Effects of Phenotypic Plasticity and Genetic Variation on Plant Growth and Litter Decomposition in a Widespread Wetland Grass
Abstract
1. Introduction
2. Materials and Methods
2.1. Common Garden Experiment
2.2. Litter Collection
2.3. Decomposition Experiment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McLaughlin, D.L.; Cohen, M.J. Realizing Ecosystem Services: Wetland Hydrologic Function along a Gradient of Ecosystem Condition. Ecol. Appl. 2013, 23, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Means, M.M.; Ahn, C.; Korol, A.R.; Williams, L.D. Carbon Storage Potential by Four Macrophytes as Affected by Planting Diversity in a Created Wetland. J. Environ. Manag. 2016, 165, 133–139. [Google Scholar] [CrossRef]
- Ma, S.; Creed, I.F.; Badiou, P. New Perspectives on Temperate Inland Wetlands as Natural Climate Solutions under Different CO2-Equivalent Metrics. NPJ Clim. Atmos. Sci. 2024, 7, 222. [Google Scholar] [CrossRef]
- Kayranli, B.; Scholz, M.; Mustafa, A.; Hedmark, Å. Carbon Storage and Fluxes within Freshwater Wetlands: A Critical Review. Wetlands 2010, 30, 111–124. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, Z.; Zhao, Z.; Ning, J.; Bi, X. Dynamics of Wetlands and Their Effects on Carbon Emissions in China Coastal Region—Case Study in Bohai Economic Rim. Ocean Coast. Manag. 2014, 87, 61–67. [Google Scholar]
- Were, D.; Kansiime, F.; Fetahi, T.; Hein, T. Soil Organic Carbon Storage in a Tropical Freshwater Wetland: The Influence of Vegetation Type. Afr. J. Aquat. Sci. 2021, 46, 161–172. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, H.; Ngo, H.H.; Guo, W.; Zhang, J.; Liu, C.; Liang, S.; Hu, Z.; Yang, Z.; Zhao, C. Microbial Abundance and Community in Subsurface Flow Constructed Wetland Microcosms: Role of Plant Presence. Environ. Sci. Pollut. Res. 2016, 23, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, W.; Luo, J.; Donnison, A. Changes in Soil Organic Carbon Dynamics in an Eastern Chinese Coastal Wetland Following Invasion by a C4 Plant Spartina alterniflora. Soil Biol. Biochem. 2010, 42, 1712–1720. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.-Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan Species as Models for Ecophysiological Responses to Global Change: The Common Reed Phragmites australis. Front. Plant Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Packer, J.G.; Meyerson, L.A.; Skálová, H.; Pyšek, P.; Kueffer, C. Biological Flora of the British Isles: Phragmites australis. J. Ecol. 2017, 105, 1123–1162. [Google Scholar] [CrossRef]
- Song, H.; Jespersen, E.; Guo, X.; Du, N.; Xie, L.; Pei, L.; Ye, S.; Wang, R.; Brix, H.; Eller, F.; et al. Differences in Relative Air Humidity Affect Responses to Soil Salinity in Freshwater and Salt Marsh Populations of the Dominant Grass Species Phragmites australis. Hydrobiologia 2021, 848, 3353–3369. [Google Scholar] [CrossRef]
- Maron, J.L.; Elmendorf, S.C.; Vilà, M. Contrasting Plant Physiological Adaptation to Climate in the Native and Introduced Range of Hypericum Perforatum. Evol. Int. J. Org. Evol. 2007, 61, 1912–1924. [Google Scholar] [CrossRef]
- Ren, L.; Guo, X.; Sorrell, B.K.; Eller, F.; Brix, H. Responses to Cold Temperature Determine Clinal Patterns of Photosynthetic Acclimation of a Cosmopolitan Grass Genus and Challenge the Concept of Quantifying Phenotypic Plasticity. Funct. Ecol. 2025, 39, 583–595. [Google Scholar] [CrossRef]
- Sheng, W.; Liu, L.; Wu, Y.; Yin, M.; Yu, Q.; Guo, X.; Song, H.; Guo, W. Exploring Salt Tolerance and Indicator Traits across Four Temperate Lineages of the Common Wetland Plant, Phragmites australis. Sci. Total Environ. 2024, 912, 169100. [Google Scholar] [CrossRef] [PubMed]
- Silan, G.; Buosi, A.; Bertolini, C.; Sfriso, A. Dynamics and Drivers of Carbon Sequestration and Storage Capacity in Phragmites australis-Dominated Wetlands. Estuar. Coast. Shelf Sci. 2024, 298, 108640. [Google Scholar] [CrossRef]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F. Intraspecific Variation in Phragmites australis: Clinal Adaption of Functional Traits and Phenotypic Plasticity Vary with Latitude of Origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Zhang, Y.; Pennings, S.C.; Liu, Z.; Li, B.; Wu, J. Consistent Pattern of Higher Lability of Leaves from High Latitudes for Both Native Phragmites australis and Exotic Spartina alterniflora. Funct. Ecol. 2021, 35, 2084–2093. [Google Scholar] [CrossRef]
- Ravenscroft, C.H.; Fridley, J.D.; Grime, J.P. Intraspecific Functional Differentiation Suggests Local Adaptation to Long-term Climate Change in a Calcareous Grassland. J. Ecol. 2014, 102, 65–73. [Google Scholar] [CrossRef]
- Kahl, S.M.; Lenhard, M.; Joshi, J. Compensatory Mechanisms to Climate Change in the Widely Distributed Species Silene vulgaris. J. Ecol. 2019, 107, 1918–1930. [Google Scholar] [CrossRef]
- Sambatti, J.B.M.; Rice, K.J. Local adaptation, patterns of selection, and gene flow in the Californian serpentine sunflower (Helianthus exilis). Evolution 2006, 60, 696–710. [Google Scholar]
- Kawecki, T.J.; Ebert, D. Conceptual Issues in Local Adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Cuma Mushagalusa, F.; Bauman, D.; Mujinya Bazirake, B.; Mleci, Y.; Kalenga, M.; Shutcha, M.N.; Meerts, P. Phenotypic Plasticity, Not Ecotype Differentiation, Explains the Broad Ecological Niche of a Tree Species in African Dry Woodlands. Environ. Exp. Bot. 2020, 178, 104186. [Google Scholar] [CrossRef]
- Liu, L.; Yin, M.; Guo, X.; Wang, J.; Cai, Y.; Wang, C.; Yu, X.; Du, N.; Brix, H.; Eller, F.; et al. Cryptic Lineages and Potential Introgression in a Mixed-ploidy Species (Phragmites australis) across Temperate China. J. Syst. Evol. 2022, 60, 398–410. [Google Scholar] [CrossRef]
- Pastore, M.A. Bringing the Underground to the Surface: Climate Change Stressors Negatively Affect Plant Growth, with Contrasting above and Belowground Physiological Responses. Plant Cell Environ. 2022, 45, 2267–2270. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant Phenotypic Plasticity in a Changing Climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR Plant Functional Types: The Use of Leaf Economics and Size Traits to Classify Woody and Herbaceous Vascular Plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Zhang, Y.; Meiners, S.J.; Meng, Y.; Yao, Q.; Guo, K.; Guo, W.; Li, S. Temporal Dynamics of Grime’s CSR Strategies in Plant Communities during 60 Years of Succession. Ecol. Lett. 2024, 27, e14446. [Google Scholar] [CrossRef]
- Pei, Z.; Leppert, K.N.; Eichenberg, D.; Bruelheide, H.; Niklaus, P.A.; Buscot, F.; Gutknecht, J.L.M. Leaf Litter Diversity Alters Microbial Activity, Microbial Abundances, and Nutrient Cycling in a Subtropical Forest Ecosystem. Biogeochemistry 2017, 134, 163–181. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, D.; Zhao, G.; Chen, S.; Sun, T.; Sun, H.; Wu, C.; Li, Y.; Yu, Z.; Li, Y.; et al. The Contribution of Wetland Plant Litter to Soil Carbon Pool: Decomposition Rates and Priming Effects. Environ. Res. 2023, 224, 115575. [Google Scholar] [CrossRef]
- Yin, M.; Liu, L.; Wu, Y.; Sheng, W.; Ma, X.; Du, N.; Zhu, P.; Wang, C.; Cui, Z.; Brix, H.; et al. Effects of Litter Species and Genetic Diversity on Plant Litter Decomposition in Coastal Wetland. Ecol. Indic. 2022, 144, 109439. [Google Scholar] [CrossRef]
- Mori, A.S.; Cornelissen, J.H.C.; Fujii, S.; Okada, K.; Isbell, F. A Meta-Analysis on Decomposition Quantifies Afterlife Effects of Plant Diversity as a Global Change Driver. Nat. Commun. 2020, 11, 4547. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, Y.; Crawford, K.M.; Chen, X.; Yu, S.; Wu, J. Plant Genotypic Diversity Effects on Soil Nematodes Vary with Trophic Level. New Phytol. 2021, 229, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ma, Y.; Gan, Z.; Cai, R.; Li, Z.; Ge, G.; Wu, L. Influence of soil fauna on the litter decomposition of Lake Poyang Wetland in winter. J. Lake Sci. 2020, 32, 395–405. [Google Scholar]
- Zhang, L.; Zhang, Y.; Zou, J.; Siemann, E. Decomposition of Phragmites australis litter retarded by invasive Solidago canadensis in mixtures: An antagonistic non-additive effect. Sci Rep. 2014, 4, 5488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cai, Y.; Luo, H.; Hong, S.; Song, C.; Yan, J.; Fang, H. Effects of Abiotic Factors on Plant Community Composition and Functional Traits in the Liaohe Estuary. J. Mar. Environ. Eng. 2024, 11, 141–156. [Google Scholar] [CrossRef]
- Liu, L.; Du, N.; Eller, F.; Ye, S.; Li, X.; Wei, J.; Guo, Y.; Brix, H.; Guo, W. Ecological Mechanisms of Carbon Sequestration in Vegetated Coastal Wetland Ecosystem: Exploring the Roles of Biodiversity and Environmental Changes. J. Mar. Environ. Eng. 2025, 12, 35–47. [Google Scholar]
- Brabson, J.A. The Kjeldahl Method for Organic Nitrogen. J. Assoc. Off. Anal. Chem. 1966, 49, 481. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, L.; Yin, M.; Guo, W. Phylogenetic relationship and soil salinity shape intraspecific trait variability of Phragmites australis in the Yellow River Delta. Front. Mar. Sci. 2022, 9, 980695. [Google Scholar] [CrossRef]
- Clevering, O.A.; Brix, H.; Lukavská, J. Geographic Variation in Growth Responses in Phragmites australis. Aquat. Bot. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Mozdzer, T.J.; Caplan, J.S.; Hager, R.N.; Proffitt, C.E.; Meyerson, L.A. Contrasting Trait Responses to Latitudinal Climate Variation in Two Lineages of an Invasive Grass. Biol. Invasions 2016, 18, 2649–2660. [Google Scholar] [CrossRef]
- Oostra, V.; Saastamoinen, M.; Zwaan, B.J.; Wheat, C.W. Strong Phenotypic Plasticity Limits Potential for Evolutionary Responses to Climate Change. Nat. Commun. 2018, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Lissner, J.; Schierup, H.-H.; Comín, F.A.; Astorga, V. Effect of Climate on the Salt Tolerance of Two Phragmites australis Populations. Aquat. Bot. 1999, 64, 317–333. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Ma, X.; Li, M.; Guo, X.; Yin, M.; Cai, Y.; Yu, X.; Du, N.; Wang, R.; et al. Impacts of the Yellow River and Qingtongxia Dams on Genetic Diversity of Phragmites australis in Ningxia Plain, China. Aquat. Bot. 2021, 169, 103341. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W.; Raddatz, T.; Wirth, C. Quantifying Photosynthetic Capacity and Its Relationship to Leaf Nitrogen Content for Global-scale Terrestrial Biosphere Models. Glob. Change Biol. 2009, 15, 976–991. [Google Scholar] [CrossRef]
- He, Y.; Xu, X.; Kueffer, C.; Zhang, X.; Shi, P. Leaf Litter of a Dominant Cushion Plant Shifts Nitrogen Mineralization to Immobilization at High but Not Low Temperature in an Alpine Meadow. Plant Soil 2014, 383, 415–426. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Zak, D.R.; Talhelm, A.F.; Burton, A.J.; Eikenberry, J.R. Nitrogen Turnover in the Leaf Litter and Fine Roots of Sugar Maple. Ecology 2010, 91, 3456–3462. [Google Scholar] [CrossRef]
- Berglund, S.L.; Ågren, G.I.; Ekblad, A. Carbon and Nitrogen Transfer in Leaf Litter Mixtures. Soil Biol. Biochem. 2013, 57, 341–348. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy Shifts in Leaf Physiology, Structure and Nutrient Content between Species of High- and Low-rainfall and High- and Low-nutrient Habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Sisto, M.L.D.; Macdougall, A.H. Effect of Terrestrial Nutrient Limitation on the Estimation of the Remaining Carbon budget. Biogeosciences 2024, 21, 21. [Google Scholar] [CrossRef]
- Uddin, M.N.; Robinson, R.W. Responses of Plant Species Diversity and Soil Physical-Chemical-Microbial Properties to Phragmites australis Invasion along a Density Gradient. Sci. Rep. 2017, 7, 11007. [Google Scholar] [CrossRef]
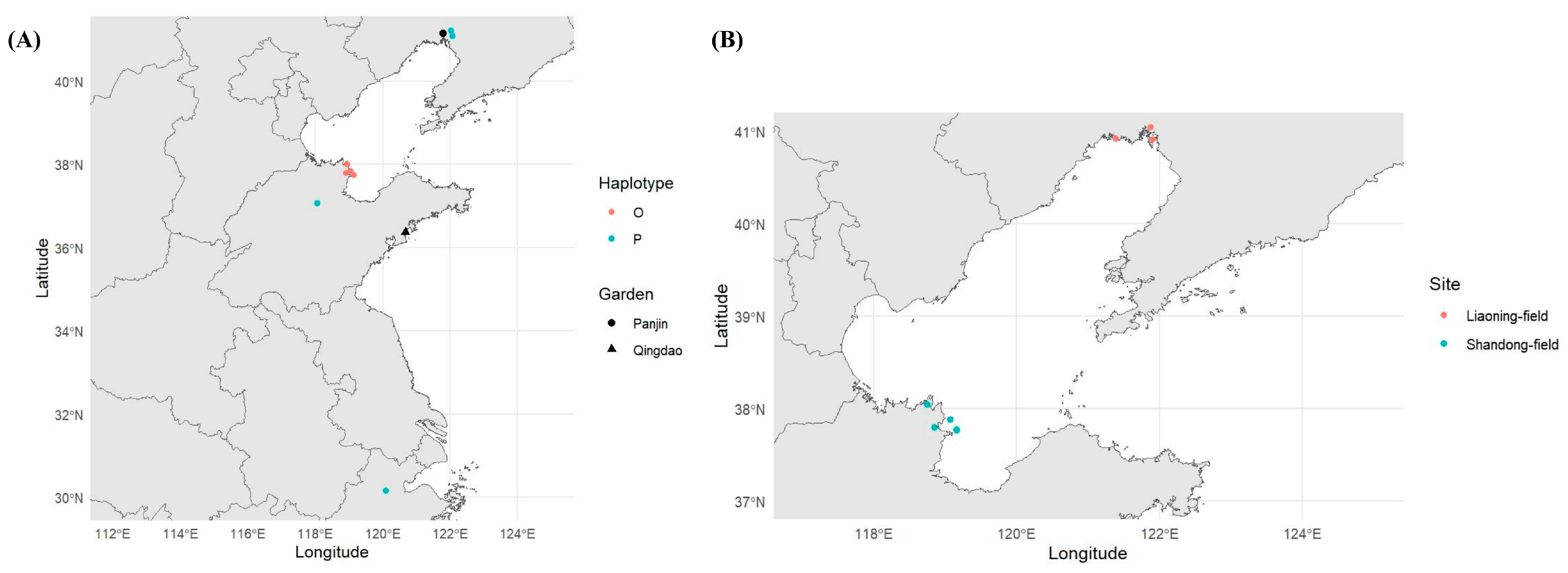

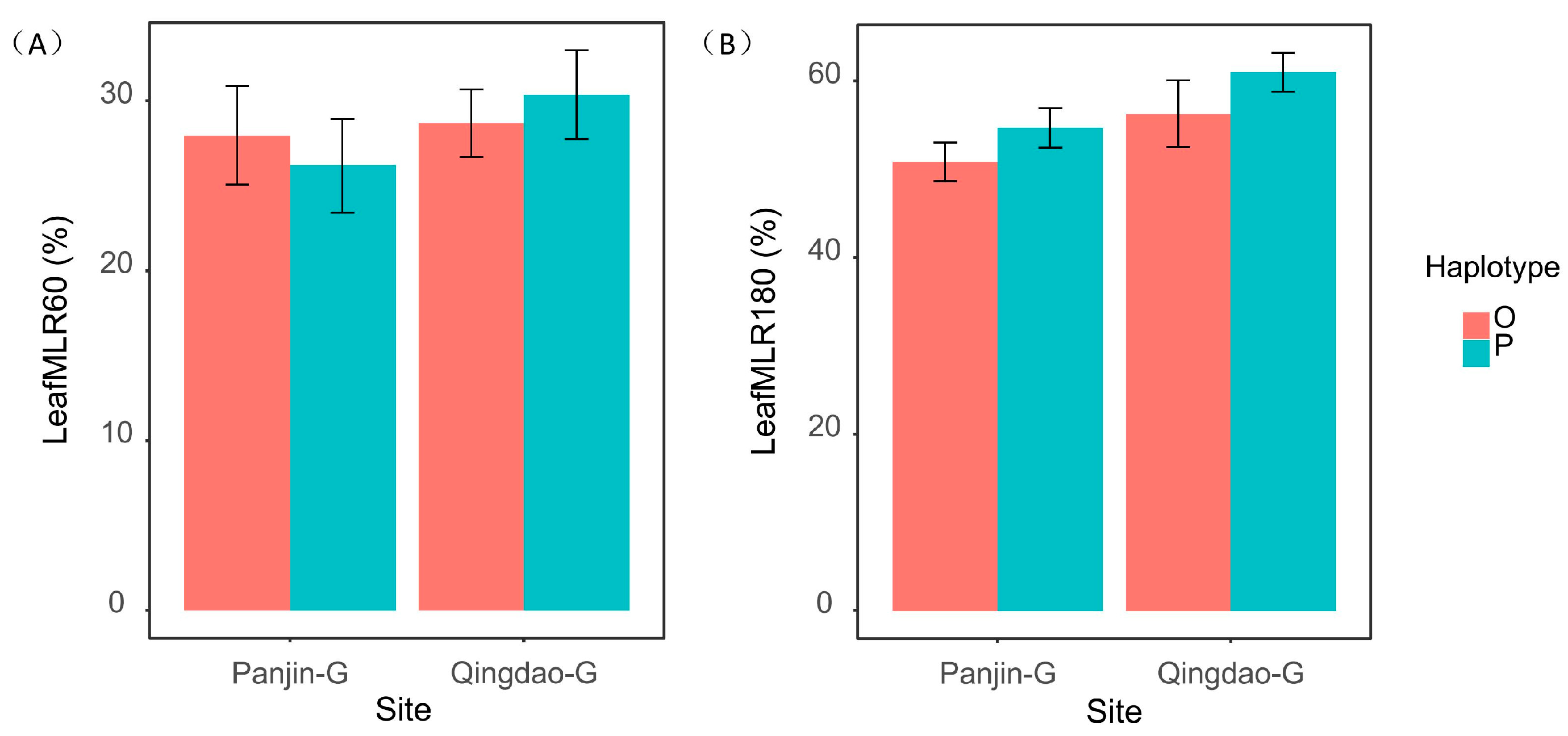
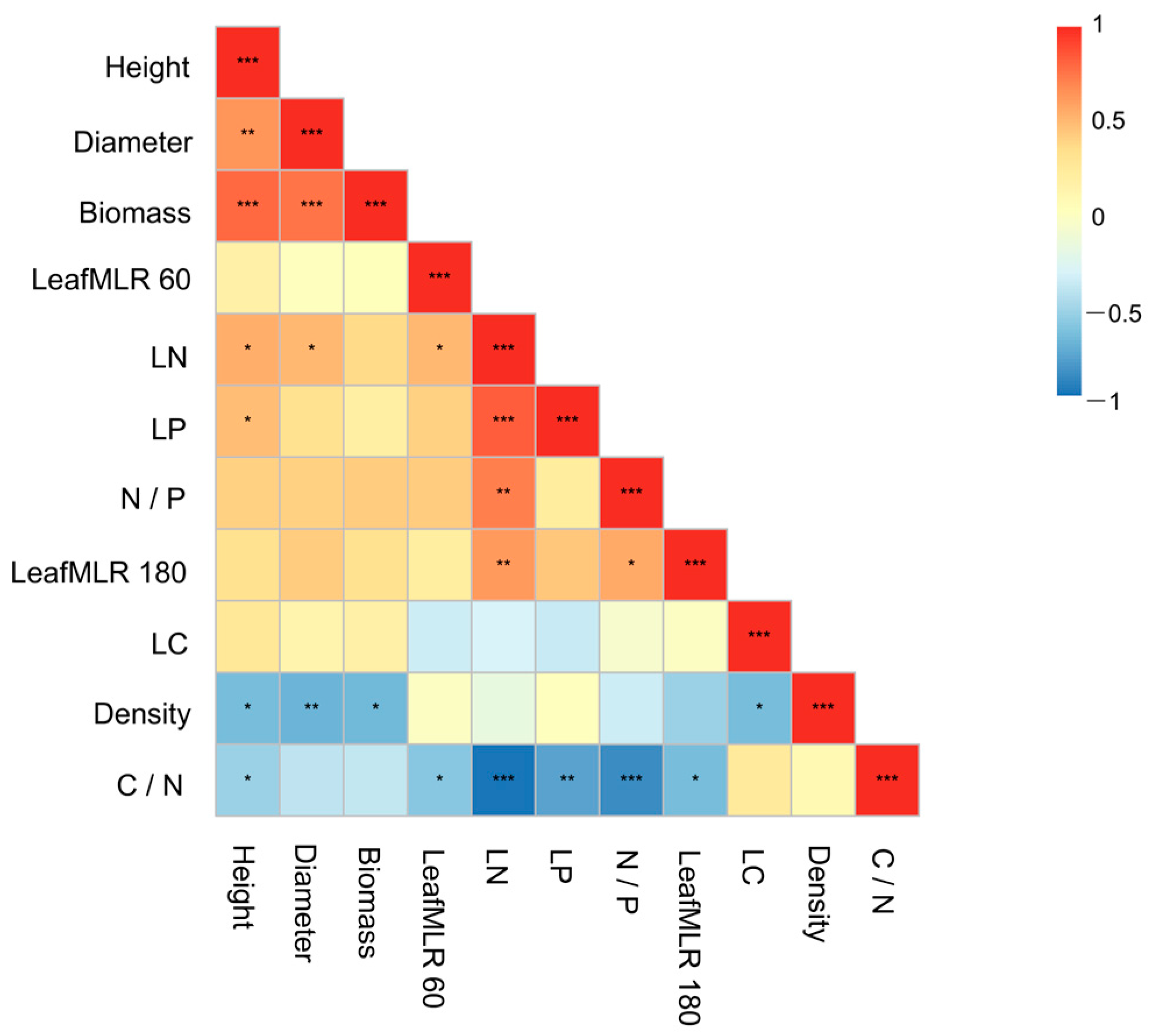
| Parameters | F (Garden) | F (Haplotype) | F (Garden × Haplotype) |
|---|---|---|---|
| Diameter | 4.867 * | 0.445 | 0.080 |
| Biomass | 4.581 | 0.008 | 0.440 |
| Height | 12.281 ** | 1.933 | 1.098 |
| Shoot Density | 0.089 | 0.291 | 0.659 |
| Leaf Litter N | 44.779 *** | 0.0000 | 0.986 |
| Leaf Litter P | 46.481 *** | 1.174 | 0.058 |
| Leaf Litter C | 1.410 | 0.908 | 1.306 |
| Leaf Litter C/N | 24.210 *** | 0.911 | 1.674 |
| Leaf Litter N/P | 3.769 | 0.677 | 0.594 |
| Leaf MLR 60 days | 1.004 | 0.005 | 0.382 |
| Leaf MLR 180 days | 6.302 * | 2.836 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Wen, Q.; Zhu, H.; Song, H.; Zhang, X.; Sheng, W.; Xie, L.; Guo, X.; Guo, Y.; Ye, S.; et al. Effects of Phenotypic Plasticity and Genetic Variation on Plant Growth and Litter Decomposition in a Widespread Wetland Grass. Diversity 2025, 17, 282. https://doi.org/10.3390/d17040282
Wei W, Wen Q, Zhu H, Song H, Zhang X, Sheng W, Xie L, Guo X, Guo Y, Ye S, et al. Effects of Phenotypic Plasticity and Genetic Variation on Plant Growth and Litter Decomposition in a Widespread Wetland Grass. Diversity. 2025; 17(4):282. https://doi.org/10.3390/d17040282
Chicago/Turabian StyleWei, Wei, Qishen Wen, Hong Zhu, Huijia Song, Xiya Zhang, Wenyi Sheng, Liujuan Xie, Xiao Guo, Yaolin Guo, Siyuan Ye, and et al. 2025. "Effects of Phenotypic Plasticity and Genetic Variation on Plant Growth and Litter Decomposition in a Widespread Wetland Grass" Diversity 17, no. 4: 282. https://doi.org/10.3390/d17040282
APA StyleWei, W., Wen, Q., Zhu, H., Song, H., Zhang, X., Sheng, W., Xie, L., Guo, X., Guo, Y., Ye, S., Wang, Y., Liu, L., & Guo, W. (2025). Effects of Phenotypic Plasticity and Genetic Variation on Plant Growth and Litter Decomposition in a Widespread Wetland Grass. Diversity, 17(4), 282. https://doi.org/10.3390/d17040282







