- Alpers, D.L.; van Vuuren, B.J.; Arctander, P.; Robinson, T.J. Population genetics of the roan antelope (Hippotragus equinus) with suggestions for conservation. Mol. Ecol. 2004, 13, 1771–1784. [Google Scholar] [CrossRef]
- Van Wyk, A.M.; Dalton, D.L.; Kotzé, A.; Grobler, J.P.; Mokgokong, P.S.; Kropff, A.S.; Jansen van Vuuren, B. Assessing introgressive hybridization in roan antelope (Hippotragus equinus): Lessons from South Africa. PloS ONE 2019, 14, e0213961. [Google Scholar] [CrossRef]
- Bomer, M. Translocation of 7 Mammal Species to Rubondo Island National Park in Tanzania; Frankfurt Zoological Society Tanzania Wildlife Conservation Project: Arusha, Tanzania, 1988. [Google Scholar]
- Gippoliti, S.; Robovský, J.; Angelici, F.M. Taxonomy and Translocations of African Mammals: A Plea for a Cautionary Approach. Conservation 2021, 1, 121–136. [Google Scholar] [CrossRef]
- Kikoti, I.A.; Mushi, R.F. Rubondo Island National Park: Overlooked World’s Largest Tropical Lake Island Protected Area in Tanzania. East Afr. J. Environ. Nat. Res. 2025, 8, 85–106. [Google Scholar] [CrossRef]
- Shivambu, N.; Shivambu, T.C.; Downs, C.T. Non-native small mammal species in the South African pet trade. Manag. Biol. Invasions 2021, 12, 294–312. [Google Scholar] [CrossRef]
- Spear, D.; Chown, S.L. The extent and impacts of ungulate translocations: South Africa in a global context. Biol. Conserv. 2009, 142, 353–363. [Google Scholar] [CrossRef]
- Spear, D.; Chown, S.L. Non-indigenous ungulates as a threat to biodiversity. J. Zool. 2009, 279, 1–17. [Google Scholar] [CrossRef]
- Cheke, A.S.; Hume, J.P. Lost Land of the Dodo: An Ecological History of Mauritius, Réunion & Rodrigues; A&C Black: London, UK, 2008. [Google Scholar]
- Cole, N. Madatyphlops cariei. In IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2021; p. e.T22607A166933641. [Google Scholar] [CrossRef]
- Mateo, J.A.; Barone, R.; Hernández-Acosta, C.N.; López-Jurado, L.F. La muerte anunciada de dos gigantes macaronésicos: El gran escinco caboverdiano, Chioninia coctei (Duméril & Bibron, 1839) y el lagarto de Salmor, Gallotia simonyi (Steindachner, 1889). Bol. Asoc. Herpetol. Esp. 2020, 31, 3–30. [Google Scholar]
- Sanchez, M. Leiolopisma ceciliae. In IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2019; p. e.T17023550A17023949. [Google Scholar] [CrossRef]
- WCMC (World Conservation Monitoring Centre). Leiolopisma mauritiana. In IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 1996; p. e.T11410A3277412. [Google Scholar] [CrossRef]
- Delgado, K.; Lopes, E.P.; Vasconcelos, R. Cabo Verde giant gecko: How many units for conservation? Amphib. Reptil. 2021, 42, 503–510. [Google Scholar] [CrossRef]
- Kehlmaier, C.; Graciá, E.; Ali, J.R.; Campbell, P.D.; Chapman, S.D.; Deepak, V.; Ihlow, F.; Jalil, N.-E.; Pierre-Huyet, L.; Samonds, K.E.; et al. Ancient DNA elucidates the lost world of western Indian Ocean giant tortoises and reveals a new extinct species from Madagascar. Sci. Adv. 2023, 9, eabq2574. [Google Scholar] [CrossRef]
- Rhodin, A.G.J.; Pritchard, P.C.H.; van Dijk, P.P.; Saumure, R.A.; Buhlmann, K.A.; Iverson, J.B.; Mittermeier, R.A. (Eds.) Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group; Chelonian Research Monographs; Chelonian Research Foundation: Arlington, VT, USA, 2015; p. 5. [Google Scholar]
- Ghestemme, T.; Salamolard, M. L’échenilleur de La Réunion, Coracina newtoni, espèce endémique en danger. Ostrich 2007, 78, 255–258. [Google Scholar] [CrossRef]
- Hume, J.P. Extinct Birds; Bloomsbury Publishing: London, UK, 2017. [Google Scholar]
- BirdLife International. Ardenna pacifica. In IUCN Red List of Threatened, Species; IUCN: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Cheke, A.S. Extinct birds of the Mascarenes and Seychelles—A review of the causes of extinction in the light of an important new publication on extinct birds. Phelsuma 2013, 21, 4–19. [Google Scholar]
- Semedo, G.; Paiva, V.H.; Militão, T.; Rodrigues, I.; Dinis, H.A.; Pereira, J.; Matos, D.; Ceia, F.R.; Almeida, N.M.; Geraldes, P.; et al. Distribution, abundance, and on-land threats to Cabo Verde seabirds. Bird Conserv. Int. 2021, 31, 53–76. [Google Scholar] [CrossRef]
- Muzaffar, S.B.; Benjamin, D.; Gubiani, R.E. The impact of fox and feral cat predation on the population viability of the threatened, endemic Socotra cormorant on Siniya Island, United Arab Emirates. Mar. Ornithol. 2013, 41, 171–177. [Google Scholar]
- Le Corre, M.; Danckwerts, D.K.; Ringler, D.; Bastien, M.; Orlowski, S.; Morey-Rubio, C.; Pinaud, D.; Micol, T. Seabird recovery and vegetation dynamics after Norway Rat eradication at Tromelin Island, western Indian Ocean. Biol. Conserv. 2015, 185, 85–94. [Google Scholar] [CrossRef]
- Hugulay, M.; Gascoigne, A.; De Deus, D.; De Lima, R.F. Notes on the breeding ecology and conservation of the Critically Endangered Dwarf Olive Ibis. Bull. Afr. Bird Club 2014, 21, 202–205. [Google Scholar] [CrossRef]
- Hansford, J.P.; Turvey, S.T. Unexpected diversity within the extinct elephant birds (Aves: Aepyornithidae) and a new identity for the world’s largest bird. R. Soc. Open Sci. 2018, 5, 181295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehtonen, J. Occurrence of the Introduced Black Rat (Rattus rattus) and Its Potential Effects on Endemic Rodents in Southeastern Madagascar. Unpublished Academic Thesis, University of Helsinki, Helsinki, Finland, 2013. [Google Scholar]
- Miljutin, A.; Lehtonen, J.T. Probability of competition between introduced and native rodents in Madagascar: An estimation based on morphological traits. Est. J. Ecol. 2008, 57, 133–152. [Google Scholar] [CrossRef]
- Godfrey, L.R.; Jungers, W.L. Subfossil Lemurs. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 1247–1252. [Google Scholar]
- Godfrey, L.R.; Rasoazanabary, E. Demise of the Bet Hedgers: A Case Study of Human Impacts on Past and Present Lemurs of Madagascar. In The Anthropology of Extinction: Essays on Culture and Species Death; Sodikoff, G.M., Ed.; Indiana University Press: Bloomington, IN, USA, 2011. [Google Scholar]
- Godfrey, L.R.; Jungers, W.L.; Burney, D.A. Subfossil Lemurs of Madagascar. In Cenozoic Mammals of Africa; Werdelin, L., Sanders, W.J., Eds.; University of California Press: Berkeley, CA, USA, 2010; pp. 351–367. [Google Scholar]
- Crowley, B.E. A refined chronology of prehistoric Madagascar and the demise of the megafauna. Quat. Sci. Rev. 2010, 29, 2591–2603. [Google Scholar] [CrossRef]
- Kimura, B.; Marshall, F.B.; Chen, S.; Rosenbom, S.; Moehlman, P.D.; Tuross, N.; Sabin, R.C.; Peters, J.; Barich, B.; Yohannes, H.; et al. Ancient DNA from Nubian and Somali wild ass provides insights into donkey ancestry and domestication. Proc. R. Soc. B Biol. Sci. 2011, 278, 50–57. [Google Scholar] [CrossRef]
- Shackelton, A. Diversity and Taxonomy of Malagasy Pygmy Hippopotamuses. M.Sc. Thesis, Northern Illinois University, DeKalb, IL, USA, 2021. [Google Scholar]
- Faith, J.T. Late Pleistocene and Holocene mammal extinctions on continental Africa. Earth-Sci. Rev. 2014, 128, 105–121. [Google Scholar] [CrossRef]
- Lucek, K.; Lemoine, M. First record of freshwater fish on the Cape Verdean archipelagos. Afr. Zool. 2012, 47, 341–344. [Google Scholar] [CrossRef][Green Version]
- Alho, M.; Granadeiro, J.P.; Rando, J.C.; Geraldes, P.; Catry, P. Characterization of an extinct seabird colony on the island of Santa Luzia (Cabo Verde) and its potential for future recolonizations. J. Ornithol. 2022, 163, 301–313. [Google Scholar] [CrossRef]
- Militão, T.; Dinis, H.A.; Zango, L.; Calabuig, P.; Stefan, L.M.; González-Solís, J. Population size, breeding biology and on-land threats of Cape Verde petrel (Pterodroma feae) in Fogo Island, Cape Verde. PLoS ONE 2017, 12, e0174803. [Google Scholar] [CrossRef]
- Peet, N.B.; Atkinson, P.W. The biodiversity and conservation of the birds of São Tomé and Príncipe. Biodivers. Conserv. 1994, 3, 851–867. [Google Scholar] [CrossRef]
- Saraf, S. Preserving the perishing endangered natural biodiversity of Socotra Island. Open J. Ecol. 2021, 11, 148–162. [Google Scholar] [CrossRef]
- Hansford, J. Diversity and Extinction in Aepyornithidae. Ph.D. Thesis, University of Southampton, Southampton, UK, 2018. [Google Scholar]
- Hansford, J.; Lister, P.; Adrian, M.; Weston, E.M.; Turvey, S.T. Simultaneous extinction of Madagascar’s megaherbivores correlates with late Holocene human-caused landscape transformation. Quat. Sci. Rev. 2011, 263, 106996. [Google Scholar] [CrossRef]
- Virah-Sawmy, M.; Willis, K.J.; Gillson, L. Evidence for drought and forest declines during the recent megafaunal extinctions in Madagascar. J. Biogeogr. 2010, 37, 506–519. [Google Scholar] [CrossRef]
- Wang, L.; Brook, G.A.; Burney, D.A.; Voarintsoa, N.R.G.; Liang, F.; Cheng, H.; Edwards, R.L. The African Humid Period, rapid climate change events, the timing of human colonization, and megafaunal extinctions in Madagascar during the Holocene: Evidence from a 2m Anjohibe Cave stalagmite. Quat. Sci. Rev. 2019, 210, 136–153. [Google Scholar] [CrossRef]
- Godfrey, L.R.; Jungers, W.L. The extinct sloth lemurs of Madagascar. Evol. Anthropol. Issues News Rev. Issues News Rev. 2003, 12, 252–263. [Google Scholar] [CrossRef]
- Pastorini, J.; Thalmann, U.; Martin, R.D. A molecular approach to comparative phylogeography of extant Malagasy lemurs. Proc. Nat. Acad. Sci. USA 2003, 100, 5879–5884. [Google Scholar] [CrossRef]
- Razafindramanana, J.; Eppley, T.M.E.; Rakotondrabe, R.; Roullet, D.; Irwin, M.; King, T. Eulemur mongoz. In IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2020; p. e.T8202A115561431. [Google Scholar] [CrossRef]
- Damme, K.V.; Banfield, L. Past and present human impacts on the biodiversity of Socotra Island (Yemen): Implications for future conservation. Zool. Middle East 2011, 54 (Suppl. S3), 31–88. [Google Scholar] [CrossRef]
- Williams, A.J.; Siegfried, W.R.; Burger, A.E.; Berruti, A. The Prince Edward Islands: A sanctuary for seabirds in the Southern Ocean. Biol. Conserv. 1979, 15, 59–71. [Google Scholar] [CrossRef]
- Bloomer, J.P.; Bester, M.N. Control of feral cats on sub-Antarctic Marion Island, Indian Ocean. Biol. Conserv. 1992, 60, 211–219. [Google Scholar] [CrossRef]
- Huntley, B.J. Marion Island: Birds, Cats, Mice and Men. In Strategic Opportunism: What Works in Africa: Twelve Fundamentals for Conservation Success; Springer Nature: Cham, Switzerland, 2023; pp. 21–37. [Google Scholar]
- Nel, D.C.; Ryan, P.G.; Crawford, R.J.; Cooper, J.; Huyser, O.A. Population trends of albatrosses and petrels at sub-Antarctic Marion Island. Polar Biol. 2002, 25, 81–89. [Google Scholar] [CrossRef]

| Scientific Species Name | Common Species Name | Family | Original Range | Distribution in Africa |
|---|---|---|---|---|
| Tenrec edacudatus | Common Tenrec | Tenrecidae | Madagascar and Comoros | Mauritius < 1970; Reunion, before 1882; Aldabra Atoll |
| Suncus madagascariensis | Madagascan Pygmy Shrew | Soricidae | Madagascar | Socotra, after 1967 |
| Suncus etruscus | Etruscan Shrew | Soricidae | Southern Palearctic | Socotra, befre 1967 |
| Suncus murinus | Asian House Shrew | Soricidae | SE Asia | E African Coast, Madagascar, before 1960, well-est.; Comoros, Mauritius, Reunion, established?; Zanzibar, Pemba before 1950; South Africa: Dyer Island 1912; Aldabra Atoll |
| Eulemur mongoz | Mongoose Lemur | Lemuridae | Madagascar | Comoros, 1665; in 2000: 45 exx./km2, 2008: 10 exx./km2, 2019: 23 ind./km2 (L. J. Ormsby) |
| Cercopithecus aethiops | Green Monkey | Cercopithecidae | Africa | Cape Verde, 1960’s |
| Cercopithecus mona | Mona Monkey | Cercopithecidae | Africa | São Tome e Principe, 1700–1800 |
| Erythrocebus patas | Patas Monkey | Cercopithecidae | Africa | South Africa: KZN; 2000–2020; crop pest |
| Macaca irus | Long-tailed Macaque | Cercopithecidae | SE Asia | Mauritius Is. 1602; very invasive |
| Macaca fascicularis | Crab-eating Macaque | Cercopithecidae | SE Asia | Mauritius 1602; South Africa: W Cape |
| Ovis aries musiomn | Muflon | Bovidae | Palearctic | South Africa |
| Ammotragus lervia | Barbary Sheep | Bovidae | N Africa | South Africa: Tsolwana Game Reserve (E Cape, early 1980’s), Northern Cape, Free State. Total popul.: c. 1000 ind. in 2015 (S.Brody) |
| Capra hircus | Feral Domestic Goat | Bovidae | Iran | South Africa: 1650, escape; Namibia (Damaraland, Namaland); Assumption Is., before 1897, 1895: 300–400 exx., 1916: only few, c. 1940 extinct; Prince Edward Is.; Aldabra, 1878, est., before 1968 extinct; Mauritius, 1512, 1950: c. 100, before 1982 extinct; São Tome e Principe, 16th cen. |
| Hemitragus jemlahicus | Himalayan Tah | Bovidae | Himalayas; S Tibet, Nepal, India | South Africa, 1930: Table Mts. (ornamental; 600 exx. 1974; 100 exx. 1981; 2000: 50–60 exx. eradicated), Cape Peninsula, Golden Gate, Free State. Vulnerable in India; but easy to keep in Zoos, reproduce efficiently |
| Boselaphus tragocamelus | Nilgai | Bovidae | India | South Africa: Eastern Cape, Free State |
| Addax nasomaculatus | Addax | Bovidae | N Africa | SA: FE, EC. |
| Bison bison | American Bison | Bovidae | N America | South Africa: Ratelfontein near Richmond, Karoo; 1990’s extinct |
| Bubalus bubalis | Asian Water Buffalo | Bovidae | SE Asia | Namibia |
| Bos frontalis | Gaur | Bovidae | SE Asia | South Africa |
| Antilope cervicapra | Indian Blackbuck | Bovidae | India | South Africa: Eastern Cape (escapee), 1985? |
| Rusa unicolor | Malayan Sambar | Cervidae | SE Asia | South Africa 1880 (hunting): Eastern Cape; Mauritius: 1639 |
| Rusa timorensis | Sunda Sambar | Cervidae | Indonesia | South Africa: EC, c.1890; Reunion |
| Cervus dama | Fallow Deer | Cervidae | S Palearctic | South Africa 1869 (escapee): Western Cape, Eastern Cape, Free State, Gauteng, KwaZulu Natal; Madagascar, 1932, extinct before 1974; Angola; Uganda |
| Cervus axis | Axis Deer | Cervidae | SE Asia | South Africa: Eastern Cape, before 1985; Free Sate; crop and forest pest |
| Cervus nippon | Sika Deer | Cervidae | Japan, China, Taiwan | South Africa: Groot Schuur, 1897, 1937: 20 exx.; Eastern Cape, Free State, Limpopo; Madagascar, 1932, now extinct; forest pest |
| Cervus elaphus | Wapiti | Cervidae | N America | Fernando Poo Isl. (Gulf of Guinea), 1954, established? South Africa: near Clocolan (Free Sate), 1895, 1930’s: 50 exx., Vereeniging (Transvaal), 1975; escapee |
| Cervus elaphus | Red Deer | Cervidae | Eurasia | South Africa: E Cape, Free State; escapee |
| Cervus timorensis | Rusa | Cervidae | SE Asia: Indonesia | Comoro Is., 1970; Madagascar, 1928, 1950’s widespread, 1990’s extinct; Mauritius, 1639, 1980’s: c. 3000 exx. |
| Cervus unicolor | Samba | Cervidae | SE Asia | South Africa: Groot Schuur, 1897, 1930’s: c. 50 exx.; 1990’s in a few enclosers in Westren Cape |
| Axis porcinus | Hog Deer | Cervidae | India | South Africa, escapee |
| Elaphurus davidianus | Père David’s dee | Cervidae | China | South Africa: Eastern Cape |
| Sus scrofa | Feral Domestic Pig | Suidae | Europe | South Africa 1926: Western Cape, Zimbabwe, Tanzania Uganda, Southern Sudan, Gabon, SE Chad; São Tome e Principe, 16th cen.; Mauritius 1606, Reunion 1629, Rodrigues c. 1792 |
| Potamochaerus porcus | Warthog | Suidae | Africa | Madagascar, before 1962; Mayotte (Comoros), before 1982 |
| Tayassus sp. | Peccari | Tayassuidae | S America | Gabon: Wonga-Wongue Presidential Reserve (WWPR), before 1986 |
| Camelus dromedarius | Feral Drome-dary Camel | Camelidae | Asia | Socotra |
| Equus asinus | Feral Donkey | Equidae | Central Asia | South Africa: 1650, escape; Namib Desert and Ovamboland; Socotra; São Tome e Principe, 16th cen. |
| Equus ferus caballus | Feral Horse | Equidae | Central Asia | South Africa: 1650, escape; Gabon: WWPR, before 1986; established?; São Tome e Principe, 16th cen. |
| Equus africanus | Feral African Wild Ass | Equidae | Africa | Socotra |
| Canis familiaris | Feral Dog | Canidae | Europe | Cape of Good Hope, before 1970; São Tome e Principe, 16th cen.; Madagascar, c.1000 BP |
| Felis catus | Feral Domestic Cat | Felidae | Europe | South Africa: 1650, escape; Marion 1949 to control rats (1977: 3409 exx.), Mauritius c.1685, Reunion c.1685, Rodrigues c.1745, Seychelles; São Tome e Principe, 16th cen.; Marion Isl. 1949 (+1991) |
| Civettictis civetta | African Civet | Viverridae | Africa | São Tome e Principe, 19th cen.? |
| Viverricula indica | Small Indian Civet | Viverridae | SE Asia | Socotra, before 1608; Zanzibar and Pemba, before 1950; Madagascar before 1950 |
| Urva auropunctatus | Small Indian Mongoose | Herpestidae | SE Asia | Tanzania: Mafia, Zanzibar, Pemba, before1950; Mauritius before 1985 (to control rats in sugar cane plantation) |
| Herpestes edwardsi | Grey Mongoose | Herpestidae | SE Asia | Mauritius, 1899 to control rats |
| Mustela nivalis | Weasel | Mustelidae | Europe | São Tome e Principe, 19th cen.? |
| Oryctolagus cuniculus | European Rabbit | Leporidae | Europe | Robben Is.: 1652 (escapee of domestic ex.); many islands around South Africa and Madagagascar, 1860, now extinct; Cape Verde c.1450, abundant, 1990’s extinct; Mauritius 1810, abundant, totally eradicated in 1987; Seychelles, 1980’s but not established; Aldabra Atoll |
| Lepus nigricollis | Black-naped Hare | Leporidae | India, Pakistan | Mauritius, late 19th cen., 1975: 650–1500 exx., 1982: 2450–2900 exx.; Cousin Is. (Seychelles): 1920’s, 1971: 120–170 exx.; Madagascar; Reunion |
| Mus musculus | House Mouse | Muridae | Middle East | South Africa: c.800, stowaway; 1500s: Namibia, Zimbabwe, Kenya, DRC, Nigeria, Benin, Niger, Senegal, Mauritius, Marion, 1800s; São Tome e Principe, 16th cen. |
| Rattus norvegicus | Brown Rat | Muridae | Far East | South Africa, 1650, stowaway; Mauritius 1735, Reunion 1735, Rodrigues before 1874 |
| Rattus rattus | House Rat | Muridae | SE Asia | South Africa: c. 800, stowaway; Madagascar, c.300 BC; Madagascar, Tanzania, Mozambique, Ethiopia, Benin, Niger, Mauritius before 1598, Reunion 1672, Rodrigues before 1691, Mayotte, Comoros; São Tome e Principe, 16th cen. |
| Rattus tanezumi | Asian House Rats | Muridae | SE Asia | Widespread in South Africa and Eswatini c. 2005 |
| Sciurus carolinensis | Grey Squirrel | Sciuridae | USA, Canada | Cape Town area: 1890‘s, 1920’s populated whole Cape Penis; Paarl: 1945; Ceres 1957; Swellendam: 1968 |
| Myocastor coypus | Coypu | Echymyidae | S America | Hanynki (140 km N of Nairobi), c. 1940; Lake Naivasha: 1965; Lake Ol Bolossat, 1970; Tanzania; Zambia |
| Hydrochoerus hydrochaeris | Capybara | Caviidae | S America | South Africa, c. 2020 |
| Species Scientific Name | Common Scientific Name | Family | RDB Status | Eff-Ects | Place | Alien Mammals | Source |
|---|---|---|---|---|---|---|---|
| AMPHIBIA | |||||||
| Sooglossus thomasetti | Thomasset’s Seychelles Frog | Sooglossidae | E, CR | P ** | Seychelles | Tenrecus ecaudatus | [70] |
| Sooglossus sechellensis | Seychelles Frog | Sooglossidae | E, EN | P ** | Seychelles | Tenrecus ecaudatus | [70] |
| Sechellophryne gardinieri | Gardiner’s Seychelles Frog | Sooglossidae | E. EX | P ** | Seychelles | Tenrecus ecaudatus | [70] |
| REPTILIA | |||||||
| Casarea dussumieri | Round Island keel-scaled Boa | Bolyeriidae | E, EX 1975 | H *** | Mauritius: Round Island | Goat, rabbit, hare | [125] |
| Madatyphlops cariei | Hoffstetter’s Worm snake | Typhlopidae | E, EX c.1800 | P *** | Mauritius | Cat, dog, other in-troduced carnivores | [126] |
| Chioninia coctei | Cape Verde Giant Skink | Scincidae | E, EX:1996 | P ** | Cape Verde | (Dog, cat) | [127] |
| Gongylomorphus bojeri | Bojer’s Skink | Scincidae | E, CR | H ** | Mauritius: Round Island | Suncus murinus (goat, rabbit) | [126] |
| Gongylomorphus fontenayi | Orange-tailed Skink | Scincidae | E, EN | P, H ** | Mauritius: Gunner’s Quoin | Rat, hare, rabbit | [126] |
| Leiolopisma ceciliae | Reunion Giant Skink | Scincidae | E, EX c.1700 | P ** | Reunion | Rats (introduced in 1670) | [128] |
| Leiolopisma mauritiana | Mauritian Giant Skink | Scincidae | E, EX? | P ** | Mauritius | Rats? | [129] |
| Leiolopisma telfairii | Telfair’s Skink | Scincidae | E, VU | H * | Mauritius: Round Is., G. Quoin | Goat and rabbit | [126] |
| Phelsuma gigas | Rodrigues Giant Day Gecko | Scincidae | E, EX 1842 | P *** | Rodrigues, Frigate Is. | Brown Rat | [129] |
| Trachylepis comorensis | Comorian Skink | Scincidae | E, LC | P *** | Comoros, Mozambique, Madagascar | Rats | a |
| Trachylepis socotrana | Socotra Skink | Scincidae | E, LC | P * | Socotra | Cat, Rat, Mouse | b |
| Nactus durrelli | Durrell’s Night gecko | Gekkonidae | E, VU | H ** | Mauritius: Round Island | Goat, rabbit | [125] |
| Nactus coindemirensis | Lesser Night Gecko | Gekkonidae | E, VU | P, H ** | Mauritius: Gunner’s quoin | Rat, hare, rabbit | [125] |
| Phelsuma guentheri | Günther’s Gecko | Gekkonidae | E, EN | H ** | Mauritius: Round Is., Il. Aigrettes | Goat, rabbit | [125] |
| Tarentola gigas | Cape Verde Giant Gecko | Phyllodactylidae | E, EN | P *** | Cape Verde | Cats, rat, mouse | [130] |
| Psammobates geometricus | Geometric Tortoise | Testudinidae | E, CR | P * | South Africa (SA): W Cape | Feral pig, cat | [14] |
| Aldabrachelys abrupta | Abrupt Giant Tortoise | Testudinidae | E, EX 1230–1315 | P * | Madagascar | Feral pig, cat | [131] |
| Aldabrachelys gigantea daudinii | Daudin’s Giant Tortoise | Testudinidae | E, EX c.1850 | P * | Seychelles: Mahe | Feral pig, cat | [132] |
| Cylindraspis indica | Réunion Giant Tortoise | Testudinidae | E, EX c.1840 | P * | Reunion | Feral pig, cat | [132] |
| Cylindraspis inept | Mauritius Giant Tortoise | Testudinidae | E, EX 1844 | P * | Mauritius | Feral pig, cat | [132] |
| AVES | |||||||
| Hirundo atrocaerulea | Blue Swallow | Hirundinidae | E, EN | H * | SA: Mpumalanga | Feral Horse | [14] |
| Terpsiphone corvina | Seychelles Black Parad. Flycatcher | Monarchidae | E, VU | P * | Seychelles: La Digue | Cat, rat | [74] |
| Copsychus sechellarum | Seychelles Magpie Robin | Muscicapidae | E, EN | P * | Seychelles: Aride Denis, Frégate, | Cat, rat | [74] |
| Humboldia flavirostris | Humbolt’s Flycatcher | Muscicapidae | E, VU | P, C * | Comoros | Black Rat, Com. Myna? | a |
| Coracina newtoni | Réunion Cuckooshrike | Campephagidae | E, CR | P, H * | Reunion | Rat, cat, (deer) | [133] |
| Zosterops modestus | Seychelles White-eye | Zosteropidae | E, VU | P * | Seychelles | Cat, rat | [74] |
| Zosterops chloronothos | Mauritius Olive White-eye | Zosteropidae | E, CR | P * | Mauritius: Ile aux Aigrettes | Rat, cat, mongoose | [125] |
| Zosterops semiflavus | Marianne White-eye | Zosteropidae | E, EX 1892 | P ** | Seychelles: Marianne Isl. | Blak rat | [134] |
| Acrocephalus seychellensis | Seychelles Warbler | Acrocephalidae | E, NT | P * | Seychelles | Cat, rat | [74] |
| Nesillas aldabrana | Aldabra Brush Warbler | Acrocepha-lidae | E, EX 1994 | P, H ** | Seychelles: Aldabra | Rat, cat, goat | [135] |
| Foudia sechellarum | Seychelles Fody | Ploceidae | E, NT | P * | Seychelles: Denis | Cat, rat | [74] |
| Foudia rubra | Mauritius Fody | Ploceidae | E, EN | P ** | Mauritius: Ile aux Aigrettes | Rat, cat, mongoose | [125] |
| Foudia delloni | Reunion Fody | Ploceidae | E, EX 1675–1680 | P * | Reunion | Rats | [136] |
| Onychognathus frater | Socotra Starling | Sturnidae | E, LC | P * | Socotra | Cat | b |
| Necropsar rodericanus | Rodrigues Starling | Sturnidae | E, EX 1726 | P * | Rodrigues | Rat | [136] |
| Fregilupus varius | Hoopoe Starling | Sturnidae | E, EX 1837 | P, H * | Reunion | Rat, cat, goat? | [135] |
| Chalcomitra balfouri | Socotra Sunbird | Nectariniidae | E, LC | P * | Socotra | Cat | b |
| Hypsipetes cowlesi | Rodrigues Bulbul | Pycnonotidae | E, EX+? | P * | Rodrigues | Rat? | [136] |
| Cisticola haesitatus | Socotra Cisticola | Cisticolidae | E, LC | P * | Socotra | Cat | b |
| Incana incana | Socotra Warbler | Cisticolidae | E, LC | P * | Socotra | Cat | b |
| Passer insularis | Socotra Sparrow | Passeridae | E, LC | P * | Socotra | Cat | b |
| Rhynchostruthus socotranus | Socotra Golden-winged Grosbeak | Fringillidae | E, LC | P * | Socotra | Cat | b |
| Emberiza socotrana | Socotra Bunting | Emberizidae | E, VU | P ** | Socotra | Cat, Rat, Indian Small Civet | b |
| Phaethon aethereus | Red-billed Tropicbird | Phaethonti-dae | LC | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Phaethon lepturus | White-tailed Tropicbird | Phaethonti-dae | LC | P * | Seychelles: Ile du Nord | Rat | [74] |
| Sula dactylatra | Masked Booby | Sulidae | LC | P * | Seychelles: Grande Ile | Rat | [74] |
| Sula leucogaster | Brown Booby | Sulidae | LC | P ** | Tromelin Island | Cat, dog, rat, mouse | [137] |
| Papasula abbottii | Abbott’s Booby | Sulidae | EN | P ** | Mascarenes, extinct c.1670 | (Monkey) | [136] |
| Hydrobates jabejabe | Cape Verde Storm-petrel | Hydrobatidae | E, NT | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Pelagodroma marina | White-faced Storm-petrel | Procellariidae | LC | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Calonectris edwardsii | Cape Verde Shearwater | Procellariidae | E, NT | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Bulweria bulwerii | Bulwer’s Petrel | Procellariidae | LC | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Pterodroma feae | Cape Verde Petrel | Procellariidae | E, NT | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Puffinus lherminieri boydi | Boyd’s Shearwater | Procellariidae | E, LC | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Fregata magnificens | Magnificent Frigatebird | Procellariidae | LC | P ** | Cape Verde | Cat, dog, rat, mouse | [137] |
| Ardenna pacifica | Wedged-tailed Shearwater | Procellariidae | LC | P * | Seychelles | Rat | [74] |
| Ardenna pacifica | Wedged-tailed Shearwater | Procellariidae | LC | H * | Mauritius: Round Island | Goat, rabbit | [125] |
| Pseudobulwaria aterrima | Mascarene Black Petrel | Procellariidae | E, CR | P * | Rodrigues | Cat? | [136] |
| Leucocarbo melanogenis | Crozet Shag | Phalacrocoracidae | NE, CR | P ** | Marion Island | Mouse, cat | [32,92] |
| Phalacrocorax africanus | African Reed Cormorant | Phalacrocoracidae | LC | P * | Mascarenes, +1710 | Rat, feral cat, pigs | [136] |
| Phalacrocorax nigrogularis | Socotra Cormorant | Phalacrocoracidae | E, VU | P * | Socotra | Cat | [138] |
| Diomedea exulans | Wandering Albatross | Diomedeidae | VU | P ** | Marion Island | Mouse, cat | [32,92] |
| Thalassarche chrysostoma | Grey-headed Albatross | Diomedeidae | NE | P ** | Marion Island | Mouse, cat | [32,92] |
| Thalassarche carteri | Indian Yellow-nosed Albatross | Diomedeidae | EN | P ** | Marion Island | Mouse, cat | [32,92] |
| Phoebetria fusca | Sooty Albatross | Diomedeidae | NE, EN | P ** | Marion Island | Mouse, cat | [32,92] |
| Phoebetria palpebrata | Light-mantled Albatross | Diomedeidae | NT | P ** | Marion Island | Mouse, cat | [76,92] |
| Gygis alba | White Tern | Laridae | LC | P ** | Tromelin Is. | Rat | [139] |
| Larus dominicanus | Kelp Gull | Laridae | LC | P * | Marion Island | Mouse, cat | [32,92] |
| Stercorarius antarcticus | Brown Skua | Laridae | LC | P * | Marion Island | Mouse, cat | [32,92] |
| Aptenodytes patagonicus | King Penguin | Spheniscidae | LC | P * | Marion Island | Mouse, cat | [32,92] |
| Eudyptes chrysolophus | Macaroni Penguin | Spheniscidae | LC | P * | Marion Island | Mouse, cat | [32,92] |
| Alopochen mauritiana | Mauritius Sheldgoose | Anatidae | E, EX c.1695 | P * | Mauritius | Rat, feral cat, pig | [136] |
| Alopochen kervazori | Reunion Sheldgoose | Anatidae | E, EX c.1700 | P ** | Reunion | Rat, feral cat, pig | [136] |
| Anas theodori | Mascarene Teal | Anatidae | E, EX c.1700 | P ** | Mauritius | Rat, feral cat, pig | [136] |
| Threskiornis solitarius | Reunion Ibis | Threskiorni-thidae | E, EX 1761 | P ** | Reunion | Rat, feral cat, pig | [136] |
| Bostrychia bocagei | São Tomé Ibis | Threskiorni-thidae | E, CR | P * | São Tomé Is. | Cat, Least Weasel, rat | [140] |
| Nycticorax mauritianus | Mauritius Night Heron | Ardeidae | E, EX 1693 | P ** | Reunion | Rat, feral cat, pig | [136] |
| Nicticorax megacephalus | Rodrigues Night Heron | Ardeidae | E, EX 1726 | P ** | Rodrigues | Rat, feral cat | [136] |
| Pelecanus rufescens | Pink-backed Pelican | Pelecanidae | LC | P * | Madagascar, extinct 1960’s | Ras, cat? | [141] |
| Aphanopteryx bonasia | Mauritius Reed Rail | Rallidae | E, EX c.1695 | P * | Mauritius | Monkey, pig, rat | [136] |
| Dryolimnas augusi | Reunion Rail | Rallidae | E, EX 1675–1705 | P * | Reunion | Rat, feral cat, pig | [136] |
| Dryolimnas cuveri abboti | Assumption White-thr. Rail | Rallidae | E, EX 1908 | P ** | Seychelles: Assumption Is. | Rats | [134] |
| Erythromachus leguati | Rodrigues Rail | Rallidae | E, EX c.1726 | P * | Rodrigues | Rat, feral cat, pig | [136] |
| Porphyrio caerulescens | Reunion Blue Gallinule | Rallidae | E, EX c.1720 | P * | Reunion | Cat, rat | [136] |
| Porphyrio sp. | Seychelles Swamphen | Rallidae | E, EX c.1730 | P * | Reunion | Cat | [134] |
| Fulica newtoni | Mascarene Coot | Rallidae | E, EX 1693 | P * | Mauritius, Reunion | Rat, pig, cat | [136] |
| Raphus cucullatus | Dodo | Columbidae | E, EX 1640’s | P, H * | Mauritius | Black Rat, pig, goat | [136] |
| Peziphaps solitarius | Rodrigue’s Solitaire | Columbidae | E, EX c.1770 | P * | Mascarenes | Cats | [136] |
| Columba thiriouxi | Mauritius Wood Pigeon | Columbidae | E, EX | P * | Mauritius | Black Rat | [136] |
| Nesoenas duboisi | Reunion Pink Pigeon | Columbidae | E, EX c.1700 | P ** | Rodrigues | Cat | [136] |
| Nesoenas rodericana | Rodrigues Turtle Dove | Columbidae | E, EX 1726–1761 | P ** | Rodrigues | Rat, cat | [136] |
| Nesoenas cicur | Mauritius Turtle Dove | Columbidae | E, EX c.1730 | P ** | Mauritius | Rat, cat | [136] |
| Nesoenas mayeri | Pink Pigeon | Columbidae | E, VU | P ** | Mauritius: Ile aux Aigrettes | Rat, cat, mongoose | [125] |
| Alectroenas nitidissima | Mauritius Blue Pigeon | Columbidae | E, EX 1826 | P * | Mauritius | Cats? | [136] |
| Alectroenas payandeei | Rodrigues Blue Pigeon | Columbidae | E, EX < 1691 | P * | Rodrigues | Rats? | [136] |
| Mascarinus mascarinus | Mascarene Parrot | Psittaculidae | E, EX 1784 | P ** | Reunion | Rats, cat | [136] |
| Necropsittacus rodericanus | Rodrigues Parrot | Psittaculidae | E, EX c.1770 | P ** | Rodrigues | Rat, cat | [136] |
| Lophopsittacus mauritianus | Broad-billed Parrot | Psittaculidae | E, EX 1670’s? | P * | Mauritius | Monkey, rat | [136] |
| Psittacula echo | Echo Parakeet | Psittaculidae | E, VU | P ** | Mauritius: Mauritius | Rat, cat, mongoose | [125] |
| Psittacula exsul | Newton’s Parakeet | Psittaculidae | E, EX 1875 | P ** | Rodrigues | Rat, cat, mongoose? | [134] |
| Otus suazieri | Mauritius Scops Owl | Strigidae | E, EX 1837 | P * | Mauritius | Rat | [136] |
| Otus grucheti | Reunion Scops Owl | Strigidae | E, EX 1700s | P * | Reunion | Cat and rat | [136] |
| Otus murivorus | Rodrigues Scops Owl | Strigidae | E, EX 1726–1761 | P * | Rodrigues | Rat | [136] |
| Otus pauliani | Grande Comore Scops Owl | Strigidae | E, EN | P ** | Comoros | Cat and rats? | 138; c |
| Otus moheliensis | Moheli Scops Owl | Strigidae | E, EN | P * | Comoros | Black Rat? | 138; a |
| Falco punctatus | Mauritius Kestrel | Falconidae | E, EN | P ** | Mauritius: Mauritius | Rat, cat, mongoose | [125] |
| Circus maillardi | Reunion Harrier | Accipitridae | E, EN | P * | Reunion, Mauritus, extinct 1606 | Rat, cat, mongoose? | [64] |
| Aepyornis maximus | Elephant Bird | Aepyornithidae | E, EX 1100–700 BP | P * | Madagascar | Dog | [141] |
| Aepyornis hildebrandti | Hildebrand’s Elephant Bird | Aepyornithidae | E, EX 1040–1380 AD | P * | Madagascar | Dog | [141] |
| Mullerornis modestus | Elephant Bird | Aepyornithidae | E, EX 680–880 AD | P * | Madagascar | Dog | [141] |
| MAMMALIA | |||||||
| Gymnuromys robertsi | Voalavoanala | Nesomyidae | E, LC | C * | Madagascar | Black Rat? | [64,142] |
| Nesomys audeberti | White-bellied Nesomys | Nesomyidae | E, LC | C * | Madagascar | Black Rat? | [143] |
| Nesomys rufus | Island Mouse | Nesomyidae | E, LC | C * | Madagascar | Black Rat? | [143] |
| Eliurus tanala | Tanala Tufted-tailed Rat | Nesomyidae | E, LC | C * | Madagascar | Black Rat? | [143] |
| Eliurub webbi | Webbie’s Tufted-tailed Rat | Nesomyidae | E, LC | C * | Madagascar | Black Rat? | [143] |
| Pachylemur insignis | Giant Lemur | Lemuridae | E, EX c.1500 | P * | Madagascar | Dog | [144,145] |
| Eulemur fulvus | Common Brow Lemur | Lemuridae | E, VU | P * | Madagascar Mayotte (introduced) | Dog | [66] |
| Propithecus verreauxi | Verreaux’s Sifaka | Indridae | E, CR | P * | Madagascar | Dog | [66] |
| Archaeolemur majori | Baboon Lemur | Archaeolemuridae | E, EX 1100–700 BP | P * | Madagascar | Dog | [66] |
| Archeolemur edwardsi | Monkey Lemur | Archaeolemuridae | E, EX, 500 BP | P * | Madagascar | Dog) | [146] |
| Hadropithecus stenognathus | Monkey Lemur | Archaeolemuridae | E, EX 444–772 | P, H ** | Madagascar | Feral cattle, pig and dog | [146] |
| Megaladapsis madagascariens. | Koala Lemur | Megaladapidae | E, EX 500–600 BP | P * | Madagascar | Dog? | [144] |
| Megaladapsis grandidieri | Koala Lemur | Megaladapidae | E, EX 500–600 BP | P * | Madagascar | Dog? | [144] |
| Megaladapsis edwardsi | Koala Lemur | Megaladapidae | E, EX 500–600 BP | P * | Madagascar | Dog? | [144] |
| Palaeopropithecus ingens | Sloth Lemur | Palaeopropi-thecidae | E, EX 1100–700 BP | P * | Madagascar | Dog | [66] |
| Mesopropithecus pithecoides | Sloth Lemur | Palaeopropi-thecidae | E, EX 570–679 CE | P * | Madagascar | Dog | [144] |
| Mesopropithecus globiceps | Sloth Lemur | Palaeopropi-thecidae | E, EX 570–679 CE | P * | Madagascar | Dog | [144] |
| Mesopropithecus dolichobrachion | Sloth Lemur | Palaeopropi-thecidae | E, EX 570–679 CE | P * | Madagascar | Dog | [144] |
| Babakotia radofilai | Sloth Lemur | Palaeopropi-thecidae | E, EX c.1000 BC | P * | Madagascar | Dog | [66] |
| Daubentonia robusta | Giant Aye-aye | Daubentonidae | E, EX 900–1150 CE | P * | Madagascar | Dog | [147] |
| Daubentonia madagascarensis | Aye-aye | Daubentoniidae | E, EN | P * | Madagascar | Black Rat? | [64] |
| Cryptoprocta ferax | Fossa | Eupleridae | E, VU | P, C * | Madagascar | Dog | [66] |
| Felis silvestris lybica | African Wild Cat | Felidae | LC | P * | Namibia, Botswana, SA | Feral Cat | [14] |
| Equus zebra | Mountain Zebra | Equidae | VU | H * | SA: N Cape; Namibia | Feral Donkey | [14] |
| Equus quagga quagga | Quagga | Equidae | EX, c.1878 | G * | SA: Cape, Free State | Feral donkey? | [72] |
| Equus africanus africanus | Nubian wild ass | Equidae | EX | G ** | Ethiopia; Eritrea: Nubian Desert | Feral Donkey | [148] |
| Hippopotamus laloumena | Malagasy Hippo | Hippopotamidae | E, EX 1670–1950 AD | P, H * | Madagascar | Dog, feral goat | [147,149] |
| Hippopotamus lemerlei | Lemerle’s Dwarf Hippopotamus | Hippopotamidae | E, EX 670–836 AD | P, H * | Madagascar | Dog, feral goat? | [147,149] |
| Hippopotamus madagascariensis | Madagascar Dwarf Hippopotamus | Hippopotamidae | E, EX 687–880 CE | P, H * | Madagascar | Dog, feral goat? | [147,149] |
| Hippotragus leucophaeus | Bluebuck | Bovidae | EX c.1800 | H * | SA: W Cape | Feral goat, deer | [150] |
| Taxa | Ex | En | O | Total | |
|---|---|---|---|---|---|
| n | n | n | n | % | |
| PISCES | 0 | 0 | 0 | 0 | 0.0 |
| AMPHIBIA | 0 | 3 | 0 | 3 | 2.1 |
| Sooglossidae | 3 | 0 | 0 | 3 | 2.1 |
| REPTILIA | 10 | 9 | 4 | 23 | 16.0 |
| Bolyeriidae | 1 | 0 | 0 | 1 | 0.7 |
| Typhlopidae | 1 | 0 | 0 | 1 | 0.7 |
| Scincidae | 4 | 3 | 4 | 11 | 7.6 |
| Gekkonidae | 0 | 3 | 0 | 3 | 2.1 |
| Phyllodactylidae | 0 | 1 | 0 | 1 | 0.7 |
| Testudinidae | 4 | 2 | 0 | 6 | 4.2 |
| AVES | 37 | 31 | 21 | 89 | 61.8 |
| Aepyornithidae | 3 | 0 | 0 | 3 | 2.1 |
| Phaethontidae | 0 | 0 | 2 | 2 | 1.4 |
| Sulidae | 0 | 0 | 2 | 2 | 1.4 |
| Hydrobatidae | 0 | 1 | 0 | 1 | 0.7 |
| Procellariidae | 0 | 3 | 6 | 9 | 6.3 |
| Phalacrocoracidae | 1 | 2 | 0 | 3 | 2.1 |
| Diomedeidae | 0 | 5 | 0 | 5 | 3.5 |
| Laridae | 0 | 0 | 3 | 3 | 2.1 |
| Spheniscidae | 0 | 0 | 2 | 2 | 1.4 |
| Anatidae | 3 | 0 | 0 | 3 | 2.1 |
| Threskiornithidae | 1 | 1 | 0 | 2 | 1.4 |
| Ardeidae | 2 | 0 | 0 | 2 | 1.4 |
| Pelecanidae | 0 | 1 | 0 | 1 | 0.7 |
| Rallidae | 7 | 1 | 0 | 8 | 5.6 |
| Columbidae | 8 | 1 | 0 | 9 | 6.3 |
| Psittaculidae | 4 | 1 | 0 | 5 | 3.5 |
| Strigidae | 3 | 2 | 0 | 5 | 3.5 |
| Falconidae | 0 | 1 | 0 | 1 | 0.7 |
| Accipitridae | 0 | 1 | 0 | 1 | 0.7 |
| Hirundinidae | 0 | 1 | 0 | 1 | 0.7 |
| Monarchidae | 0 | 1 | 0 | 1 | 0.7 |
| Muscicapidae | 0 | 2 | 0 | 2 | 1.4 |
| Campephagidae | 0 | 1 | 0 | 1 | 0.7 |
| Zosteropidae | 1 | 2 | 0 | 3 | 2.1 |
| Acrocephalidae | 1 | 1 | 0 | 2 | 1.4 |
| Ploceidae | 1 | 2 | 0 | 3 | 2.1 |
| Sturnidae | 1 | 0 | 1 | 2 | 1.4 |
| Nectariniidae | 0 | 0 | 1 | 1 | 0.7 |
| Pycnonotidae | 1 | 0 | 0 | 1 | 0.7 |
| Cisticolidae | 0 | 0 | 2 | 2 | 1.4 |
| Passeridae | 0 | 0 | 1 | 1 | 0.7 |
| Emberizidae | 0 | 1 | 0 | 1 | 0.7 |
| Fringillidae | 0 | 0 | 1 | 1 | 0.7 |
| MAMMALIA | 18 | 5 | 6 | 29 | 20.1 |
| Nesomyidae | 0 | 0 | 5 | 5 | 3.5 |
| Lemuridae | 1 | 1 | 0 | 2 | 1.4 |
| Indrididae | 0 | 1 | 0 | 1 | 0.7 |
| Archeolemuridae | 3 | 0 | 0 | 3 | 2.1 |
| Megaladapidae | 3 | 0 | 0 | 3 | 2.1 |
| Palaeopropithecidae | 5 | 0 | 0 | 5 | 3.5 |
| Daubentonidae | 1 | 1 | 0 | 2 | 1.4 |
| Eupleridae | 0 | 1 | 0 | 1 | 0.7 |
| Felidae | 0 | 0 | 1 | 1 | 0.7 |
| Equidae | 1 | 1 | 0 | 2 | 1.4 |
| Hippopotamidae | 3 | 0 | 0 | 3 | 2.1 |
| Bovidae | 1 | 0 | 0 | 1 | 0.7 |
| Total number of species | 65 | 45 | 31 | 144 | 100.0 |
| Places | Reptilia | Aves | Mammalia | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ex | En | O | Ex | En | O | Ex | En | O | Ex | En | O | |
| AFRICA: continent | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 1 | 1 | 3 | 3 | 1 |
| Ethiopia/Eritrea | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Southern Africa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| South Africa | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 2 | 0 |
| AFRICA: islands | 9 | 10 | 2 | 42 | 29 | 24 | 16 | 4 | 5 | 68 | 43 | 32 |
| Cape Verde | 1 | 1 | 0 | 0 | 3 | 5 | 0 | 0 | 0 | 1 | 4 | 5 |
| Gulf of Guinea islands | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Marion Isl. | 0 | 0 | 0 | 0 | 6 | 4 | 0 | 0 | 0 | 0 | 6 | 4 |
| Madagascar | 1 | 0 | 0 | 3 | 1 | 0 | 16 | 4 | 5 | 20 | 5 | 5 |
| Mauritius | 3 | 9 | 0 | 11 | 7 | 2 | 0 | 0 | 0 | 14 | 16 | 2 |
| Reunion | 2 | 0 | 0 | 12 | 1 | 2 | 0 | 0 | 0 | 14 | 1 | 2 |
| Rodrigues | 2 | 0 | 0 | 12 | 1 | 0 | 0 | 0 | 0 | 14 | 1 | 1 |
| Other Mascarenes | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| Comoros | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 1 |
| Seychelles | 1 | 0 | 0 | 3 | 5 | 3 | 0 | 0 | 0 | 4 | 5 | 3 |
| Socotra | 0 | 0 | 1 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 1 | 8 |
| Total | 10 | 11 | 2 | 42 | 30 | 24 | 19 | 5 | 6 | 71 | 46 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopij, G. Alien Mammals in the Afrotropical Region and Their Impact on Vertebrate Biodiversity: A Review. Diversity 2025, 17, 286. https://doi.org/10.3390/d17040286
Kopij G. Alien Mammals in the Afrotropical Region and Their Impact on Vertebrate Biodiversity: A Review. Diversity. 2025; 17(4):286. https://doi.org/10.3390/d17040286
Chicago/Turabian StyleKopij, Grzegorz. 2025. "Alien Mammals in the Afrotropical Region and Their Impact on Vertebrate Biodiversity: A Review" Diversity 17, no. 4: 286. https://doi.org/10.3390/d17040286
APA StyleKopij, G. (2025). Alien Mammals in the Afrotropical Region and Their Impact on Vertebrate Biodiversity: A Review. Diversity, 17(4), 286. https://doi.org/10.3390/d17040286






