Diversity and Composition of Soil Microbes Associated with Barringtonia racemosa Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sample Collection
2.2. Analysis of Soil Physicochemical Properties
2.3. Analysis of Plant Traits
2.4. PLFA Analysis to Identify the Rhizosphere Microorganisms
2.5. Soil Microbial Analysis
2.5.1. Sequencing and Data Analysis
2.5.2. Alpha Diversity Index
2.5.3. Species Composition Analysis
2.5.4. Principle Coordinates Analysis (PCoA)
2.6. Statistical Analyses
3. Results
3.1. Differences in Soil Physicochemical Characteristics Among the B. Racemosa Pure and Associated Communities
3.2. Differences in Leaf Traits Among the B. racemosa Pure and Associated Communities
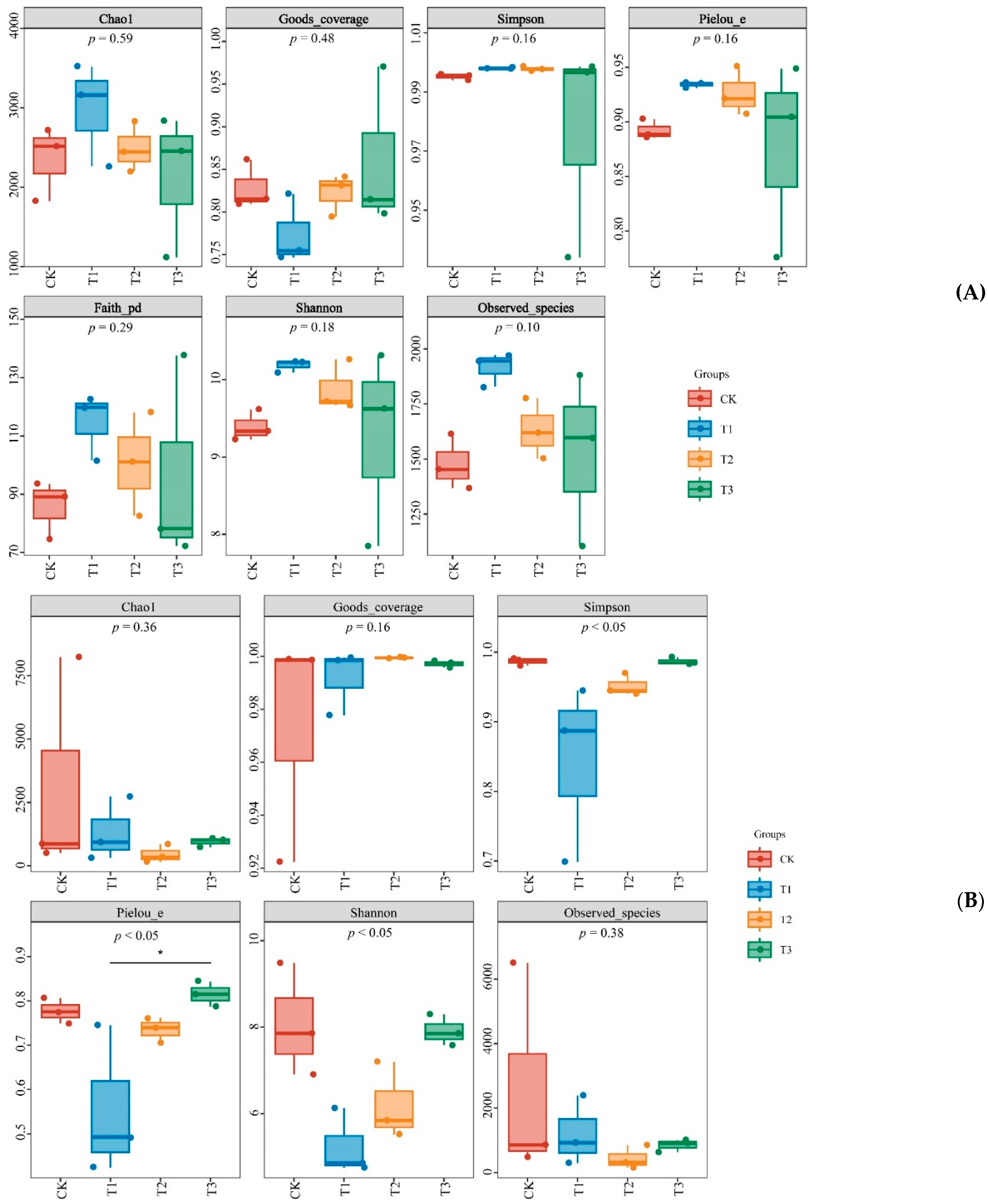
3.3. Composition and Diversity of Soil Microbes Associated with B. Racemosa Pure and Associated Communities
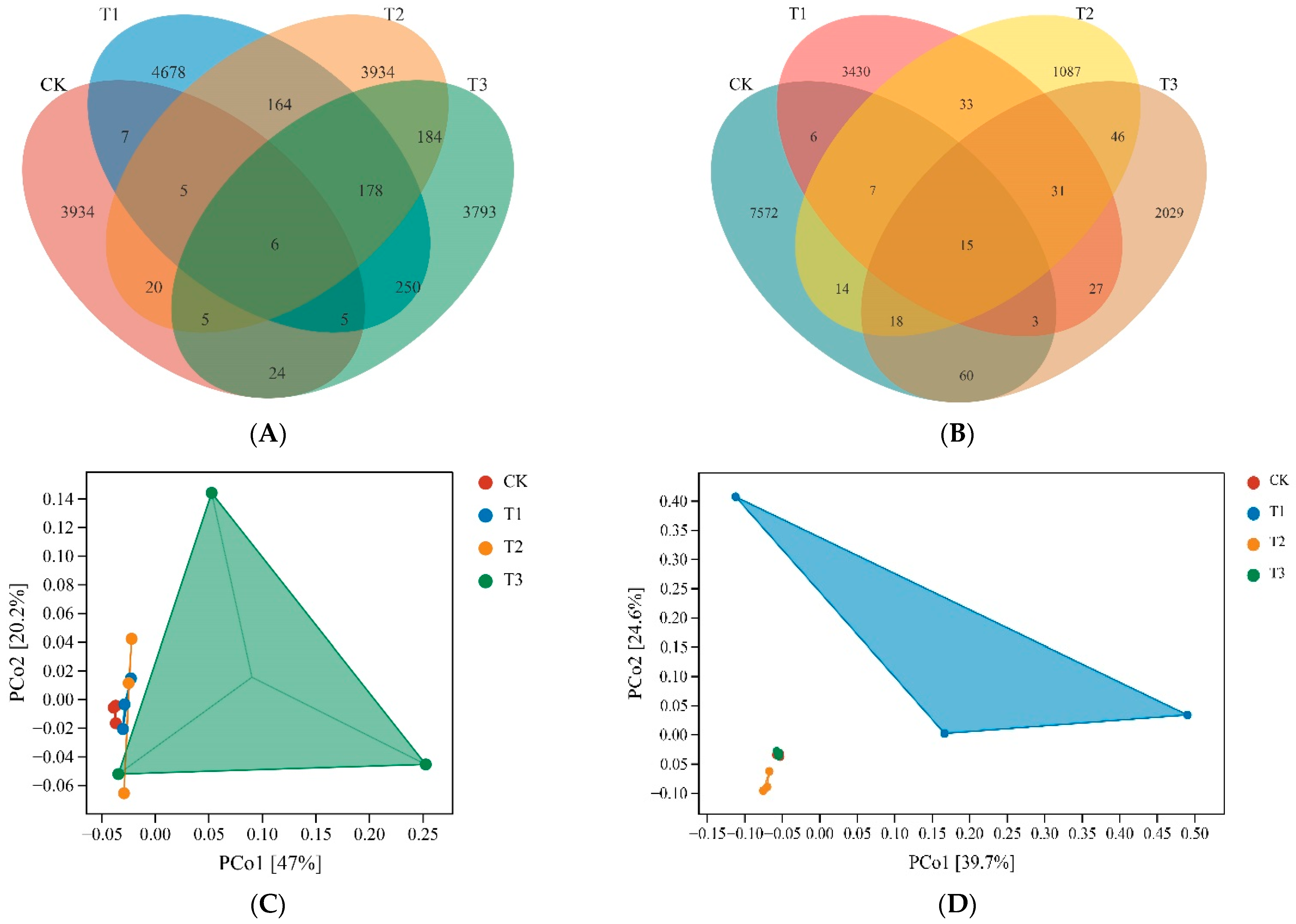
3.3.1. Taxonomy and Composition of Soil Microbes
3.3.2. Microbial Diversity
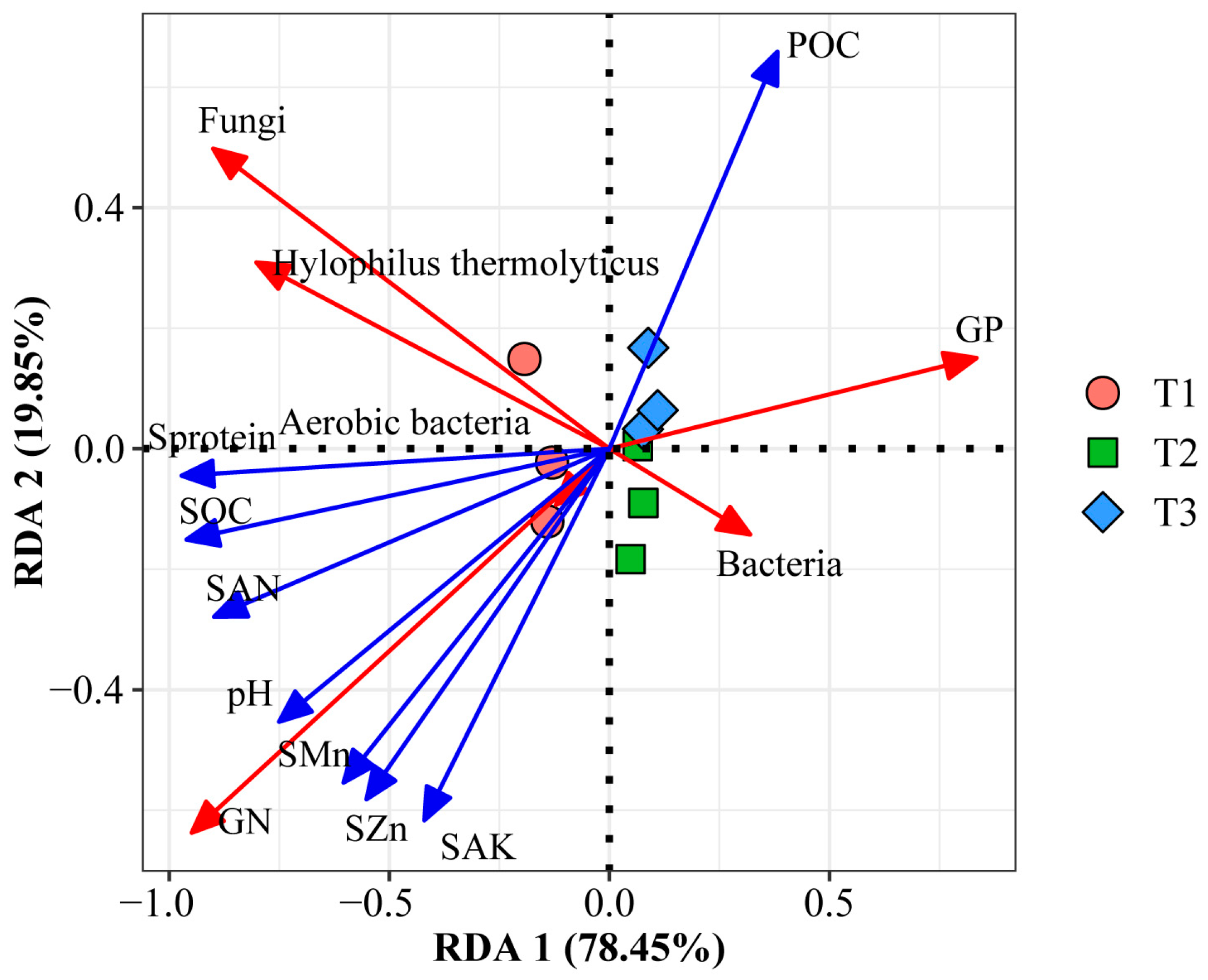
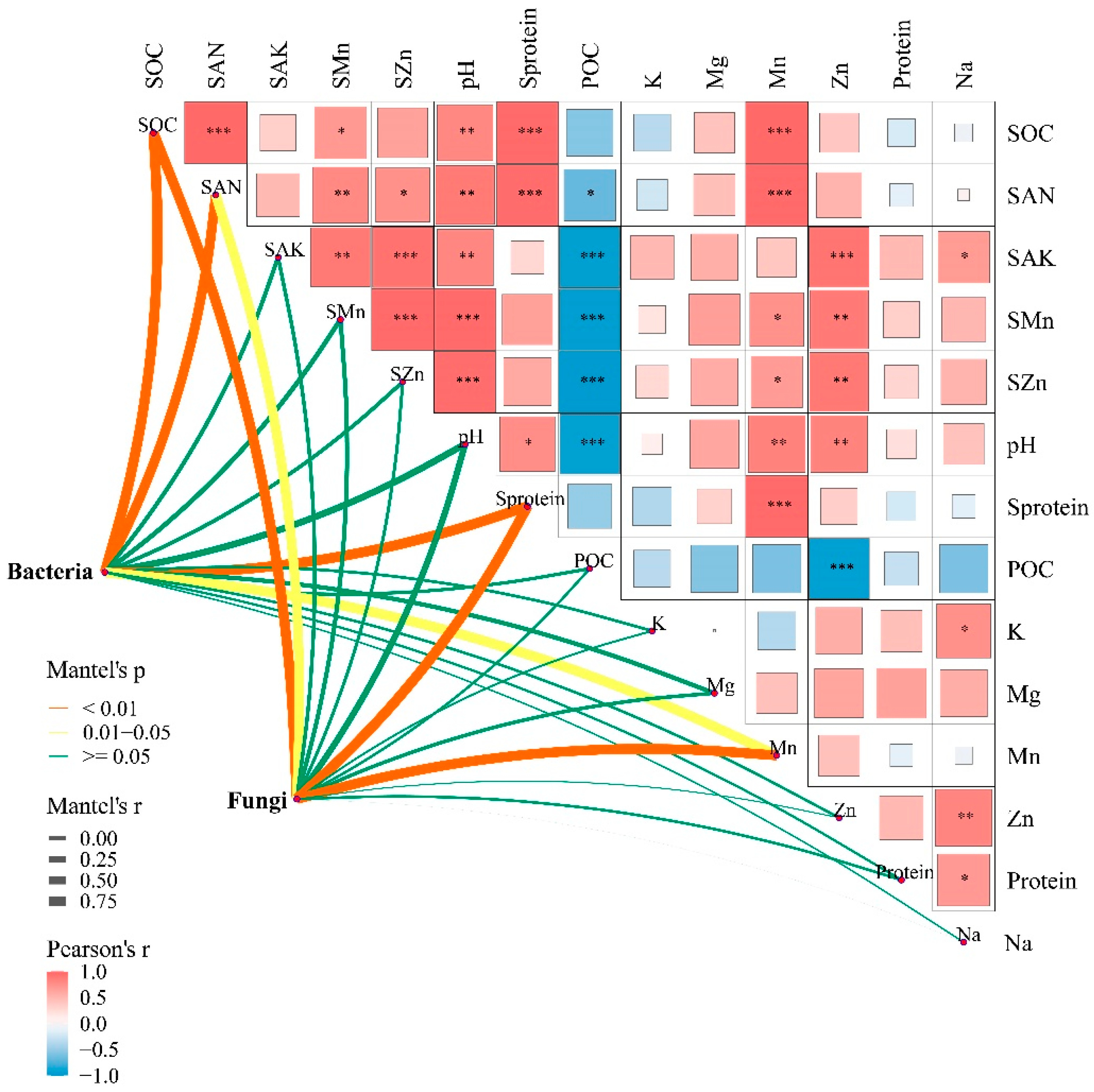
3.4. Major Factors Regulating the Composition of Soil Microbes of the B. Racemosa Pure and Associated Communities
4. Discussion
4.1. Soil Physicochemical Characteristics and Plant Traits Influence Soil Microbial Community in the B. Racemosa Pure and Associated Communities
4.2. Factors Driving the Soil Bacterial and Fungal Communities in B. racemosa Pure and Associated Communities
4.3. Limitations and Future Directions in Plant-Microbe Interaction Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andreote, F.D.; Jiménez, D.J.; Chaves, D.; Dias, A.C.F.; Luvizotto, D.M.; Dini-Andreote, F.; Fasanella, C.C.; Lopez, M.V.; Baena, S.; Taketani, R.G.; et al. The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PLoS ONE 2012, 7, e38600. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Jusoff, K. Malaysian mangrove forests and their significance to the coastal marine environment. Pol. J. Environ. Stud. 2013, 22, 979–1005. [Google Scholar]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Arfi, Y.; Buée, M.; Marchand, C.; Levasseur, A.; Record, E. Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees Avicennia marina and Rhizophora stylosa. FEMS Microbiol. Ecol. 2012, 79, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Gao, A.H.; Xie, Q.Y.; Gao, H.; Zhuang, L.; Lin, H.P.; Yu, H.P.; Li, J.; Yao, X.S.; Goodfellow, M.; et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Liu, L.; Li, T.; Dou, Y.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Nitrogen fertilization weakens the linkage between soil carbon and microbial diversity: A global meta-analysis. Glob. Change Biol. 2022, 28, 6446–6461. [Google Scholar] [CrossRef]
- Banerjee, S.; van der Heijden, M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Wu, Y.T.; Gutknecht, J.; Nadrowski, K.; Geißler, C.; Kühn, P.; Scholten, T.; Both, S.; Erfmeier, A.; Böhnke, M.; Bruelheide, H.; et al. Relationships between soil microorganisms, plant communities, and soil characteristics in Chinese subtropical forests. Ecosystems 2012, 15, 624–636. [Google Scholar] [CrossRef]
- Smith, M.E.; Facelli, J.M.; Cavagnaro, T.R. Interactions between soil properties, soil microbes and plants in remnant-grassland and old-field areas: A reciprocal transplant approach. Plant Soil 2018, 433, 127–145. [Google Scholar] [CrossRef]
- Hawkins, A.P.; Crawford, K.M. Interactions between plants and soil microbes may alter the relative importance of intraspecific and interspecific plant competition in a changing climate. AoB Plants 2018, 10, ply039. [Google Scholar] [CrossRef]
- Meng, F.; Hu, A.; Wang, J. Microbial traits shed light on species distributions, assembly processes and ecosystem functions. Acta Microbiol. Sin. 2020, 60, 1784–1800. [Google Scholar] [CrossRef]
- Lan, G.; Quan, F.; Yang, C.; Sun, R.; Chen, B.; Zhang, X.; Wu, Z. Driving factors for soil fungal and bacterial community assembly in topical forest of China. Appl. Soil Ecol. 2022, 177, 104520. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Lin, Z.a.; Li, Y.; Yang, X.; Zhao, B. Soil microbial community structure and function are significantly affected by long-term organic and mineral fertilization regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Barreto, C.R.; Morrissey, E.M.; Wykoff, D.D.; Chapman, S.K. Co-occurring mangroves and salt marshes differ in microbial community composition. Wetlands 2018, 38, 497–508. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, B.; Peng, Y.; Lin, G.; Sun, D.; Ye, W. Responses of microbial communities and greenhouse gas production to land use change in mangrove wetland sediments. Limnol. Oceanogr. 2025. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Fu, Q.; Qiu, Y.; Zhao, J.; Li, J.; Wu, X.; Yang, Y.; Liu, H.; Yang, X.; et al. Critical transition of soil microbial diversity and composition triggered by plant rhizosphere effects. Front. Plant Sci. 2023, 14, 1252821. [Google Scholar] [CrossRef]
- Li, Q.; Feng, J.; Wu, J.; Jia, W.; Zhang, Q.; Chen, Q.; Zhang, D.; Cheng, X. Spatial variation in soil microbial community structure and its relation to plant distribution and local environments following afforestation in central China. Soil Tillage Res. 2019, 193, 8–16. [Google Scholar] [CrossRef]
- Muwawa, E.M.; Makonde, H.M.; Obieze, C.C.; de Oliveira, I.G.; Jefwa, J.M.; Kahindi, J.H.P.; Khasa, D.P. Diversity and assembly patterns of mangrove rhizosphere mycobiome along the coast of Gazi Bay and Mida Creek in Kenya. PLoS ONE 2024, 19, e0298237. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Y.; Dong, S.; He, Q.; Jia, Y.; Zhao, L.; Yu, S.; Liu, H.; Liu, B.; Yan, Y.; et al. Threatened species list of China’s higher plants. Biodivers. Sci. 2017, 25, 696–744. [Google Scholar] [CrossRef]
- Liang, F.; Tan, X.; Deng, X.; Wu, Y.; Wu, M.; Yang, X.; Li, J. Growth and physiological responses of semi-mangrove plant Barringtonia racemosa to waterlogging and salinity stress. Guihaia 2021, 41, 872–882. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, X.; Zhou, Y.; Liu, N. Effects of a heavy metal (cadmium) on the responses of subtropical coastal tree species to drought stress. Environ. Sci. Pollut. Res. 2023, 30, 12682–12694. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Hu, J.; Lin, Y.; Li, L.; Yu, Y.; Liu, B.; Meng, Z.; Xiang, Z.; Tan, X. Interspecific competition and survival pressures in endangered Barringtonia racemosa populations of Mainland China. Sci. Rep. 2024, 14, 31190. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Q.; Xu, S.; Chen, H.; Li, W.; Pan, T. The disturbance mechanism and potential ecological loss of the Barringtonia racemosa community on the east coast of Leizhou Peninsula. J. South China Norm. Univ. (Nat. Sci. Ed.) 2019, 51, 67–75. [Google Scholar] [CrossRef]
- Lin, X.; Hou, L.; Liu, M.; Li, X.; Zheng, Y.; Yin, G.; Gao, J.; Jiang, X. Nitrogen mineralization and immobilization in sediments of the East China Sea: Spatiotemporal variations and environmental implications. J. Geophys. Res. Biogeosciences 2016, 121, 2842–2855. [Google Scholar] [CrossRef]
- Jiang, L.; Geng, Z.; Li, S.; She, D.; He, X.; Zhang, Q.; Liang, C.; Liu, D.; Jing, W.; Wang, S. Soil cation exchange capacity and exchangeable base cation content in the profiles of four typical soils in the Xi-Shui Forest Zone of the Qilian Mountains. Acta Ecol. Sin. 2012, 32, 3368–3377. [Google Scholar] [CrossRef]
- Bao, X.; Focke, M.; Pollard, M.; Ohlrogge, J. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 2000, 22, 39–50. [Google Scholar] [CrossRef]
- Yu, W.; Gao, H.; Kang, H. Ester Linked Fatty Acid (ELFA) method should be used with caution for interpretating soil microbial communities and their relationships with environmental variables in forest soils. PLoS ONE 2021, 16, e0251501. [Google Scholar] [CrossRef]
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Landesman, W.J.; Dighton, J. Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biol. Biochem. 2010, 42, 1751–1758. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Y.; Zhang, H.; Li, Q.; Zhao, X.; Guo, X.; Liu, W.; Wan, F. Asymmetric succession in soil microbial communities enhances the competitive advantage of invasive alien plants. Microbiome 2024, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Kivlin, S.N.; Mann, M.A.; Lynn, J.S.; Melanie, R.K.; Taylor, D.L.; Jennifer, A.R. Grass species identity shapes communities of root and leaf fungi more than elevation. ISME Commun. 2022, 2, 25. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; de Siqueira Ferreira, A.; Barboza, A.D.; Roesch, L.F. Changes in diversity, abundance, and structure of soil bacterial communities in Brazilian Savanna under different land use systems. Microb. Ecol. 2013, 66, 593–607. [Google Scholar] [CrossRef]
- Lasa, A.V.; Fernández-González, A.J.; Villadas, P.J.; Toro, N.; Fernández-López, M. Metabarcoding reveals that rhizospheric microbiota of Quercus pyrenaica is composed by a relatively small number of bacterial taxa highly abundant. Sci. Rep. 2019, 9, 1695. [Google Scholar] [CrossRef]
- Gschwend, F.; Hartmann, M.; Mayerhofer, J.; Hug, A.-S.; Enkerli, J.; Gubler, A.; Meuli, R.G.; Frey, B.; Widmer, F. Site and land-use associations of soil bacteria and fungi define core and indicative taxa. FEMS Microbiol. Ecol. 2021, 97, fiab165. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.J.A.; da Cruz Silva, A.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2020, 457, 117696. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, Y.; Zhang, W.; Hu, G.; Xie, H.; Yan, J.; Han, S.; He, H.; Zhang, X. Linkage of microbial residue dynamics with soil organic carbon accumulation during subtropical forest succession. Soil Biol. Biochem. 2017, 114, 114–120. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, W. Plant–soil microbe feedbacks drive seedling establishment during secondary forest succession: The ‘successional stage hypothesis’. J. Plant Ecol. 2023, 16, rtad021. [Google Scholar] [CrossRef]
- Tang, J.; Yin, J.; Davy, A.J.; Pan, F.; Miao, R.; Han, X. Changes in soil fertility and microbial communities following cultivation of native grassland in Horqin Sandy Land, China: A 60-year chronosequence. Ecol. Process. 2023, 12, 18. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, J.; Lu, H.; Yang, C.; Yang, Y.; Zhou, J.; Li, D. An integrated study to analyze soil microbial community structure and metabolic potential in two forest types. PLoS ONE 2014, 9, e93773. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Hu, Y.; Lu, X. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- Ona, L.; Kost, C. Cooperation increases robustness to ecological disturbance in microbial cross-feeding networks. Ecol. Lett. 2022, 25, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
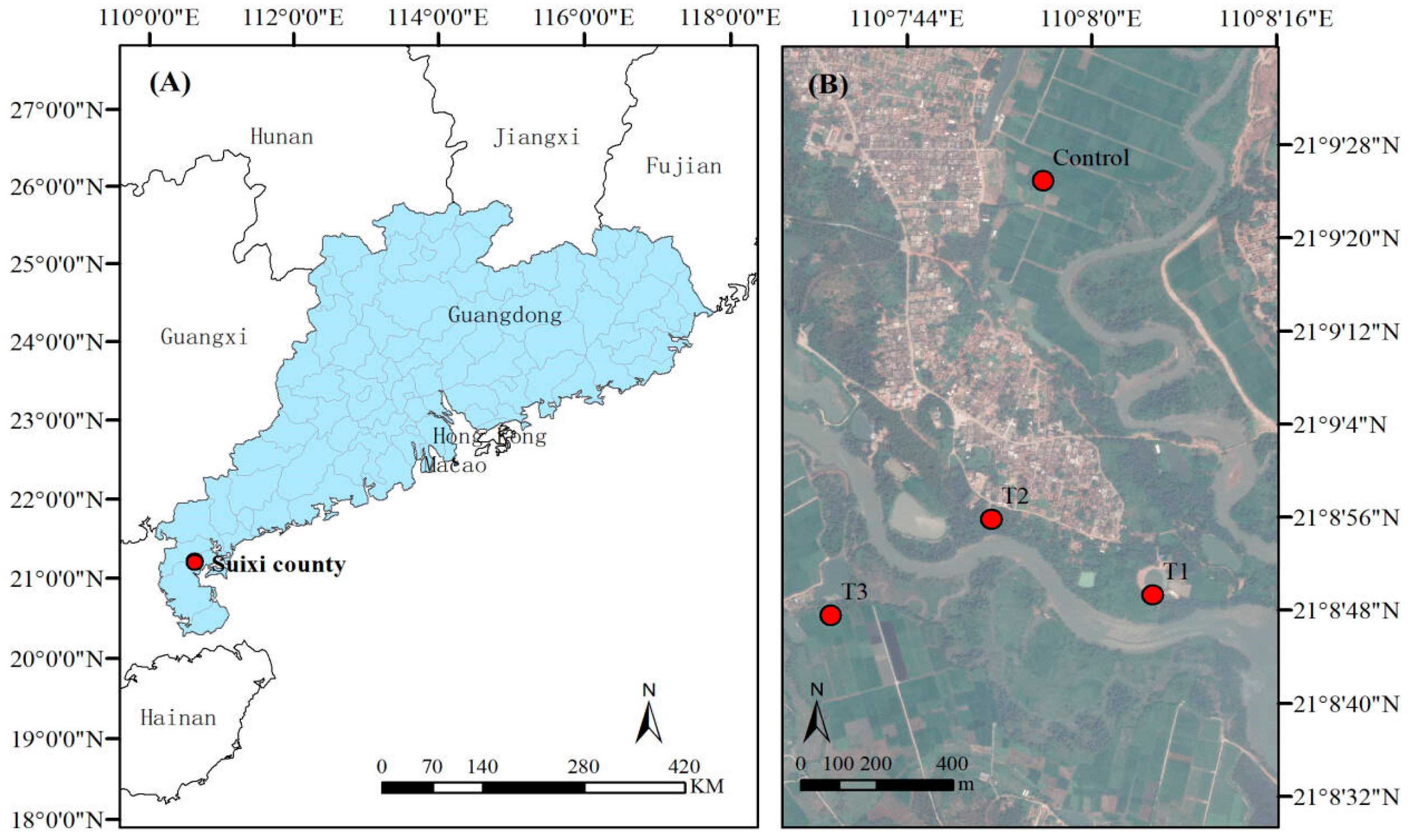
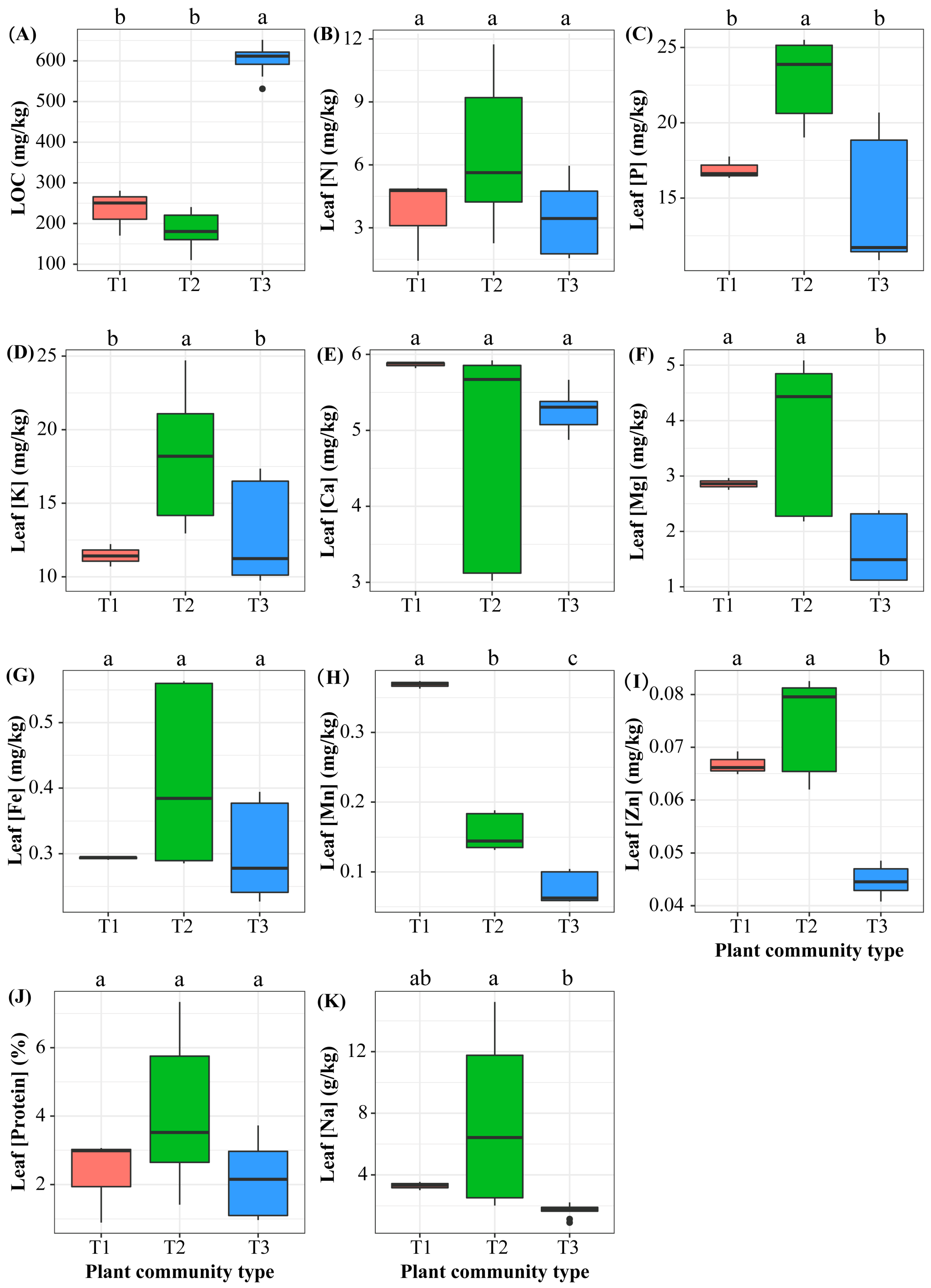

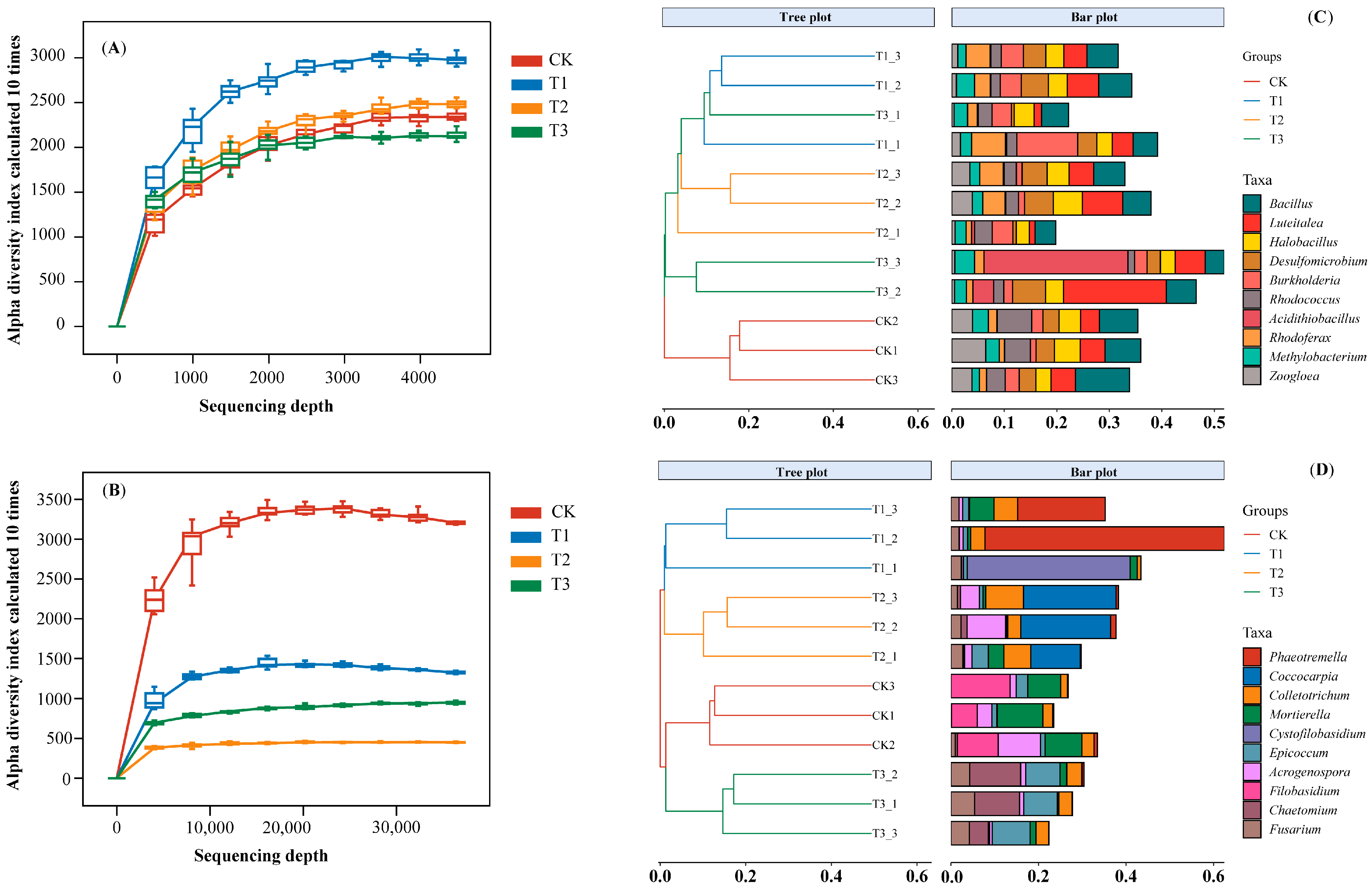

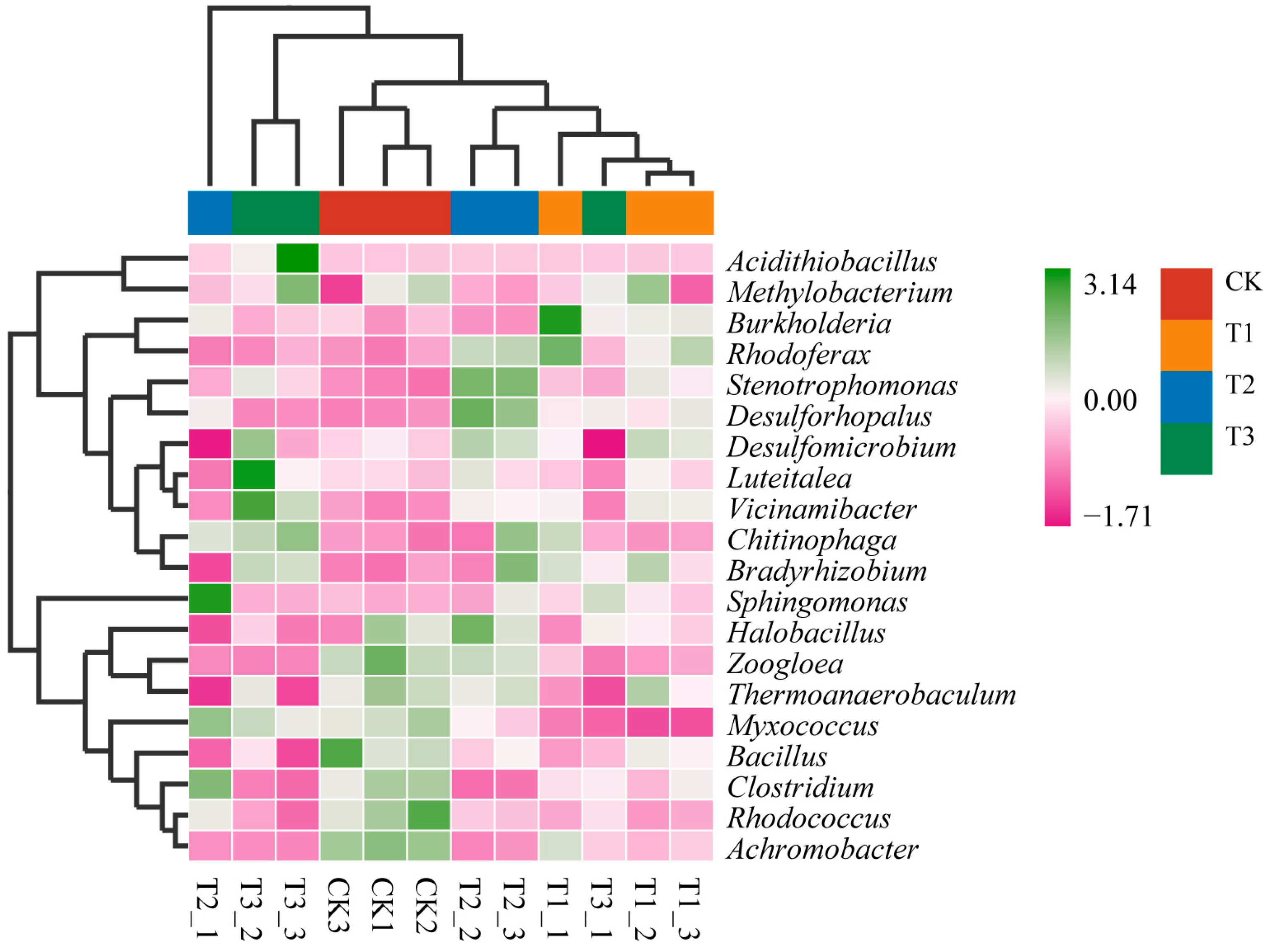
| Plot Number | Community Types | Longitude and Latitude | Slope Position | Slope | Slope Direction | Elevation |
|---|---|---|---|---|---|---|
| CK | Rice field | 21°9′26.20″ N/110°7′55″ E | Middle | 1.72 | Northwest | 3 m |
| T1 | B. racemosa pure forest | 21°8′50.00″ N/110°8′2.00″ E | Uphill | 0.75 | North | 6 m |
| T2 | B. racemosa + Acanthus ilicifolius | 21°9′10.30″ N/110°8′2.90″ E | Middle | 3.37 | North | 3 m |
| T3 | B. racemosa + Talipariti tiliaceum + Pongamia pinnata | 21°8′50.00″ N/110°7′34.00″ E | Middle | 2.13 | Southwest | 4 m |
| Soil Physicochemical Characteristics | CK | T1 | T2 | T3 |
|---|---|---|---|---|
| SOC (mg kg−1) | 28.20 ± 0.94 d | 74.32 ± 0.48 a | 50.93 ± 0.40 b | 44.91 ± 1.01 c |
| SOM (%) | 4.86 ± 0.16 d | 12.81 ± 0.08 a | 8.78 ± 0.07 b | 7.74 ± 0.17 c |
| SN (mg kg−1) | 8.20 ± 0.64 c | 22.00 ± 0.72 a | 13.26 ± 0.53 b | 11.63 ± 0.54 b |
| SAN (g kg−1) | 0.13 ± 0.01 d | 0.81 ± 0.01 a | 0.47 ± 0.02 b | 0.29 ± 0.01 c |
| SP (mg kg−1) | 0.78 ± 0.01 d | 26.24 ± 0.30 a | 19.69 ± 0.06 b | 8.32 ± 0.08 c |
| SAP (mg kg−1) | 0.26 ± 0.00 a | 0.11 ± 0.00 c | 0.14 ± 0.00 b | 0.06 ± 0.00 d |
| SK (g kg−1) | 5.42 ± 0.06 d | 10.57 ± 0.38 b | 1.07 ± 0.01 a | 7.84 ± 0.16 c |
| SAK (mg kg−1) | 1.06 ± 0.03 a | 0.72 ± 0.01 b | 12.55 ± 0.29 a | 0.15 ± 0.00 c |
| WSM (g kg−1) | 1.74 ± 0.08 b | 3.02 ± 0.16 a | 2.79 ± 0.14 a | 0.92 ± 0.04 c |
| SNa (g kg−1) | 2.09 ± 0.14 b | 4.08 ± 0.14 a | 3.65 ± 0.23 a | 2.15 ± 0.11 b |
| SCa (g kg−1) | 1.12 ± 0.08 c | 2.54 ± 0.18 a | 2.13 ± 0.12 b | 1.12 ± 0.09 c |
| SMg (g kg−1) | 1.74 ± 0.12 d | 4.51 ± 0.14 b | 5.03 ± 0.10 a | 2.29 ± 0.15 c |
| SMn (g kg−1) | 0.13 ± 0.01 b | 1.76 ± 0.07 a | 1.65 ± 0.07 a | 0.22 ± 0.01 b |
| SFe (g kg−1) | 24.49 ± 0.52 c | 51.75 ± 2.27 a | 53.92 ± 1.48 a | 29.26 ± 0.58 b |
| SZn (mg kg−1) | 34.35 ± 1.16 b | 105.43 ± 1.72 a | 107.95 ± 2.32 a | 36.46 ± 2.32 b |
| SWC (%) | 20.42 ± 0.21 d | 65.65 ± 1.17 a | 58.07 ± 1.29 b | 30.27 ± 2.31 c |
| Soil pH | 5.80 ± 0.01 d | 6.29 ± 0.01 a | 6.23 ± 0.01 b | 6.04 ± 0.01 c |
| SProtein (%) | 5.13 ± 0.40 c | 13.75 ± 0.45 a | 8.29 ± 0.33 b | 7.27 ± 0.34 b |
| CK | T1 | T2 | T3 | |
|---|---|---|---|---|
| Bacteria (nmol g−1) | 29.84 ± 2.82 b | 92.72 ± 6.86 a | 37.73 ± 4.95 b | 38.46 ± 4.15 b |
| Fungi (nmol g−1) | 5.37 ± 0.72 b | 17.28 ± 2.85 a | 4.99 ± 0.79 b | 5.49 ± 0.43 b |
| F:B | 0.18 ± 0.01 a | 0.19 ± 0.02 a | 0.13 ± 0.01 b | 0.14 ± 0.01 b |
| Hylophilus thermolyticus (nmol g−1) | 2.15 ± 0.18 b | 11.02 ± 1.54 a | 3.03 ± 0.41 b | 3.35 ± 0.43 b |
| Aerobic bacteria (nmol g−1) | 1.11 ± 0.06 b | 2.33 ± 0.17 a | 0.91 ± 0.15 bc | 0.79 ± 0.09 c |
| Gram-positive bacteria (nmol g−1) | 24.31 ± 2.11 b | 70.99 ± 6.05 a | 30.48 ± 3.92 b | 32.17 ± 3.61 b |
| Gram-negative bacteria (nmol g−1) | 5.53 ± 0.76 b | 21.72 ± 0.87 a | 7.25 ± 1.16 b | 6.29 ± 0.60 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Tan, X.; Hu, J.; Yu, Y.; Yang, X.; Li, L.; Tan, Y.; Dong, Z.; Wei, Y.; Liang, F. Diversity and Composition of Soil Microbes Associated with Barringtonia racemosa Communities. Diversity 2025, 17, 249. https://doi.org/10.3390/d17040249
Lin Y, Tan X, Hu J, Yu Y, Yang X, Li L, Tan Y, Dong Z, Wei Y, Liang F. Diversity and Composition of Soil Microbes Associated with Barringtonia racemosa Communities. Diversity. 2025; 17(4):249. https://doi.org/10.3390/d17040249
Chicago/Turabian StyleLin, Yutong, Xiaohui Tan, Ju Hu, Yanping Yu, Xiuling Yang, Lin Li, Yanfang Tan, Zeting Dong, Yilan Wei, and Fang Liang. 2025. "Diversity and Composition of Soil Microbes Associated with Barringtonia racemosa Communities" Diversity 17, no. 4: 249. https://doi.org/10.3390/d17040249
APA StyleLin, Y., Tan, X., Hu, J., Yu, Y., Yang, X., Li, L., Tan, Y., Dong, Z., Wei, Y., & Liang, F. (2025). Diversity and Composition of Soil Microbes Associated with Barringtonia racemosa Communities. Diversity, 17(4), 249. https://doi.org/10.3390/d17040249









