Spatiotemporal Ecology of an Imperiled Cushion Plant Assemblage at a North American Rocky Mountain Summit: Implications for Diversity Conservation
Abstract
1. Introduction
2. Materials and Methods
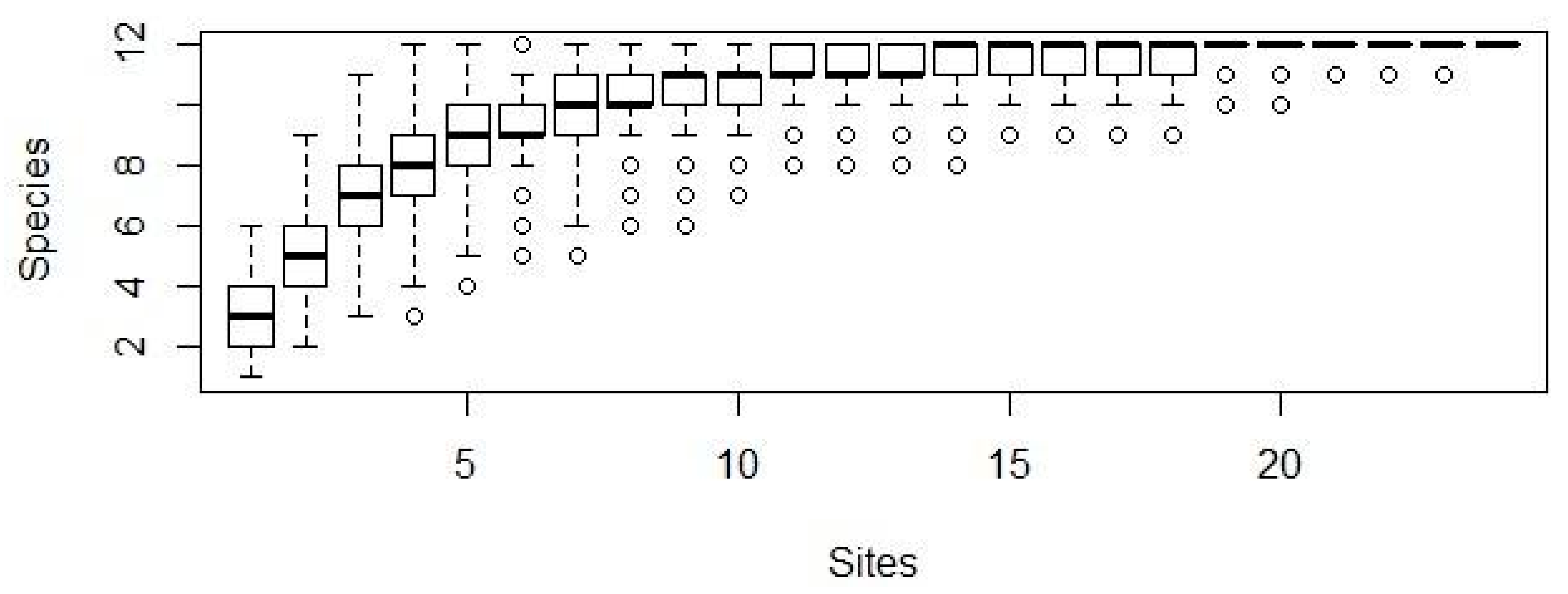
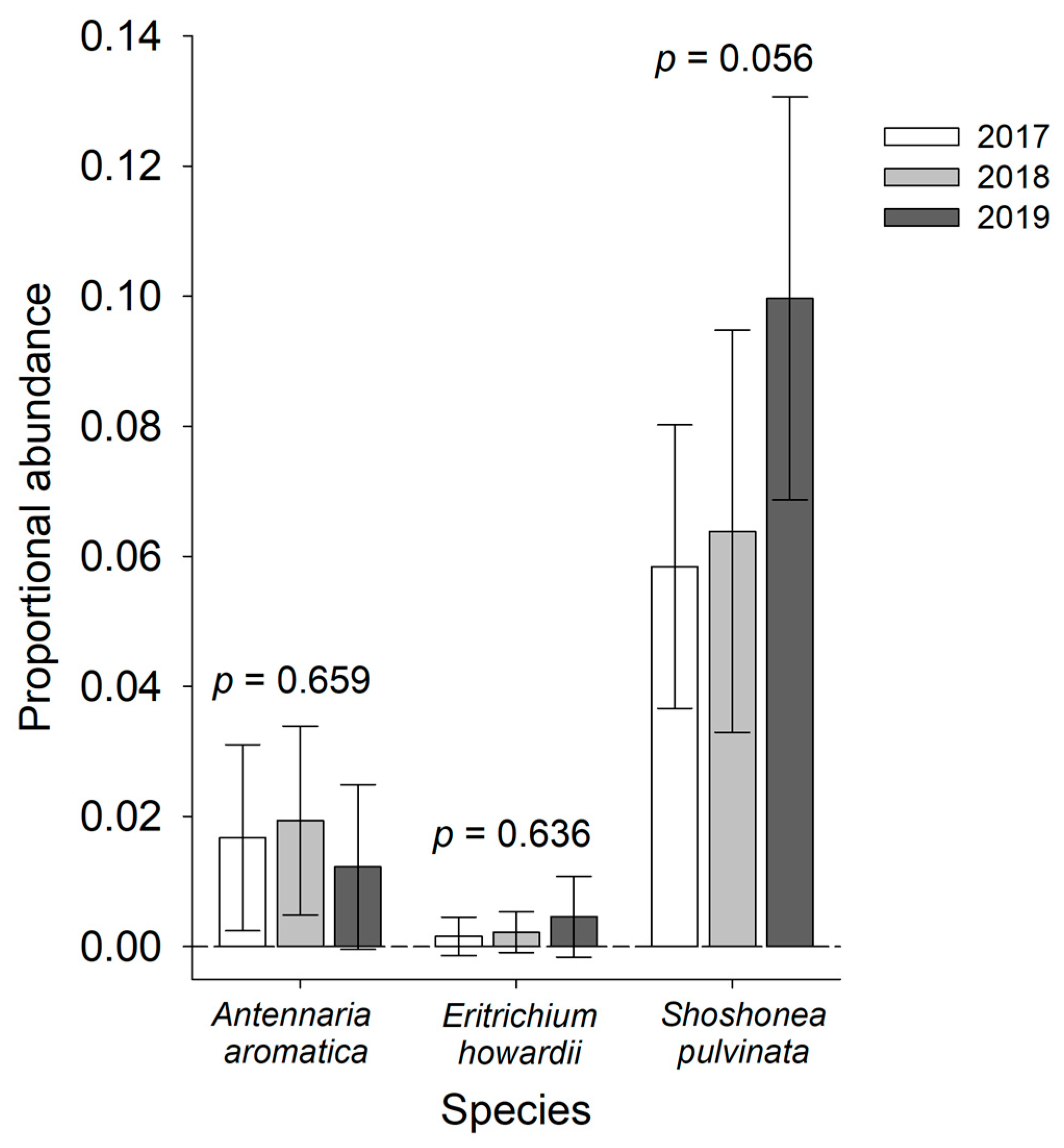
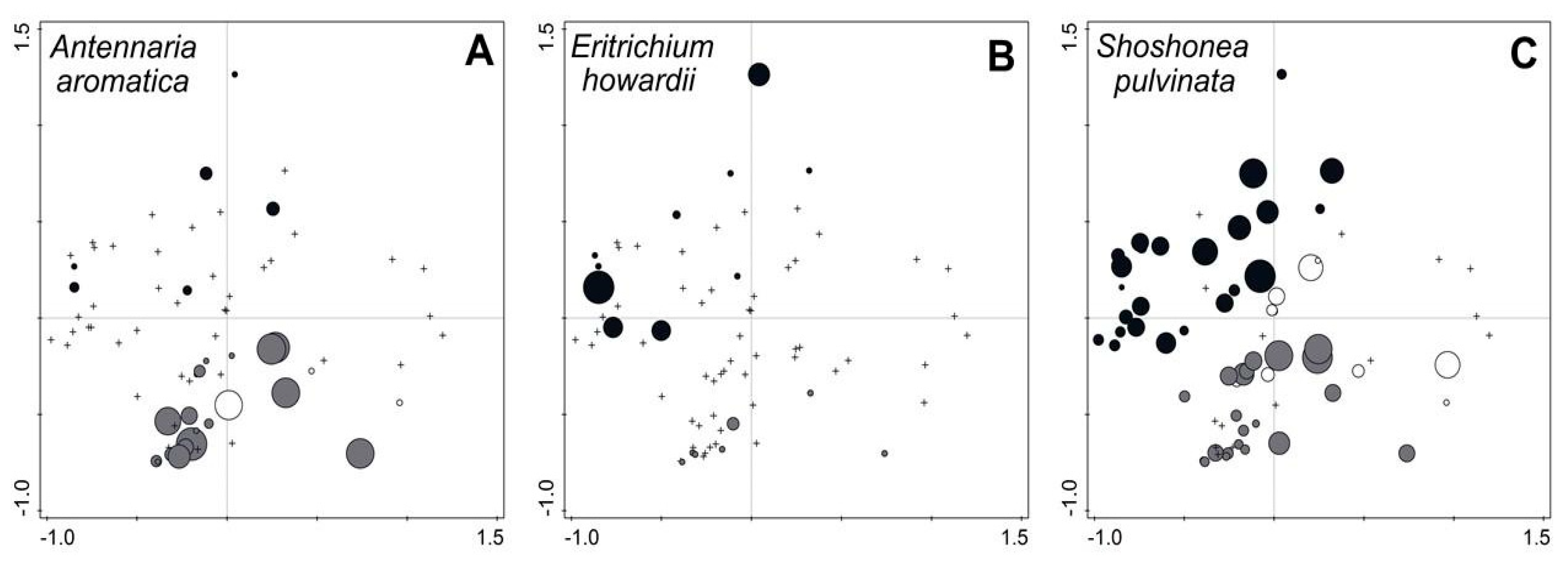
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monz, C.A.; Marion, J.L.; Goonan, K.A.; Manning, R.E.; Wimpey, J.; Carr, C. Assessment and monitoring of recreation impacts and resource conditions on mountain summits: Examples from the Northern Forest, USA. Mt. Res. Dev. 2010, 30, 332–343. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, NY, USA, 1989. [Google Scholar]
- Ulrich, R.S.; Parsons, R. Chapter 15 Influences of passive experiences with plants on individual well-being and health. In The Role of Horticulture in Human Well-Being and Social Development; Relf, D., Ed.; Timber Press: Portland, OR, USA, 1992; pp. 93–105. [Google Scholar]
- Herzog, T.R.; Black, A.M.; Fountaine, K.A.; Knotts, D.J. Reflection and attentional recovery as distinctive benefits of restorative environments. J. Environ. Psychol. 1997, 17, 165–170. [Google Scholar] [CrossRef]
- Hawkins, D.E.; Lamoureux, K. Global growth and magnitude of ecotourism. In The Encyclopedia of Ecotourism; CABI Publishing: Wallingford, UK, 2001; pp. 63–72. [Google Scholar] [CrossRef]
- Bednar-Friedl, B.; Behrens, D.A.; Getzner, M. Optimal dynamic control of visitors and endangered species in a national park. Environ. Resour. Econ. 2012, 52, 1–22. [Google Scholar] [CrossRef]
- Hoffmann, A.J.; Alliende, C. Impact of trampling upon the vegetation of Andean areas in Central Chile. Mt. Res. Dev. 1982, 2, 189–194. [Google Scholar] [CrossRef]
- Scott, J.J.; Kirkpatrick, J.B. Effects of human trampling on the sub-Antarctic vegetation of Macquarie Island. Polar Rec. 1994, 30, 207–220. [Google Scholar] [CrossRef]
- Liddle, M.J. Recreation Ecology; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Buckley, R. Environmental Impacts of Tourism; CABI International: New York City, NY, USA, 2004. [Google Scholar]
- Pickering, C.M.; Hill, W. Impacts of recreation and tourism on plant biodiversity and vegetation in protected areas in Australia. J. Environ. Manag. 2007, 85, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Monz, C.A.; Pickering, C.M.; Hadwen, W.L. Recent advances in recreation ecology and the implications of different relationships between recreation use and ecological impacts. Front. Ecol. Environ. 2013, 11, 441–446. [Google Scholar] [CrossRef]
- Krüger, O. The role of ecotourism in conservation: Panacea or Pandora’s box? Biodivers. Conserv. 2005, 14, 579–600. [Google Scholar] [CrossRef]
- Buckley, R. Tourism and environment. Annu. Rev. Environ. Resour. 2011, 36, 397–416. [Google Scholar] [CrossRef]
- Spanou, S.; Tsegenidi, K.; Georgiadis, T. Perception of visitors’ environmental impacts of ecotourism: A case study in The Valley of Butterflies protected area, Rhodes Island, Greece. Int. J. Environ. Res. 2012, 6, 245–258. [Google Scholar]
- Hensen, I.; Kilian, C.; Wagner, V.; Durka, W.; Pusch, J.; Wesche, K. Low genetic variability and strong differentiation among isolated populations of the rare steppe grass Stipa capillata L. in Central Europe. Plant Biol. 2010, 12, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Vogler, F.; Reisch, C. Vital survivors: Low genetic variation but high germination in glacial relict populations of the typical rock plant Draba aizoides. Biodivers. Conserv. 2013, 22, 1301–1316. [Google Scholar] [CrossRef]
- Kelly, C.L.; Pickering, C.M.; Buckley, R.C. Impacts of tourism on threatened plant taxa and communities in Australia. Ecol. Manag. Restor. 2003, 4, 37–44. [Google Scholar] [CrossRef]
- Beutner, E.C.; Gerbi, G.P. Catastrophic emplacement of the Heart Mountain block slide, Wyoming and Montana, USA. Geol. Soc. Am. Bull. 2005, 117, 724–735. [Google Scholar] [CrossRef]
- Craddock, J.P.; Malone, D.H.; Magloughlin, J.; Cook, A.L.; Rieser, M.E.; Doyle, J.R. Dynamics of the emplacement of the Heart Mountain allochthon at White Mountain: Constraints from calcite twinning strains, anisotropy of magnetic susceptibility, and thermodynamic calculations. Geol. Soc. Am. Bull. 2009, 121, 919–938. [Google Scholar] [CrossRef]
- Heidel, B. Wyoming Plant Species of Concern. Wyoming Natural Diversity Database; University of Wyoming: Laramie, WY, USA, 2012; Available online: https://www.uwyo.edu/wyndd/_files/docs/soc-plants/2012_Plant_SOC.pdf (accessed on 11 March 2024).
- Evert, E.F. A new species of Antennaria (Asteraceae) from Montana and Wyoming. Madroño 1984, 31, 109–112. Available online: https://www.jstor.org/stable/41424480 (accessed on 1 March 2025).
- Chmielewski, J.G.; Chinnappa, C.C. Range extension of Antennaria aromatica Evert (Asteraceae: Inuleae). SIDA Contrib. Bot. 1988, 13, 256–258. Available online: https://www.jstor.org/stable/41966785 (accessed on 1 March 2025).
- Bayer, R.J. A systematic and phytogeographic study of Antennaria aromatica and A. densifolia (Asteraceae: Inuleae) in the western North American cordillera. Madroño 1989, 36, 248–259. Available online: https://www.jstor.org/stable/41424771 (accessed on 1 March 2025).
- Evert, E.F.; Constance, L. Shoshonea pulvinata, a new genus and species of Umbelliferae from Wyoming. Syst. Bot. 1982, 7, 471–475. [Google Scholar] [CrossRef]
- Lesica, P.; Achuff, P.L. Monitoring Populations of Shoshonea Pulvinata in the Pryor and Beartooth Mountains, Carbon County, Montana: Establishment Report; Montana Natural Heritage Program: Helena, MT, USA, 1991; Available online: https://archive.org/details/monitoringpopula00lesirich (accessed on 11 March 2024).
- Lesica, P. Monitoring Populations of Shoshonea Pulvinata in the Pryor and Beartooth Mountains, Carbon County, Montana: Progress Report; Montana Natural Heritage Program: Helena, MT, USA, 1992; Available online: https://www.biodiversitylibrary.org/bibliography/25361 (accessed on 11 March 2024).
- Heidel, B.L. Monitoring Shoshonea Pulvinata in the Pryor and Beartooth Mountains, Carbon County, Montana: 1999 Trend Report; Montana Natural Heritage Program: Helena, MT, USA, 2001; Available online: https://mtnhp.org/plants/reports/Sho_pul.pdf (accessed on 11 March 2024).
- Murray, D.F. Notes on Eritrichium (Boraginaceae) in North America II. J. Bot. Res. Inst. Tex. 2015, 9, 311–315. Available online: https://www.jstor.org/stable/24621282 (accessed on 1 March 2025).
- Wiser, S.K.; Peet, R.K.; White, P.S. Prediction of rare-plant occurrence: A southern Appalachian example. Ecol. Appl. 1998, 8, 909–920. [Google Scholar] [CrossRef]
- PRISM Climate Group. Oregon State University. 2014. Available online: https://prism.oregonstate.edu (accessed on 4 February 2024).
- Dunnewald, T.J.; Pearson, C.; Thorp, J.; Carpenter, E.J.; Fitzpatrick, E.G. Soil Survey of the Shoshone Area, Wyoming. United States Department of Agriculture. 1927. Available online: https://archive.org/details/shoshoneWY1927 (accessed on 11 March 2024).
- Elzinga, C.L.; Salzer, D.W.; Willoughby, J.W. Measuring & Monitoring Plant Populations. US Department of the Interior, Bureau of Land Management, 1998, Technical Reference 1730-1. Available online: https://www.ntc.blm.gov/krc/legacy/course/265 (accessed on 11 March 2024).
- Dorn, R.D. Vascular Plants of Wyoming, 3rd ed.; Mountain West Publishing: Cheyenne, WY, USA, 2001. [Google Scholar]
- Braun-Blanquet, J. Fitossociología: Bases para el Estudio de las Comunidades Vegetales; Blume: Madrid, Spain, 1979. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. The Vegan Package. Community Ecology Package 2007, 10(631–637), 719. R Package Version 2.3-3. Available online: https://CRAN.R-project.org/package=vegan (accessed on 11 March 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 October 2024).
- Dean, A.; Voss, D.; Draguljić, D.; Dean, A.; Voss, D.; Draguljić, D. Chapter 18—Nested Models. In Design and Analysis of Experiments, 2nd ed.; Springer Texts in Statistics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- ter Braak, C.J.; Smilauer, P. CANOCO Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Wageningen University: Wageningen, The Netherlands, 2012. [Google Scholar]
- Levine, J.M.; Rees, M. Effects of temporal variability on rare plant persistence in annual systems. Am. Nat. 2004, 164, 350–363. [Google Scholar] [CrossRef]
- Gauthier, P.; Pons, V.; Letourneau, A.; Klesczewski, M.; Papuga, G.; Thompson, J.D. Combining population monitoring with habitat vulnerability to assess conservation status in populations of rare and endangered plants. J. Nat. Conserv. 2017, 37, 83–95. [Google Scholar] [CrossRef]
- Kulonen, A.; Imboden, R.A.; Rixen, C.; Maier, S.B.; Wipf, S. Enough space in a warmer world? Microhabitat diversity and small-scale distribution of alpine plants on mountain summits. Divers. Distrib. 2018, 24, 252–261. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Davies, T.J.; Schaefer, H.; Cleland, E.E.; Cook, B.I.; Travers, S.E.; Willis, C.G.; Davis, C.C. Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am. J. Bot. 2013, 100, 1407–1421. [Google Scholar] [CrossRef]
- Pauchard, A.; Milbau, A.; Albihn, A.; Alexander, J.; Burgess, T.; Daehler, C.; Englud, G.; Essl, F.; Evengård, B.; Greenwood, G.B.; et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biol. Invasions 2016, 18, 345–353. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Dolezal, J.; Dvorsky, M.; Kopecky, M.; Altman, J.; Mudrak, O.; Capkova, K.; Rehakova, K.; Macek, M.; Liancourt, P. Functionally distinct assembly of vascular plants colonizing alpine cushions suggests their vulnerability to climate change. Ann. Bot. 2019, 123, 569–578. [Google Scholar] [CrossRef]
- Ballantyne, M.; Pickering, C.M. Tourism and recreation: A common threat to IUCN red-listed vascular plants in Europe. Biodivers. Conserv. 2013, 22, 3027–3044. [Google Scholar] [CrossRef]
- Queiroz, R.E.; Ventura, M.A.; Silva, L. Plant diversity in hiking trails crossing Natura 2000 areas in the Azores: Implications for tourism and nature conservation. Biodivers. Conserv. 2014, 23, 1347–1365. [Google Scholar] [CrossRef]
- Pickering, C.M.; Buckley, R.C. Swarming to the summit. Mt. Res. Dev. 2003, 23, 230–233. [Google Scholar] [CrossRef]
- Willard, B.E.; Marr, J.W. Recovery of alpine tundra under protection after damage by human activities in the Rocky Mountains of Colorado. Biol. Conserv. 1971, 3, 181–190. [Google Scholar] [CrossRef]
- Lozano, P.; Cabrera, O.; Peyre, G.; Cleef, A.; Toulkeridis, T. Plant diversity and composition changes along an altitudinal gradient in the isolated volcano sumaco in the ecuadorian amazon. Diversity 2020, 12, 229. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Tian, Y.; Zhao, Z. Bryophyte flora in alpine grasslands of the Qinghai–Tibet Plateau based on plot sampling. Diversity 2024, 16, 143. [Google Scholar] [CrossRef]
- Bonanomi, G.; Stinca, A.; Chirico, G.B.; Ciaschetti, G.; Saracino, A.; Incerti, G. Cushion plant morphology controls biogenic capability and facilitation effects of Silene acaulis along an elevation gradient. Funct. Ecol. 2016, 30, 1216–1226. [Google Scholar] [CrossRef]




| Macroplot | Latitude | Longitude | Altitude (m) | Area (m²) | Quadrats (n) |
|---|---|---|---|---|---|
| 1 | 46°40.035′ | 111°06.996′ | 2304 | 295 | 6 * |
| 2 | 45°39.914′ | 110°06.840′ | 2334 | 2250 | 9 |
| 3 | 45°39.961′ | 110°07.192′ | 2478 | 700 | 9 |
| Family | Scientific Name | Common Name | T 1 | Conservation Status 2 |
|---|---|---|---|---|
| Asteraceae | Antennaria aromatica | scented pussytoes | F | HSR S3, FS SPLC, WYNDD SOC |
| Asteraceae | Antennaria monocephala | pygmy pussytoes | F | HSR S2, WYNDD SOC |
| Caryophyllaceae | Arenaria hookeri 3 | Hooker’s sandwort | F | Species not ranked |
| Fabaceae | Astragalus spatulatus | tufted milkvetch | F | HSR S5 |
| Scrophulariaceae | Castilleja angustifolia 4 | northwestern indian paintbrush | F | HSR S5 |
| Boraginaceae | Eritrichium howardii | Howard’s alpine forget-me-not | F | HSR S2, FS SPLC, WYNDD SOC |
| Asteraceae | Erigeron ochroleucus | buff fleabane | F | HSR S4 |
| Poaceae | Festuca idahoensis | Idaho fescue | G | HSR S4/S5 |
| Saxifragaceae | Heuchera parvifolia | littleleaf alumroot | F | HSR S5 |
| Rosaceae | Kelseya uniflora | oneflower kelseya | S | HSR S2 |
| Poaceae | Leucopoa kingii 5 | spike fescue | G | HSR S5 |
| Apiaceae | Lomatium attenuatum | tapertip desertparsley | F | HSR S2, WYNDD SOC |
| Caryophyllaceae | Minuartia nuttallii 6 | Nuttall’s sandwort | F | HSR S4/S5 |
| Asteraceae | Packera cana | woolly groundsel | F | HSR S5 |
| Asteraceae | Packera werneriifolia | hoary groundsel | F | HSR S2 |
| Polemoniaceae | Phlox hoodii | spiny phlox | F | HSR S5 |
| Pinaceae | Pinus flexilis | limber pine | T | HSR S4, FS SPLC, WYNDD SPOC |
| Poaceae | Poa glauca | glaucous bluegrass | G | HSR S3/S4 |
| Apiaceae | Shoshonea pulvinata | Shoshone carrot | F | HSR S2-WY S1-MT; FS-R2, WYNDD SOC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furquim, F.F.; Scasta, J.D. Spatiotemporal Ecology of an Imperiled Cushion Plant Assemblage at a North American Rocky Mountain Summit: Implications for Diversity Conservation. Diversity 2025, 17, 248. https://doi.org/10.3390/d17040248
Furquim FF, Scasta JD. Spatiotemporal Ecology of an Imperiled Cushion Plant Assemblage at a North American Rocky Mountain Summit: Implications for Diversity Conservation. Diversity. 2025; 17(4):248. https://doi.org/10.3390/d17040248
Chicago/Turabian StyleFurquim, Fernando Forster, and John Derek Scasta. 2025. "Spatiotemporal Ecology of an Imperiled Cushion Plant Assemblage at a North American Rocky Mountain Summit: Implications for Diversity Conservation" Diversity 17, no. 4: 248. https://doi.org/10.3390/d17040248
APA StyleFurquim, F. F., & Scasta, J. D. (2025). Spatiotemporal Ecology of an Imperiled Cushion Plant Assemblage at a North American Rocky Mountain Summit: Implications for Diversity Conservation. Diversity, 17(4), 248. https://doi.org/10.3390/d17040248






