First Marine Fossil Otoliths (Teleostei) from East Africa (Tanzania) †
Abstract
1. Introduction
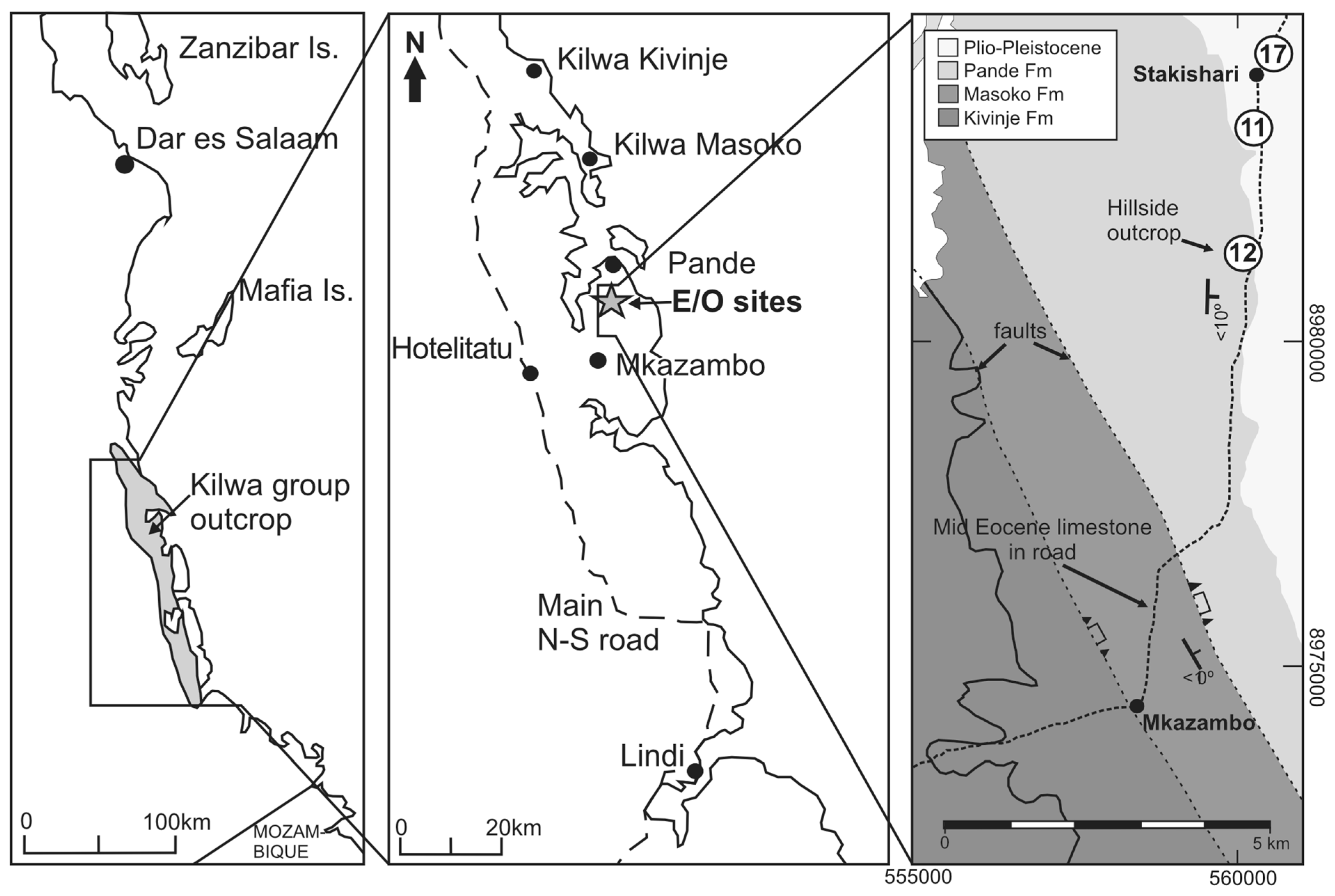
Regional Geology and Locations
2. Materials and Methods
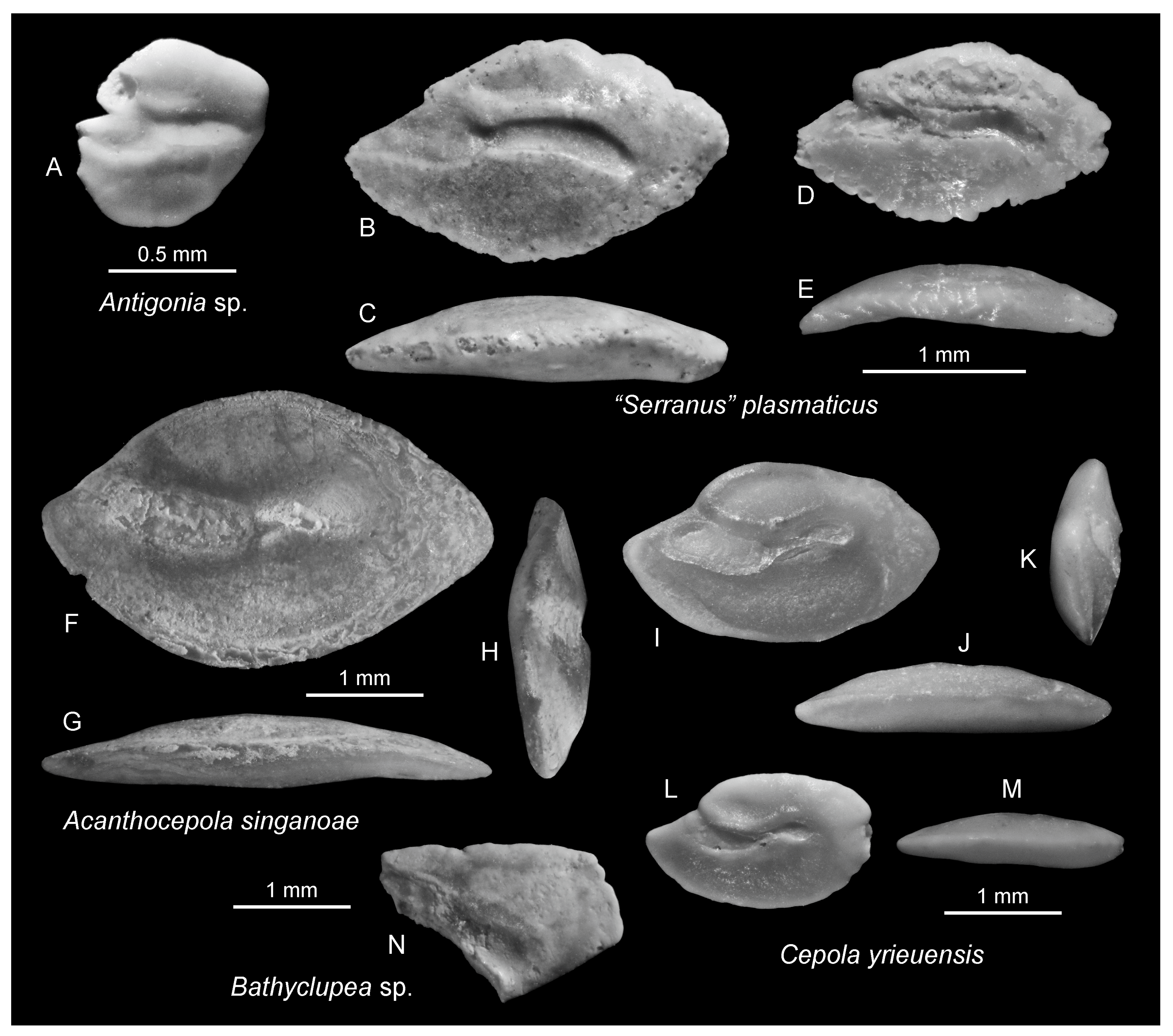
3. Systematic Paleontology
4. Discussion
4.1. Faunal Evaluation
4.2. Correlation with Events of the EOT
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHMD | Natural History Museum Denmark |
| TDP | Tanzania Drilling Project |
| OL | Otolith length |
| OH | Otolith height |
| OT | Otolith thickness |
| OsL | Ostium length |
| CaL | Cauda length |
| OCL | Length of ostial colliculum |
| CCL | Length of caudal colliculum |
| SuL | Sulcus length |
References
- Altner, M.; Reichenbacher, B. A small cichlid species flock from the Upper Miocene (9–10 MYA) of Central Kenya. Hydrobiologia 2021, 848, 3613–3637. [Google Scholar] [CrossRef]
- Nicholas, C.J.; Pearson, P.N.; Bown, P.R.; Jones, T.D.; Huber, B.T.; Karega, A.; Lees, J.A.; McMillan, I.K.; O’Halloran, A.; Singano, J.M.; et al. Stratigraphy and sedimentology of the Upper Cretaceous to Paleogene Kilwa Group, southern coastal Tanzania. J. Afr. Earth Sci. 2006, 45, 431–466. [Google Scholar] [CrossRef]
- Pearson, P.N.; McMillan, I.K.; Wade, B.S.; Jones, T.D.; Coxall, H.K.; Bown, P.R.; Lear, C.H. Extinction and environmental change across the Eocene-Oligocene boundary in Tanzania. Geology 2008, 36, 179–182. [Google Scholar] [CrossRef]
- Coxall, H.; Pearson, P.N. The Eocene–Oligocene transition. In Deep-Time Perspectives on Climate Change: Marrying the Signal from Computer Models and Biological Proxies; Williams, M., Haywood, A.M., Gregory, F.J., Schmidt, D.N., Eds.; The Micropalaeontological Society, Special Publications: London, UK, 2007; pp. 351–387. [Google Scholar]
- Katz, M.E.; Miller, K.G.; Wright, J.D.; Wade, B.S.; Browning, J.V.; Cramer, B.S.; Rosenthal, Y. Stepwise transition from the Eocene greenhouse to the Oligocene icehouse. Nat. Geosci. 2008, 1, 329–334. [Google Scholar] [CrossRef]
- Hutchinson, D.K.; Coxall, H.K.; Lunt, D.J.; Steinthorsdottir, M.; de Boer, A.M.; Baatsen, M.; von der Heydt, A.; Huber, M.; Kennedy-Asser, A.T.; Kunzmann, L.; et al. The Eocene–Oligocene transition: A review of marine and terrestrial proxy data, models and model-data comparisons. Clim. Past Discuss. 2020, 17, 269–315. [Google Scholar] [CrossRef]
- Lear, C.H.; Bailey, T.R.; Pearson, P.N.; Coxall, H.K.; Rosenthal, Y. Cooling and ice growth across the Eocene-Oligocene transition. Geology 2008, 36, 251–254. [Google Scholar] [CrossRef]
- Coxall, H.K.; Wilson, P.A. Early Oligocene glaciation and productivity in the eastern equatorial Pacific: Insights into global carbon cycling. Paleoceanography 2011, 26, PA2221. [Google Scholar] [CrossRef]
- Coxall, H.K.; Jones, T.D.; Jones, A.P.; Lunt, P.; MacMillan, I.; Marliyani, G.I.; Nicholas, C.J.; O’Halloran, A.; Piga, E.; Sanyoto, P.; et al. The Eocene−Oligocene transition in Nanggulan, Java: Lithostratigraphy, biostratigraphy and foraminiferal stable isotopes. J. Geol. Soc. 2021, 178, jgs2021-006. [Google Scholar] [CrossRef]
- Aubry, M.-P.; Bord, D. Reshuffling the cards in the photic zone at the Eocene-Oligocene boundary. In The Late Eocene Earth—Hothouse, Icehouse, and Impacts; Geological Society of America: Boulder, CO, USA, 2009; Special Paper 452; pp. 279–301. [Google Scholar]
- McCraney, W.T.; Thacker, C.E.; Faircloth, B.C.; Harrington, R.C.; Near, T.J.; Alfaro, M.E. Explosion of Goby Fish Diversity at the Eocene-Oligocene Transition. SSRN 2024. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Reichenbacher, B. Reconstruction of Oligocene and Neogene freshwater fish faunas—An actualistic study on cypriniforms otoliths. Acta Paleontol. Pol. 2006, 51, 283–304. [Google Scholar]
- Bown, P.R.; Jones, T.D.; Lees, J.; Randell, R.D.; Mizzi, J.A.; Pearson, P.N.; Coxall, H.K.; Young, J.R.; Nicholas, C.J.; Karega, A.; et al. A Paleogene calcareous microfossil Konservat-Lagerstatte from the Kilwa Group of coastal Tanzania. Geol. Soc. Am. Bull. 2008, 120, 3–12. [Google Scholar] [CrossRef]
- Dunkley Jones, T.; Bown, P.R.; Pearson, P.N.; Wade, B.S.; Coxall, H.K.; Lear, C.H. Major shifts in calcareous phytoplankton assemblages through the Eocene-Oligocene transition of Tanzania and their implications for low-latitude primary production. Paleoceanography 2008, 23, PA4204. [Google Scholar]
- Wade, B.S.; Pearson, P.N. Planktonic foraminiferal turnover, diversity fluctuations and geochemical signals across the Eocene/Oligocene boundary in Tanzania. Mar. Micropaleontol. 2008, 68, 244–255. [Google Scholar] [CrossRef]
- Dunkley Jones, T.; Bown, P.R.; Pearson, P.N. Exceptionally well preserved upper Eocene to lower Oligocene calcareous nannofossils (Prymnesiophyceae) from the Pande Formation (Kilwa Group), Tanzania. J. Syst. Palaeontol. 2009, 7, 359–411. [Google Scholar]
- Cotton, L.J.; Pearson, P.N. Extinction of larger benthic foraminifera at the Eocene/Oligocene boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 311, 281–296. [Google Scholar]
- Di Martino, E.D.; Taylor, P.D.; Cotton, L.J.; Pearson, P.N. First bryozoan fauna from the Eocene–Oligocene transition in Tanzania. J. Syst. Palaeontol. 2018, 16, 225–243. [Google Scholar]
- Koorapati, R.K.; Moon, B.-C.; Cotton, L.J. Morphological trends in reticulate Nummulites across the Eocene-Oligocene transition. Palaeontology 2025, 68, e70003. [Google Scholar]
- Koken, E. Über Fisch-Otolithen, insbesondere über diejenigen der norddeutschen Oligocän-Ablagerungen. Z. Dtsch. Geol. Ges. 1884, 36, 500–565. [Google Scholar]
- Chaine, J.; Duvergier, J. Recherches sur les otolithes des poissons. Etude descriptive et comparative de la sagitta des Teléostéens. Actes Soc. Linnéenne Bordx. 1934, 86, 5–256. [Google Scholar]
- Schwarzhans, W. Otolith-morphology and its usage for higher systematical units with special reference to the Myctophiformes s.l. Meded. Werkgr. Voor Tert. Kwartaire Geol. 1978, 15, 167–185. [Google Scholar]
- Near, T.J.; Thacker, C.E. Phylogenetic classification of living and fossil ray-finned fishes (Actinopterygii). Bull. Peabody Mus. Nat. Hist. 2024, 65, 3–302. [Google Scholar]
- Van der Laan, R.; Eschmeyer, W.N.; Fricke, R. Family-group names of Recent fishes. Zootaxa 2014, 3882, 1–230. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. (Eds.) Eschmeyer’s Catalog of Fishes: Genera, Species, References; World Wide Web Electronic Publication. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 30 January 2025).
- Schwarzhans, W.W.; Stringer, G.L.; Takeuchi, G.T. The middle Eocene bony fish fauna of California, USA, reconstructed by means of otoliths. Riv. Ital. Paleontol. Stratigr. 2024, 130, 373–473. [Google Scholar]
- Nolf, D.; Stringer, G.L. Late Eocene (Priabonian) fish otoliths from the Yazoo Clay at Copenhagen, Louisiana. In Geological Pamphlet; Louisiana Geological Survey: Baton Rouge, LA, USA, 2003; Volume 13, pp. 1–23. [Google Scholar]
- Šulc, J. Les otolithes du Paléogène des environs de Biarritz. In Rozpravy Státniho Geologického Ustavu Ceskolovenské Republiky; Státní Geologický Ústav Československé Republiky: Prague, Czech Republic, 1932; Volume 7, pp. 1–94. [Google Scholar]
- Frost, G.A. Otoliths of fishes from the lower Tertiary formations of southern England. V. Anacanthini, Heterosomata, Ostariophysi. Ann. Mag. Nat. Hist. 1934, 14, 500–505. [Google Scholar]
- Nolf, D. Les otolithes de téléostéens éocènes d’Aquitaine (sud-ouest de la France) et leur intérêt stratigraphique. Mémoire L’académie R. Belg. Cl. Sci. 1988, 19, 1–147. [Google Scholar]
- Schwarzhans, W. Die tertiäre Teleosteer-Fauna Neuseelands, rekonstruiert anhand von Otolithen. Berl. Geowiss. Abh. 1980, 26, 1–211. [Google Scholar]
- Schwarzhans, W.; Ohe, F.; Ando, Y. An early Oligocene fish-fauna from Japan reconstructed from otoliths. Zitteliana 2017, 90, 3–26. [Google Scholar]
- Schwarzhans, W.; Aguilera, O. Otoliths of the Gobiidae from the Neogene of tropical America. Swiss J. Palaeontol. 2024, 143, 13. [Google Scholar] [CrossRef]
- Steurbaut, E. Les otolithes de téléostéens de l’Oligo-Miocène d’Aquitaine (Sued-Ouest de la France). Palaeontogr. A 1984, 186, 1–162. [Google Scholar]
- Schwarzhans, W.; Carnevale, G. Fish otoliths from the upper Oligocene and Lower Miocene of the Monferrato and Turin Hills, northern Italy. Riv. Ital. Paleontol. E Stratigr. 2024, 130, 663–709. [Google Scholar]
- Lin, C.-H.; Chang, C.-W. Otolith Atlas of Taiwan Fishes; NMMBA Atlas Series; National Museum of Marine Biology & Aquarium: Pin-Tung, Taiwan, 2012; Volume 12, 415p. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.se/search.php (accessed on 30 January 2025).
- Přikryl, T.; Brzobohatý, R.; Gregorová, R. Diversity and distribution of fossil codlets (Teleostei, Gadiformes, Bregmacerotidae): Review and commentary. Palaeobiodivers. Palaeoenviron. 2016, 96, 13–39. [Google Scholar] [CrossRef]
- Wade, B.S.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth-Sci. Rev. 2011, 104, 111–142. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarzhans, W.W.; Cotton, L.J. First Marine Fossil Otoliths (Teleostei) from East Africa (Tanzania). Diversity 2025, 17, 255. https://doi.org/10.3390/d17040255
Schwarzhans WW, Cotton LJ. First Marine Fossil Otoliths (Teleostei) from East Africa (Tanzania). Diversity. 2025; 17(4):255. https://doi.org/10.3390/d17040255
Chicago/Turabian StyleSchwarzhans, Werner W., and Laura J. Cotton. 2025. "First Marine Fossil Otoliths (Teleostei) from East Africa (Tanzania)" Diversity 17, no. 4: 255. https://doi.org/10.3390/d17040255
APA StyleSchwarzhans, W. W., & Cotton, L. J. (2025). First Marine Fossil Otoliths (Teleostei) from East Africa (Tanzania). Diversity, 17(4), 255. https://doi.org/10.3390/d17040255







