Characterizing the Seed Coat in the Subtribe Angraecinae (Orchidaceae, Vandeae) and Its Taxonomic Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Microscopic Study
2.3. Cluster and Principal Component Analysis
3. Results
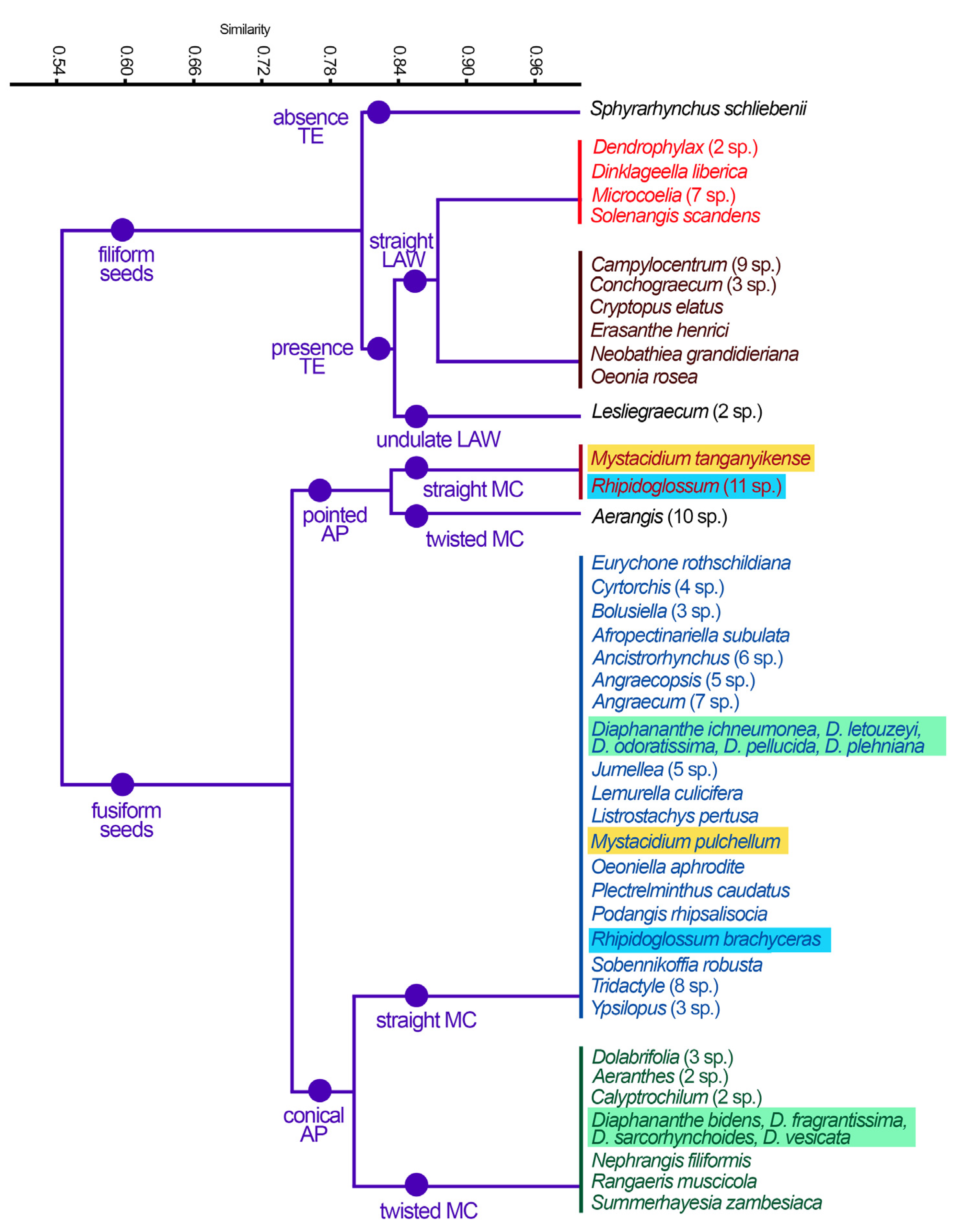
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molvray, M.; Chase, M.W. Seed morphology. In Genera Orchidacearum; Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 1999; Volume 1, pp. 59–66. [Google Scholar]
- Arditti, J.; Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid Seed Diversity: A Scanning Electron Microscopy Survey; Englera; Botanic Garden and Botanical Museum: Berlin, Germany, 2014; Volume 32, p. 245. [Google Scholar]
- Clifford, H.T.; Smith, W.K. Seed morphology and classification of Orchidaceae. Phytomorphology 1969, 19, 133–139. [Google Scholar]
- Gamarra, R.; Ortúñez, E.; Sanz, E.; Esparza, I.; Galán Cela, P. Seeds in subtribe Orchidinae (Orchidaceae): The best morphological tool to support molecular analyses. In Tools for Identifying Biodiversity: Progress and Problems; Nimis, P.L., Vignes Lebbe, R., Eds.; Edizioni Università di Trieste: Trieste, Italy, 2010; pp. 323–326. [Google Scholar]
- Gamarra, R.; Ortúñez, E.; Galán Cela, P.; Guadaño, V. Anacamptis versus Orchis (Orchidaceae): Seed micromorphology and its taxonomic significance. Plant Syst. Evol. 2012, 298, 597–607. [Google Scholar] [CrossRef]
- Gamarra, R.; Galán Cela, P.; Pedersen, H.A.; Ortúñez, E.; Sanz, E. Seed micromorphology in Dactylorhiza Necker ex Nevski (Orchidaceae) and allied genera. Turk. J. Bot. 2015, 39, 298–309. [Google Scholar] [CrossRef]
- Chomicki, G.; Bidel, L.P.; Ming, F.; Coiro, M.; Zhang, X.; Wang, Y.; Baissac, Y.; Jay-Allemand, C.; Renner, S.S. The velamen protects photosynthetic orchid roots against UV-B damage, and a large dated phylogeny implies multiple gains and losses of this function during the Cenozoic. New Phytol. 2015, 205, 1330–1341. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Clements, M.A.; Arroyo, M.T.K.; Leebens-Mack, J.; Endara, L.; et al. Orchid phylogenomics and multiple drivers of extraordinary diversification. Proc. R. Soc. B Biol. Sci. 2015, 282, 171–180. [Google Scholar] [CrossRef]
- Givnish, T.J.; Spalink, D.; Ames, M.; Lyon, S.P.; Hunter, S.J.; Zuluaga, A.; Doucette, A.; Girlado Caro, G.; McDaniel, J.; Clements, M.A.; et al. Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. J. Biogeogr. 2016, 43, 1905–1916. [Google Scholar] [CrossRef]
- Vij, S.P.; Kaur, P.; Kaur, S.; Kaushal, P.S. The orchid seeds: Taxonomic, evolutionary, and functional aspects. J. Orchid. Soc. India 1992, 6, 91–107. [Google Scholar]
- Gamarra, R.; Ortúñez, E.; Galán Cela, P.; Merencio, Á. Seed micromorphology of Orchidaceae in the Gulf of Guinea (West Tropical Africa). Plant Syst. Evol. 2018, 304, 665–677. [Google Scholar] [CrossRef]
- Freudenstein, J.V.; Chase, M.W. Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: Progressive specialization and diversification. Ann. Bot. 2015, 115, 665–681. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Dodsworth, S.; Bogarín, D.; Bellot, S.; Balbuena, J.A.; Schley, R.J.; Kikuchi, I.A.; Morris, S.K.; Epitawalage, N.; Cowan, R.; et al. Hundreds of nuclear and plastic loci yield novel insights into orchid relationships. Amer. J. Bot. 2021, 108, 1166–1180. [Google Scholar] [CrossRef]
- Pridgeon, A.; Cribb, P.; Chase, M.; Rasmussen, F.N. Genera Orchidacearum–Volume 6. Epidendroideae (Part Three); Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 23 January 2025).
- Carlsward, B.S.; Whitten, W.M.; Williams, N.H.; Bytebier, B. Molecular phylogenetics of Vandeae (Orchidaceae) and the evolution of leafless. Amer. J. Bot. 2006, 93, 770786. [Google Scholar] [CrossRef] [PubMed]
- Simó-Droissart, M.; Plunkett, G.M.; Droissart, V.; Edwards, M.B.; Farminhão, J.N.; Ječmenica, V.; D’haijère, T.; Lowry, P.P.; Sonké, B.; Micheneau, C.; et al. New phylogenetic insights toward developing a natural generic classification of African angraecoid orchids (Vandeae, Orchidaceae). Molec. Phylogen. Evol. 2018, 126, 241–249. [Google Scholar] [CrossRef]
- Szlachetko, D.L.; Tukałło, P.; Mytnik-Ejsmont, J.; Grochocka, E. Reclassification of the Angraecum-alliance (Orchidaceae, Vandoideae) based on molecular and morphological data. Biodiv. Res. Conserv. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Farminhão, J.N.; Verlynde, S.; Kaymak, E.; Droissart, V.; Simó-Droissart, M.; Collobert, G.; Martos, F.; Stévart, T. Rapid radiation of angraecoids (Orchidaceae, Angraecinae) in tropical Africa characterised by multiple karyotypic shifts under major environmental instability. Molec. Phylogen. Evol. 2021, 159, 105–107. [Google Scholar] [CrossRef]
- Martos, F.; Le Péchon, T.; Johnson, S.D.; Bytebier, B. A reassessment of Angraecopsis, Mystacidium and Sphyrarhynchus (Orchidaceae: Vandeae) based on molecular and morphological evidence. Bot. J. Linn. Soc. 2017, 186, 1–17. [Google Scholar] [CrossRef]
- Farminhão, J.N.; Meerts, P.; Descourvières, P.; Droissart, V.; Simó-Droissart, M.; Stévart, T. A revised concept of Rhipidoglossum (Angraecinae, Orchidaceae). Phytotaxa 2018, 349, 247–256. [Google Scholar] [CrossRef]
- D’haijère, T.; Mardulyn, P.; Dong, L.; Plunkett, G.M.; Simó-Droissart, M.; Droissart, V.; Stévart, T. Molecular phylogeny and taxonomic synopsis of the angraecoid genus Ypsilopus (Orchidaceae, Vandeae). Taxon 2019, 68, 455–470. [Google Scholar] [CrossRef]
- Szlachetko, D.L.; Romowicz, A. Dolabrifolia, un nouveau genre d’orchidées de l’alliance Angraecum. Richardiana 2007, 7, 53–54. [Google Scholar]
- Simó-Droissart, M.; Stévart, T.; Sonké, B.; Mayogo, S.; Kamdem, N.; Droissart, V. New taxonomic and conservation status of Ossiculum (Vandeae, Orchidaceae), a highly threatened and narrow-endemic angraecoid orchid from Central Africa. Phytokeys 2018, 98, 85–97. [Google Scholar] [CrossRef]
- Szlachetko, D.L.; Grochocka, E.; Oledrzynska, N.; Mytnik, J. Taxonomical notes on Angraecoid orchids from Africa, with a new genus and new combinations. Richardiana. Nouv. Série 2018, 2, 87–96. [Google Scholar]
- Ziegler, B. Mikromorphologie der Orchidaceen-Samen Unter Berücksichtigung Taxonomischer Aspekte. Ph.D. Thesis, Ruprecht-Karls-Universität, Heidelberg, Germany, 1981. [Google Scholar]
- Ortúñez, E.; Gamarra, R. Seed morphology, life form and distribution in three Bromheadia species (Epidendroideae, Orchidaceae). Diversity 2023, 15, 195. [Google Scholar] [CrossRef]
- Fan, X.-L.; Chomicki, G.; Hao, K.; Liu, Q.; Xiong, Y.-Z.; Renner, S.S.; Gao, J.-Y.; Huang, S.Q. Transitions between the terrestrial and epiphytic habit drove the evolution of seed-aerodynamic traits in orchids. Amer. Nat. 2020, 195, 275–283. [Google Scholar] [CrossRef]
- Vafaee, Y.; Mohammadi, G.; Nazari, F.; Fatahi, M.; Kaki, A.; Gholami, S.; Ghorbani, A.; Khadivi, A. Phenotypic characterization and seed-micromorphology diversity of the threatened terrestrial orchids: Implications for conservation. S. Afr. J. Bot. 2021, 137, 386–309. [Google Scholar] [CrossRef]
- Alfaro Pinto, A.; McGill, C.; Nadarajan, J.; Archila Morales, F.; Clavijo McCormick, A. Seed morphology of three neotropical orchid species of the Lycaste genus. Seeds 2023, 2, 331–339. [Google Scholar] [CrossRef]
- Gamarra, R.; Ortúñez, E. Endocarpic trichomes in Vandeae (Orchidaceae). Flora 2021, 280, 151844. [Google Scholar] [CrossRef]
- Verma, J.; Sharma, K.; Thakur, K.; Sembi, J.K.; Vij, S.P. Study on seed morphometry of some threatened Western Himalayan orchids. Turk. J. Bot. 2014, 38, 234–251. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Chase, M.W.; Pippen, J.S. Seed morphology in the Onciidinae and related subtribes (Orchidaceae). Syst. Bot. 1988, 13, 313–323. [Google Scholar] [CrossRef]
- Diantina, S.; McGill, C.; Millner, J.; Nadarajan, J.; Pritchard, H.W.; Clavijo McCormick, A. Comparative seed morphology of tropical and temperate orchid species with different growth habits. Plants 2020, 9, 161. [Google Scholar] [CrossRef]
- Andriananjamanantsoa, H.N.; Engberg, S.; Louis, E.E.; Brouillet, L. Diversification of Angraecum (Orchidaceae, Vandeae) in Madagascar: Revised phylogeny reveals species accumulation through time rather than rapid radiation. PLoS ONE 2016, 11, e0163194. [Google Scholar] [CrossRef] [PubMed]
- Kolanowska, M.; Grochocka, E.; Konowalik, K. Phylogenetic climatic niche conservatism and evolution of climatic suitability in Neotropical Angraecinae (Vandeae, Orchidaceae) and their closest African relatives. PeerJ 2017, 5, e3328. [Google Scholar] [CrossRef] [PubMed]
- Micheneau, C.; Carlsward, B.S.; Fay, M.F.; Bytebier, B.; Pailler, T.; Chase, M.W. Phylogenetics and biogeography of Mascarene angraecoid orchids (Vandeae, Orchidaceae). Molec. Phylogen. Evol. 2008, 46, 908–922. [Google Scholar] [CrossRef] [PubMed]
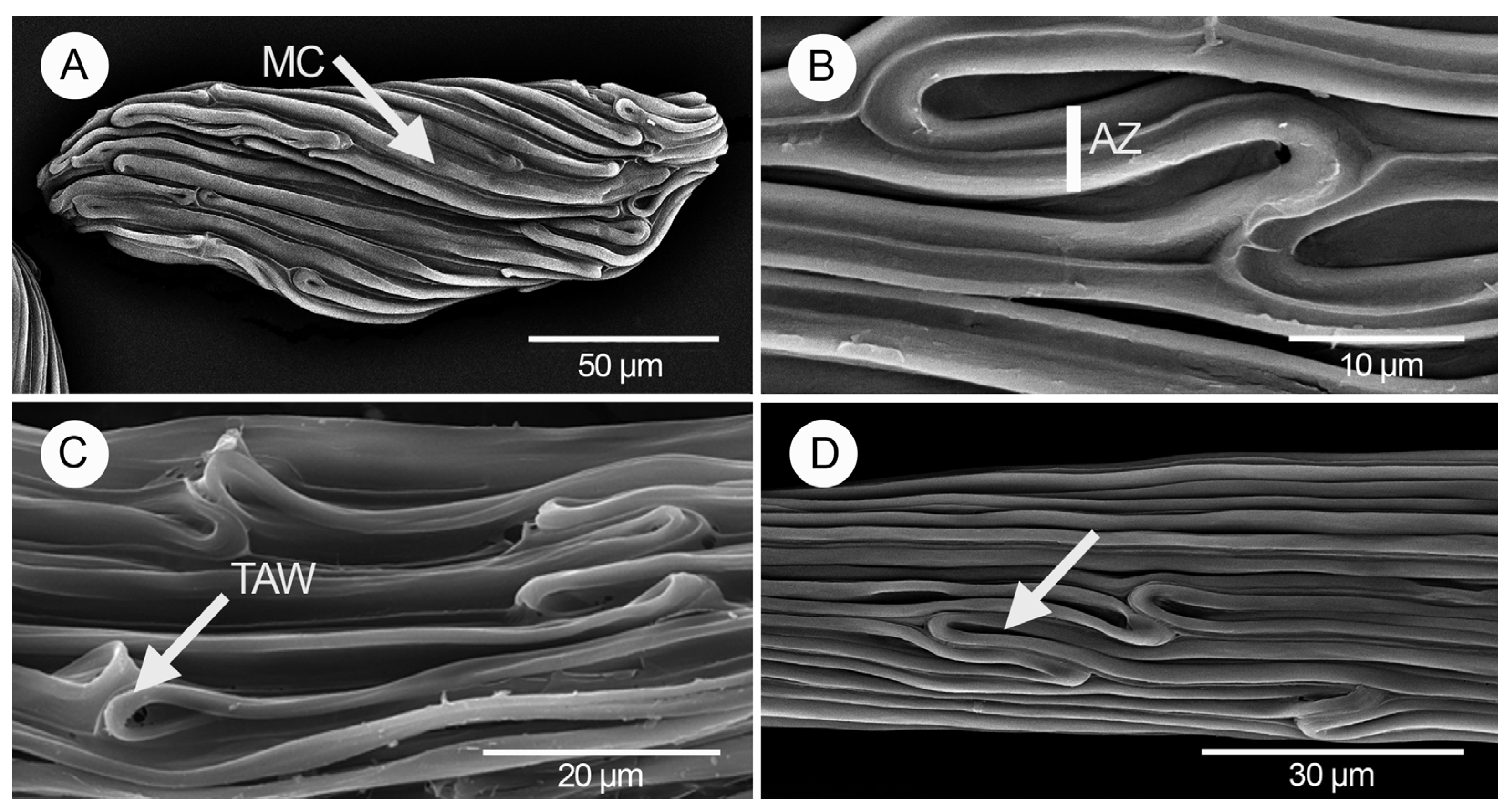

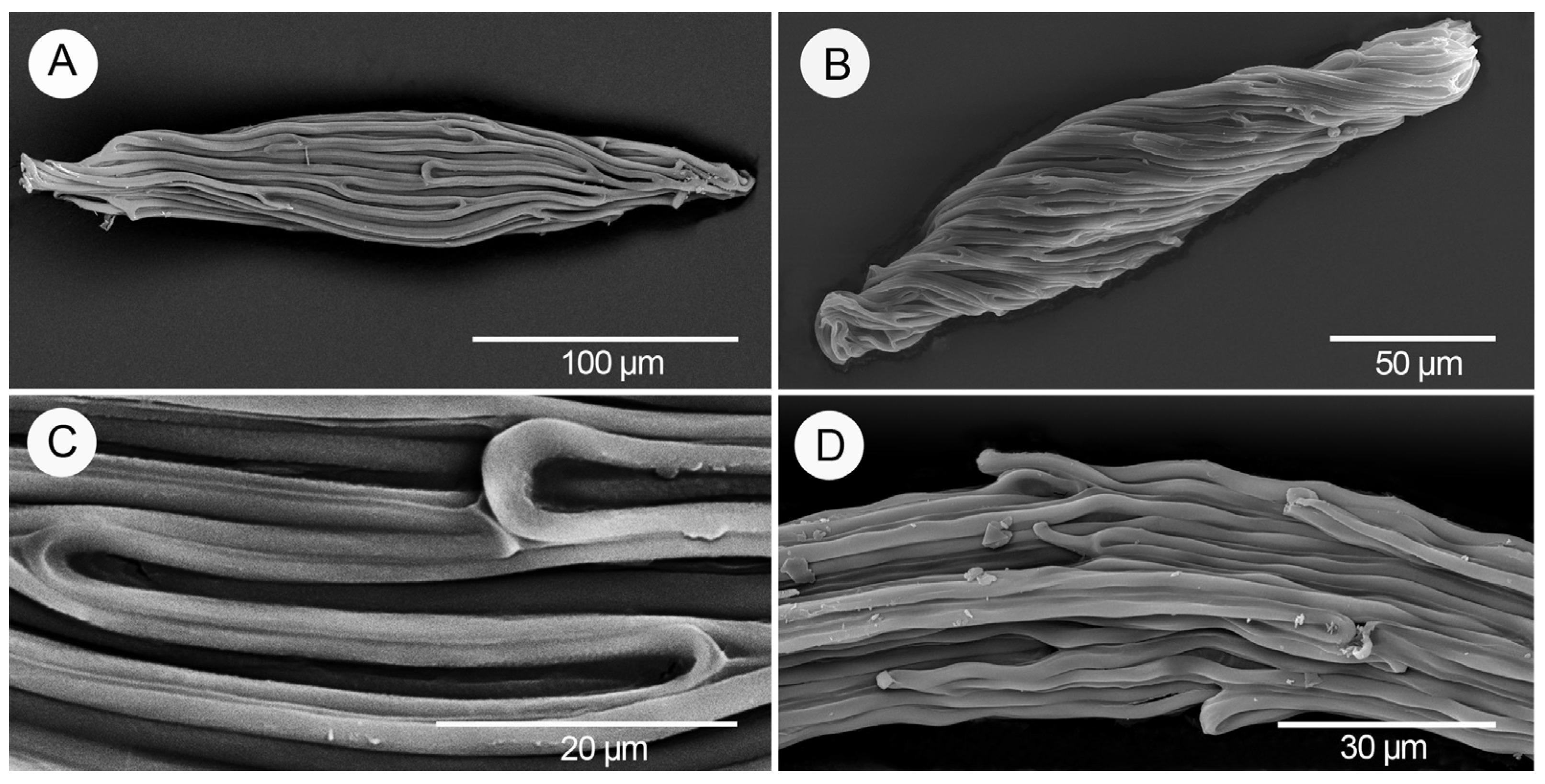
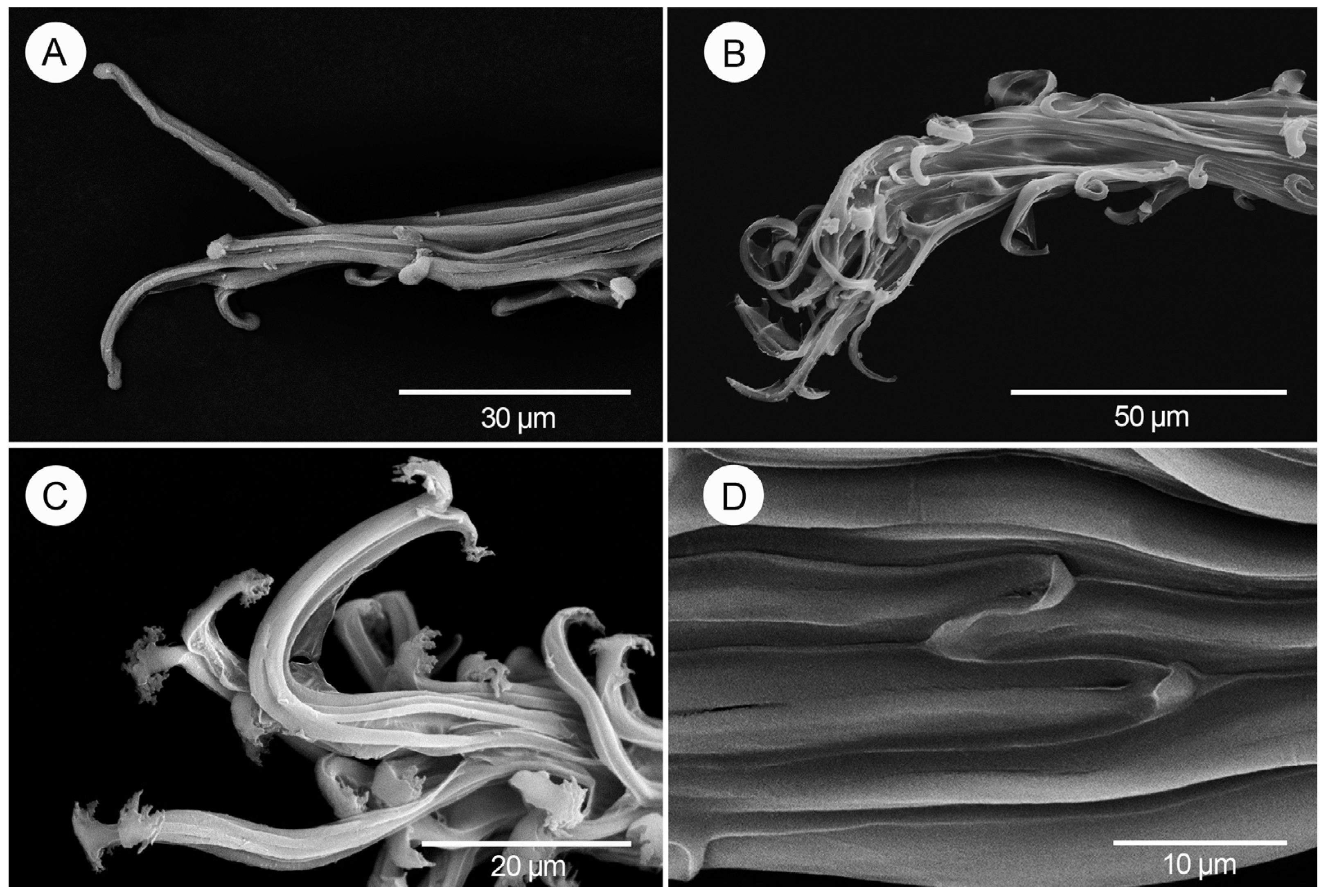
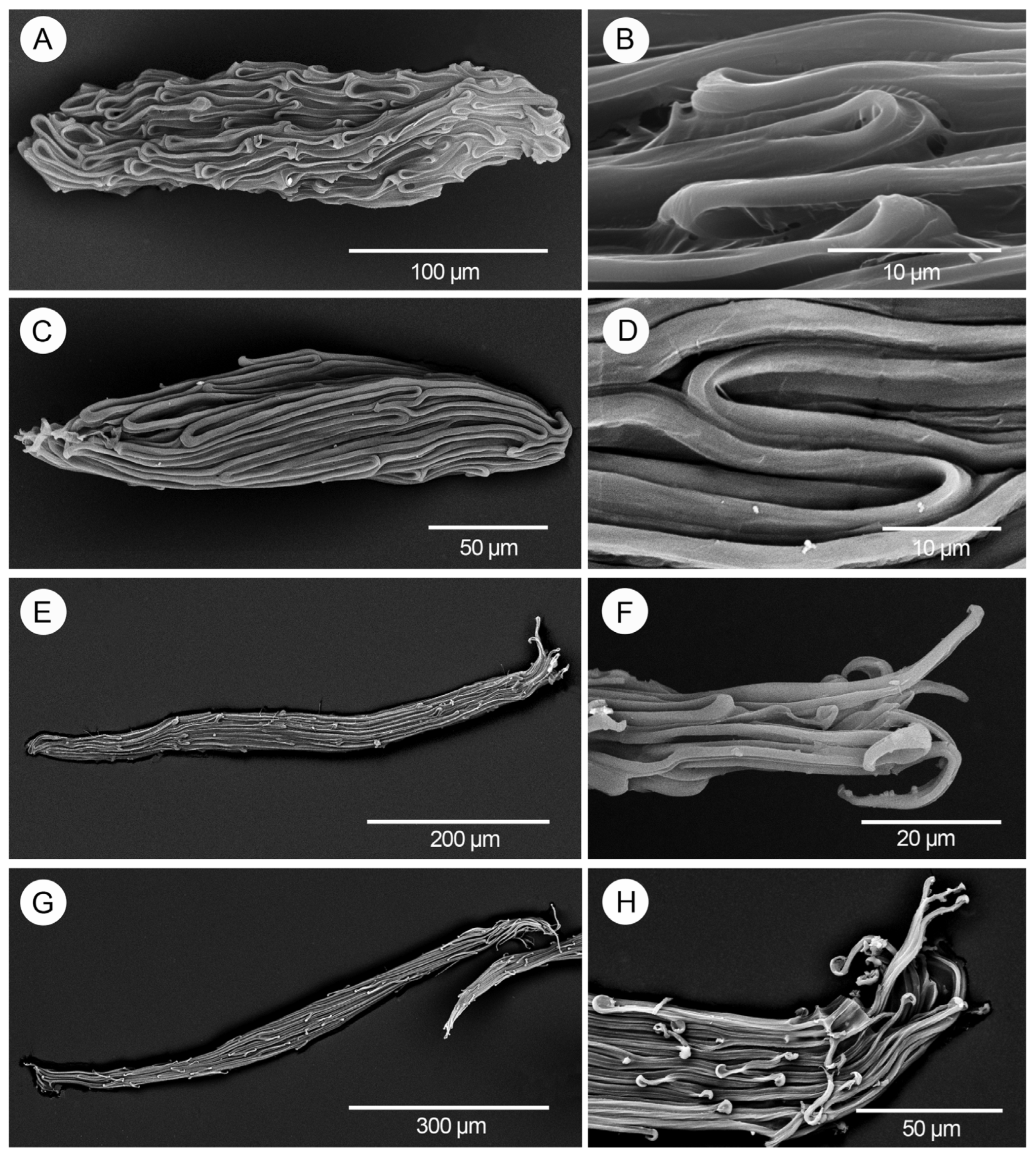

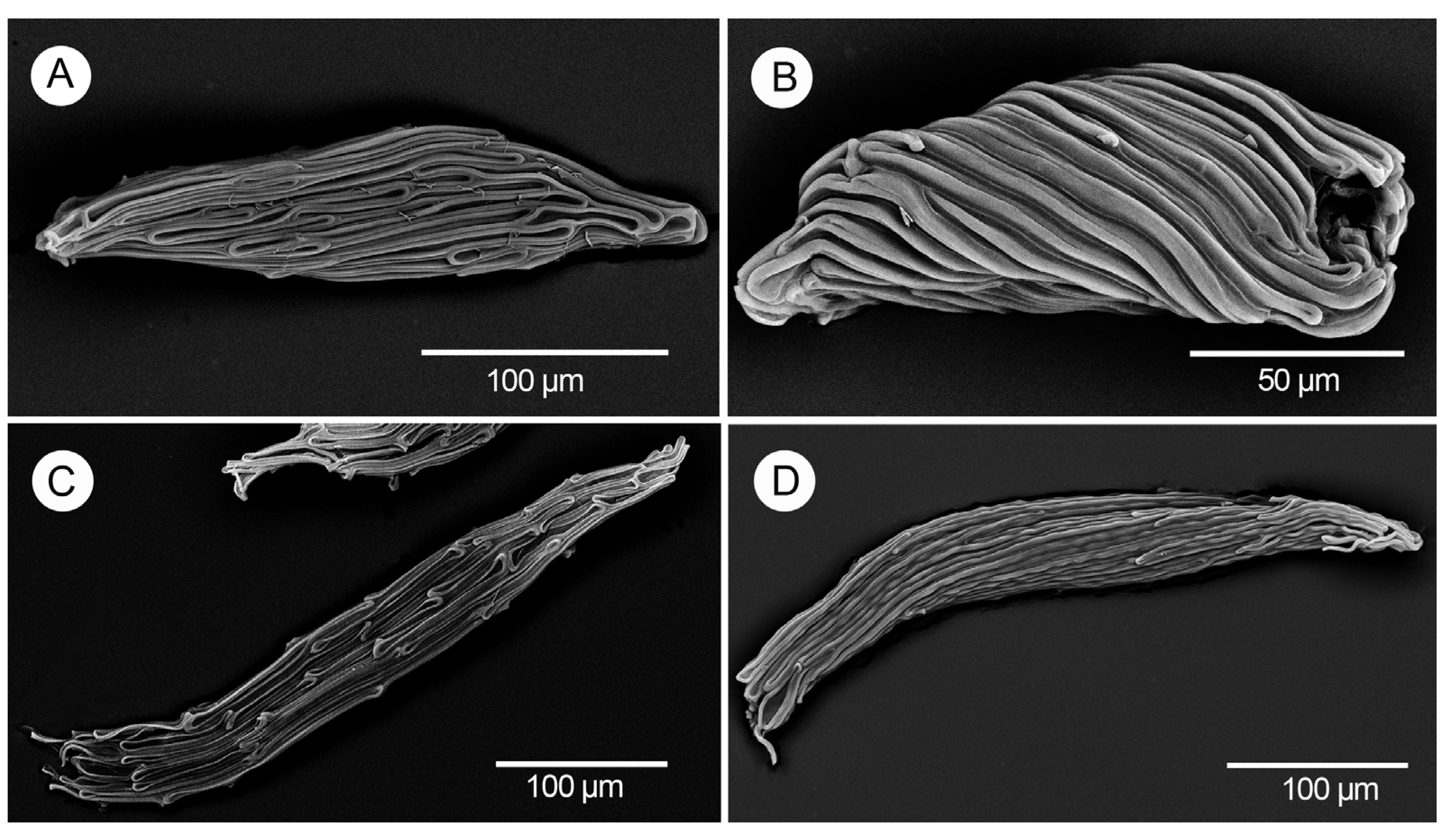
| Taxa | Seed Shape | Apical Pole | Basal Pole | Arrangement of the Medial Cells | Longitudinal Anticlinal Walls | Testa Extensions |
|---|---|---|---|---|---|---|
| Aerangis (10 sp.) | Fusiform | Pointed | Truncated | Twisted | Straight | Absent |
| Aeranthes (2 sp.) | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Afropectinariella subulata | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Ancistrorhynchus (6 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Angraecopsis (5 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Angraecum (7 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Bolusiella (3 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Calyptrochilum (2 sp.) | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Campylocentrum (9 sp.) | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Conchograecum (3 sp.) | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Cryptopus elatus | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Cyrtorchis (4 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Dendrophylax (2 sp.) | Filiform | Pointed | Pointed | Straight | Straight | Present |
| Diaphananthe (5 sp.) except | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| D. bidens, D. fragrantissima, D. sarcorhynchoides, D. vesicata | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Dinklagella liberica | Filiform | Pointed | Pointed | Straight | Straight | Present |
| Dolabrifolia (3 sp.) | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Erasanthe henrici | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Eurychone rothschildiana | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Jumellea (5 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Lemurella culicifera | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Lesliegraecum (2 sp.) | Filiform | Pointed | Truncated | Straight | Undulating | Present |
| Listrostachys pertusa | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Microcoelia (7 sp.) | Filiform | Pointed | Pointed | Straight | Straight | Present |
| Mystacidium pulchellum | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| M. tanganyikense | Fusiform | Pointed | Truncated | Straight | Straight | Absent |
| Neobathiea grandidieriana | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Nephrangis filiformis | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Oeonia rosea | Filiform | Pointed | Truncated | Straight | Straight | Present |
| Oeoniella aphroditae | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Plectrelminthus caudatus | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Podangis rhipsalisocia | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Rangaeris muscicola | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Rhipidoglossum (11 sp.) | Fusiform | Pointed | Truncated | Straight | Straight | Absent |
| R. brachyceras | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Sobennikoffia robusta | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Solenangis scandens | Filiform | Pointed | Pointed | Straight | Straight | Present |
| Sphyrarhynchus schliebenii | Filiform | Pointed | Truncated | Straight | Straight | Absent |
| Summerhayesia zambesiaca | Fusiform | Conical | Truncated | Twisted | Straight | Absent |
| Tridactyle (8 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Ypsilopus (3 sp.) | Fusiform | Conical | Truncated | Straight | Straight | Absent |
| Trait | Variability |
|---|---|
| Seed shape | 0 (fusiform); 1 (filiform) |
| Apical pole | 0 (conical); 1 (pointed) |
| Basal pole | 0 (truncated); 1 (pointed) |
| Arrangement of medial cells | 0 (straight); 1 (twisted) |
| Longitudinal anticlinal walls | 0 (straight); 1 (undulating) |
| Testa extensions | 0 (absent); 1 (present) |
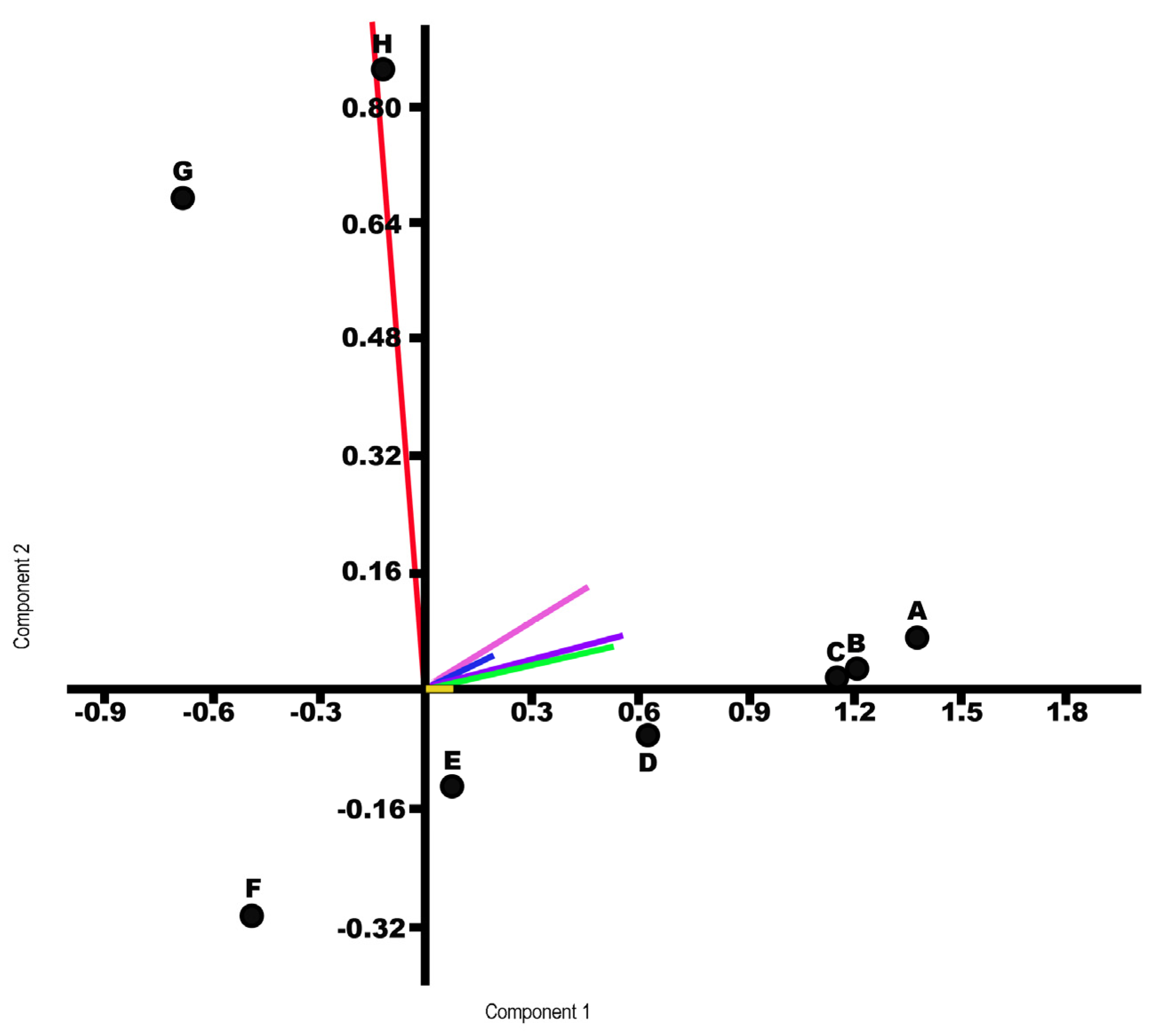
| PC | Eigenvalue | %Variance |
|---|---|---|
| 1 | 0.6375 | 68.783 |
| 2 | 0.142784 | 15.406 |
| 3 | 0.0690271 | 7.4477 |
| 4 | 0.0461135 | 4.9754 |
| 5 | 0.0206128 | 2.224 |
| 6 | 0.0107919 | 1.1644 |
| Component 1 | Component 2 | |
|---|---|---|
| Seed shape | 0.5590 | 0.0697 |
| Apical pole | 0.5596 | 0.1740 |
| Basal pole | 0.2155 | 0.0518 |
| Arrangement of medial cells | −0.1949 | 0.9776 |
| Longitudinal anticlinal walls | 0.0472 | 0.0047 |
| Testa extensions | 0.5854 | 0.0798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamarra, R.; Ortúñez, E.; De La Fuente, P.; Valdelvira, G.; Hernando, Á. Characterizing the Seed Coat in the Subtribe Angraecinae (Orchidaceae, Vandeae) and Its Taxonomic Value. Diversity 2025, 17, 280. https://doi.org/10.3390/d17040280
Gamarra R, Ortúñez E, De La Fuente P, Valdelvira G, Hernando Á. Characterizing the Seed Coat in the Subtribe Angraecinae (Orchidaceae, Vandeae) and Its Taxonomic Value. Diversity. 2025; 17(4):280. https://doi.org/10.3390/d17040280
Chicago/Turabian StyleGamarra, Roberto, Emma Ortúñez, Pablo De La Fuente, Guillermo Valdelvira, and Álvaro Hernando. 2025. "Characterizing the Seed Coat in the Subtribe Angraecinae (Orchidaceae, Vandeae) and Its Taxonomic Value" Diversity 17, no. 4: 280. https://doi.org/10.3390/d17040280
APA StyleGamarra, R., Ortúñez, E., De La Fuente, P., Valdelvira, G., & Hernando, Á. (2025). Characterizing the Seed Coat in the Subtribe Angraecinae (Orchidaceae, Vandeae) and Its Taxonomic Value. Diversity, 17(4), 280. https://doi.org/10.3390/d17040280






