Multimarker Analysis Reveals Ecological Islands in Hybrid Complexes: The Case of Quercus castanea × Q. crassipes Complex (Fagaceae) in Central Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Species Description
2.3. Leaf Macro-Morphological Data
2.4. Leaf Micro-Morphological Data
2.5. Geometric Morphometrics
2.6. Foliar Chemical Composition of Oak Taxa
2.6.1. Preparation of Extracts from Quercus
2.6.2. Chromatographic Analysis
2.6.3. GC-MS Analysis
2.6.4. Nutritional Chemistry
2.7. Statistical Analysis
3. Results
3.1. Expression of Macro- and Micro-Foliar Morphology
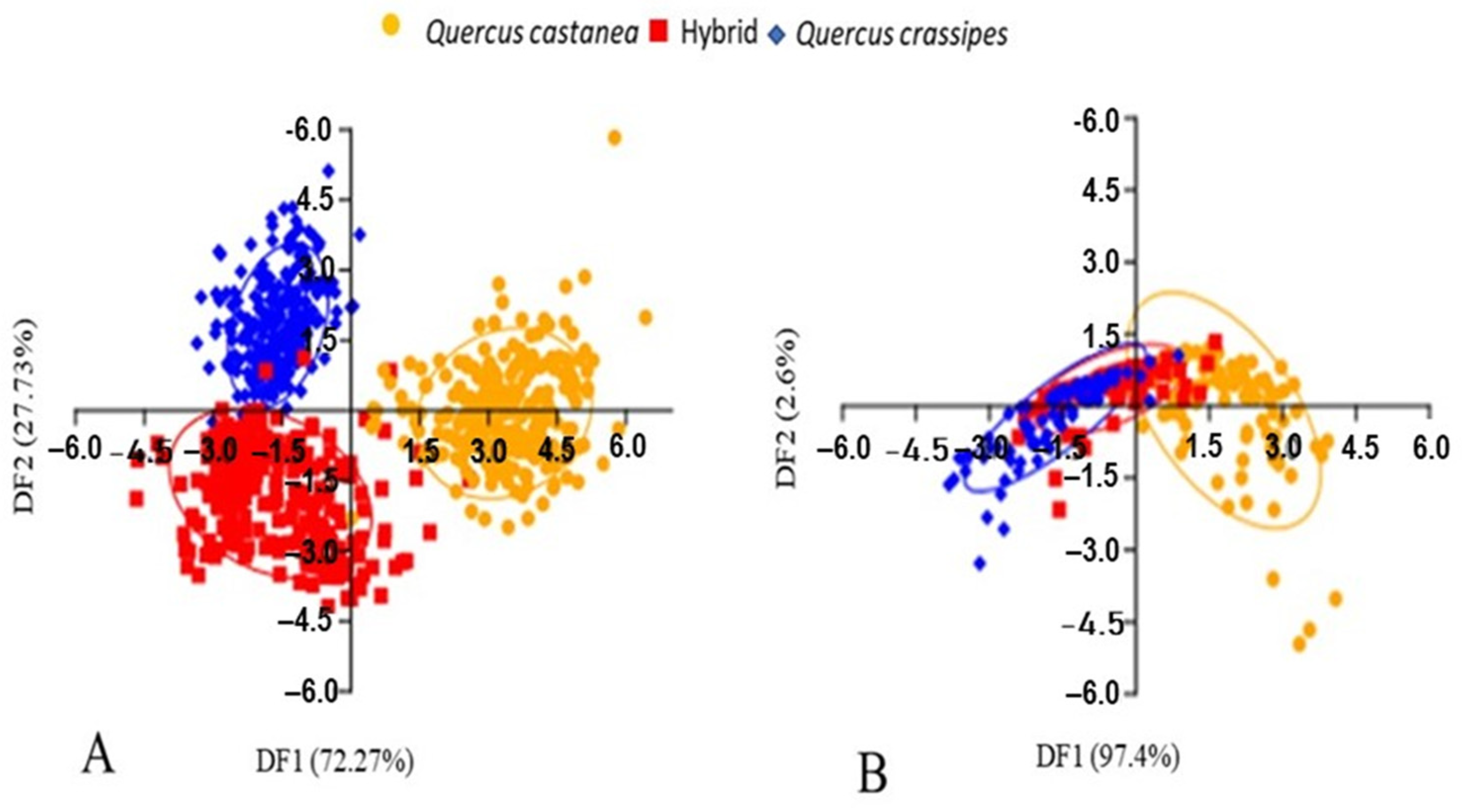
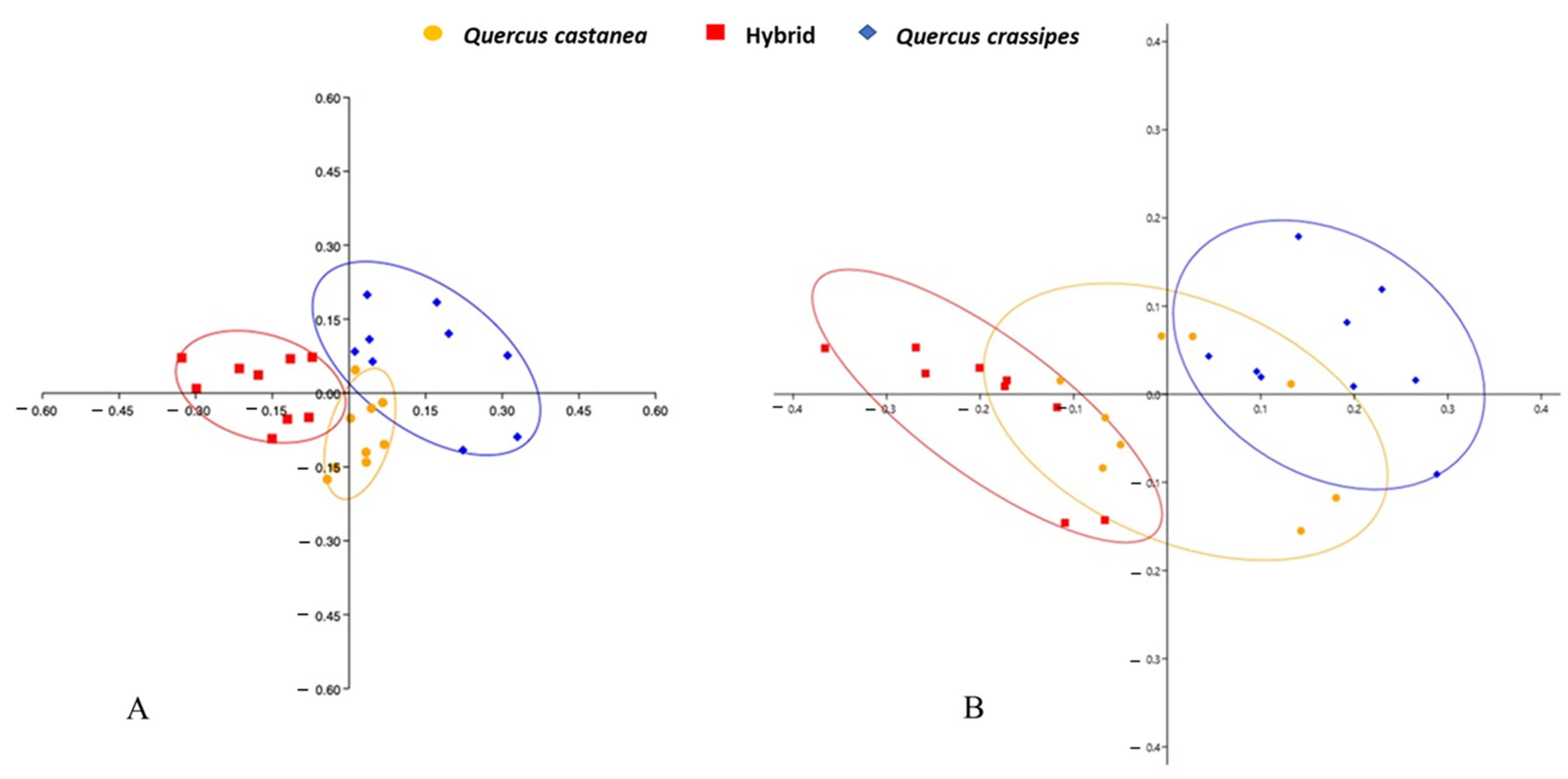
3.2. Expression of Foliar Shape
3.3. Qualitative and Quantitative Production of Secondary Metabolites
3.4. Nutritional Chemistry
4. Discussion
4.1. Expression of Macro- and Micro-Morphology and Shape Foliar in the Quercus castanea × Q. crassipes Complex
4.2. Production of Secondary Metabolites and Nutritional Chemicals
4.3. Ecological Consequences of Hybridization Between Q. castanea and Q. crassipes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
Appendix A.2
Appendix B
Appendix B.1
Appendix B.2
References
- Orians, C.M. The effects of hybridization in plants on secondary chemistry: Implications for the ecology and evolution of plant–herbivore interactions. Am. J. Bot. 2000, 87, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L. Evolution Through Genetic Exchange; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Todesco, M.; Pascual, M.A.; Owens, G.L.; Ostevik, K.L.; Moyers, B.T.; Hübner, S.; Heredia, S.M.; Hahn, M.A.; Caseys, C.; Bock, D.G.; et al. Hybridization and extinction. Evol. Appl. 2016, 9, 892–908. [Google Scholar] [CrossRef]
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in plants: Old ideas, new techniques. Plant Physiol. 2017, 173, 65–78. [Google Scholar] [CrossRef]
- Ocampo-Bautista, F.; Mussali-Galante, P.; Alvarez, L.; Marquina-Bahena, S.; Valencia-Cuevas, L.; Valencia-A, S.; Tovar-Sánchez, E. Natural Hybridization between Bursera bicolor × B. glabrifolia (Burseraceae) Complex: Molecular and Chemical Evidence. Forest 2023, 14, 1382. [Google Scholar] [CrossRef]
- Shang, H.; Yan, Y. Natural Hybridization and Biodiversity Conservation. Biodivers. Sci. 2017, 25, 683. [Google Scholar] [CrossRef][Green Version]
- Whitham, T.G.; Gehring, C.A.; Lamit, L.J.; Wojtowicz, T.; Evans, L.M.; Keith, A.R.; Smith, D.S. Community specificity: Life and afterlife effects of genes. Trends Plant Sci. 2012, 17, 271–281. [Google Scholar] [CrossRef]
- López-Caamal, A.; Tovar-Sánchez, E. Genetic, morphological, and chemical patterns of plant hybridization. Rev. Chil. Hist. Nat. 2014, 87, 16. [Google Scholar] [CrossRef]
- Porretta, D.; Canestrelli, D. The ecological importance of hybridization. Trends Ecol. Evol. 2023, 38, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Whitham, T.G.; Martinsen, G.D.; Keim, P.; Floate, K.D.; Dungey, H.S.; Potts, B.M. Plant hybrid zones affect biodiversity: Tools for a genetic-based understanding of community structure. Ecology 1999, 80, 416–428. [Google Scholar] [CrossRef]
- Soltis, P.S. Hybridization, speciation and novelty. J. Evol. Biol. 2023, 26, 291–293. [Google Scholar] [CrossRef]
- Whitham, T.G.; Morrow, P.A.; Potts, B.M. Plant hybrid zones as centers of biodiversity: The herbivore community of two endemic Tasmanian eucalypts. Oecologia 1994, 97, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, E.; Oyama, K. Effect of hybridization of the Quercus crassifolia × Quercus crassipes complex on the community structure on endophagous insects. Oecologia 2006, 147, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.; White, P.; Imm, D. Introduction to ecology and management of rare plants of the Southeast. Nat Area J. 2001, 21, 4–11. [Google Scholar]
- Loehle, C. Endemic plant distributions in eastern North America: Implications for conservation. J. For. 2006, 104, 415–418. [Google Scholar] [CrossRef]
- Cartwright, J. Ecological islands: Conserving biodiversity hotspots in a changing climate. Front. Ecol. Environ. 2019, 17, 331–340. [Google Scholar] [CrossRef]
- Hughes, J.M.; Schmidt, D.J.; McLean, A.; Wheatley, A. Population genetic structure in stream insects: What have we learned? In Aquatic Insects: Challenges to Populations; Lancaster, J., Briers, R.A., Eds.; CAB International: Wallingford, UK, 2008; pp. 268–288. [Google Scholar] [CrossRef]
- Whitham, T.G.; Bailey, J.K.; Scheweitzer, J.A.; Shuster, S.M.; Bangert, R.K.; LeRoy, C.J.; Lonsdorf, E.V.; Allan, G.J.; DiFazio, S.P.; Potts, B.M.; et al. A framework for community and ecosystem genetics: Form genes to ecosystems. Nature 2006, 7, 510–523. [Google Scholar] [CrossRef]
- Dungey, H.S.; Potts, B.M.; Whitham, T.G.; Li, H.-F. Plant genetics affects arthropod community richness and composition: Evidence from a synthetic eucalypt hybrid population. Evolution 2000, 54, 1938–1946. [Google Scholar]
- Bangert, R.K.; Turek, R.J.; Martinsen, G.D.; Wimp, G.M.; Bailey, J.K.; Whitham, T.G. Benefits of conservation of plant genetic diversity to arthropod diversity. Conserv. Biol. 2005, 19, 379–390. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Tovar-Sánchez, E. Oak canopy arthropod communities: Which factors shape its structure? Rev. Chil. Hist. Nat. 2015, 88, 15. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Bass, E.; Corrigan, A.; Kessler, A.; Poveda, K. Interaction diversity explains the maintenance of phytochemical diversity. Ecol. Lett. 2021, 24, 1205–1214. [Google Scholar] [CrossRef]
- Valencia, S. Diversidad del género Quercus en México. Bol. Soc. Bot. Méx. 2004, 75, 33–53. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Piñero, D.; Mussali-Galante, P.; Valencia, S.; Tovar-Sánchez, E. Effect of a red oak species gradient on genetic structure and diversity of Quercus castanea (Fagaceae) in Mexico. Tree Genet. Genomes 2014, 10, 641–652. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Mussali-Galante, P.; Piñero, D.; Castillo-Mendoza, E.; Rangel-Altamirano, G.; Tovar-Sánchez, E. Hybridization of Quercus castanea (Fagaceae) across a red oak species gradient in Mexico. Plant Syst. Evol. 2015, 301, 1085–1097. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Valencia-Cuevas, L.; Mussali-Galante, P.; Cano-Santana, Z.; Pujade-Villar, J.; Equihua-Martínez, A.; Tovar-Sánchez, E. Genetic variation in foundation species governs the dynamics of trophic interactions. Curr. Zool. 2018, 64, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.D.; Varley, G.C.; Gradwell, G.R. Estimating the relative roles of top-down and bottom-up forces on insect herbivore populations: A classic study revisited. Proc. Natl. Acad. Sci. USA 1997, 94, 9176–9181. [Google Scholar] [CrossRef]
- Tovar-Sánchez, E.; Oyama, K. Natural hybridization and hybrid zones between Quercus crassifolia and Quercus crassipes (Fagaceae) in Mexico: Morphological and molecular evidence. Am. J. Bot. 2004, 91, 1352–1363. [Google Scholar] [CrossRef]
- Cheng, D.; Vrieling, K.; Klinkhammer, P.G.L. The effect of hybridization on secondary metabolites and herbivore resistance: Implications for the evolution of chemical diversity in plants. Phytochem. Rev. 2011, 10, 107–117. [Google Scholar] [CrossRef][Green Version]
- Wimp, G.M.; Wooley, S.; Bangert, R.K.; Young, W.P.; Martinsen, G.D.; Keim, P.; Rehill, B.; Lindroth, R.L.; Whitham, T.G. Plant genetics predicts intra-annual variation in phytochemistry and arthropod community structure. Mol. Ecol. 2007, 16, 5057–5069. [Google Scholar] [CrossRef]
- Diario Oficial de la Federación: Decreto de Expropiación: pp. 31–39. 28 de junio, 1989. 1989. Available online: https://dof.gob.mx/nota_to_pdf.php?fecha=10/07/1989&edicion=MAT (accessed on 1 January 2025).
- Tovar-Sanchez, E. Canopy arthropods community within and among oak species in central Mexico. Current Zool. 2009, 55, 132–144. [Google Scholar] [CrossRef]
- White, E.S.; Reyes, C.M.; Ortega, R.J.; Valastro, S., Jr. El Ajusco: Geomorfología Volcánica y Acontecimientos Glaciales Durante el Pleistoceno Superior y Comparación con las Series Glaciales Mexicanas y de las Montañas Rocallosas. Colección Científica 212; INAH: Mexico City, Mexico, 1990; p. 77. [Google Scholar]
- Rzedowski, J. Vegetación del Pedregal de San Ángel, Distrito Federal México. In Reserva Ecológica del Pedregal de San Ángel: Ecología, Historia Natural y Manejo; Rojo, A., Ed.; UNAM: Mexico City, Mexico, 1994; pp. 9–65. [Google Scholar]
- Valencia, S. Contribución al Conocimiento del Género Quercus (Fagaceae) en el Estado de Guerrero, México; Contribuciones del Herbario de la Facultad de Ciencias, UNAM: Mexico City, Mexico, 1995; p. 154. [Google Scholar]
- Vázquez, M.L. Trichome morphology in selected Mexican red oak species (Quercus section Lobatae). Sida Contribut. Bot. 2006, 22, 1091–1110. [Google Scholar]
- Rzedowski, G.C.D.; Rzedowski, J. Flora Fanerogámica del Valle de México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2001. [Google Scholar]
- Vähä, J.P.; Primmer, C.R. Efficiency of model-based Bayesian methods for detecting hybrid individuals under different hybridization scenarios and with different numbers of loci. Mol. Ecol. 2006, 15, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.L. Plant epidermal sections and imprints using cyanoacrylate adhesives. Can J. Plant Sci. 1981, 61, 781–783. [Google Scholar] [CrossRef]
- Rohlf, F.J. tpsDig; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA, 2010. [Google Scholar]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef]
- Fried, B.; Sherma, J. Practical Thin-Layer Chromatography: A Multidisciplinary Approach; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Kjeldahl, C. A new method for the determination of nitrogen in organic matter. Fresenius J. Anal. Chem. 1883, 22, 366. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Schwarzbach, A.E.; Donovan, L.A.; Rieseberg, L.H. Transgressive character expression in a hybrid sunflower species. Am. J. Bot. 2001, 88, 270–277. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.og (accessed on 1 January 2025).
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Warwick, R.M.; Clarke, K.R. A statistical analysis of coral community responses to the 1982–1983 El Nino in the thousand islands, Indonesia. Coral Reefs 1990, 8, 171–179. [Google Scholar] [CrossRef]
- Howard, D.J.; Preszler, R.W.; Williams, J.; Fenchel, S.; Boecklen, W.J. How discrete are oaks species? Insights from a hybrid zone between Quercus grisea and Quercus gambelii. Evolution 1997, 51, 747–755. [Google Scholar] [CrossRef]
- Statsoft, Inc. STATISTICA for Windows; Statsoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Craft, K.J.; Ashley, M.V.; Koenig, W.D. Limited hybridization between Quercus lobata and Quercus douglasii (Fagaceae) in a mixed stand in central coastal California. Am. J. Bot. 2002, 89, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, T.; Stiling, P. Perfect is best: Low leaf fluctuating asymmetry reduces herbivory by leaf miners. Oecologia 2005, 142, 46–56. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Small variations over large scales: Fluctuating asymmetry over the range of two oak species. Inter. J. Plant Scie. 2010, 171, 303–309. [Google Scholar]
- Cattell, M.V.; Stilling, P. Tritrophic interactions and trade-offs in herbivore fecundity on hybridising host plants. Ecol. Entomol. 2004, 29, 255–263. [Google Scholar] [CrossRef]
- Albarrán-Lara, A.L.; Mendoza-Cuenca, L.; Valencia-Avalos, S.; Gonzalez-Rodriguez, A.; Oyama, K. Leaf fluctuating asymmetry increases with hybridization and introgression between Quercus magnoliifolia and Quercus resinosa (Fagaceae) through an altitudinal gradient in Mexico. Int. J. Plant Sci. 2010, 171, 310–322. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Haukioja, E.; Koricheva, J.; Sulkinoja, M. Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology 1998, 79, 2092–2099. [Google Scholar] [CrossRef]
- Leary, R.F.; Allendorf, F.W. Fluctuating asymmetry as an indicator of stress: Implications for conservation biology. Trends Ecol. Evol. 1989, 4, 214–217. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Canché-Delgado, A.; Maldonado-López, Y.; Fernandes, G.W.; Oyama, K.; González-Rodríguez, A. Patterns of herbivory and leaf morphology in two Mexican hybrid oak complexes: Importance of fluctuating asymmetry as indicator of environmental stress in hybrid plants. Ecol. Indic. 2018, 90, 164–170. [Google Scholar] [CrossRef]
- Hochwender, C.G.; Fritz, S. Fluctuating asymmetry in a Salix hybrid system: The importance of genetic versus environmental causes. Evolution 1999, 53, 408–416. [Google Scholar]
- Huber, D.P.W.; Bohlmann, J. Terpene synthases and the mediation of plant–insect ecological interactions by terpenoids: A mini-review. In Plant Adaptation: Molecular Genetics and Ecology, Proceedings of an International Workshop Vancouver, British Columbia, Canada, 11–13 December 2002; Cronk, Q.C.B., Whitton, J., Ree, H.E., Taylor, I.E.P., Eds.; NRC Research Press: Ottawa, ON, Canada, 2004; pp. 70–81. [Google Scholar]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Li, T.; Weng, J.; Jhan, Y.; Lin, S.; Chou, C. The Role of Pentacyclic Triterpenoids in the Allelopathic Effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mendoza, E.; Salinas-Sánchez, D.; Valencia-Cuevas, L.; Zamilpa, A.; Tovar-Sánchez, E. Natural hybridisation among Quercus glabrescens, Q. rugosa and Q. obtusata (Fagaceae): Microsatellites and secondary metabolites markers. Plant Biol. 2019, 21, 110–121. [Google Scholar] [CrossRef] [PubMed]
- El-Hagrassi, A.M.; Ali, M.M.; Osman, A.F.; Shaaban, M. Phytochemical investigation and biological studies of Bombax malabaricum flowers. Nat. Product Res. 2011, 25, 141–151, ISSN 1029-2349. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.; Palazon, J.; Navarro-Ocaña, A. The Pentacyclic Triterpenes α, β-amyrins: A Review of Sources and Biological Activities. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; IntechOpen: London, UK, 2012; pp. 487–502. ISBN 978-953-51-0296-0. [Google Scholar]
- Volf, M.; Renoult, S.A.; Panthee, S.; van Dam, N.M. Quantifying various aspects of chemical diversity in hybrid plants can help understanding ecological consequences of hybridization. Am. J. Bot. 2024, 111, e16283. [Google Scholar] [CrossRef]
- Costantini, D. Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage Between Mechanistic and Evolutionary Approaches, 1st ed.; Springer Science & Business Media: Berlin, Germany, 2014. [Google Scholar]
- Barbehenn, R.V.; Niewiadomski, J.; Pecci, C. Physiological benefits of feeding in the spring by Lymantria dispar caterpillars on red oak and sugar maple leaves: Nutrition versus oxidative stress. Chemoecology 2013, 23, 59–70. [Google Scholar] [CrossRef]
- Schädler, M.; Jung, G.; Auge, H.; Brandl, R. Does the Fretwell–Oksanen model apply to invertebrates? Oikos 2003, 100, 203–207. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Kirk, H.; Choi, Y.H.; Kim, H.K.; Verpoorte, R.; Van Der Maijden, E. Comparing metabolomes: The chemical consequences of hybridization in plants. New Phytol. 2005, 167, 613–622. [Google Scholar] [CrossRef]
- Lamit, L.J.; Wojtowicz, T.; Kovacs, Z.; Wooley, S.C.; Zinkgraf, M.; Whitham, T.G.; Lindroth, R.L.; Gehring, C.A. Hybridization among foundation tree species influences the structure of associated understory plant communities. Botany 2011, 89, 165–174. [Google Scholar] [CrossRef]
- Wimp, G.M.; Martinsen, G.D.; Floate, K.D.; Bangert, R.K.; Whitham, T.G. Plant genetic determinants of arthropod community structure and diversity. Evolution 2005, 59, 6–169. [Google Scholar] [CrossRef]
- Bailey, J.K.; Deckert, R.; Schweitzer, J.A.; Rehill, B.J.; Lindroth, R.L.; Gehring, C.; Whitham, T.G. Host plant genetics affect hidden ecological players: Links among Populus, condensed tannins, and fungal endophyte infection. Canad. J. Bot. 2005, 83, 356–361. [Google Scholar]
- LeRoy, C.J.; Whitham, T.G.; Keim, P. Plant genes link forests and streams. Ecology 2006, 87, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, G.; González-Rodríguez, A.; Oyama, K.; Cuevas-Reyes, P. Effects of plant hybridization on the structure and composition of a highly rich community of cynipid gall wasps: The case of the oak hybrid complex Quercus magnoliifolia x Quercus resinosa in Mexico. Biodivers. Conserv. 2016, 25, 633–651. [Google Scholar] [CrossRef]
- Bangert, R.K.; Turek, R.J.; Rehill, B.; Wimp, G.M.; Schweitzer, J.A.; Allan, G.J.; Bailey, J.K.; Martinsen, G.D.; Leim, P.; Lindroth, R.L.; et al. A genetic similarity rule determines arthropod community structure. Mol. Ecol. 2006, 15, 1379–2139. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.S.; Moulia, C.; Newcombe, G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Ann. Rev. Ecol. Syst. 1999, 35, 565–591. [Google Scholar]
- Caseys, C.; Stritt, C.; Glauser, G.; Blanchard, T.; Lexer, C. Effects of hybridization and evolutionary constraints on secondary metabolites: The genetic architecture of phenylpropanoids in European Populus species. PLoS ONE 2015, 10, e0128200. [Google Scholar] [CrossRef]

| Abbreviations | Description |
|---|---|
| Macro-morphological characters | |
| PL | Length of petiole |
| LL | Length of lamina |
| TLL | Total leaf length (LL + LP) |
| MWL | Maximal width of lamina |
| HMW | Height of maximal width (length of lamina from base to widest part) |
| PD | Petiole diameter |
| MD | Midvein diameter |
| NV | Number of veins |
| LWB | Leaf width at basal 1/3 of leaf |
| LWA | Leaf width at apical 1/3 of leaf |
| NA | Number of aristae |
| LLBW | Length of lamina from base to widest part (LL—HMW) |
| Combinations of characters | |
| P (%) | Length of petiole × 100/total leaf length |
| HW (%) | Height of maximal width × 100/total leaf length |
| DW (%) | Length of lamina from base to widest part × 100/total leaf length |
| LL/MWL | Length of lamina/maximal width of lamina |
| LLBW/MWL | Length of lamina from base to widest part/maximal width of lamina |
| Micro-morphological characters | |
| (stomata) | |
| SL | Stomata length |
| SW | Stomata width |
| SC | Stomata coverture [(Stoma length + Stoma width)/4]2 π |
| SD | Stomata density at 40x |
| Character | Units | Q. castanea | Hybrid | Q. crassipes | F Taxa (df = 2807) | Hybrid Phenotype |
|---|---|---|---|---|---|---|
| Macro-morphological characters | ||||||
| PL | cm | 0.45 ± 0.01 a | 0.43 ± 0.01 a | 0.34 ± 0.07 b | 30.86 *** | Q. castanea-like |
| LL | cm | 6.24 ± 0.09 a | 5.70 ± 0.09 ab | 4.73 ± 0.05 b | 87.40 ** | intermediate |
| TLL | cm | 6.69 ± 0.09 a | 6.13 ± 0.09 ab | 5.08 ± 0.06 b | 90.20 ** | intermediate |
| MWL | cm | 2.19 ± 0.04 a | 1.67 ± 0.02 ab | 1.39 ± 0.01 b | 226.49 ** | intermediate |
| HMW | cm | 4.17 ± 0.07 a | 2.91 ± 0.04 ab | 2.67 ± 0.04 b | 225.10 ** | intermediate |
| PD | mm | 1.14 ± 0.06 a | 0.90 ± 0.01 b | 0.92 ± 0.00 b | 11.87 *** | Q. crassipes-like |
| MD | mm | 0.08 ± 0.01 a | 0.05 ± 0.01 c | 0.07 ± 0.01 b | 398.49 ** | transgressive (−) |
| NV | No. | 18.31 ± 0.28 a | 22.1 ± 0.28 ab | 24.09 ± 0.21 b | 161.10 ** | intermediate |
| LWB | cm | 1.79 ± 0.02 a | 1.41 ± 0.02 ab | 1.30 ± 0.01 b | 134.95 ** | intermediate |
| LWA | cm | 2.05 ± 0.03 a | 1.58 ± 0.02 ab | 1.27 ± 0.01 b | 229.50 ** | intermediate |
| NA | No. | 8.60 ± 0.16 a | 2.21 ± 0.11 ab | 1.00 ± 0.00 b | 1514.98 ** | intermediate |
| LLBW | cm | 3.89 ± 0.06 a | 2.54 ± 0.04 b | 2.40 ± 0.04 b | 256.83 ** | Q. crassipes-like |
| Combinations of characters | ||||||
| P | % | 6.77 ± 0.15 a | 7.19 ± 0.13 a | 6.79 ± 0.10 a | 3.42 ns | parental-like |
| HW | % | 62.54 ± 0.57 a | 47.86 ± 0.33 ab | 52.63 ± 0.49 b | 264.51 ** | intermediate |
| DW | % | 58.19 ± 0.52 a | 41.66 ± 0.34 c | 47.19 ± 0.50 b | 322.69 ** | transgressive (−) |
| LL/MWL | 1.53 ± 0.02 a | 1.96 ± 0.01 ab | 2.36 ± 0.05 b | 148.68 ** | intermediate | |
| LLBW/MWL | 1.78 ± 0.02 a | 1.52 ± 0.01 c | 1.72 ± 0.02 b | 52.09 ** | transgressive (−) | |
| Micro-morphological characters | ||||||
| SL | µm | 27.44 ± 0.29 a | 23.03 ± 0.29 ab | 20.57 ± 0.29 b | 135.10 ** | intermediate |
| SW | µm | 18.24 ± 0.27 a | 14.61 ± 0.27 b | 14.02 ± 0.27 b | 68.18 ** | Q. crassipes-like |
| SC | µm2 | 415.20 ± 7.85 a | 281.30 ± 7.85 ab | 237.80 ± 7.85 b | 138.60 ** | intermediate |
| SD | No./µm2 | 3.30 ± 0.02 a | 3.18 ± 0.02 b | 3.12 ± 0.02 b | 16.74 *** | Q. crassipes-like |
| Taxa | Source | df | SS | MS | Rsq | F | Z | Pr (>F) |
|---|---|---|---|---|---|---|---|---|
| Q. castanea | ||||||||
| individual | 8 | 0.18075 | 0.022594 | 0.18831 | 5.4951 | 7.291 | 0.001 | |
| side | 1 | 0.15413 | 0.154135 | 0.16058 | 37.4876 | 6.6267 | 0.001 | |
| individual: side | 152 | 0.62497 | 0.004112 | 0.65111 | ||||
| Total | 161 | 0.95985 | ||||||
| Q. crassipes | ||||||||
| individual | 7 | 0.16757 | 0.023939 | 0.29704 | 14.375 | 13.8155 | 0.001 | |
| side | 1 | 0.16509 | 0.165087 | 0.29263 | 99.131 | 7.8166 | 0.001 | |
| individual: side | 139 | 0.23148 | 0.001665 | 0.41033 | ||||
| Total | 147 | 0.56414 | ||||||
| Hybrid | ||||||||
| individual | 8 | 0.29237 | 0.036547 | 0.27383 | 9.6728 | 9.4556 | 0.001 | |
| side | 1 | 0.18592 | 0.185924 | 0.17413 | 49.2085 | 7.6401 | 0.001 | |
| individual: side | 156 | 0.58941 | 0.003778 | 0.55203 | ||||
| Total | 165 | 1.06771 | ||||||
| Compound Name | Compound Type | Retention Time | Chemical Formula |
|---|---|---|---|
| Q. castanea | |||
| Oleic acid | Fatty acid | 20.98 | 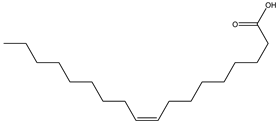 |
| 2,6,10,14,18,22-tetracosahexane, 2,6,10,15,19,23-hexamethyl-. (all-E)- | Triterpene | 29.31 | 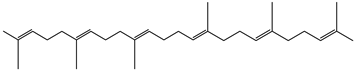 |
| Friedelin | Triterpene | 41.52 | 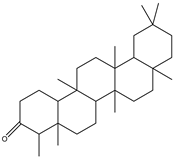 |
| Q. crassipes | |||
| Nonacosanol | Alcohol | 28.616 |  |
| 2-Cyclohexen-1-one,4-(3-hydroxybutyl)-3,5,5-trimethyl | Monoterpene | 16.62 | 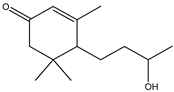 |
| 10-Octadecenoic acid, methyl ester | Fatty acid | 20.33 | 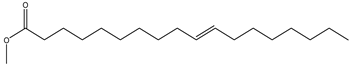 |
| Hybrids | |||
| Pregnenolone | Steroid | 27.82 | 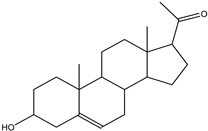 |
| Furan,2,5-bis(3,4-dimethoxyphenyl)tetrahydro-3,4-dimethyl-,[2R-(2a,3b,4b,5a)] | Lignan | 30.75 | 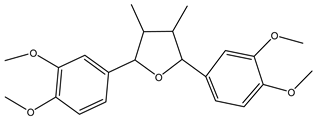 |
| 2-Pentadecanone,6,10,14- trimethyl- | Isoprenoide | 17.82 |  |
| 8,11-Ocatdecadienoic acid, methyl ester | Fatty acid | 20.26 | 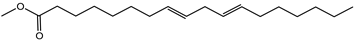 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | Diterpene alcohol | 19.68 |  |
| Benzenepropanol,4-hydroxy-α-methyl-,(R)- | Monoterpene | 15.03 | 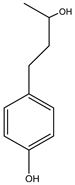 |
| 3,7,11,15-Tetramethylhexadeca-1,6,10,14-tetraen-3-ol | Diterpene alcohol | 19.68 |  |
| D;B-Friedo-B;A:-neogammacer-5-en-3-ol,(3B)- | Triterpene | 38.11 |  |
| 2-Naphthalenol,2,3,4,4a,5,6,7-octahydro-1,4a-dimethyl-7-(2-hydroxy-1-methylethyl) | Sesquiterpene | 18.204 | 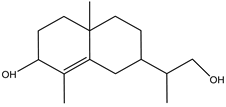 |
| Nutritional Character | Units | Q. castanea | Hybrid | Q. crassipes | FTaxa (df = 2, 24) | Hybrid Phenotype |
|---|---|---|---|---|---|---|
| Carbon | % | 21.53 ± 0.91 a | 23.34 ± 0.89 a | 21.56 ± 0.74 a | 1.269 ns | parental-like |
| Nitrogen | % | 1.03 ± 0.06 a | 0.97 ± 0.14 a | 1.34 ± 0.07 b | 3.542 * | Q. castanea-like |
| Total phosphorous | ppm | 3.39 ± 0.18 a | 4.37 ± 0.18 ab | 4.53 ± 0.18 b | 10.97 *** | intermediate |
| C/N ratio | 21.06 ± 1.92 a | 29.42 ± 5.54 b | 15.49 ± 1.78 c | 5.197 * | Transgressive (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Cuevas, L.; Ocampo-Bautista, F.; Alvarez, L.; Marquina-Bahena, S.; De Luna-Bonilla, O.Á.; Tovar-Sánchez, E. Multimarker Analysis Reveals Ecological Islands in Hybrid Complexes: The Case of Quercus castanea × Q. crassipes Complex (Fagaceae) in Central Mexico. Diversity 2025, 17, 264. https://doi.org/10.3390/d17040264
Valencia-Cuevas L, Ocampo-Bautista F, Alvarez L, Marquina-Bahena S, De Luna-Bonilla OÁ, Tovar-Sánchez E. Multimarker Analysis Reveals Ecological Islands in Hybrid Complexes: The Case of Quercus castanea × Q. crassipes Complex (Fagaceae) in Central Mexico. Diversity. 2025; 17(4):264. https://doi.org/10.3390/d17040264
Chicago/Turabian StyleValencia-Cuevas, Leticia, Fidel Ocampo-Bautista, Laura Alvarez, Silvia Marquina-Bahena, Oscar Ángel De Luna-Bonilla, and Efraín Tovar-Sánchez. 2025. "Multimarker Analysis Reveals Ecological Islands in Hybrid Complexes: The Case of Quercus castanea × Q. crassipes Complex (Fagaceae) in Central Mexico" Diversity 17, no. 4: 264. https://doi.org/10.3390/d17040264
APA StyleValencia-Cuevas, L., Ocampo-Bautista, F., Alvarez, L., Marquina-Bahena, S., De Luna-Bonilla, O. Á., & Tovar-Sánchez, E. (2025). Multimarker Analysis Reveals Ecological Islands in Hybrid Complexes: The Case of Quercus castanea × Q. crassipes Complex (Fagaceae) in Central Mexico. Diversity, 17(4), 264. https://doi.org/10.3390/d17040264







