Species Diversity of Benthic Marine Diatoms from a Natural Protected Area in Cuba
Abstract
1. Introduction
2. Materials and Methods
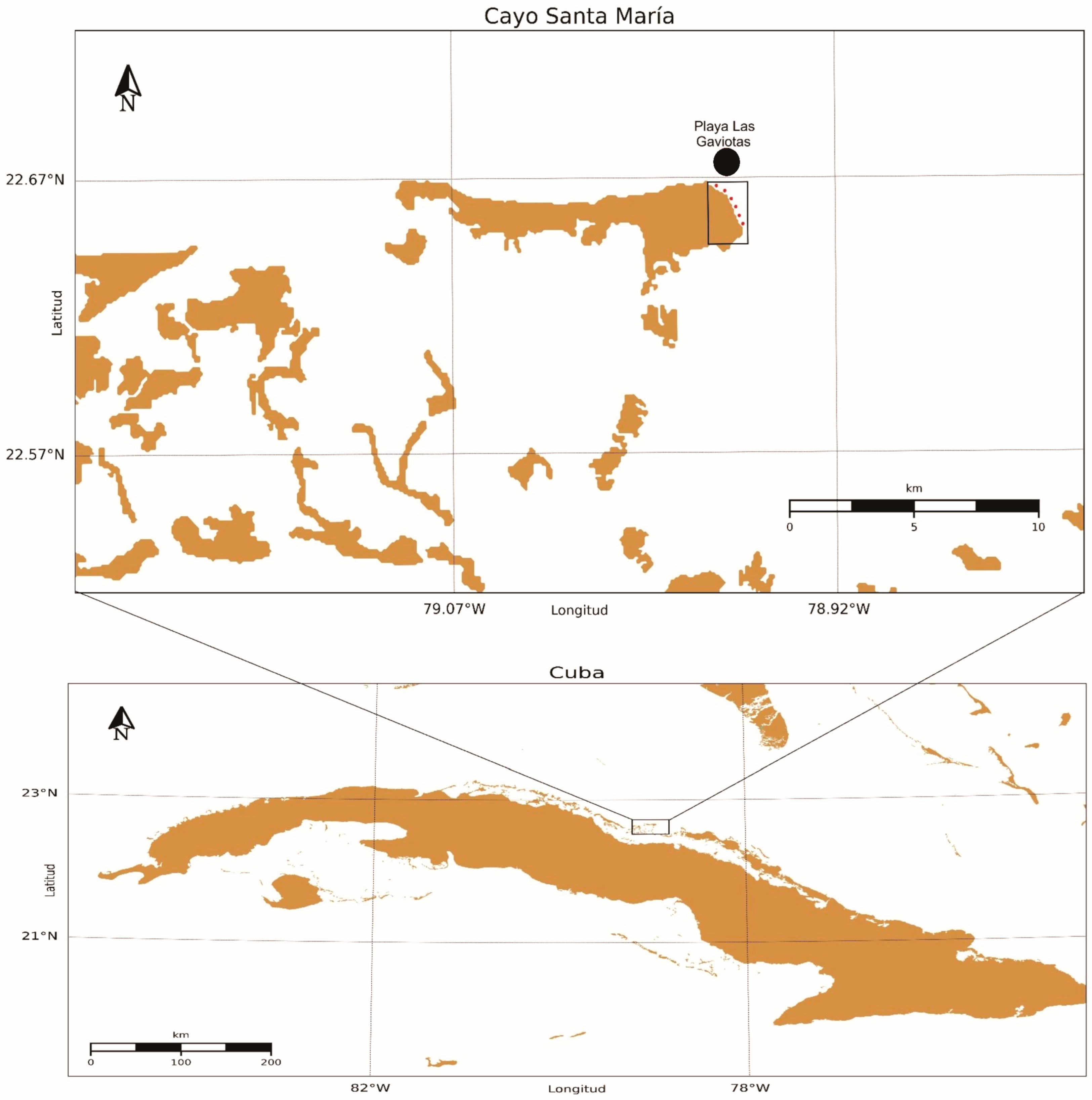
2.1. Study Area
2.2. Sampling and Processing of Samples
2.3. Qualitative and Quantitative Analyses of Diatom Samples
3. Results
3.1. Diatom Flora
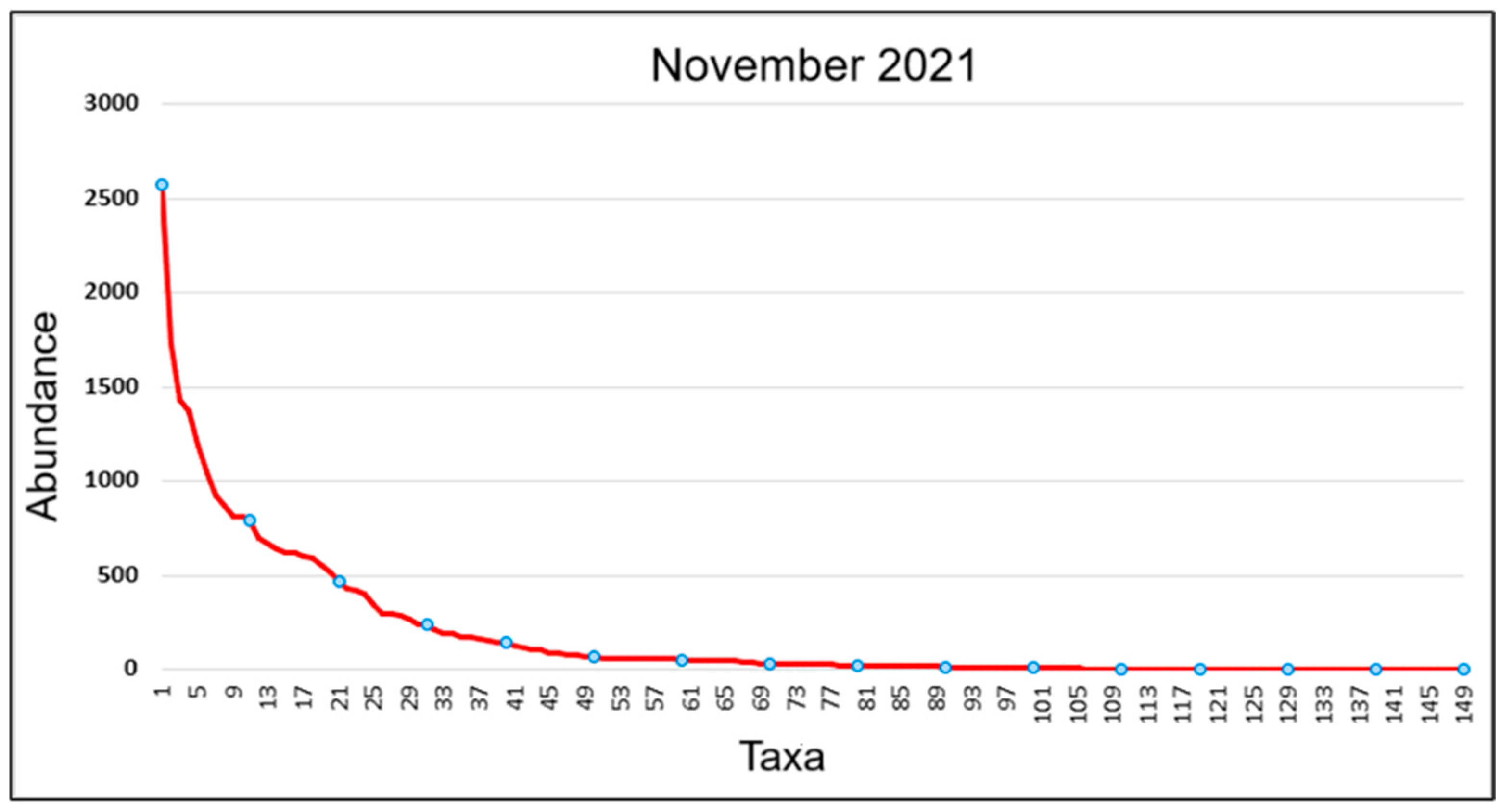
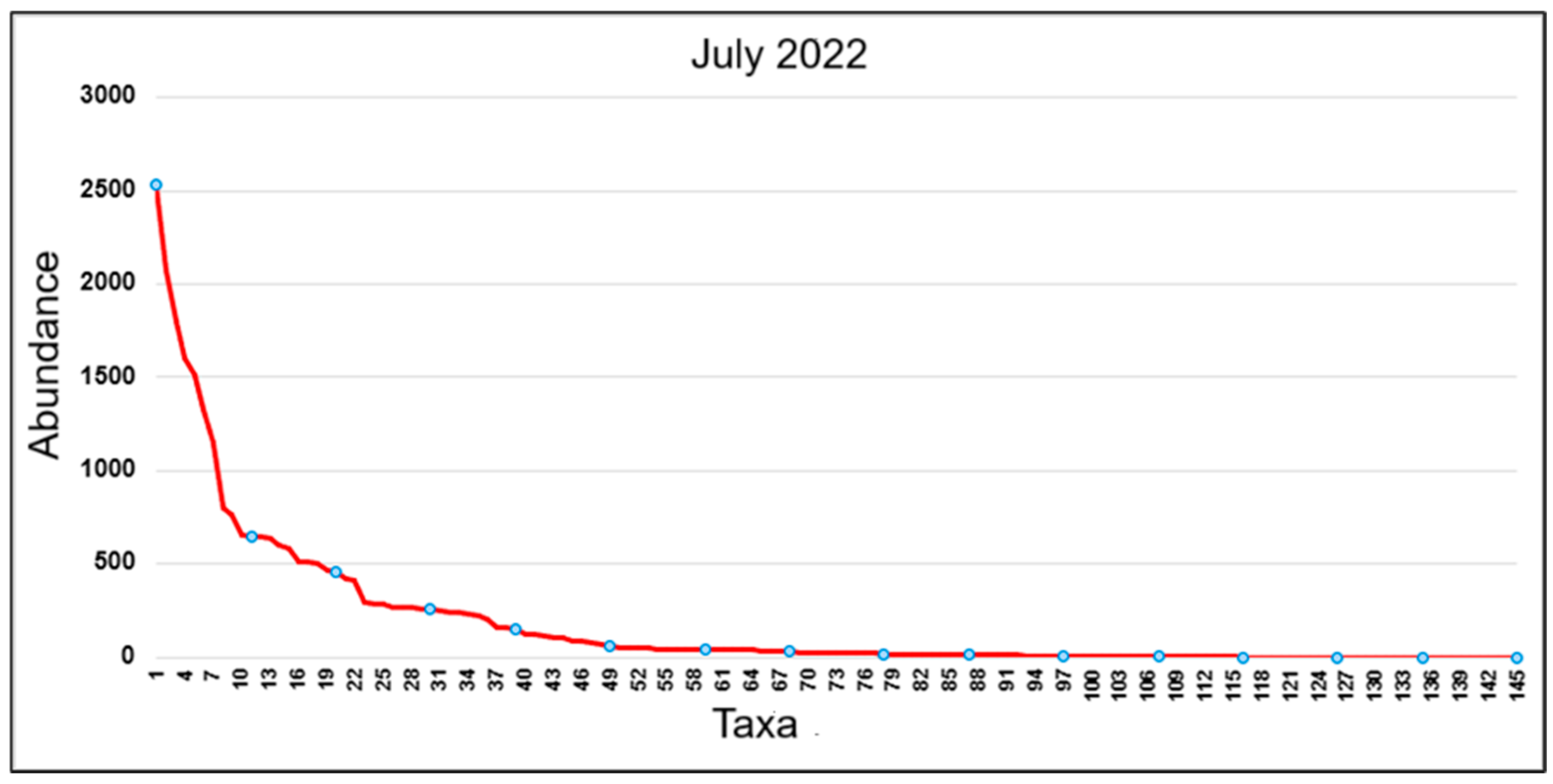
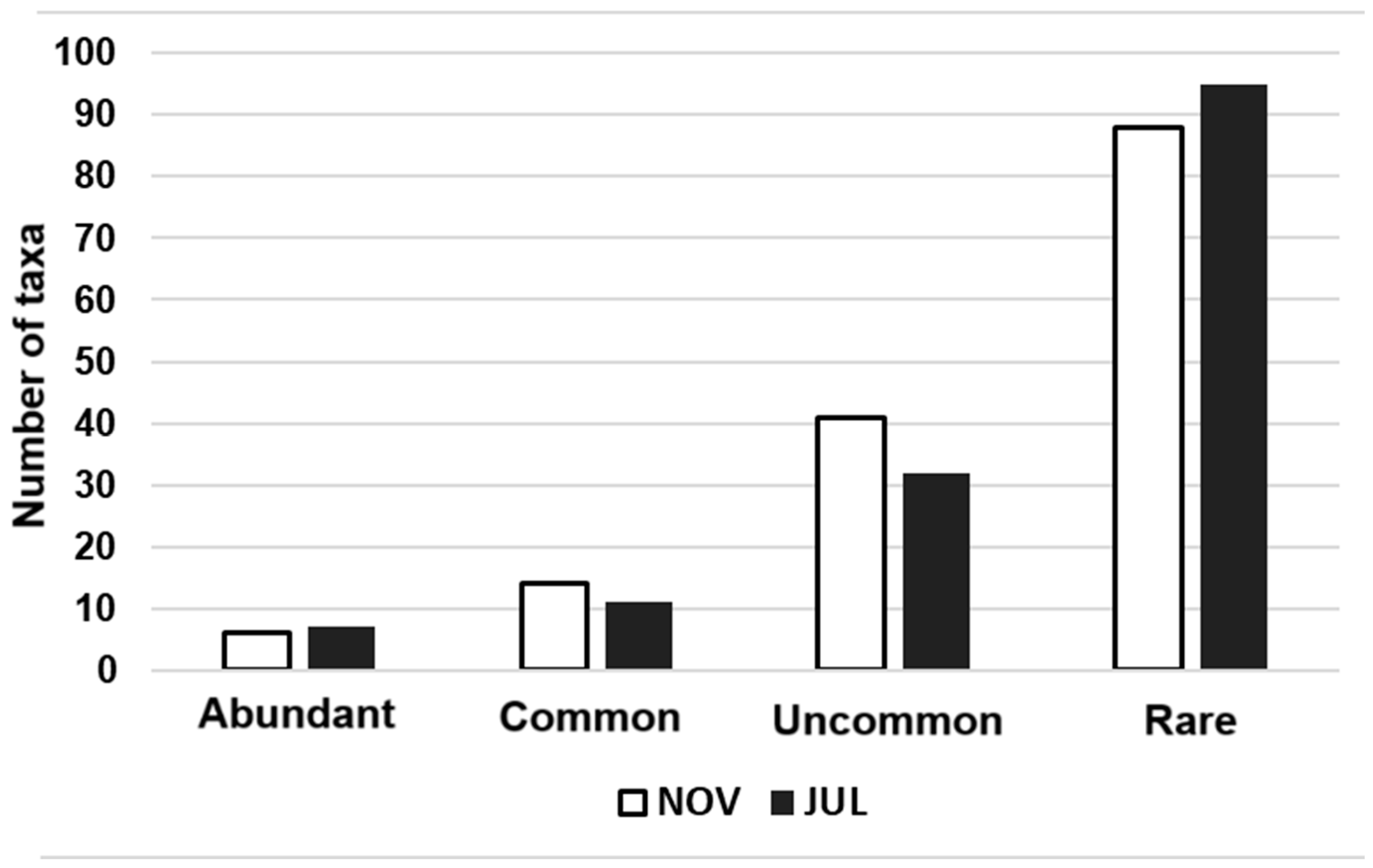
3.1.1. Structure of Epipelic Diatom Taxocenoses in Playa Las Gaviotas, Cuba
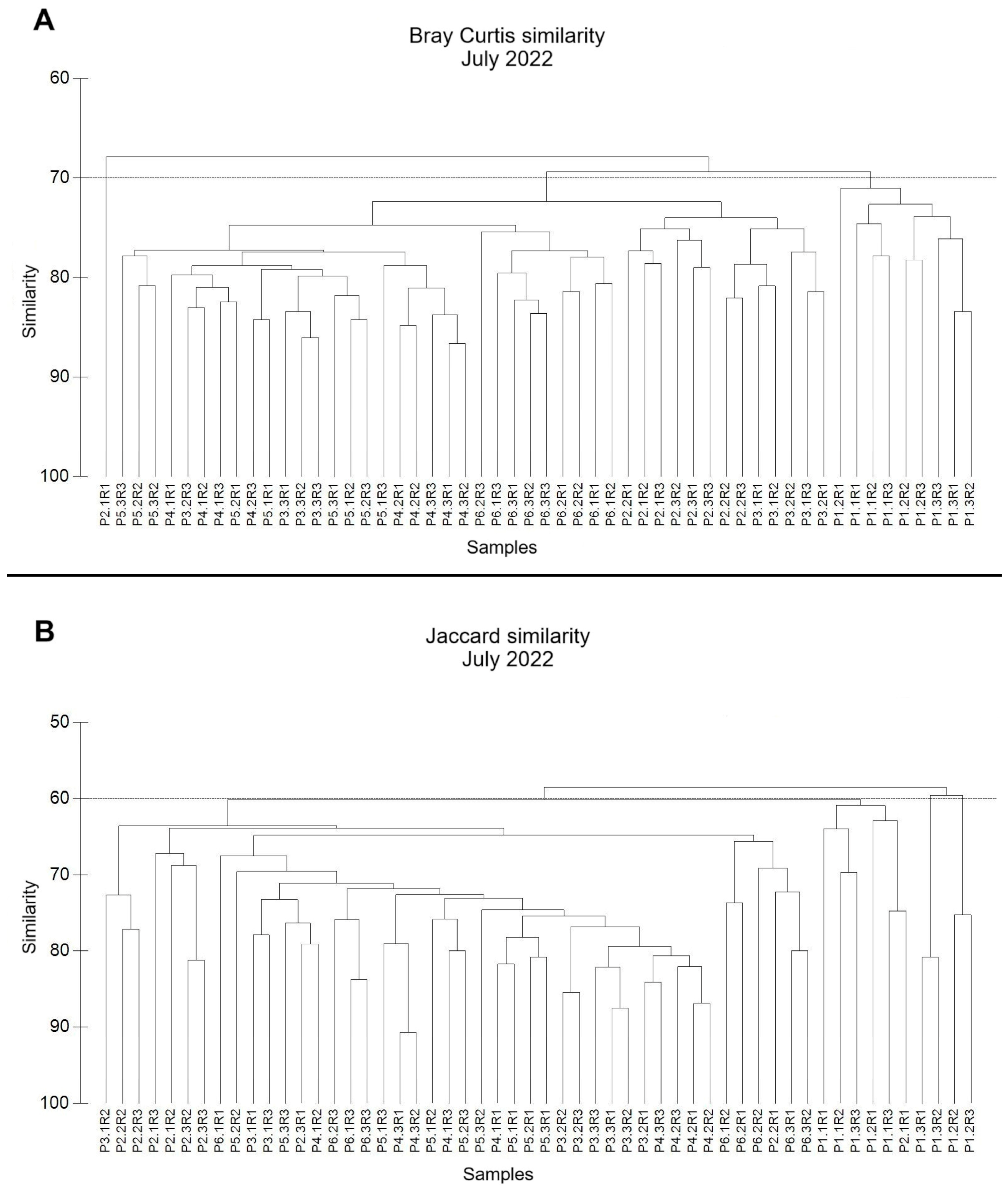
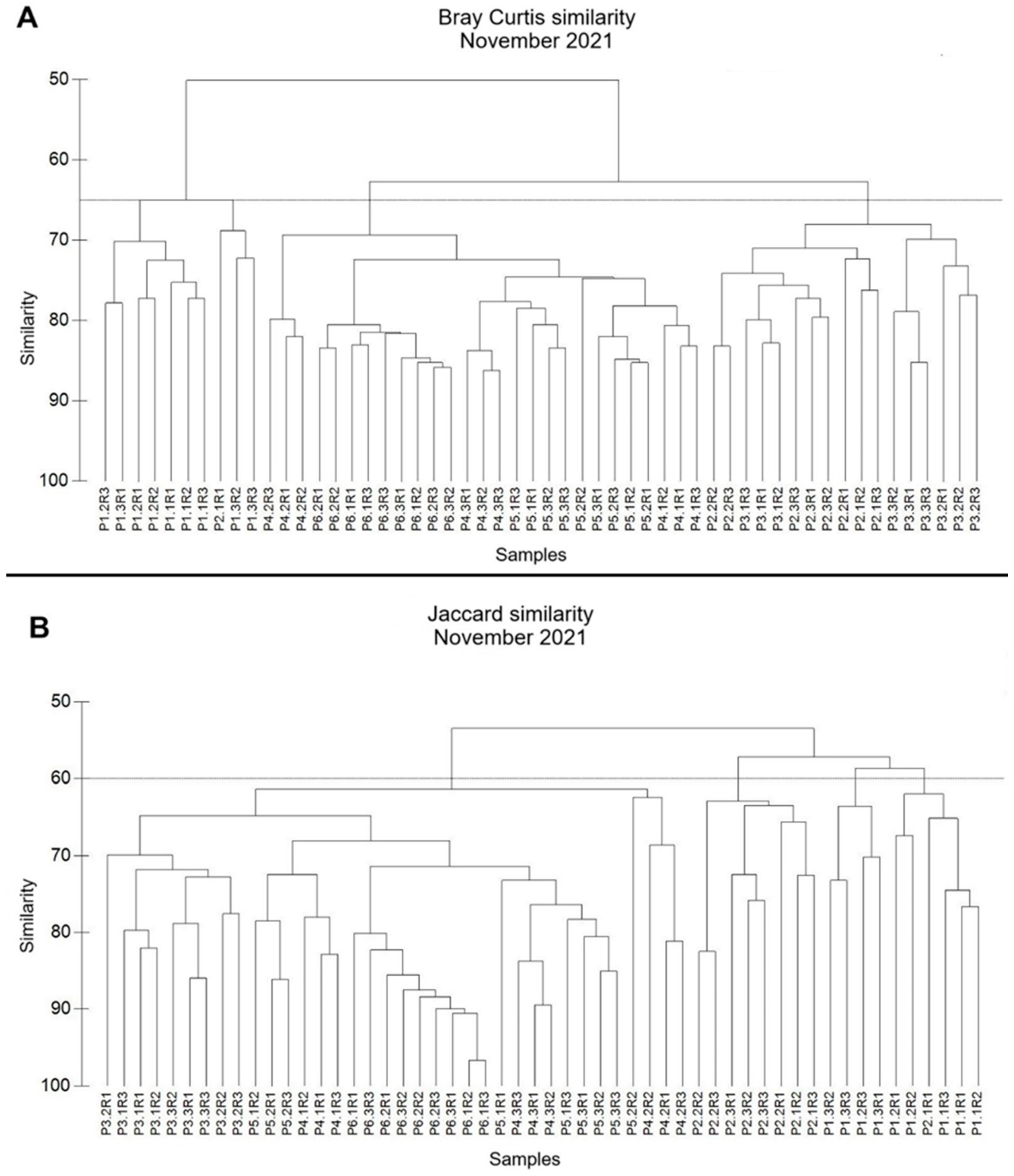
3.1.2. Similarity
4. Discussion
4.1. Species Composition of the BDA in Playa Las Gaviotas, Cuba
4.2. Structure of the BDA in Playa Las Gaviotas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxon | NR | NU |
|---|---|---|
| Achnanthes biasolettiana (Kützing) Grunow (Figure A20y) | x | |
| Achnanthes longipes Agardh | x | |
| Achnanthes javanica f. subconstricta (Meister) Hustedt | ||
| Actinocyclus crassus (W.Smith) Ralfs ex Pritchard | ||
| Actinocyclus octonarius var. sparsus (Gregory) Hustedt (Figure A1a,d) | x | |
| Amphicocconeis discrepans (A.W.F.Schmidt) Riaux-Gobin, Witkowski, Ector & Igersheim (Figure A9n) | x | |
| Amphipentas pentacrinus Ehrenberg (Figure A2e, Figure A3g–i and Figure A4a–d,g–j,m) | x | |
| Amphora alaeziarum Alvarez-Blanco & S. Blanco | x | |
| Amphora arenaria Donkin (Figure A23a–d,g) | x | |
| Amphora bigibba var. interrupta (Grunow) Cleve (Figure A24a–c) | ||
| Amphora biundulata Bérard-Therriault, Cardinal & Poulin (Figure A26k) | x | |
| Amphora capensis A.W.F.Schmidt | x | |
| Amphora clevei Grunow | ||
| Amphora cf. copulata (Kützing) Schoeman & R.E.M.Archibald (Figure A25o–t) | x | |
| Amphora corpulenta Cleve & Grove (Figure A22c) | x | |
| Amphora corpulenta var. capitata Tempère & Peragallo (Figure A22d) | x | |
| Amphora farcimen Grunow (Figure A22h,i) | x | |
| Amphora floridae A.H.Wachnicka & E.E.Gaiser (Figure A23v) | x | |
| Amphora gigantea var. fusca (Schmidt 1875 in Schmidt et al. 1874–1959) Cleve (Figure A25k) | x | |
| Amphora graeffeana Hendey (Figure A25f–h) | ||
| Amphora graffeana Hendey var.? (Figure A24n,o) | x | |
| Amphora graeffei Grunow (Figure A25d,e) | x | |
| Amphora hamata Heiden & Kolbe | x | |
| Amphora immarginata Nagumo (Figure A25a–c) | x | |
| Amphora laevissima W.Gregory (Figure A23e,f) | x | |
| Amphora montgomeryi A.H.Wachnicka & E.E.Gaiser | x | |
| Amphora proteus W.Gregory (Figure A25l–n,u,v) | x | |
| Amphora sp. 1 (Figure A23p–r,m–o,u) | x | |
| Amphora sp. 2 (Figure A24k) | ||
| Amphora spectabilis W.Gregory (Figure A26i) | ||
| Amphora tegetum A.H.Wachnicka & E.E.Gaiser | ||
| Amphora tomiakae Witkowski, Lange-Bertalot & Metzeltin | ||
| Anaulus minutus Grunow (Figure A6g,h) | x | |
| Anorthoneis eurystoma Cleve (Figure A9k-l) | x | |
| Ardissonea formosa (Hantzsch) Grunow (Figure A8a) | x | |
| Asteromphalus robustus Castracane (Figure A1e) | ||
| Auricula intermedia (F.W.Lewis) Cleve (Figure A22a) | x | |
| Azpeitia nodulifer (Schmidt) Fryxell & Sims (Figure A1j,k) | x | |
| Bacillaria socialis (W.Gregory) Ralfs | x | |
| Berkeleya scopulorum (Brébisson ex Kützing) | ||
| Biddulphia biddulphiana (J.E.Smith) Boyer | x | |
| Biddulphia subaequa (Kützing) Ralfs | x | |
| Biddulphiella regina (W.Smith) P.A.Sims & M.P.Ashworth | x | |
| Biremis ambigua (Cleve) D.G.Mann | x | |
| Brassierea vivens M.K.Hein & B.M.Winsborough | x | |
| Caloneis linearis (Cleve) Boyer (Figure A15l) | x | |
| Caloneis bacillum (Grunow) Cleve | x | |
| Caloneis egena (A.W.F.Schmidt) Cleve (Figure A15m) | ||
| Caloneis excentrica (Grunow) Boyer (Figure A15j) | ||
| Caloneis liber (W.Smith) (Figure, 22a–e,h) | ||
| Caloneis sectilis (A.W.F.Schmidt) Cleve (Figure A15i–k) | x | |
| Caloneis sp. 1 | x | |
| Campylodiscus cordatus Hagelstein | ||
| Campylodiscus giffenii Lobban & J.S.Park | ||
| Campylodiscus hodgsonii W.Smith | x | |
| Campylodiscus intermedius Grunow | x | |
| Campylodiscus neofastuosus Ruck & Nakov (Figure A31a,c,e,f) | ||
| Campylodiscus ralfsii W.Smith | x | |
| Campylodiscus sp. 1 (Figure A28f,g) | ||
| Campylodiscus subangularis Cleve & Möller (Figure A28a–c) | x | |
| Catacombas gaillonii (Bory) Williams & Round (Figure A8h–j) | ||
| Climaconeis lorenzii Grunow (Figure A27w) | x | |
| Climacosphenia cf. moniligera Ehrenberg | x | |
| Cocconeiopsis fraudulenta (A.W.F.Schmidt) Witkowski, Lange-Bertalot & Metzeltin (Figure A27r,v) | x | |
| Cocconeiopsis orthoneoides (Hustedt) Witkowski, Lange-Bertalot & Metzeltin (Figure A9f)) | x | |
| Cocconeiopsis patrickiae (Hustedt) A.Witkowski, Lange-Bertalot & Metzeltin (Figure A20w,x) | ||
| Cocconeiopsis pullus (Hustedt) Witkowski, Lange-Bertalot, Metzeltin (Figure A20u) | x | |
| Cocconeiopsis regularis (Hustedt) Witkowski, Lange-Bertalot, Metzeltin | x | |
| Cocconeiopsis sp. 1 (Figure A9m) | x | |
| Cocconeiopsis sp. 2 (Figure A20s) | x | |
| Cocconeis guttata Hustedt & Aleem | x | |
| Cocconeis pseudodiruptoides Foged | x | |
| Cocconeis sp. 1 (Figure A9i,j) | x | |
| Cocconeis britannica Naegeli ex Kützing (Figure A9a–c) | x | |
| Cocconeis caribensis O.E.Romero & J.N.Navarro (Figure A9h) | x | |
| Cocconeis clandestina A. Schmidt (Figure A6a) | x | |
| Cocconeis comis A.W.F.Schmidt | x | |
| Cocconeis dirupta var. flexella (Janisch & Rabenhorst) Grunow | x | |
| Cocconeis heteroidea Hantzsch | ||
| Cocconeis singularis R.Hagelstein (Figure A9d,e) | x | |
| Coscinodiscus asteromphalus Ehrenberg | x | |
| Cymatosira lorenziana Grunow (Figure A6i) | x | |
| Cymbella sp. 1 | x | |
| Dictyoneis marginata (F.W.Lewis) Cleve (Figure A10a–d) | ||
| Dictyoneis marginata var. elongata (Cleve) Fricke (Figure A10e) | ||
| Dictyoneis marginata var. spectatissima (Greville) Cleve (Figure A10f–i) | x | |
| Dimeregramma sp. 1 | x | |
| Dimeregramma fulvum (W.Gregory) Ralfs | x | |
| Dimeregramma marinum (W.Gregory) Ralfs | ||
| Diploneis advena (A. Schmidt) Cleve | x | |
| Diploneis bomboides var. media (Grunow) Hustedt | ||
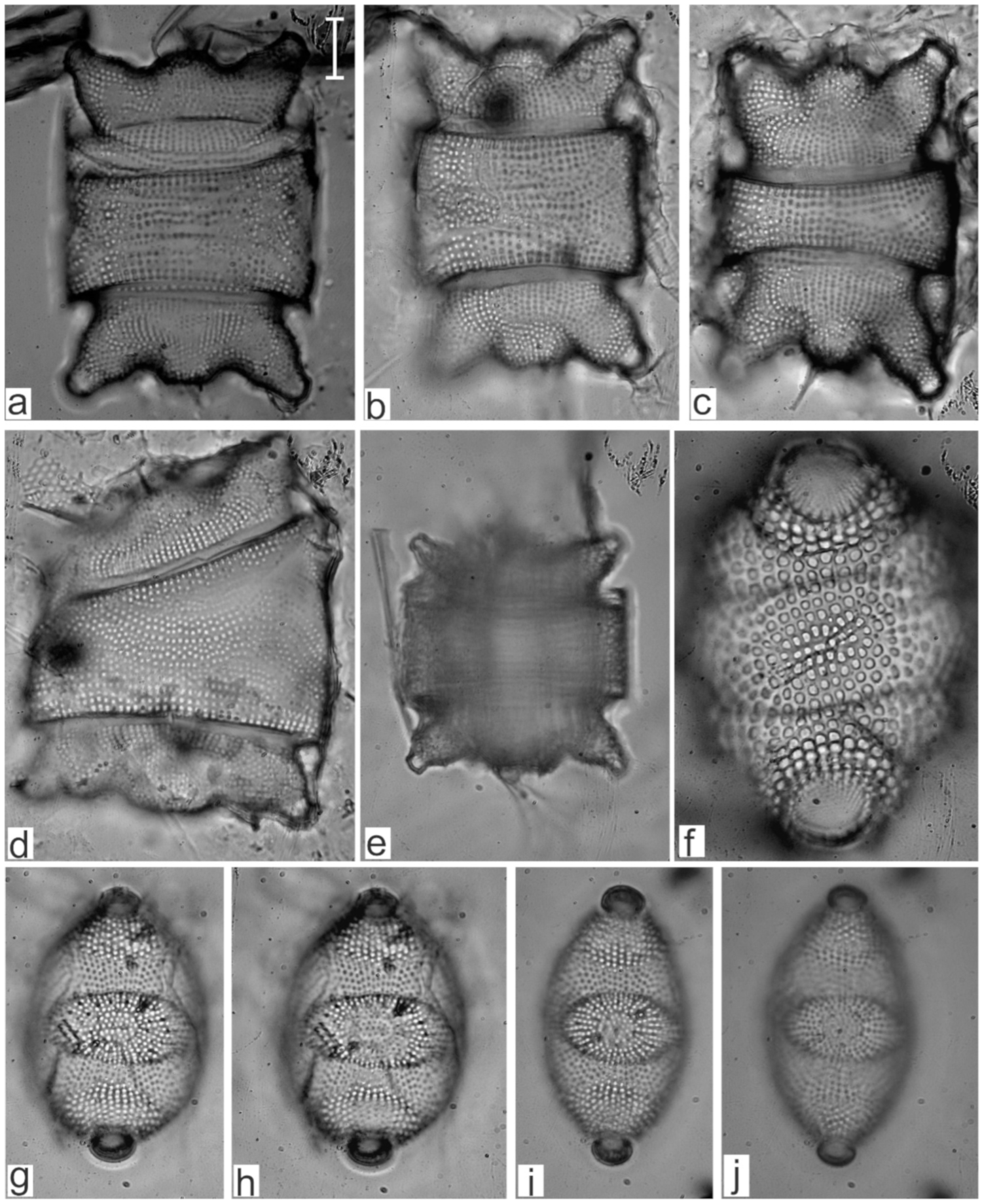
| Diploneis coffeiformis (A.W.F.Schmidt) Cleve (Figure A14d–g) | x | |
| Diploneis chersonensis (Grunow) Cleve (Figure A12f–h and Figure A13b,d,e,h,i) | x | |
| Diploneis crabro (Ehrenberg) Ehrenberg (Figure A12a–e) | x | |
| Diploneis fusca (Greg.) Cleve (Figure A14a–c) | x | |
| Diploneis fusca var. amphigomphus Hustedt | x | |
| Diploneis gemmatula (Grunow) Cleve (Figure A13a) | x | |
| Diploneis littoralis var. littoralis (Donkin) Cleve | ||
| Diploneis littoralis var. clathrata (Østrup) Cleve (Figure A11a,b,j) | ||
| Diploneis nitescens (Gregory) Cleve (Figure A11s,t) | x | |
| Diploneis obliqua (Brun) Hustedt (Figure A14h,i) | x | |
| Diploneis oblongella var. gibbosa McCall | x | |
| Diploneis papula (A.W.F.Schmidt) Cleve (Figure A11o–q) | ||
| Diploneis papula var. constricta Hustedt (Figure A13f,g,l) | ||
| Diploneis parca (A.W.F. Schmidt) Boyer | x | |
| Diploneis smithi var. pumila (Grun.) Hustedt (Figure A11m,n,u) | ||
| Diploneis smithii var. smithii (Brébisson in Wm. Smith) Cleve (Figure A11r) | ||
| Diploneis suborbicularis (Gregory) Cleve (Figure A14e,f) | ||
| Diploneis vacillans var. renitens (A.W.F.Schmidt) Cleve | ||
| Diploneis weissflogii (A.W.F.Schmidt) Cleve (Figure A13h–k) | x | |
| Epithemia guettingeri (Krammer) Lobban & J.S.Park (Figure A28l) | ||
| Fallacia floriniae (M.Møller) Witkowski (Figure A11l) | ||
| Fallacia forcipata (Greville) Stickle & D.G.Mann | ||
| Fallacia litoricola (Hustedt) D.G.Mann | x | |
| Fallacia ny (Cleve) D.G.Mann | ||
| Fallacia nyella (Hustedt) D.G.Mann in Round, R.M.Crawford & D.G.Mann | x | |
| Fallacia oculiformis Hustedt D.G.Mann (Figure A11f,g,k) | x | |
| Frustulia sp. 1 | x | |
| Glyphodesmis eximia Greville (Figure A6e,f) | x | |
| Gomphonemopsis lindae Witkowski, Metzeltin & Lange-Bertalot | x | |
| Grammatophora macilenta W.Smith | x | |
| Grammatophora oceanica Ehrenberg | x | |
| Grammatophora serpentina Ehrenberg (Figure A6j) | x | |
| Grammatophora undulata Ehrenberg | x | |
| Grunowago bacillaris (Grunow) Lobban & Ashworth | x | |
| Gyrosigma cf. balticum (Ehrenberg) Rabenhorst | x | |
| Gyrosigma beaufortianum Hustedt (Figure A27g) | ||
| Gyrosigma hummii Hustedt (Figure A27f) | x | |
| Gyrosigma simile (Grunow) Boyer (Figure A27e) | ||
| Gyrosigma tenuissimum (W.Smith) J.W.Griffith & Henfrey | ||
| Halamphora turgida (W.Gregory) Levkov (Figure A24d–j) | ||
| Halamphora capitata (R.Hagelstein) Álvarez-Blanco & S.Blanco | x | |
| Halamphora coffeiformis (C.Agardh) Mereschkowsky | ||
| Halamphora cymbifera (W.Gregory) Levkov | x | |
| Halamphora eunotia (Cleve) Levkov | x | |
| Halamphora wisei (M.M.Salah) I.Álvarez-Blanco & S.Blanco (Figure A24l,m) | ||
| Hantzschia pseudomarina Hustedt (Figure A28n) | x | |
| Hendeyella dubia (Grunow) Li, Witkowski & Ashworth, nom. inval. | x | |
| Homoeocladia angularis (W.Smith) Kuntze | x | |
| Homoeocladia distans (W.Gregory) Kuntze (Figure A29g,h) | x | |
| Hyalosynedra laevigata (Grunow) D.M.Williams & Round (Figure A8g) | x | |
| Navicula cf. sovereignae Hustedt (Figure A20l–p) | x | |
| Karayevia cf. carissima (Lange-Bertalot) Bukhtiyarova (Figure A27m) | x | |
| Karayevia sp. 1 (Figure A27n) | x | |
| Licmophora gracilis (Ehrenberg) Grunow | x | |
| Licmophora remulus (Grunow) Grunow (Figure A8k) | x | |
| Lyrella approximata (Greville) D.G.Mann | x | |
| Lyrella clavata var. indica J.L.Moreno (Figure A19a) | ||
| Lyrella diffluens (A.W.F.Schmidt) D.G.Mann (Figure A18e,f) | ||
| Lyrella lyra var. lyra (Ehrenberg) Karayeva (Figure A16a,c and Figure A17a,d–f) | x | |
| Lyrella lyroides Hendey | x | |
| Lyrella praetexta (Ehrenberg) D.G.Mann (Figure A19b) | x | |
| Lyrella subforcipata (Hustedt) Gusliakov & Karayeva | x | |
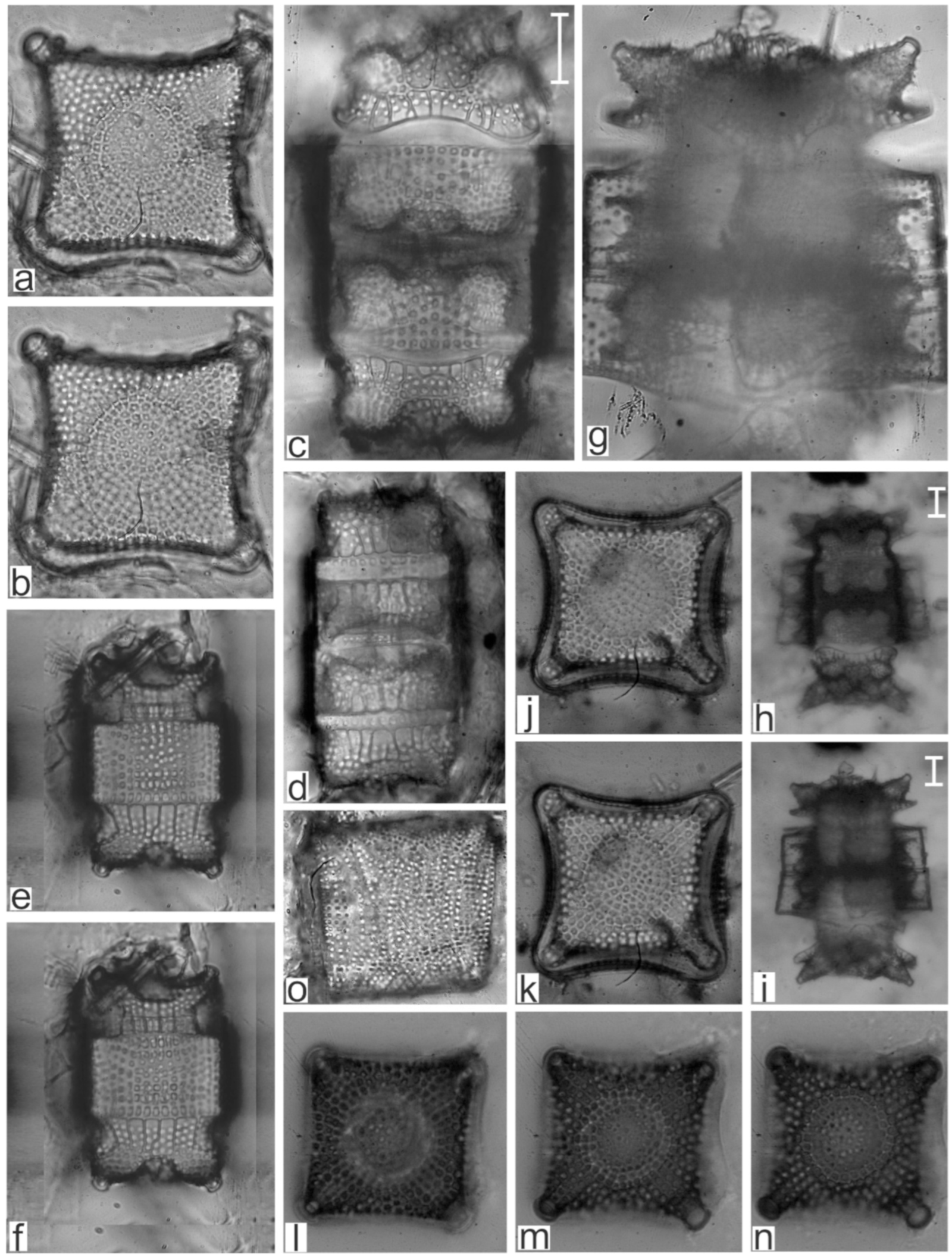
| Mastogloia achnanthioides var. elliptica Hustedt | x | |
| Mastogloia adriatica Voight | x | x |
| Mastogloia affinis Cleve | x | |
| Mastogloia angulata F.W.Lewis | x | |
| Mastogloia apiculata W.Smith | x | |
| Mastogloia (Orthoneis) aspera H. Peragallo & M. Peragallo | x | |
| Mastogloia asperula Grunow | x | |
| Mastogloia asperuloides Hustedt | x | |
| Mastogloia bahamensis Cleve | x | |
| Mastogloia beaufortiana Hustedt | ||
| Mastogloia biapiculata Hustedt | ||
| Mastogloia binotata (Grunow) Cleve | ||
| Mastogloia biocellata (Grunow) G.Novarino & A.R.Muftah | x | |
| Mastogloia bisulcata Grunow | x | |
| Mastogloia (Orthoneis) clevei Grunow | x | |
| Mastogloia cocconeiformis Grunow | ||
| Mastogloia corsicana (Grunow) H.Peragallo & M.Peragallo | x | |
| Mastogloia corsicana var. capitata Meister | x | |
| Mastogloia cribrosa Grunow | ||
| Mastogloia crucicula (Grunow) Cleve | x | |
| Mastogloia crucicula var. alternans Zanon | x | |
| Mastogloia cuneata (Meister) Simonsen | x | |
| Mastogloia decipiens Hustedt | ||
| Mastogloia delicatissima Hustedt | x | |
| Mastogloia elegans Lewis | x | |
| Mastogloia emarginata Hustedt | ||
| Mastogloia erythraea Grunow | ||
| Mastogloia fallax f. biloculis Foged | ||
| Mastogloia fimbriata (T.Brightwell) Grunow | x | |
| Mastogloia floridensis Lobban & Franckovich | ||
| Mastogloia funafutensis A.W.F.Schmidt | x | |
| Mastogloia gilberti A. Schmidt | ||
| Mastogloia gracillima Hustedt | ||
| Mastogloia graciloides Hustedt | ||
| Mastogloia cf. horvathiana Grunow | ||
| Mastogloia jelineckii var. jelineckii Grunow (Figure A19e) | ||
| Mastogloia jelineckii var. extensa (Grunow) Voigt | x | |
| Mastogloia lacrimata Voigt | x | |
| Mastogloia lancetula Cleve (Figure A21p) | x | |
| Mastogloia latecostata Hustedt | ||
| Mastogloia laterorostrata Hustedt | x | |
| Mastogloia lineata var. albifrons (Brun) Hustedt | x | |
| Mastogloia lucayensis Lobban & Franckovich | x | |
| Mastogloia manokwariensis Cholnoki | ||
| Mastogloia matthaei Pennesi & Poulin | x | |
| Mastogloia mauritiana Brun | x | |
| Mastogloia mauritiana var. capitata Voigt | x | |
| Mastogloia minuta Grevillei | x | |
| Mastogloia ovata Grunow | ||
| Mastogloia parva Hustedt | x | |
| Mastogloia peracuta Janisch | x | |
| Mastogloia pisciculus Cleve | x | |
| Mastogloia pseudoelegans Hustedt | x | |
| Mastogloia pseudolacrimata T.A.Yohn & R.A.Gibson | x | |
| Mastogloia pseudolatecostata Yohn & R.A.Gibson | x | |
| Mastogloia punctatissima (Greville) Ricard | ||
| Mastogloia punctifera Brun | x | |
| Mastogloia rigida Hustedt | ||
| Mastogloia rimosa Cleve | x | |
| Mastogloia rostellata Grun | ||
| Mastogloia sigillata Voigt | x | |
| Mastogloia similis Hustedt | x | |
| Mastogloia sp. 1 | x | |
| Mastogloia staurophora Hustedt (Figure A27l) | ||
| Mastogloia stephensiana Yohn & R.A.Gibson | x | |
| Mastogloia subaffirmata Hustedt | x | |
| Mastogloia sublatericia Hustedt | ||
| Mastogloia tenera Hustedt | ||
| Mastogloia tenuis Hustedt | x | |
| Mastogloia umbra Paddock & Kemp | x | |
| Mastogloia varians Hustedt | x | |
| Navicula abunda Hustedt (Figure A21k) | ||
| Navicula agnita Hustedt (Figure A21h,m,n) | ||
| Navicula apta Hustedt (Figure A21l) | x | |
| Navicula cancellata Donkin | ||
| Navicula cf. bipustulata A.Mann | ||
| Navicula cf. sovereignae Hustedt | x | |
| Navicula digitoradiata (Gregory) Ralfs in Pritchard | x | |
| Navicula directa (W.Smith) Brébisson | x | |
| Navicula (Lyrella) irrorata f. mexicana Cleve (Figure A16b,d and Figure A17b,c) | x | |
| Navicula irroratoides f. elliptica Hustedt | x | |
| Navicula johanrossii Giffen (Figure A21e) | x | |
| Navicula longa var. longa (W.Gregory) Ralfs (Figure A21a) | x | |
| Navicula longa var. irregularis Hustedt (Figure A21c,d) | ||
| Navicula lusoria M.F.Giffen | ||
| Navicula lyra (Lyrella) var. elliptica A.W.F.Schmidt (Figure A16e–h) | x | |
| Navicula microdigitoradiata Lange-Bertalot (Figure A21j) | x | |
| Navicula palpebralis Brébisson ex W.Smith (Figure A20d and Figure A21r) | ||
| Navicula pennata A. Schmidt | ||
| Navicula ponticula Giffen (Figure A20t) | x | |
| Navicula sp. 1 (Figure A27x,y) | x | |
| Navicula sp. 2 (Figure A21k) | x | |
| Navicula sp. 3 (Figure A27s–u) | x | |
| Navicula subrostellata Hustedt | ||
| Navicula torifera Hustedt | x | |
| Navicula viminea Hustedt (Figure A20f–h) | ||
| Navicula zostereti Grunow (Figure A21b) | ||
| Neodelphineis silenda (M.H. Hohn & J. Hellerman) N. Desianti & M. Potapova | x | |
| Neohuttonia reichardtii (Grunow) Kuntze | x | |
| Nitzschia cf. hummi Hustedt | x | |
| Nitzschia cf. pseudofonticola Hustedt | ||
| Nitzschia fusiformis Grunow in Cleve & Grunow | x | |
| Nitzschia lorenziana Grunow | ||
| Nitzschia pellucida Grunow | x | |
| Nitzschia sp. 1 | ||
| Nitzschia amphibia Grunow | ||
| Nitzschia fluminensis Grunow (Figure A29e) | x | |
| Nitzschia fusoides Ehrlich (Figure A28m) | ||
| Nitzschia grossestriata Hustedt | x | |
| Nitzschia longissima (Brébisson) Ralfs | ||
| Nitzschia majuscula Grunow | x | |
| Nitzschia marginulata var. didyma Grunow (Figure A30j–m) | x | |
| Nitzschia miserabilis Cholnoky | ||
| Nitzschia parvula W.Smith | x | |
| Nitzschia scabra Cleve (Figure A29c,d) | ||
| Nitzschia scalpelliformis Grunow | x | |
| Nitzschia sigma (Kützing) W.Smith (Figure A29a,b,f) | ||
| Odontella aurita (Lyngbye) C.Agardh (Figure A2a–d,g–j) | x | |
| Oestrupia ergadensis (W.Gregory) Witkowski, Lange-Bertalot & Metzeltin (Figure A21g,s–u) | x | |
| Oestrupia powelii (F.W.Lewis) Heiden | x | |
| Opephora marina (W.Gregory) Petit (Figure A6k–m) | x | |
| Opephora mutabilis Sabbe & Wyverman, nom. inval. | x | |
| Opephora pacifica (Grunow) Petit (Figure A6o) | ||
| Paralia sulcata (Ehrenberg) Cleve | ||
| Parlibellus bennikei Witkowski, Metzeltin & Lange-Bertalot | ||
| Parlibellus delognei (Van Heurck) E.J.Cox | ||
| Parlibellus grevilleoides (Hustedt) E.J.Cox | ||
| Parlibellus phoebeae Witkowski, Metzeltin & Lange-Bertalot (Figure A20e) | ||
| Perissonoë crucifera (Kitton) Desikachary & al. (Figure A7g–j) | ||
| Petroneis granulata D.G.Mann (Figure A18b,c) | x | |
| Petroneis plagiostoma (Grunow) D.G.Mann (Figure A18a–d) | x | |
| Plagiogramma constrictum Greville | x | |
| Plagiogramma minus var. minus (Gregory) Chunlian Li, Ashworth & Witkowski (Figure A6c,d) | x | |
| Plagiogramma minus var. nanum (Gregory) Chunlian Li, Ashworth & Witkowski | ||
| Plagiogramma pulchellum var. pygmaeum (Greville) H.Peragallo & M.Peragallo | x | |
| Plagiogramma wallichianum Greville | x | |
| Plagiotropis lepidoptera (W.Gregory) Kuntze (Figure A31g,h) | x | |
| Plagiotropis pusilla (W.Gregory) Kuntze (Figure A28p) | ||
| Plagiotropis vitrea (W.Smith) Grunow (Figure A28o,q) | x | |
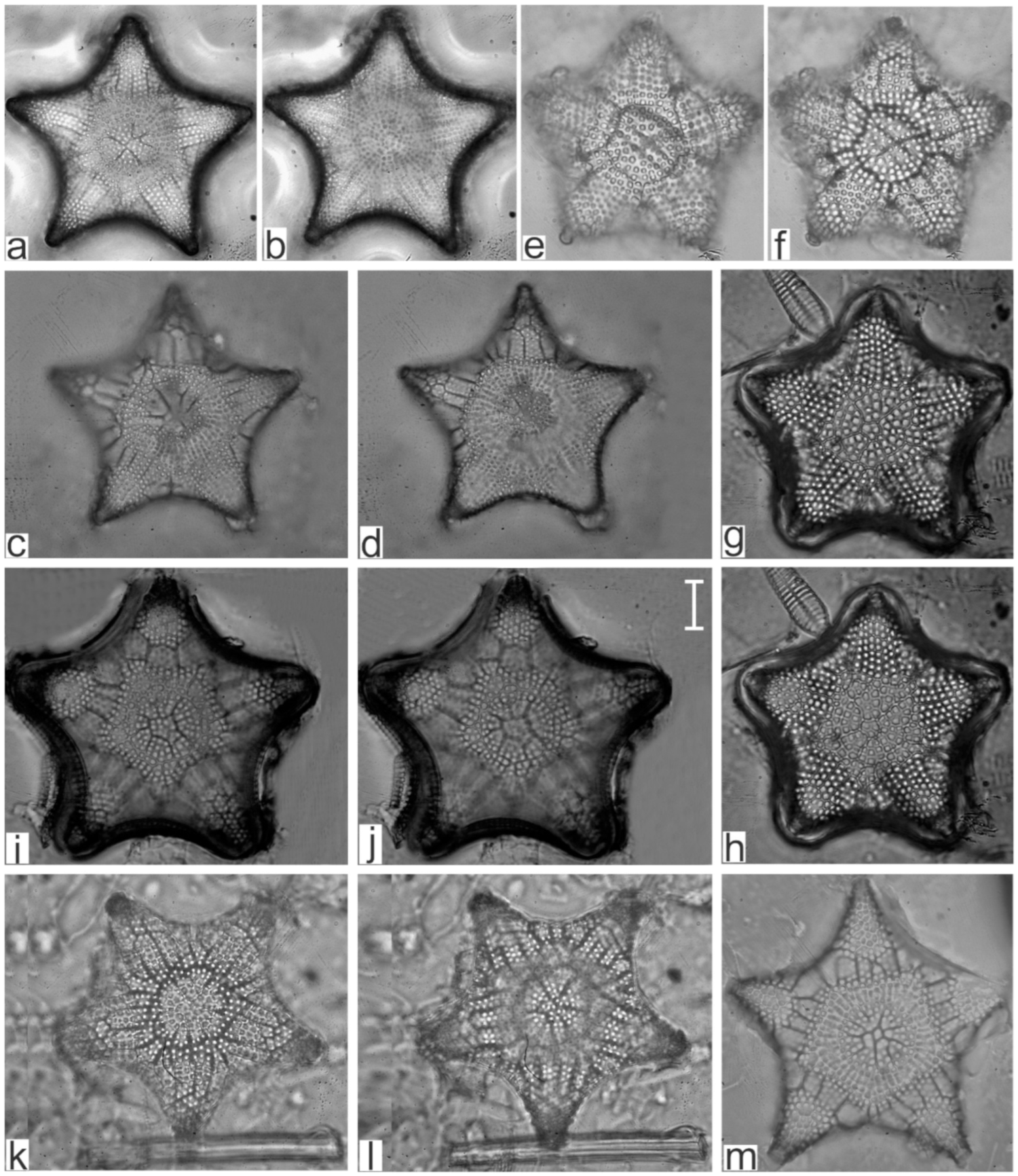
| Pleurosigma affine Grunow (Figure A19f,g) | x | |
| Pleurosigma latiusculum H.Peragallo | x | |
| Pleurosigma salinarum Grunow (Figure A27a–d) | x | |
| Podocystis adriatica (Kützing) Ralfs (Figure A7a,d) | x | |
| Podocystis spathulata (Shadbolt) Van Heurck (Figure A7e,f) | x | |
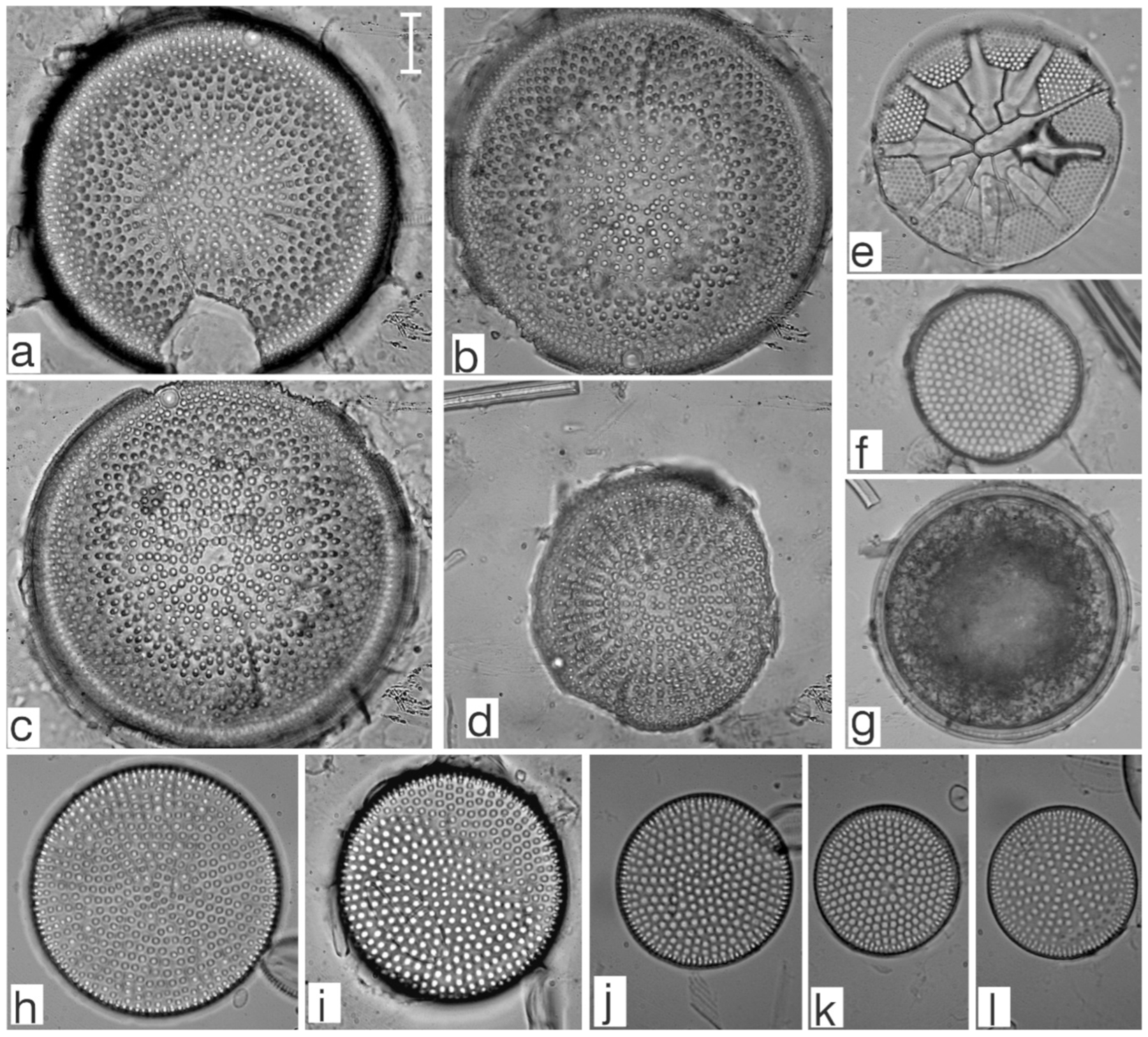
| Podosira hormoides var. adriatica (Kützing) Grunow (Figure A1g) | x | |
| Prestauroneis cf. crucicula (W.Smith) Genkal & Yarushina | ||
| Protoraphis hustedtiana Simonsen | x | |
| Psammodictyon panduriforme (W.Gregory) D.G.Mann | x | |
| Psammodictyon rudum (Cholnoky) D.G.Mann | x | |
| Psammodiscus calceatus T. Watanabe, T. Nagumo & J.Tanaka (Figure A1l) | x | |
| Psammodiscus nitidus (W.Gregory) Round & D.G.Mann (Figure A1h,i) | ||
| Rhaphoneis amphiceros (Ehrenberg) Ehrenberg | x | |
| Rhoikoneis sp. (Figure A20k) | x | |
| Rhopalodia gibberula (Ehrenberg) O.Müller (Figure A28h,i) | x | |
| Rhopalodia musculus var. musculus (Kützing) O.Müller | x | |
| Rhopalodia musculus var. producta (Grunow) Peragallo & Peragallo (Figure A28j,k) | ||
| Rossia linearis Voigt | ||
| Rutilaria cf. obesa (unidentified) Greville ex Cleve (Figure A8l,m) | x | |
| Schizostauron citronella (A.Mann) Górecka, Riaux-Gobin & Witkowski | x | |
| Seminavis barbara Witkowski, Lange-Bertalot & Metzeltin | x | |
| Seminavis eulensteinii (Grunow) Danielidis, Ford & Kennett | ||
| Seminavis delicatula Wachnicka & Gaiser (Figure A23s,t) | x | |
| Seminavis lunulata (A.H.Wachnicka & E.E.Gaiser) C.H.Li, Y.H,Gao & C.P.Chen (Figure A23h–l) | x | |
| Seminavis latior (Schmidt) Danielidis & Mann (Figure A22f) | x | |
| Seminavis robusta Danielidis & Mann (Figure A22g) | x | |
| Shionodiscus oestrupii (Ostenfeld) A.J.Alverson, S.-H.Kang & E.C.Theriot | x | |
| Staurophora salina (W.Smith) Mereschkowsky (Figure A19c,d) | x | |
| Staurophora sp. 1 (Figure A27o) | x | |
| Surirella platyloba F.Meister (Figure A31b–d) | x | |
| Synedra sp. 2 | x | |
| Synedra sp. 3 | x | |
| Synedra pulcherrima Hantzsch | x | x |
| Synedrosphenia cf. crystallina (C.Agardh) Lobban & Ashworth | x | x |
| Synedrosphenia fulgens (Greville) Lobban & Ashworth | x | |
| Synedrosphenia gomphonema (Janisch & Rabenhorst) Hustedt (Figure A8b,c) | ||
| Tabularia affinis var. acuminata (Grunow) Aboal | ||
| Talaroneis furcigera (Grunow) Sterrenburg | ||
| Tephanocyclus meneghinianus (Kützing) Kulikovskiy, Genkal & Kociolek | x | |
| Terpsinoë musica Ehrenberg | x | |
| Tetramphora sulcata (Brébisson) Stepanek & Kociolek | ||
| Tetramphora intermedia (Cleve) Stepanek & Kociolek (Figure A22e) | x | |
| Tetramphora ostrearia (Brébisson) Mereschkowsky (Figure A22b) | x | |
| Tetramphora rhombica (Kitton) Stepanek & Kociolek | x | |
| Thalassiosira decipiens (Grunow ex Van Heurck) Jørgensen (Figure A1f) | x | |
| Toxarium hennedyanum (W.Gregory) Pelletan | x | |
| Toxarium undulatum Bailey | ||
| Trachyneis aspera var. aspera (Ehrenberg) Cleve (Figure A27j) | ||
| Trachyneis aspera var. elliptica Hendey | ||
| Trachyneis aspera var. oblonga (Bailey) Cleve (Figure A27h) | x | |
| Trachyneis velata (A.W.F.Schmidt) Cleve (Figure A27k) | ||
| Triceratium balearicum f. quadratum Hustedt (Figure A3a,b,j–n) | ||
| Triceratium balearicum Cleve & Grunow (Figure A3c–f and Figure A4e,f,k,l) | ||
| Triceratium reticulum Ehrenberg (Figure A3o) | x | |
| Trigonium formosum var. pentagonale (A.W.F.Schmidt) Desikachary & Prema | x | |
| Tryblionella cf. graeffei (Grunow ex Cleve) D.G.Mann (Figure A30i) | x | |
| Tryblionella coarctata (Grunow) D.G.Mann (Figure A30f,g) | x | |
| Tryblionella jelineckii (Grunow) D.G.Mann | x | |
| Tryblionella levidensis W.Smith | x | |
| Tryblionella parvula (W.Smith) T.Ohtsuka & Y.Fujita (Figure A30o,p) | ||
| Tryblionella persuadens (Cholnoki) K.P.Cavalcante, P.I.Tremarin & T.A.V.Ludwig | ||
| Tryblionella punctata W.Smith | x | |
| Ulnaria ulna (Nitzsch) Compère | x | |
| Diatom sp. (Figure A5a,f) | x |
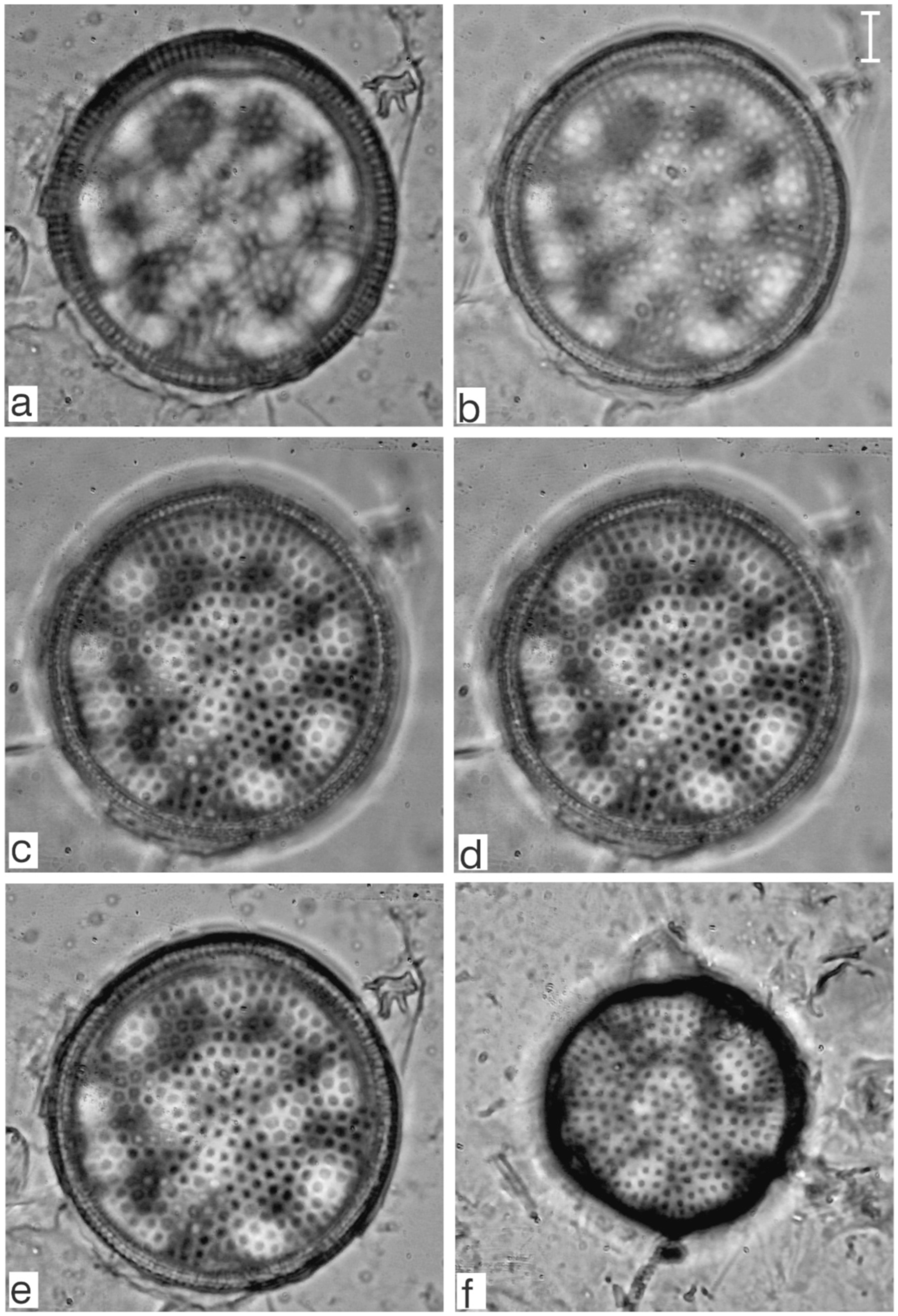
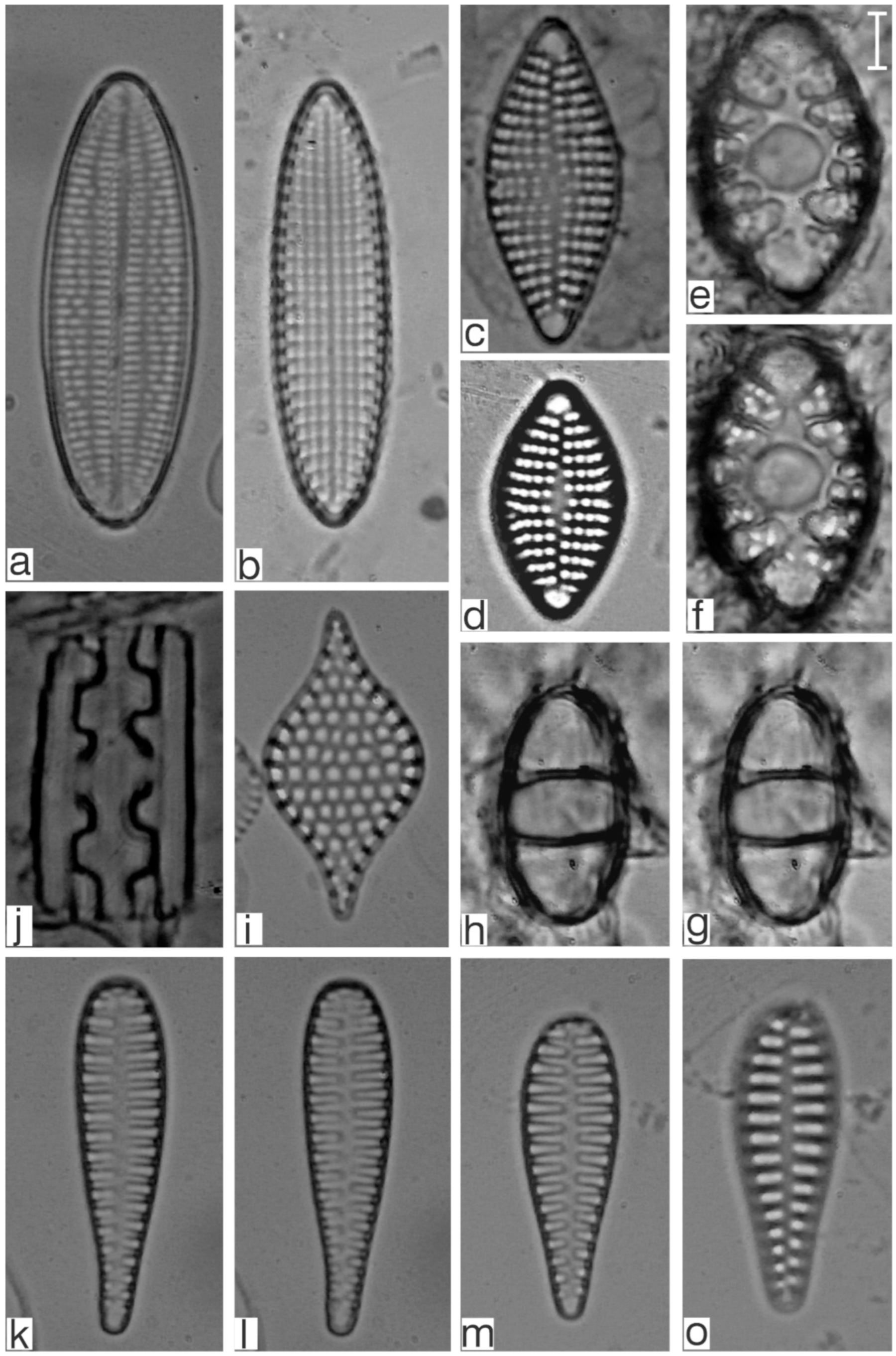
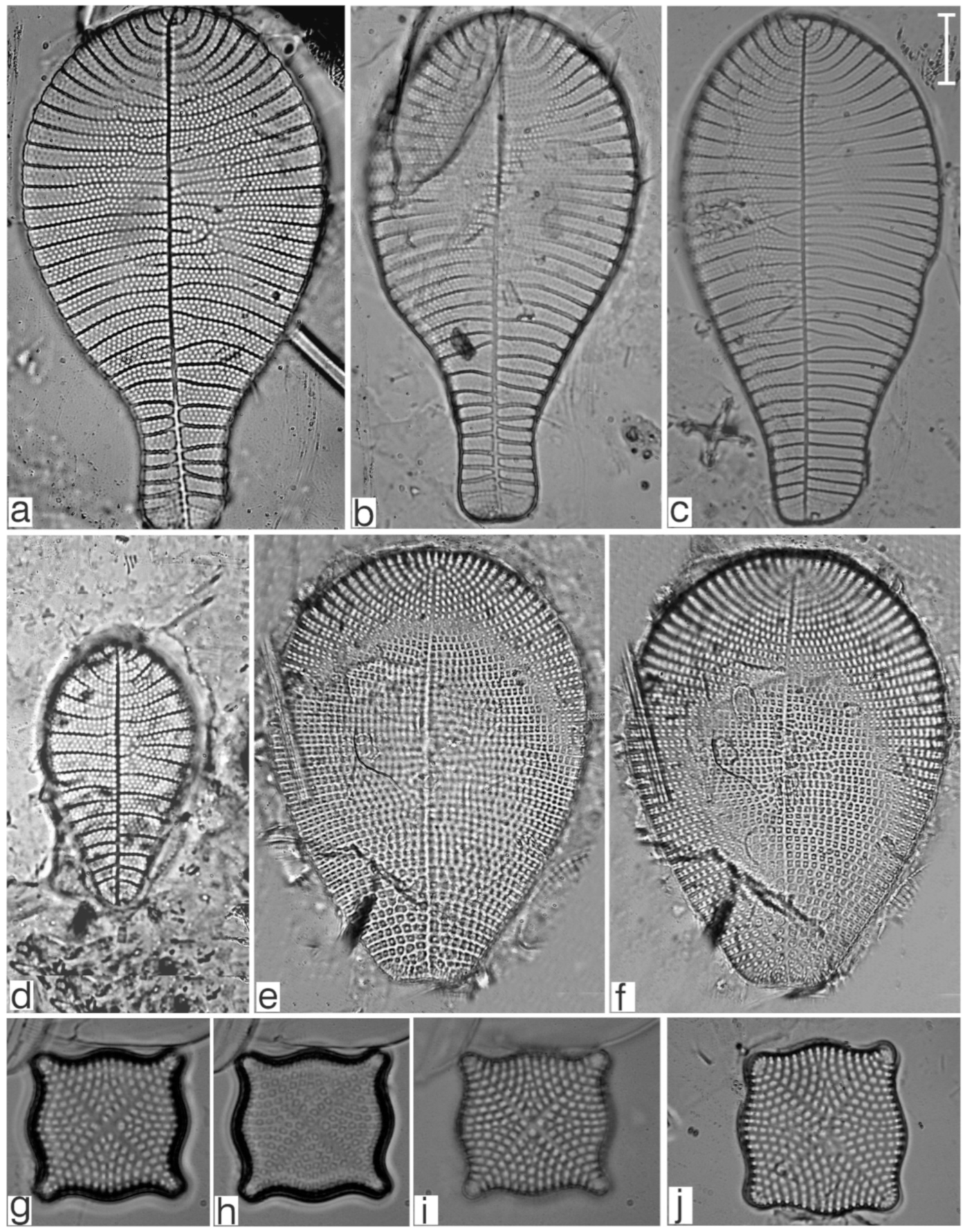
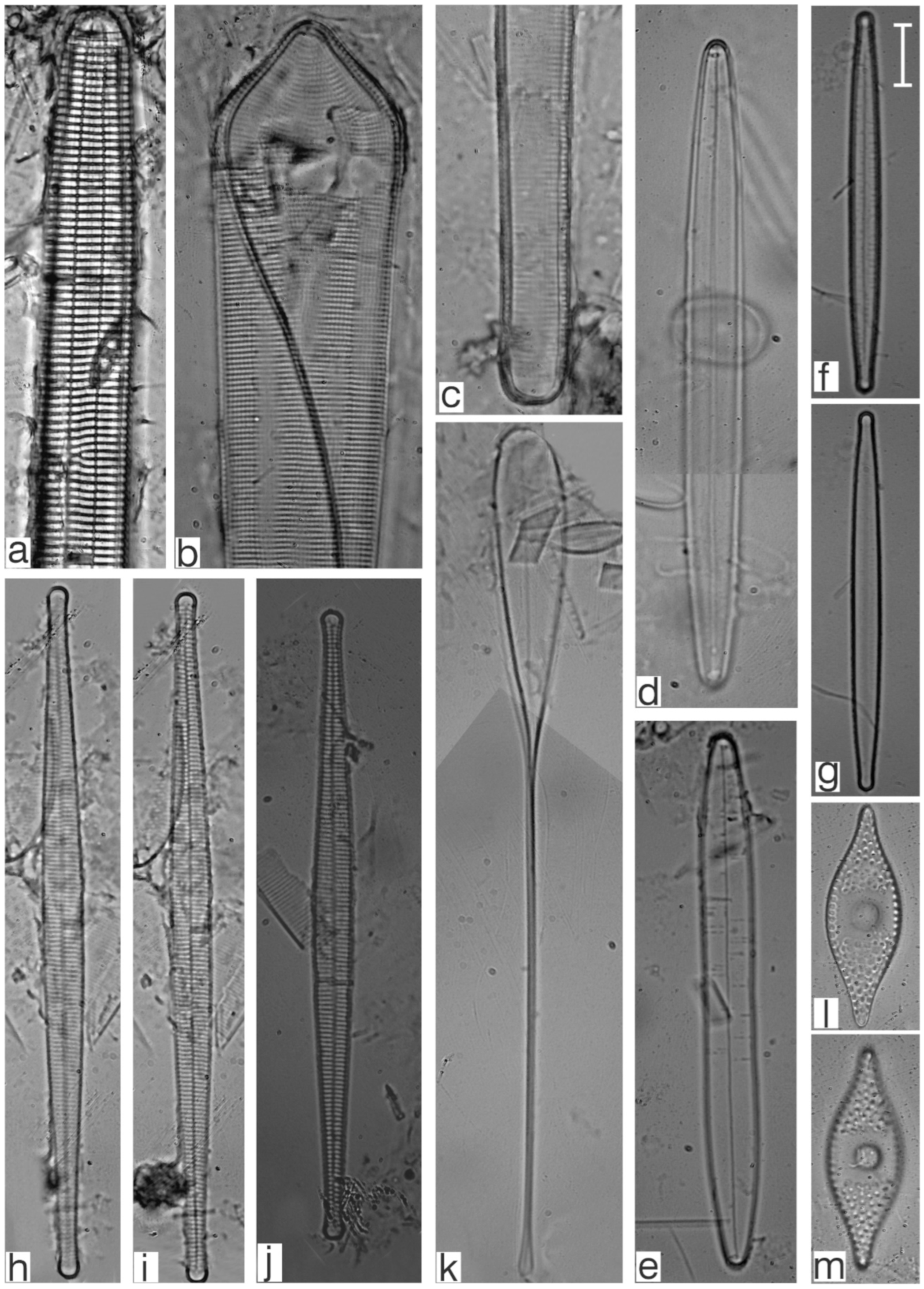
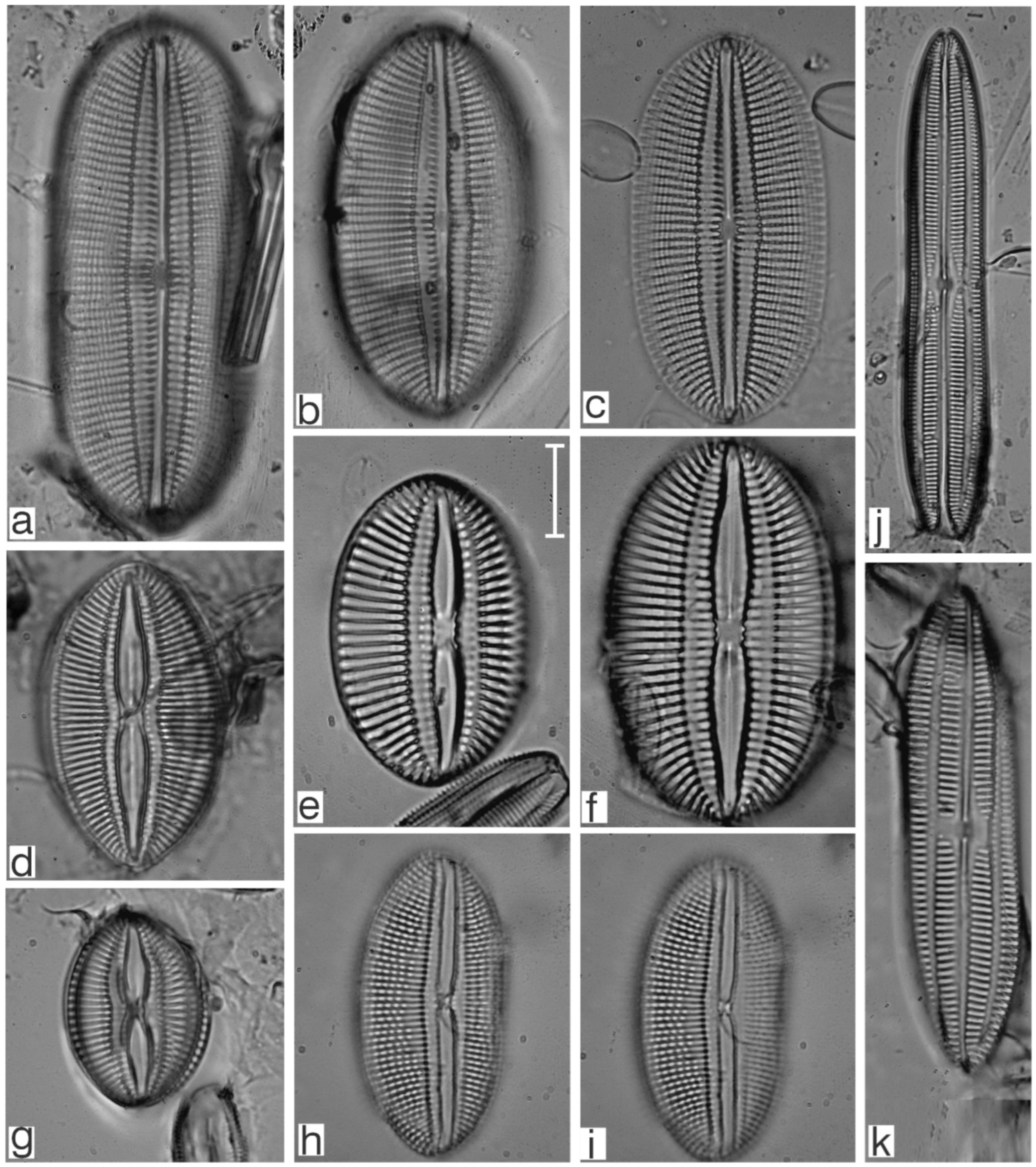
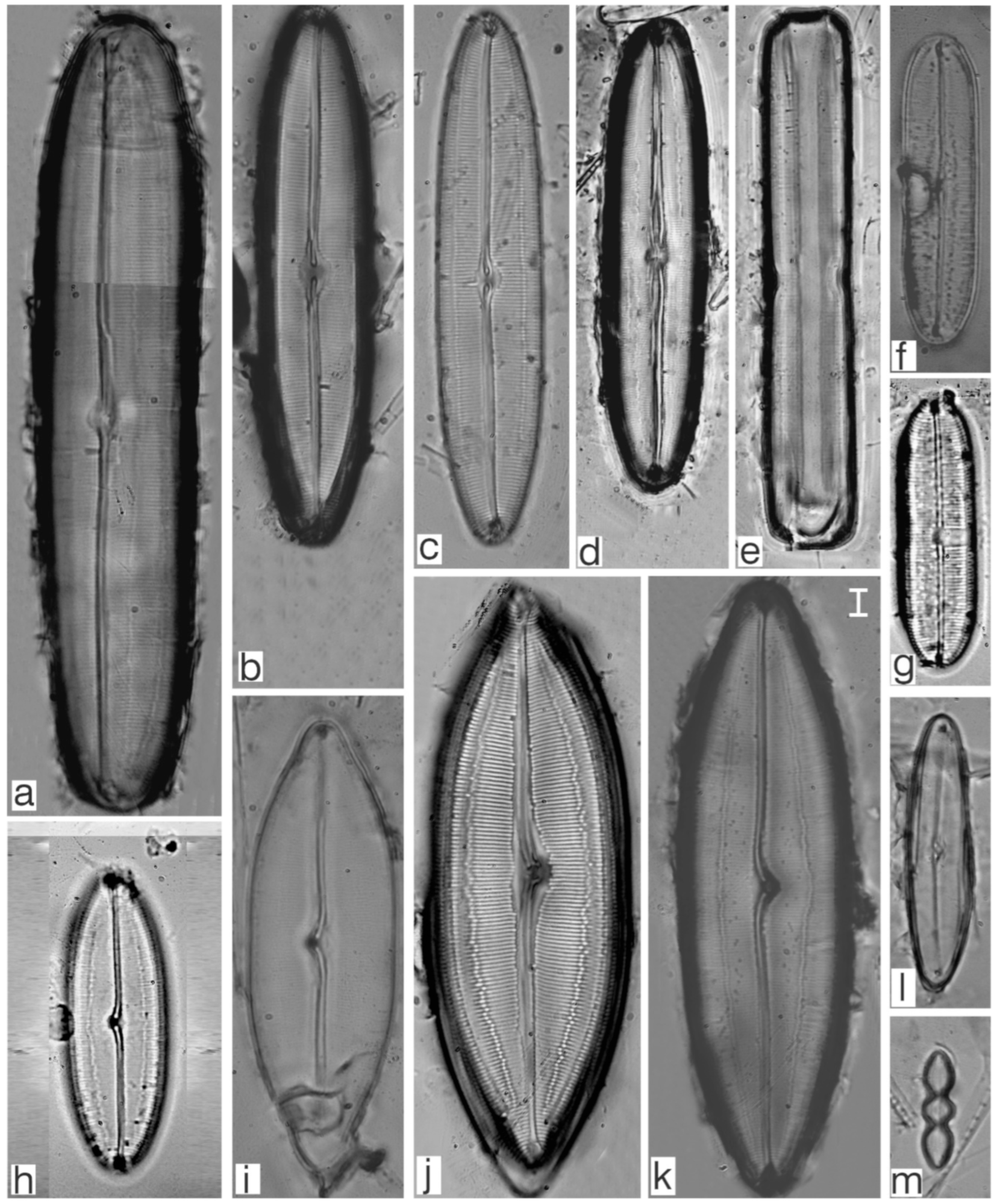
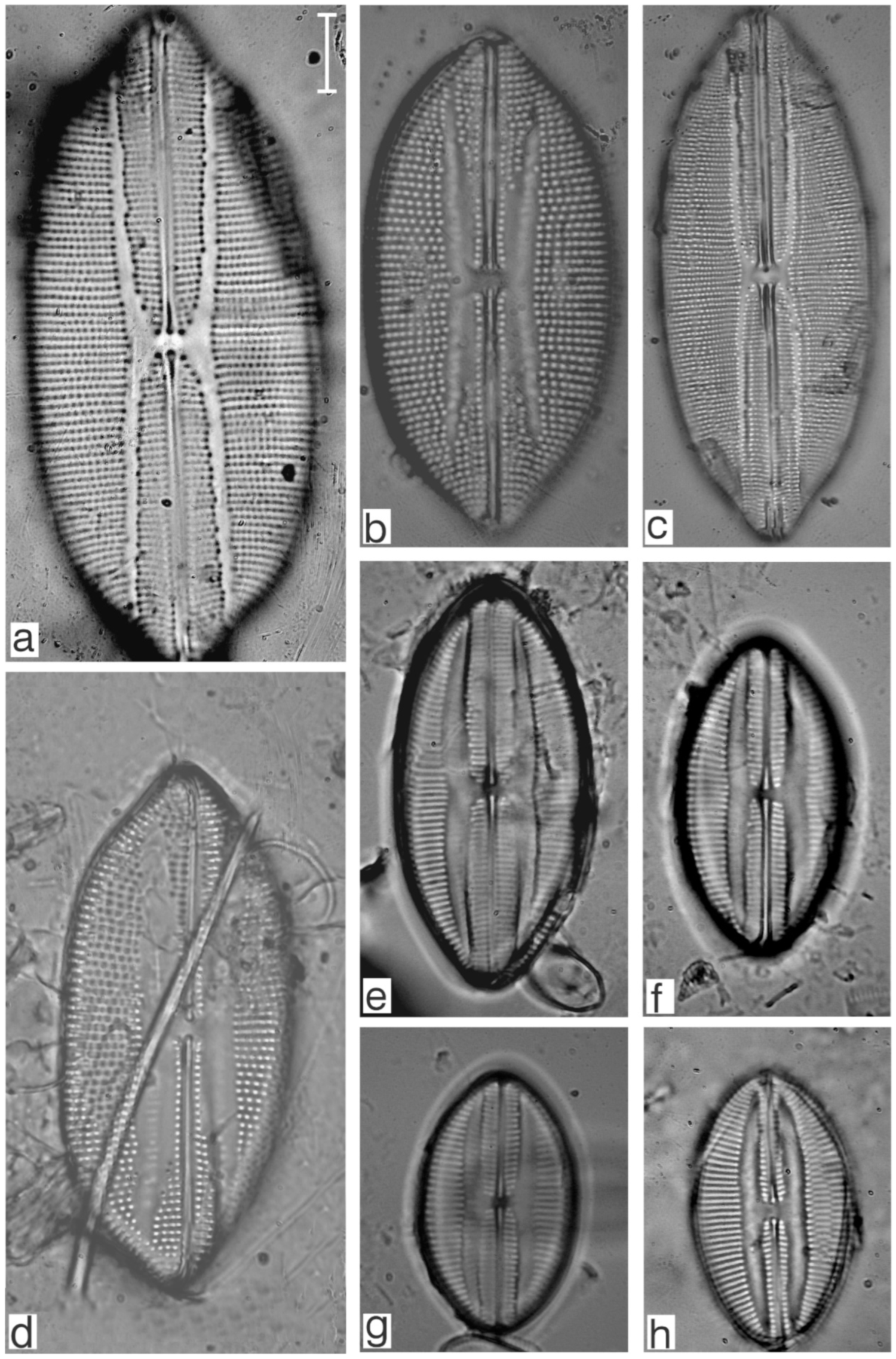
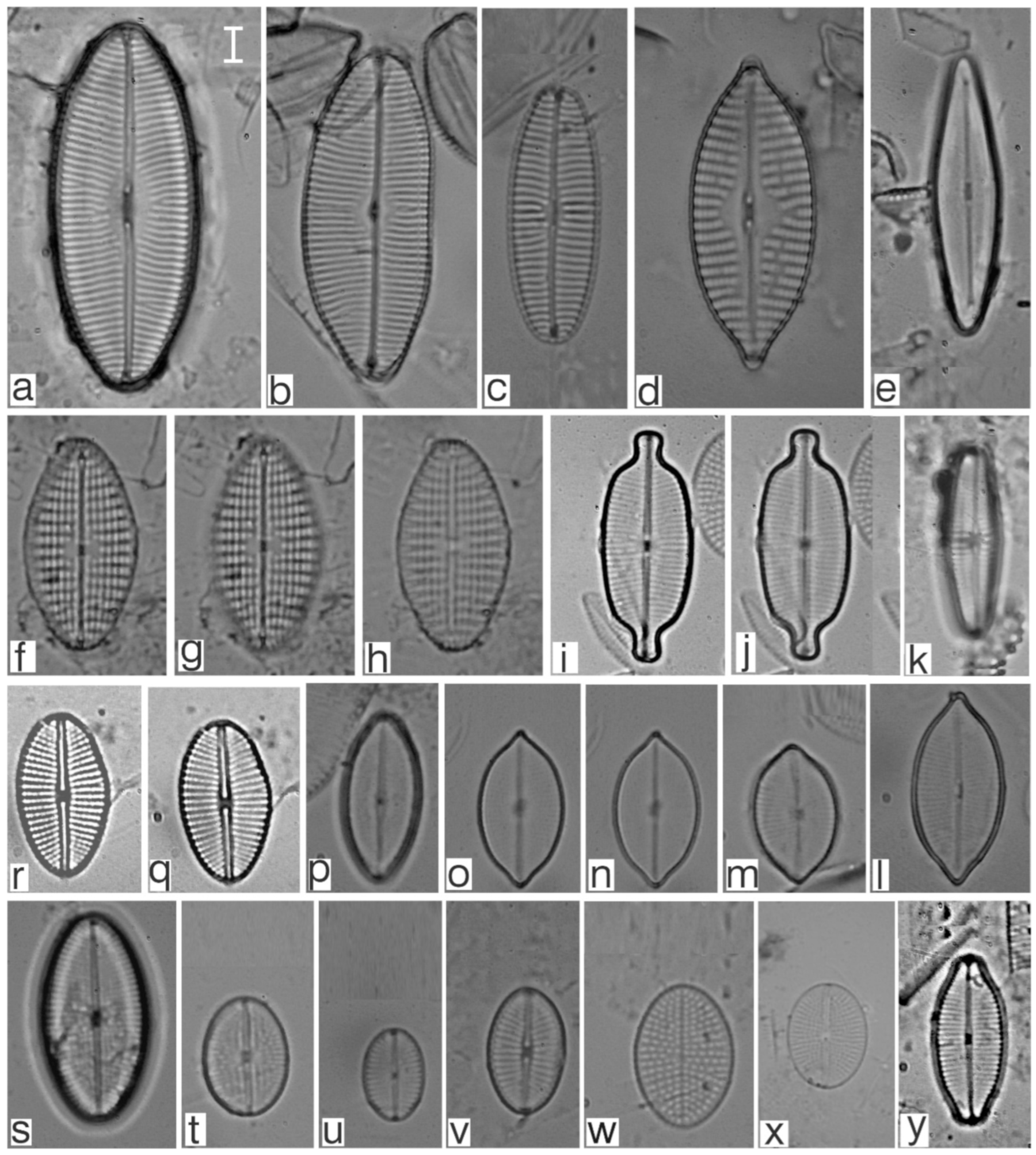
Appendix B. Iconographic Catalog of Representative Diatom Taxa Found in Sediments of Playa Las Gaviotas, Cuba

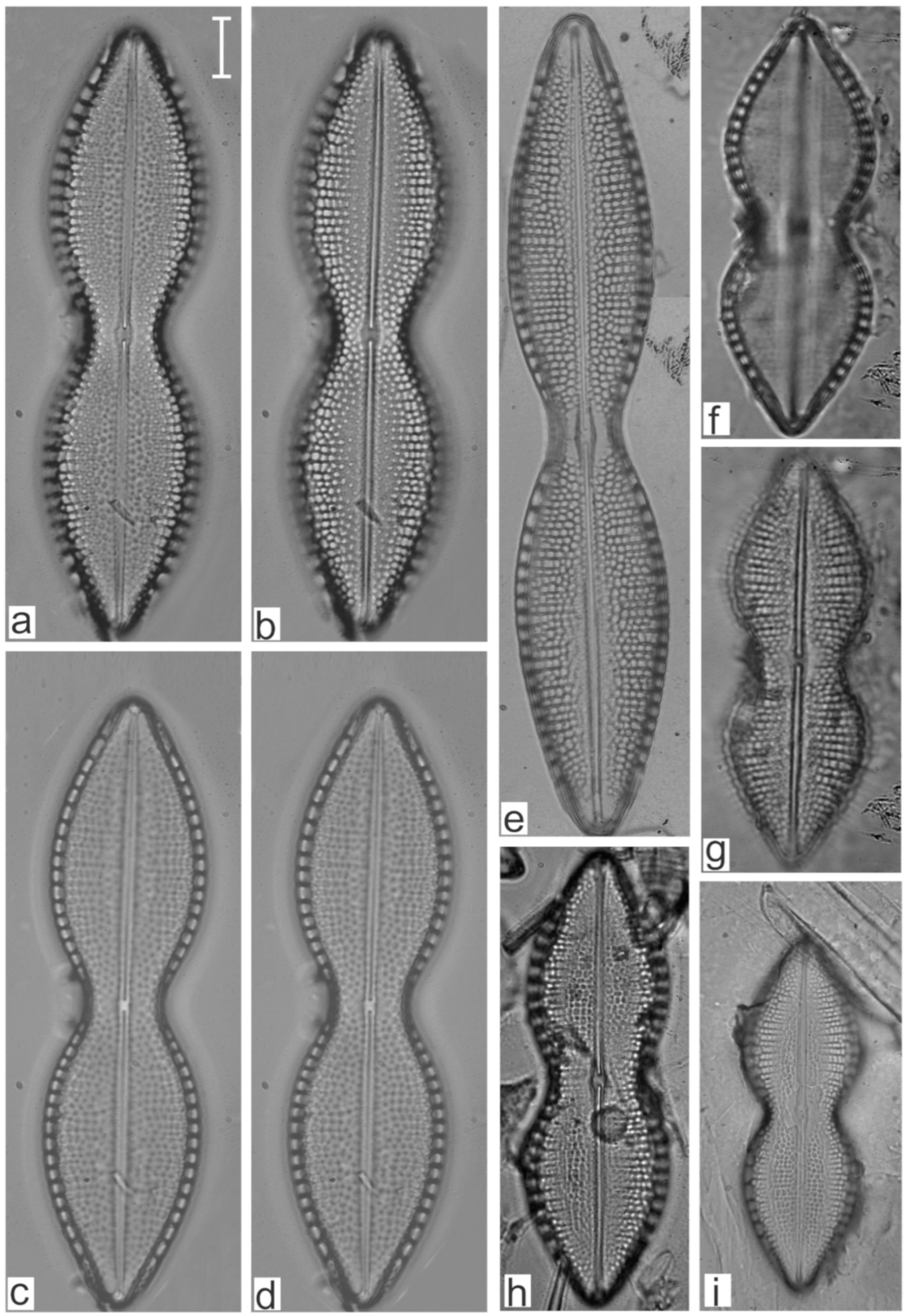
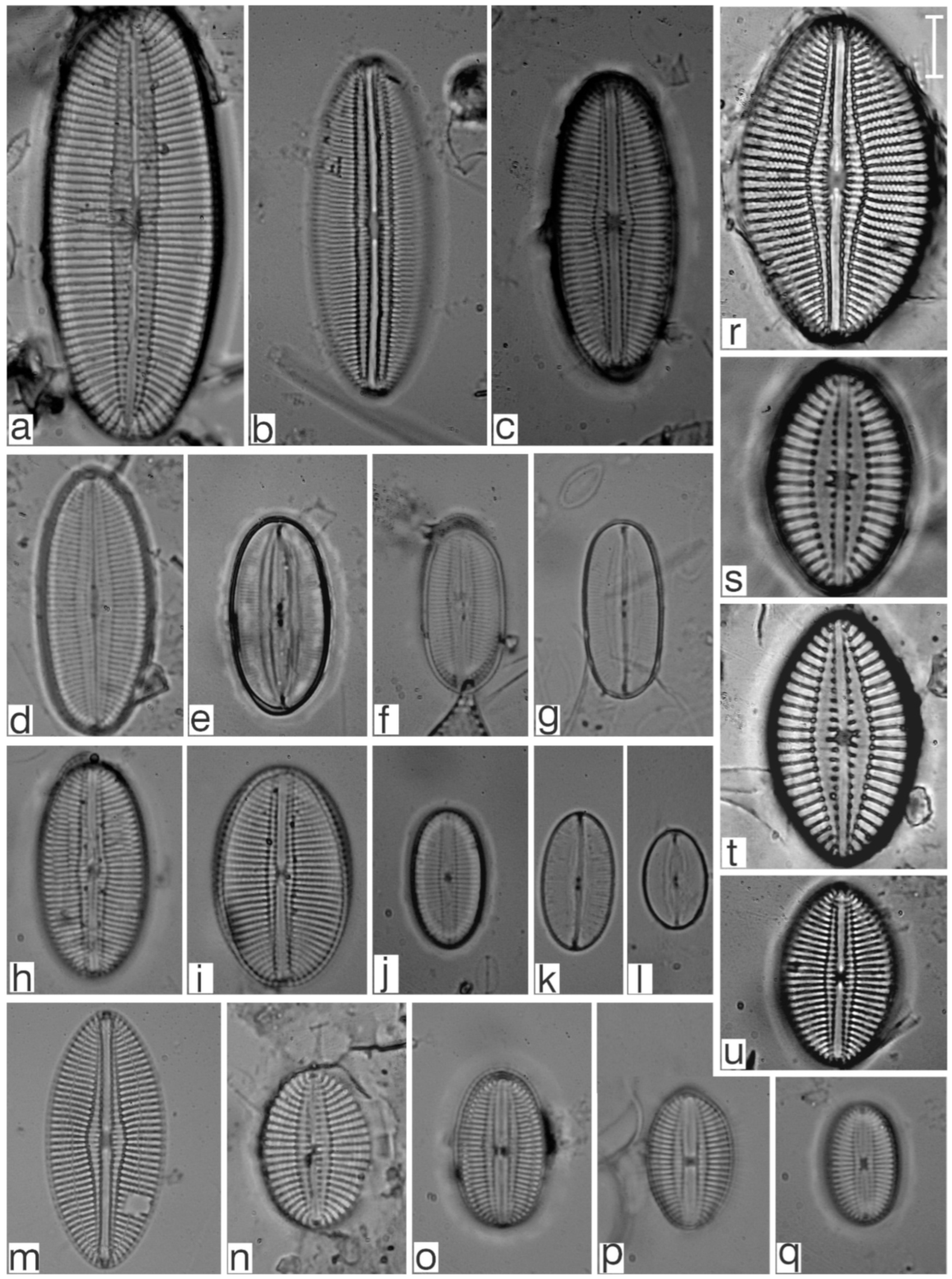
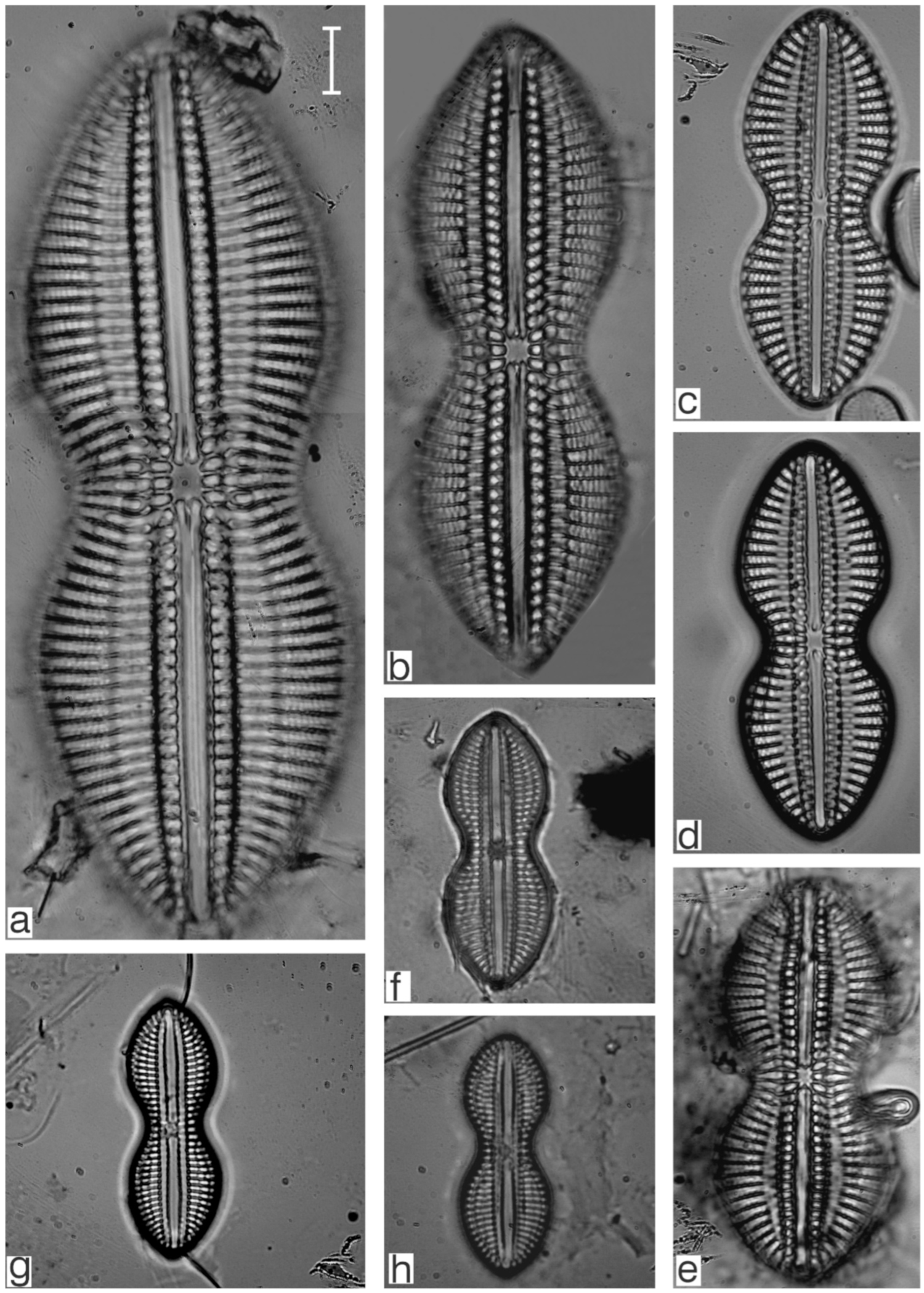
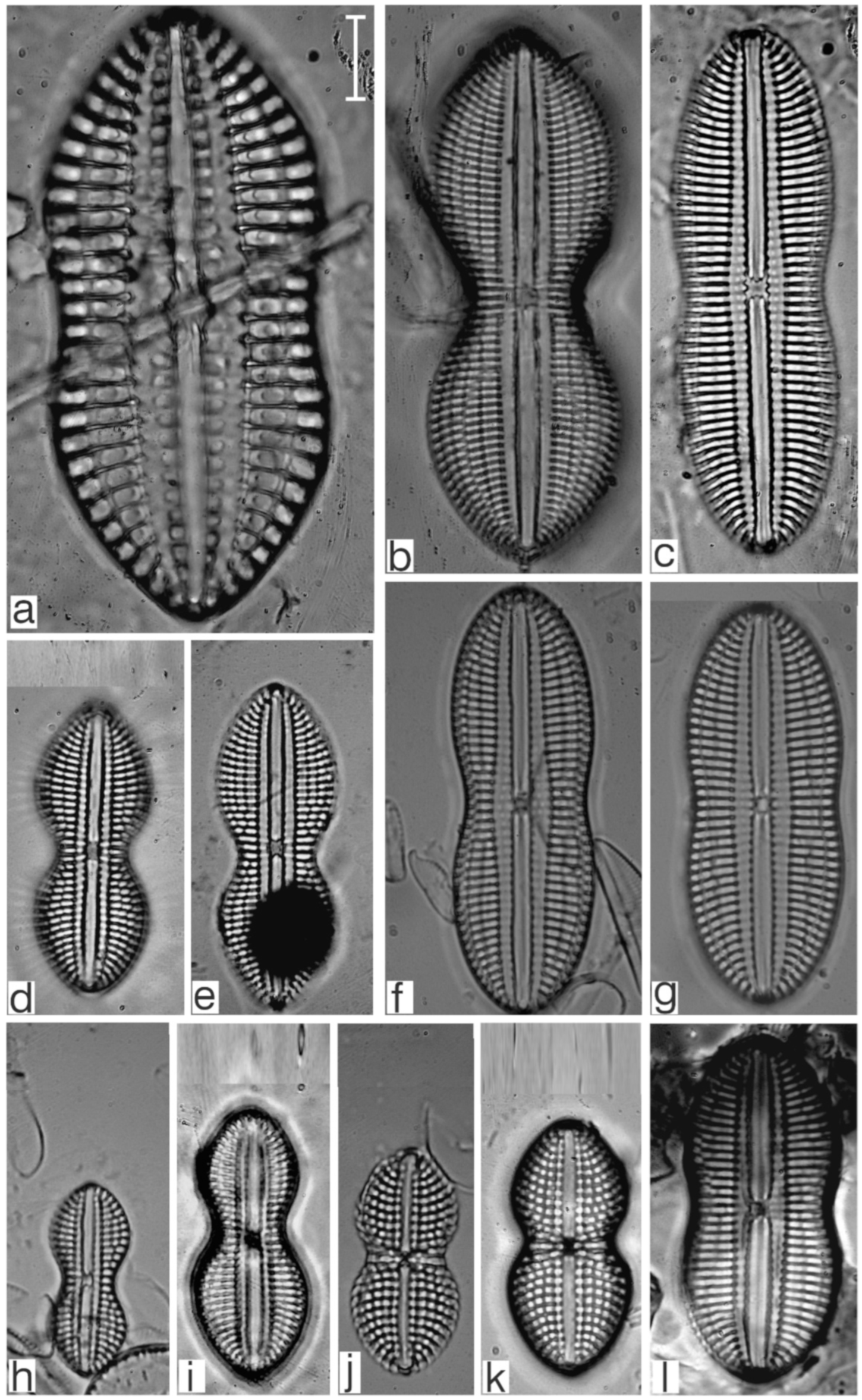




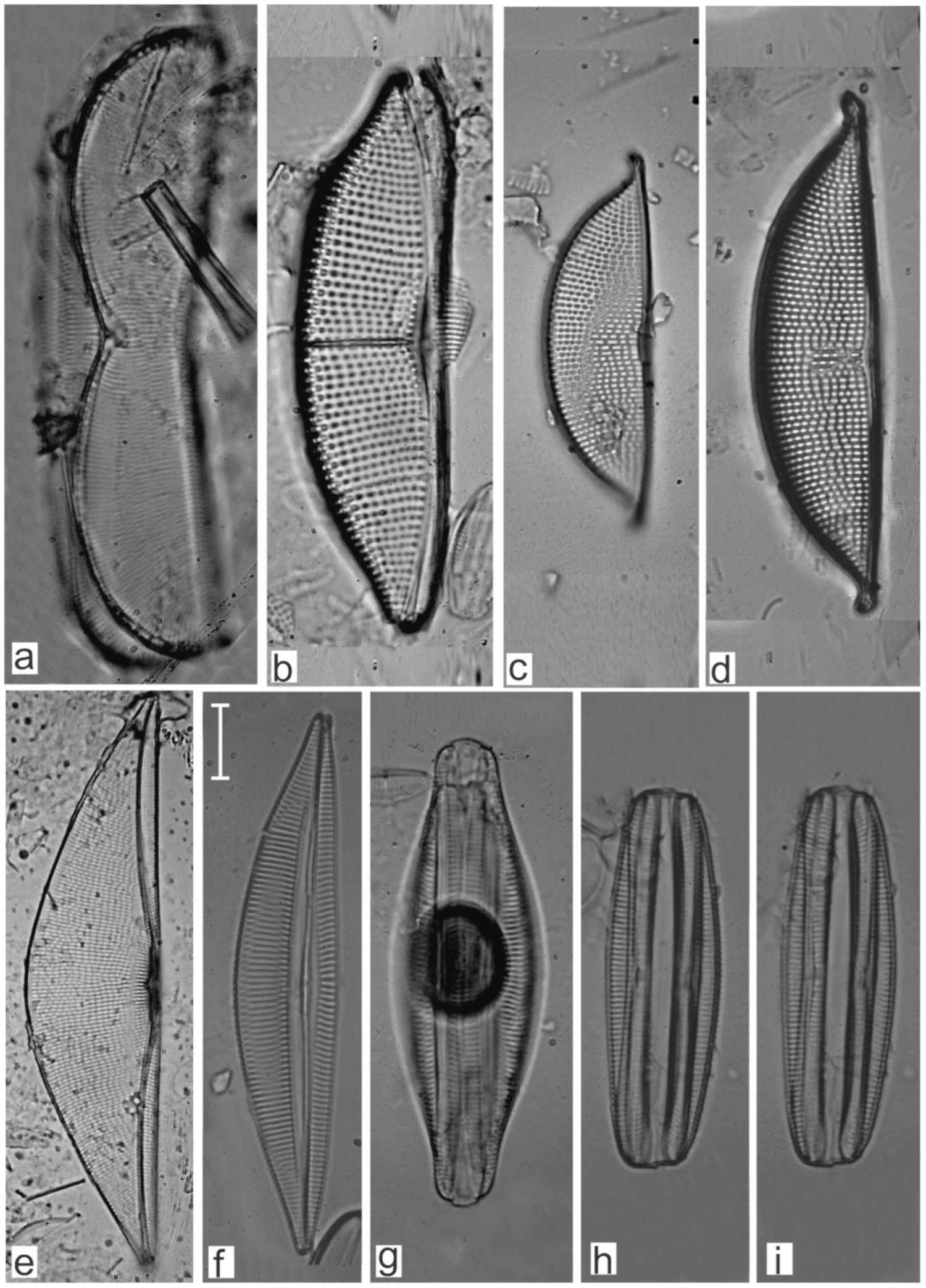
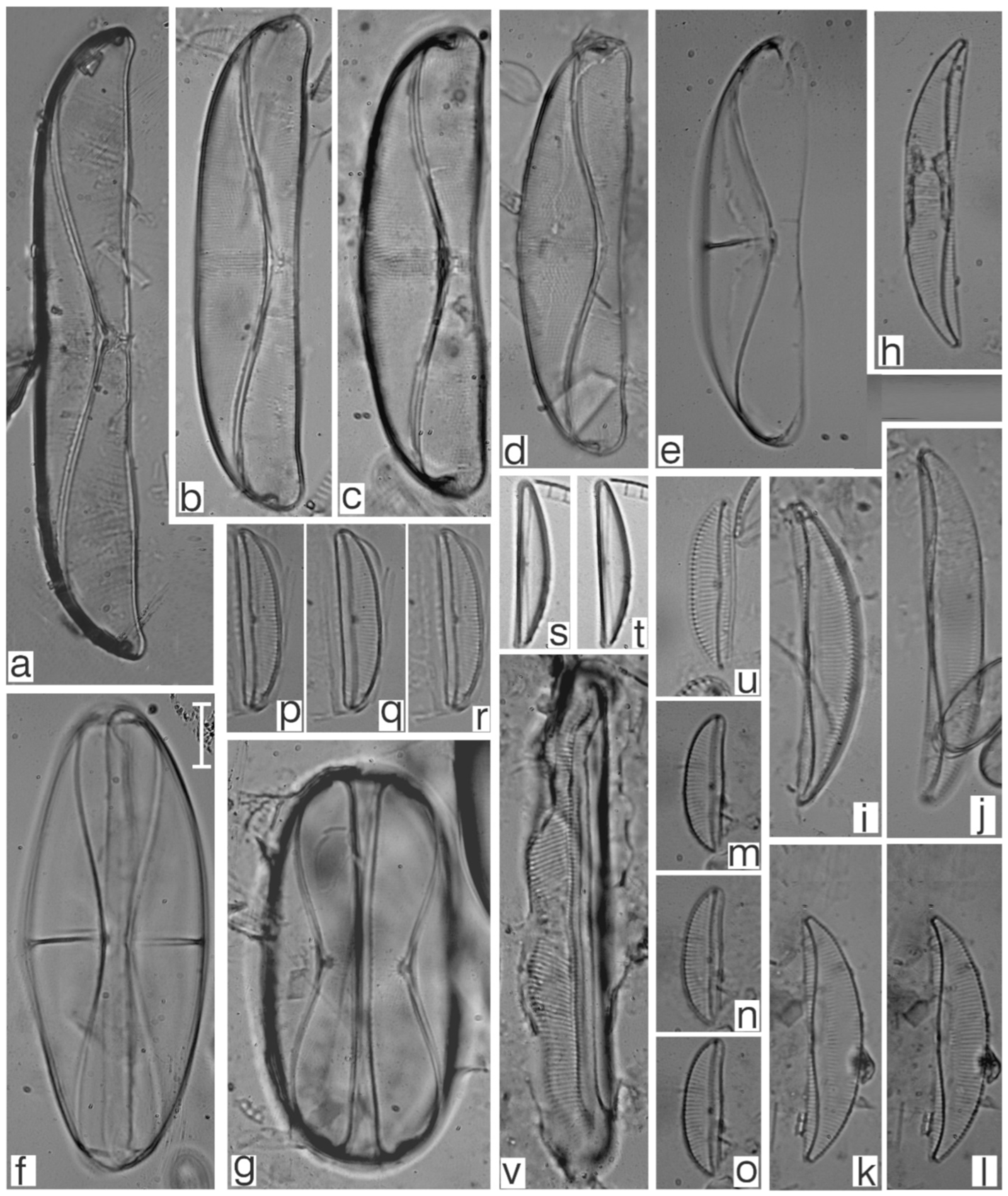
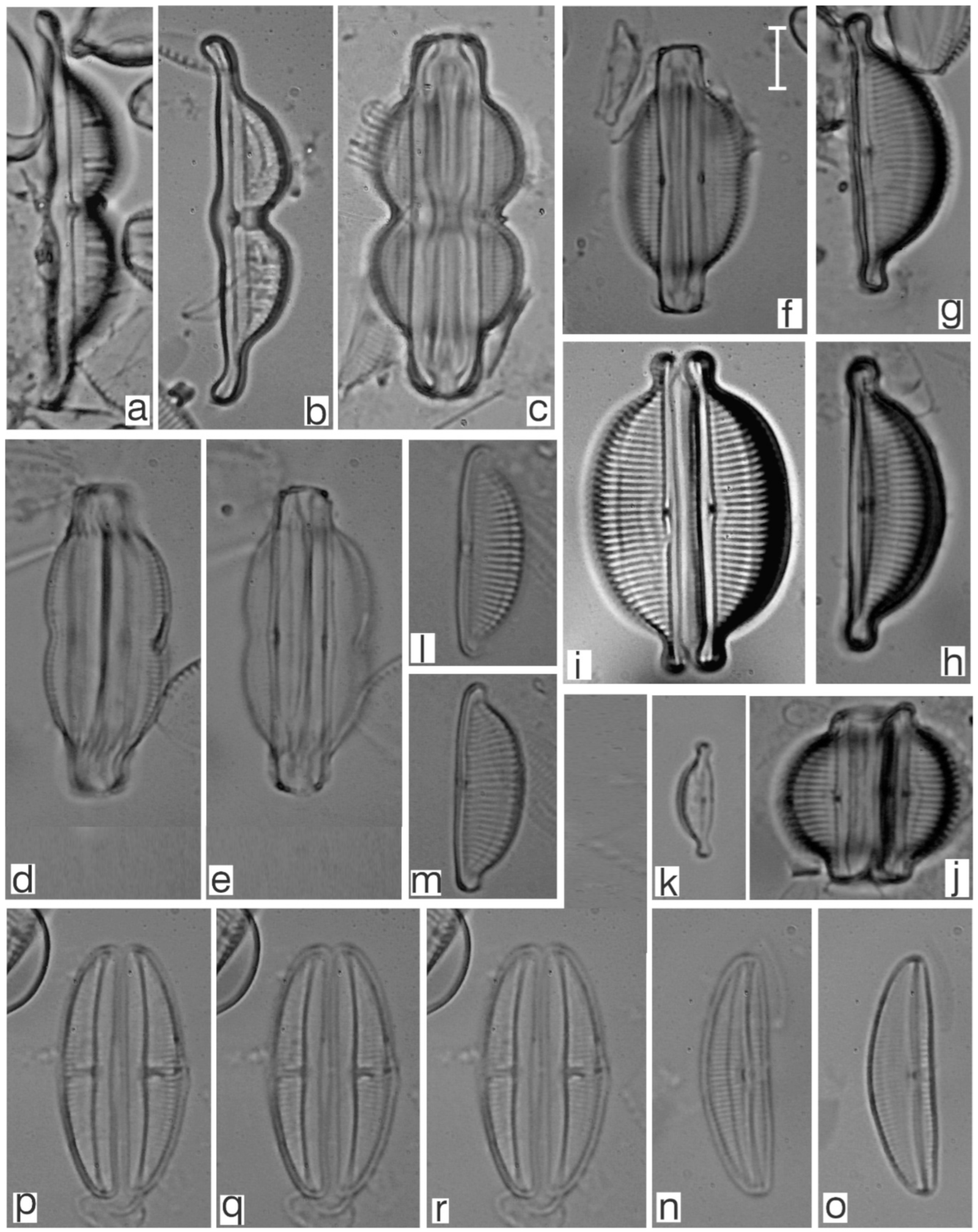
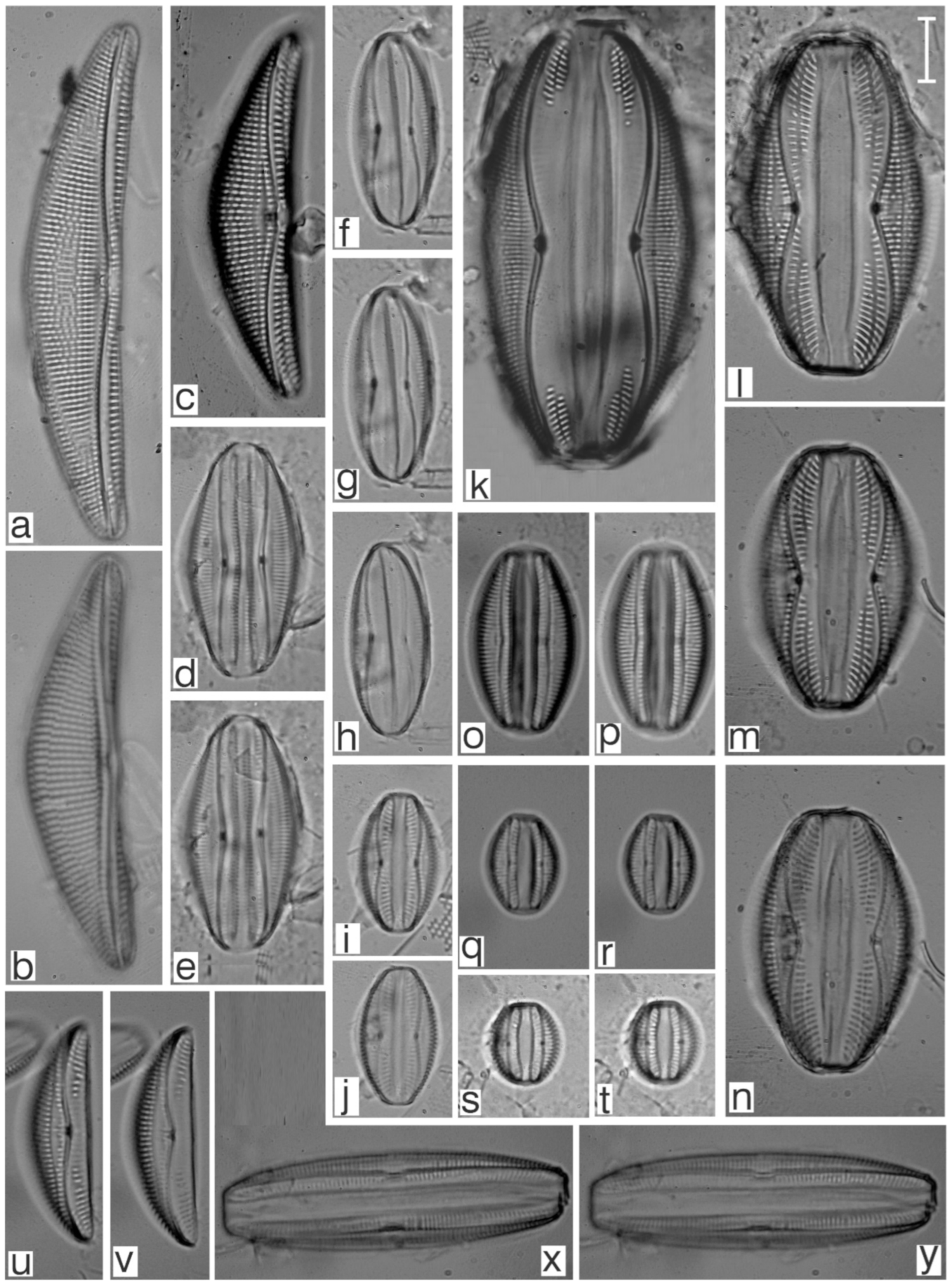

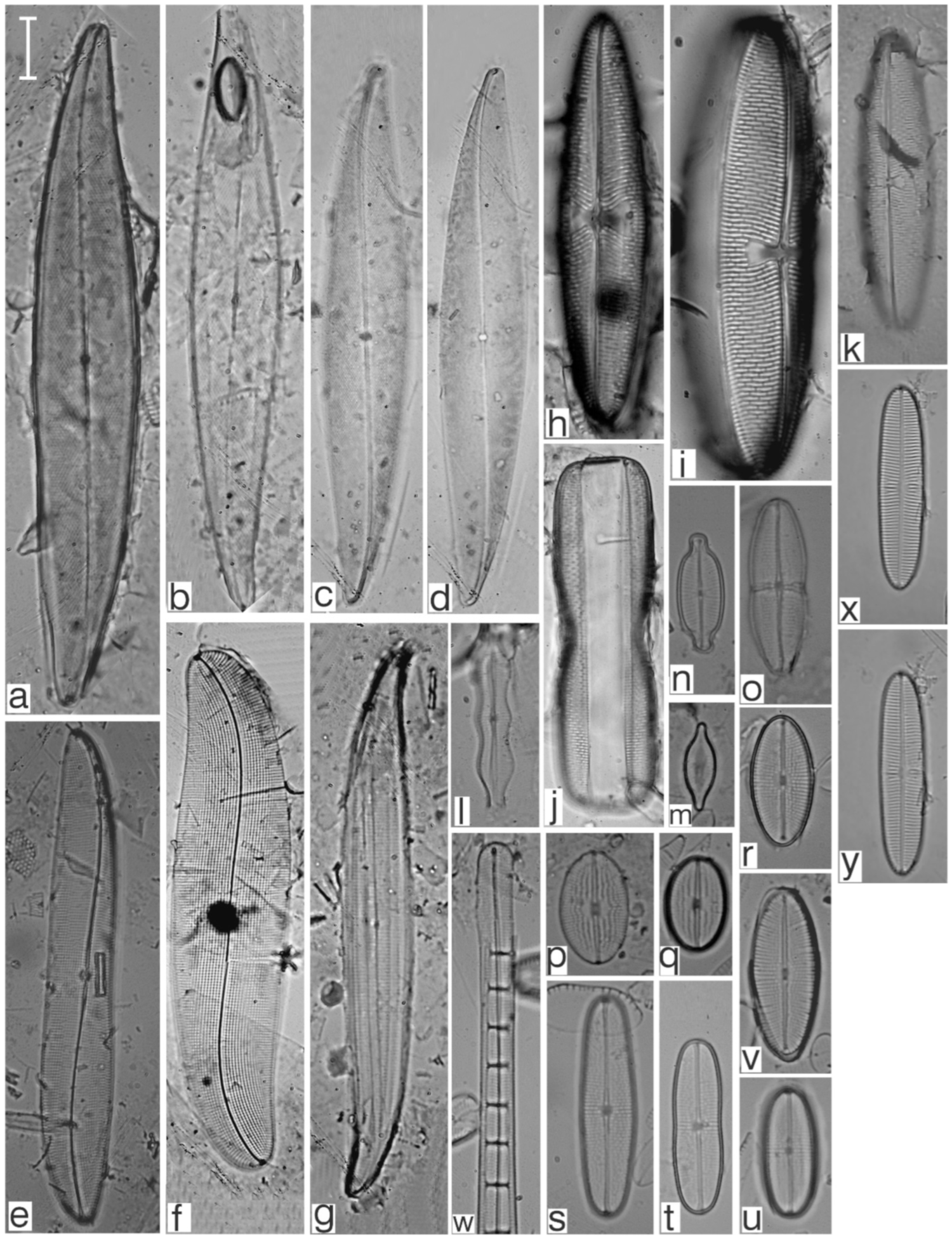
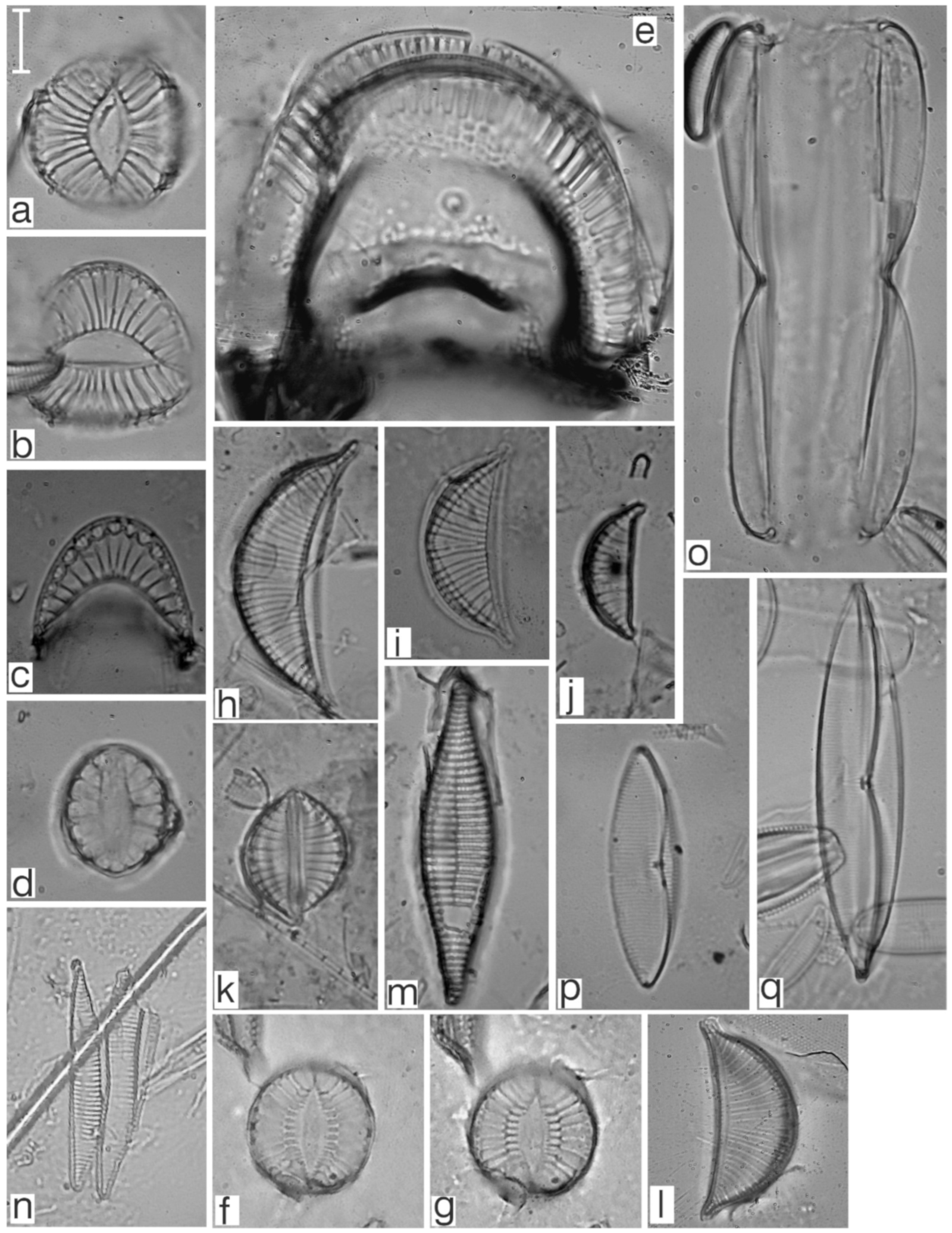
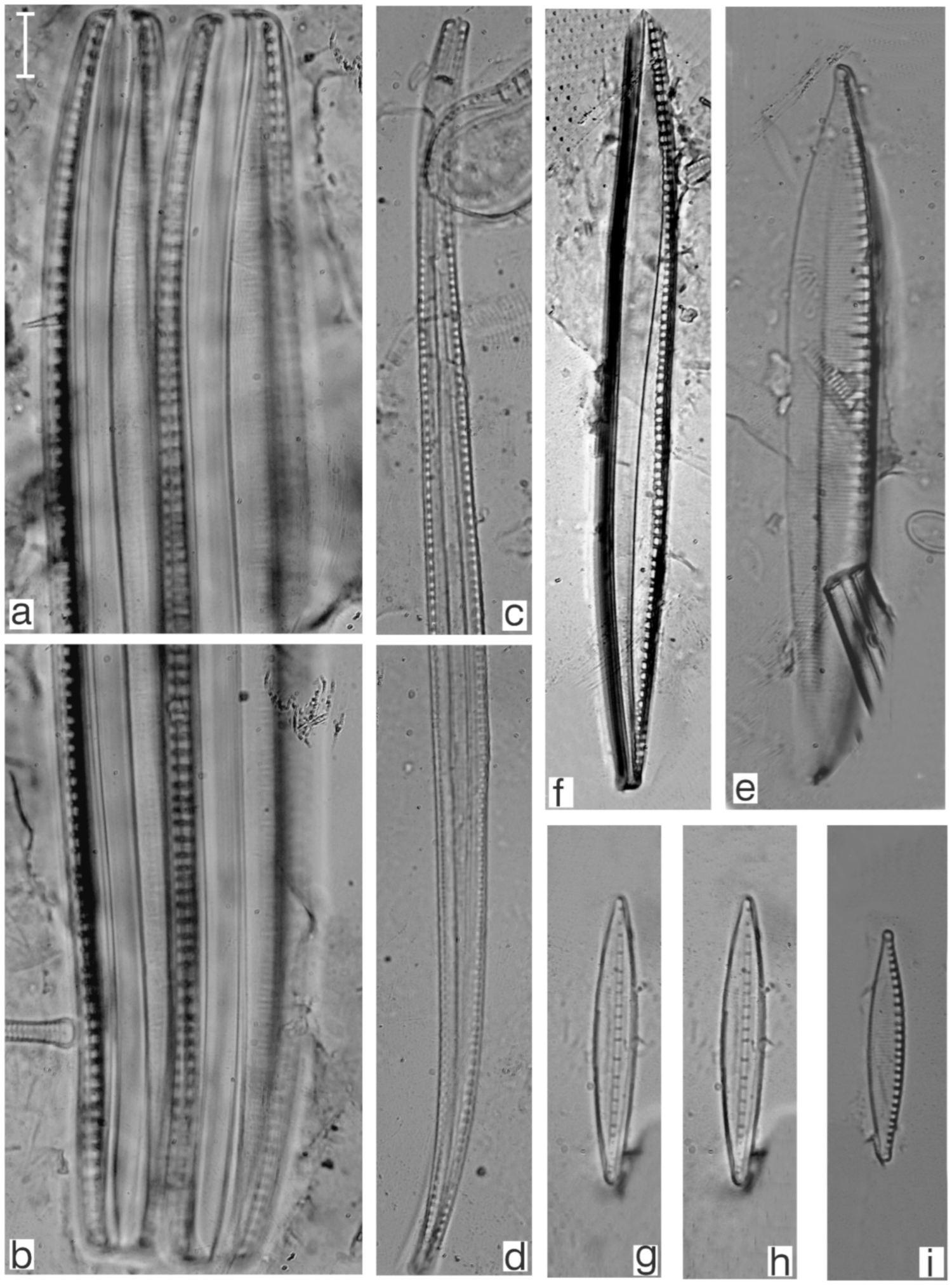
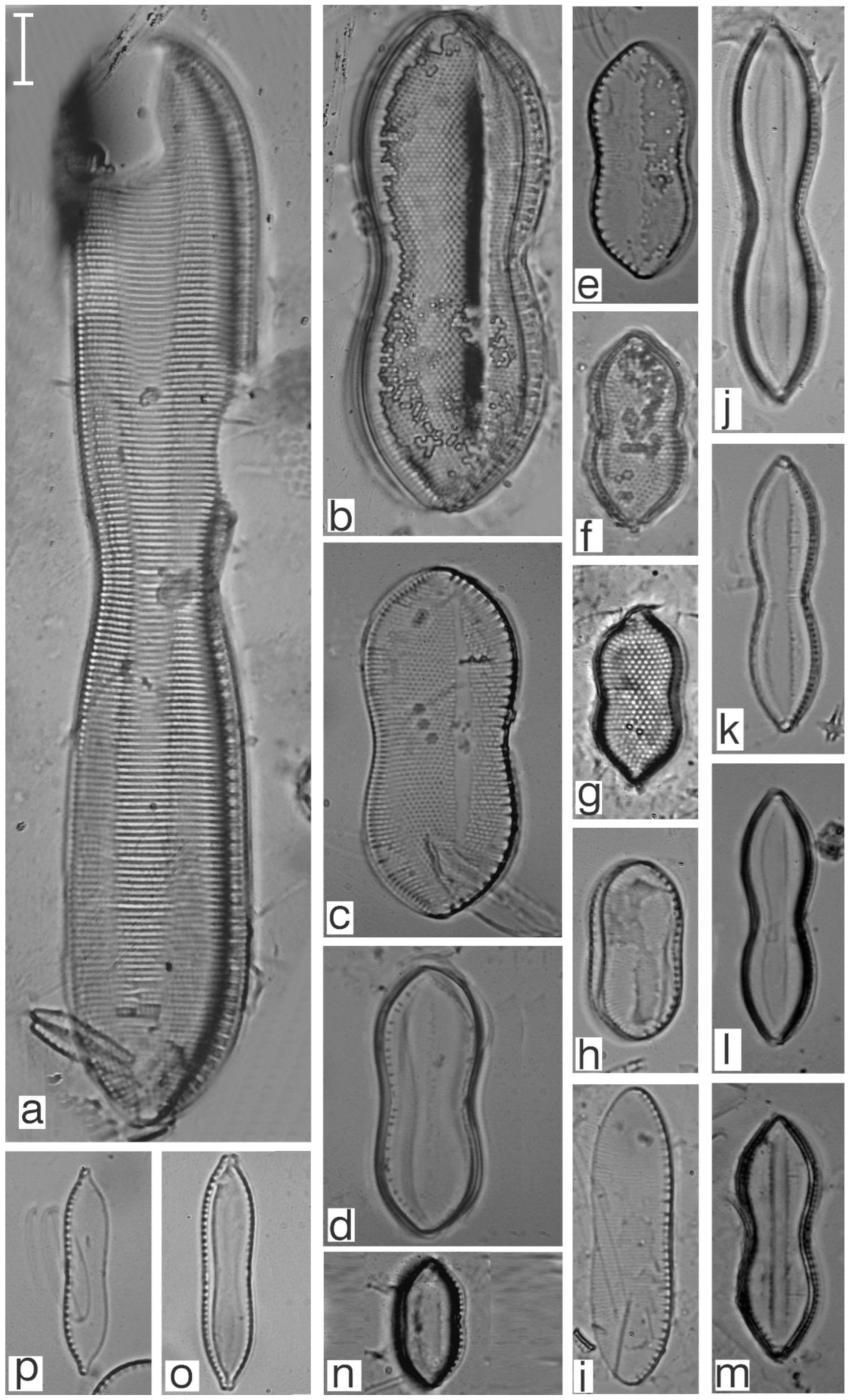

References
- Siqueiros Beltrones, D.A.; Martínez, Y.J.; López-Fuerte, F.O. Epiphytic diatoms from the central region of the Gulf of California: Floristics and biogeographic remarks. Diversity 2023, 15, 510. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; Argumedo Hernández, U.; Hernández Almeida, O.U. High species diversity (H) of benthic diatoms in a coastal lagoon located within a natural protected area. Hidrobiológica 2017, 27, 293–300. [Google Scholar] [CrossRef]
- Lobban, C.S.; Schefter, M.; Jordan, R.W.; Arai, Y.; Sasaki, A.; Theriot, E.C.; Ashworth, M.; Ruck, E.C.; Pennesi, C. Coral-reef diatoms (Bacillariophyta) from Guam: New records and preliminary checklist, with emphasis on epiphytic species from farmer-fish territories. Micronesica 2012, 43, 237–479. [Google Scholar]
- Park, J.S.; Lobban, C.S.; Kyun-Woo, L. Diatoms associated with seaweeds from Moen Island in Chuuk Lagoon, Micronesia. Phytotaxa 2018, 351, 101–140. [Google Scholar] [CrossRef]
- Park, J.S.; Lobban, C.S.; Lee, K.W.; Jung, S.W. Additional floristic study of planktonic and seaweed-associated diatoms in Chuuk, Micronesia. J. Mar. Biol. Assoc. U. K. 2022, 102, 27–61. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; López-Fuerte, F.O.; Martínez, Y.J.; Altamirano-Cerecedo, M.C. A first estimate of species diversity for benthic diatom assemblages from the Revillagigedo Archipelago, México. Diversity 2021, 13, 458. [Google Scholar] [CrossRef]
- López-Fuerte, F.O.; Siqueiros Beltrones, D.A.; Martínez, Y.J. Floristics and Biogeographical Affinity of Diatoms Attached to Sargassum fluitans (Børgesen) Børgesen and Sargassum natans (Linnaeus) Gaillon Arriving on Mexico’s Caribbean Coasts. Diversity 2022, 14, 758. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; Martínez, Y.J.; Aldana-Moreno, A. Exploratory floristics of epiphytic diatoms from Revillagigedo Islands (Mexico). Cymbella 2019, 5, 98. [Google Scholar]
- Siqueiros Beltrones, D.A.; Echevarría Herrera, E.; López-Fuerte, F.O. Additions to the Marine Mastogloia (Bacillariophyceae) from Cuban Coasts; Remarks on Misidentified Taxa. Diversity 2024, 16, 747. [Google Scholar] [CrossRef]
- Cibic, T.; Comici, C.; Bussani, A.; Del Negro, P. Benthic diatom response to changing environmental conditions, Estuarine. Coast. Shelf Sci. 2012, 115, 158–169. [Google Scholar] [CrossRef]
- Gray, C.L.; Samantha, L.L.; Newbold, T.; Hudson, N.L.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Schariemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef]
- Foged, N. Freshwater and littoral diatoms from Cuba. Bibl. Diatomol. 1984, 5, 243. [Google Scholar]
- Loza, S.M.; Sánchez, M.; Carmenat Siqueiros Beltrones, D.A. Adición a la microflora de diatomeas de las aguas marinas de Cuba. Ser. Oceanol. 2011, 8, 47–52. [Google Scholar]
- Hein, M.K.; Winsborough, B.M.; Sullivan, M.J. Bacillariophyta (Diatoms) of the Bahamas; A.R.G. Gantner/Koeltz Scientific Books: Köenigstein, Germany, 2008. [Google Scholar]
- Hendey, N.I. An Introductory Account of The Smaller Algae of British Coastal Waters. Part V: Bacillariophyceae (Diatoms). In Fishery Investigations, Series IV; Her Majesty´s Stationery Office: London, UK, 1964; p. 317. [Google Scholar]
- Admiraal, W.; Peletier, H. Influence of seasonal variations of temperature and light on the growth rate of cultures and natural populations of intertidal diatoms. Mar. Ecol. Prog. 1980, 2, 35–43. [Google Scholar] [CrossRef]
- Anonymous. Estudio de los Grupos Insulares y zonas Litorales del Archipiélago Cubano con fines Turísticos. Cayos: Francés, Cobos, Las Brujas, Ensenachos y Santa María. La Habana, Cuba: Científico-Técnica, 1990; Academia de Ciencias de Cuba e Instituto Cubano de Geodesia y Cartografía: La Habana, Cuba, 1990. [Google Scholar]
- Monzón, A.N.; Noa, I.C.; Mederos, J.M. Flora y Vegetación de Cayo Santa María (Archipiélago Sabana-Camagüey). Rev. Jardín Botánico Nac. 2001, 1, 67–84. [Google Scholar]
- Hernández Albernas JRuiz Rojas, E.; González Amador, Y.; Aborrezco Pérez, P. Plan de Manejo Refugio de Fauna Cayo Santa María; Grupo de Turismo Gaviota S.A.: Villa Clara, Cuba, 2020; p. 187. [Google Scholar]
- Rodríguez-Portal, J.P.; Ramírez, J.R. Las mareas en las costas cubanas. Acad. Cien. Cuba. Rep. Invest. Oceanol. 1983, 6, 1–37. [Google Scholar]
- Blair, T.C.; McPherson, J.G. Grain-size and textural classification of coarse sedimentary particles. J. Sediment. Res. 1999, 69, 6–19. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A. Diatomeas Bentónicas de la Península de Baja California; Diversidad y Potencial Ecológico; Centro Interdisciplinario de Ciencias Marinas-Instituto Politécnico Nacional: La Paz, BCS, Mexico; Universidad Autónoma de Baja California Sur: La Paz, BCS, Mexico, 2002; p. 205. [Google Scholar]
- Schmidt, A. Atlas der Diatomaceenkunde’, OR Reisland, Aschersleben, Leipzig and Berlin. (1874–1959). Reprint; Koeltz Scientific Books: Koenigstein, Germany, 1984. [Google Scholar]
- Peragallo, H.; Peragallo, M. Diatomées Marines de France; Tempère, M.J., Ed.; Micrographe-Editeur: Grez-sur-Loing, France, 1897; p. 491. [Google Scholar]
- Moreno, J.L.; Licea, S.; Santoyo, H. Diatomeas del Golfo de California; Universidad Autónoma de Baja California Sur.: La Paz, Mexico, 1996. [Google Scholar]
- Witkowski, A. Diatom flora of marine coasts I. Lange-Bertalot, Iconographia Diatomologica. Annot. Diatom Microgr. 2000, 7, 925. [Google Scholar]
- Wachnicka, A.H.; Gaise, E.E. Characterization of Amphora and Seminavis from South Florida. USA Diatom. Res. 2007, 22, 387455. [Google Scholar]
- Stidolph, S.R.; Stidolph, S.R.; Sterrenburg, F.A.S.; Smith, K.E.L.; Kraberg, A. Stuart R. Stidolph Diatom Atlas; Open-File Report 2012-1163; U.S. Geological Survey: Reston, VA, USA, 2012; Volume 8.
- Loir, M.; Novarino, G. Marine Mastogloia Thwaites ex W. Sm. and Stigmaphora Wallich Species from the French Lesser Antilles; Diatom Monographs Köningstein, Koeltz Scientific Books: Königstein, Germany, 2013; Volume 16. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. Algaebase. Available online: http://www.algaebase.org (accessed on 20 November 2024).
- Fountanier, E.; Kociolek, J.P. WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 20 November 2024).
- Brower, J.E.; Zar, J.H.; Von Ende, C.N. Field and Laboratory Methods for General Ecology; WCB McGraw-Hill Boston: Geneva, Switzerland, 1998. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; p. 179. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- McIntire, C.D.; Overton, W.S. Distributional patterns in assemblages of attached diatoms from Yaquina Bay Estuary, Oregon. Ecology 1971, 52, 758–777. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; Hernández Almeida, O.U. Diversity measurement of benthic diatom taxocoenoses based on information theory (H′) using genus-to-species ratio. Hidrobiológica 2022, 32, 245–249. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biostatistics; Francise & Co: New York, NY, USA, 1987; p. 10. [Google Scholar]
- Ehrlich, A. Atlas of the Inland-Water Diatom Flora of Israel. In Flora Palaestina; The Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1975; p. 165. [Google Scholar]
- Lobban, C.S.; Tharngan, B.G. Benthic Marine Diatom Flora (Bacillariophyta) of Yap, Micronesia: Preliminary Annotated List, with Some New Mangrove Species. Diversity 2025, 17, 34. [Google Scholar] [CrossRef]
- Cunningham, L.; McMinn, A. The influence of natural environmental factors on benthic diatom communities from the Windmill Islands, Antarctica. Phycologia 2004, 43, 744–755. [Google Scholar] [CrossRef]
- Whitehead, J.M.; McMinn, A. Paleodepth determination from Antarctic benthic diatom assemblages. Mar. Micropaleontol. 1997, 29, 301–318. [Google Scholar] [CrossRef]
| Sampling | Kolmogorov–Smirnov | Shapiro–Wilks | ||
|---|---|---|---|---|
| November | D = 0.09 | p = 0.31 | W = 0.97 | p = 0.45 |
| July | D = 0.09 | p = 0.29 | W = 0.98 | p = 0.54 |
| Non-Lithified Fraction | Class | Grade | Size (µm) | SITES | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Sand | Sand | Very coarse | 2000–1000 | 0.8 | 0.7 | 5.0 | 3.5 | 0 | 1.0 |
| Coarse | 999–500 | 18.6 | 5.2 | 23.0 | 13.0 | 4.6 | 9.5 | ||
| Medium | 499–250 | 39.4 | 39.6 | 37.7 | 50.0 | 42.8 | 37.0 | ||
| Fine | 249–125 | 23.6 | 38.3 | 21.7 | 23.6 | 36.0 | 25.6 | ||
| Very fine | 124–63 | 1.5 | 2.1 | 1.3 | 0.0 | 2.3 | 0.7 | ||
| Total sand | 83.9 | 85.77 | 88.84 | 90.02 | 85.68 | 73.81 | |||
| Mudd | Silt | Coarse | 62–31 | 5.7 | 4.8 | 3.7 | 3.2 | 4.9 | 9.8 |
| Medium | 30–15 | 5.2 | 4.5 | 3.5 | 3.5 | 4.7 | 8.4 | ||
| Fine | 14–8 | 2.0 | 1.9 | 1.5 | 1.3 | 1.7 | 3.0 | ||
| Very fine | 7–4 | 1.5 | 1.4 | 1.1 | 1.1 | 1.4 | 2.5 | ||
| Total silt | 14.4 | 12.51 | 9.81 | 9.14 | 12.65 | 23.62 | |||
| Clay | ≤3 | 1.7 | 1.7 | 1.3 | 0.9 | 1.7 | 2.6 | ||
| Genus | No. spp. | Genus | No. spp. | Genus | No. spp. |
|---|---|---|---|---|---|
| Mastogloia | 70 | Odontella | 2 | Grunowago | 1 |
| Navicula | 26 | Oestrupia | 2 | Hantzschia | 1 |
| Amphora | 25 | Perissonoë | 2 | Haslea | 1 |
| Diploneis | 20 | Petroneis | 2 | Hendeyella | 1 |
| NItzschia | 18 | Pleurosigma | 2 | Hyalosynedra | 1 |
| Cocconeis | 10 | Podocystis | 2 | Neohuttonia | 1 |
| Halamphora | 8 | Psammodiscus | 2 | Orthoneis | 1 |
| Campylodiscus | 8 | Toxarium | 2 | Paralia sulcata | 1 |
| Caloneis | 7 | Trachyneis | 4 | Podosira | 1 |
| Lyrella | 7 | Amphicocconeis | 1 | Prestauroneis | 1 |
| Seminavis | 6 | Amphipentas | 1 | Protoraphis | 1 |
| Plagiogramma | 5 | Anaulus | 1 | Psammodictyon | 1 |
| Fallacia | 5 | Anorthoneis | 1 | Rhaphoneis | 1 |
| Cocconeiopsis | 4 | Ardissonea | 1 | Rhopalodia | 1 |
| Dimeregramma | 4 | Auricula | 1 | Rutilaria | 1 |
| Grammatophora | 4 | Bacillaria | 1 | Schizostauron | 1 |
| Gyrosigma | 4 | Berkeleya | 1 | Shionodiscus | 1 |
| Tetramphora | 4 | Biddulphiella | 1 | Staurophora | 2 |
| Tryblionella | 4 | Biremis | 1 | Surirella | 1 |
| Achnanthes | 3 | Brassierea | 1 | Tabularia | 1 |
| Opephora | 3 | Climaconeis | 1 | Talaroneis | 1 |
| Parlibellus | 3 | Climacosphenia | 1 | Tephanocyclus | 1 |
| Plagiotropis | 3 | Coscinodiscus | 1 | Terpsinoë | 1 |
| Synedra | 3 | Cymatosira | 1 | Thalassiosira | 1 |
| Synedrosphenia | 3 | Cymbella | 1 | Trigonium | 1 |
| Triceratium | 3 | Dictyoneis | 1 | Ulnaria | 1 |
| Actinocyclus | 2 | Epithemia | 1 | ||
| Biddulphia | 2 | Frustulia | 1 | ||
| Homoeocladia | 2 | Glyphodesmis | 1 | ||
| Licmophora | 2 | Gomphonemopsis | 1 |
| Sample | S | N | H′ | J′ | 1 − λ | λ |
|---|---|---|---|---|---|---|
| P1.1R1 | 44 | 500 | 4.76 | 0.87 | 0.95 | 0.05 |
| P1.1R2 | 50 | 500 | 5.01 | 0.89 | 0.96 | 0.04 |
| P1.1R3 | 47 | 500 | 5.02 | 0.90 | 0.96 | 0.04 |
| P1.2R1 | 48 | 500 | 5.02 | 0.90 | 0.96 | 0.04 |
| P1.2R2 | 47 | 500 | 5.01 | 0.90 | 0.96 | 0.04 |
| P1.2R3 | 45 | 500 | 4.76 | 0.87 | 0.95 | 0.05 |
| P1.3R1 | 49 | 500 | 4.93 | 0.88 | 0.96 | 0.04 |
| P1.3R2 | 56 | 500 | 5.21 | 0.90 | 0.97 | 0.03 |
| P1.3R3 | 56 | 500 | 5.25 | 0.90 | 0.97 | 0.03 |
| P2.1R1 | 43 | 500 | 5.11 | 0.94 | 0.97 | 0.03 |
| P2.1R2 | 51 | 500 | 5.21 | 0.92 | 0.97 | 0.03 |
| P2.1R3 | 51 | 500 | 5.05 | 0.89 | 0.96 | 0.04 |
| P2.2R1 | 45 | 500 | 5.03 | 0.92 | 0.96 | 0.04 |
| P2.2R2 | 51 | 500 | 5.21 | 0.92 | 0.97 | 0.03 |
| P2.2R3 | 46 | 500 | 4.92 | 0.89 | 0.96 | 0.04 |
| P2.3R1 | 49 | 500 | 4.97 | 0.88 | 0.96 | 0.04 |
| P2.3R2 | 49 | 500 | 4.91 | 0.87 | 0.96 | 0.04 |
| P2.3R3 | 46 | 500 | 4.77 | 0.86 | 0.95 | 0.05 |
| P3.1R1 | 38 | 500 | 4.74 | 0.90 | 0.95 | 0.05 |
| P3.1R2 | 40 | 500 | 4.85 | 0.91 | 0.96 | 0.04 |
| P3.1R3 | 50 | 500 | 4.99 | 0.88 | 0.96 | 0.04 |
| P3.2R1 | 56 | 500 | 5.26 | 0.91 | 0.96 | 0.04 |
| P3.2R2 | 51 | 500 | 5.17 | 0.91 | 0.97 | 0.03 |
| P3.2R3 | 47 | 500 | 5.03 | 0.90 | 0.96 | 0.04 |
| P3.3R1 | 56 | 500 | 5.36 | 0.92 | 0.97 | 0.03 |
| P3.3R2 | 52 | 500 | 5.24 | 0.92 | 0.97 | 0.03 |
| P3.3R3 | 58 | 500 | 5.35 | 0.91 | 0.97 | 0.03 |
| P4.1R1 | 48 | 500 | 4.85 | 0.87 | 0.95 | 0.05 |
| P4.1R2 | 48 | 500 | 4.96 | 0.89 | 0.96 | 0.04 |
| P4.1R3 | 51 | 500 | 4.88 | 0.86 | 0.96 | 0.04 |
| P4.2R1 | 34 | 500 | 4.39 | 0.86 | 0.94 | 0.06 |
| P4.2R2 | 34 | 500 | 4.34 | 0.85 | 0.94 | 0.06 |
| P4.2R3 | 35 | 500 | 4.56 | 0.89 | 0.95 | 0.05 |
| P4.3R1 | 38 | 500 | 4.71 | 0.90 | 0.95 | 0.05 |
| P4.3R2 | 38 | 500 | 4.64 | 0.88 | 0.95 | 0.05 |
| P4.3R3 | 36 | 500 | 4.59 | 0.89 | 0.95 | 0.05 |
| P5.1R1 | 40 | 500 | 4.65 | 0.87 | 0.95 | 0.05 |
| P5.1R2 | 43 | 500 | 4.72 | 0.87 | 0.95 | 0.05 |
| P5.1R3 | 39 | 500 | 4.87 | 0.92 | 0.96 | 0.04 |
| P5.2R1 | 32 | 500 | 4.55 | 0.91 | 0.94 | 0.06 |
| P5.2R2 | 33 | 500 | 4.44 | 0.88 | 0.94 | 0.06 |
| P5.2R3 | 40 | 500 | 4.77 | 0.90 | 0.95 | 0.05 |
| P5.3R1 | 36 | 500 | 4.66 | 0.90 | 0.95 | 0.05 |
| P5.3R2 | 42 | 500 | 4.86 | 0.90 | 0.96 | 0.04 |
| P5.3R3 | 45 | 500 | 5.01 | 0.91 | 0.96 | 0.04 |
| P6.1R1 | 37 | 500 | 4.69 | 0.90 | 0.95 | 0.05 |
| P6.1R2 | 44 | 500 | 4.98 | 0.91 | 0.96 | 0.04 |
| P6.1R3 | 45 | 500 | 4.98 | 0.91 | 0.96 | 0.04 |
| P6.2R1 | 52 | 500 | 5.03 | 0.88 | 0.96 | 0.04 |
| P6.2R2 | 41 | 500 | 4.88 | 0.91 | 0.96 | 0.04 |
| P6.2R3 | 48 | 500 | 4.97 | 0.89 | 0.96 | 0.04 |
| P6.3R1 | 45 | 500 | 5.03 | 0.92 | 0.96 | 0.04 |
| P6.3R2 | 40 | 500 | 4.88 | 0.92 | 0.96 | 0.04 |
| P6.3R3 | 46 | 500 | 5.03 | 0.91 | 0.96 | 0.04 |
| Sample | S | N | H′ | J′ | 1 − λ | λ |
|---|---|---|---|---|---|---|
| P1.1R1 | 43 | 500 | 4.63 | 0.85 | 0.95 | 0.05 |
| P1.1R2 | 50 | 500 | 4.91 | 0.87 | 0.96 | 0.05 |
| P1.1R3 | 52 | 500 | 4.86 | 0.85 | 0.95 | 0.05 |
| P1.2R1 | 49 | 500 | 4.65 | 0.83 | 0.94 | 0.06 |
| P1.2R2 | 52 | 500 | 4.90 | 0.86 | 0.95 | 0.05 |
| P1.2R3 | 49 | 500 | 4.97 | 0.89 | 0.96 | 0.04 |
| P1.3R1 | 50 | 500 | 4.94 | 0.88 | 0.96 | 0.04 |
| P1.3R2 | 49 | 500 | 4.82 | 0.86 | 0.95 | 0.05 |
| P1.3R3 | 39 | 500 | 4.78 | 0.90 | 0.96 | 0.04 |
| P2.1R1 | 47 | 500 | 5.06 | 0.91 | 0.96 | 0.04 |
| P2.1R2 | 44 | 500 | 4.98 | 0.91 | 0.96 | 0.04 |
| P2.1R3 | 44 | 500 | 4.80 | 0.88 | 0.95 | 0.05 |
| P2.2R1 | 38 | 500 | 4.78 | 0.91 | 0.96 | 0.04 |
| P2.2R2 | 37 | 500 | 4.70 | 0.90 | 0.95 | 0.05 |
| P2.2R3 | 46 | 500 | 4.92 | 0.89 | 0.96 | 0.04 |
| P2.3R1 | 40 | 500 | 4.73 | 0.89 | 0.95 | 0.05 |
| P2.3R2 | 52 | 500 | 4.97 | 0.87 | 0.96 | 0.04 |
| P2.3R3 | 49 | 500 | 4.91 | 0.87 | 0.96 | 0.04 |
| P3.1R1 | 41 | 500 | 4.91 | 0.92 | 0.96 | 0.04 |
| P3.1R2 | 48 | 500 | 5.00 | 0.90 | 0.96 | 0.04 |
| P3.1R3 | 54 | 500 | 5.16 | 0.90 | 0.96 | 0.04 |
| P3.2R1 | 42 | 500 | 4.96 | 0.92 | 0.96 | 0.04 |
| P3.2R2 | 50 | 500 | 5.10 | 0.90 | 0.96 | 0.04 |
| P3.2R3 | 53 | 500 | 5.26 | 0.92 | 0.97 | 0.03 |
| P3.3R1 | 45 | 500 | 5.06 | 0.92 | 0.96 | 0.04 |
| P3.3R2 | 51 | 500 | 5.10 | 0.90 | 0.96 | 0.04 |
| P3.3R3 | 53 | 500 | 5.24 | 0.91 | 0.97 | 0.03 |
| P4.1R1 | 45 | 500 | 4.94 | 0.90 | 0.96 | 0.04 |
| P4.1R2 | 51 | 500 | 5.06 | 0.89 | 0.96 | 0.04 |
| P4.1R3 | 45 | 500 | 4.92 | 0.90 | 0.96 | 0.04 |
| P4.2R1 | 52 | 500 | 4.96 | 0.87 | 0.96 | 0.04 |
| P4.2R2 | 47 | 500 | 4.97 | 0.89 | 0.96 | 0.04 |
| P4.2R3 | 42 | 500 | 4.85 | 0.90 | 0.96 | 0.04 |
| P4.3R1 | 50 | 500 | 5.00 | 0.89 | 0.96 | 0.04 |
| P4.3R2 | 47 | 500 | 4.87 | 0.88 | 0.96 | 0.04 |
| P4.3R3 | 46 | 500 | 4.90 | 0.89 | 0.96 | 0.04 |
| P5.1R1 | 48 | 500 | 4.83 | 0.87 | 0.95 | 0.05 |
| P5.1R2 | 46 | 500 | 5.00 | 0.90 | 0.96 | 0.04 |
| P5.1R3 | 49 | 500 | 4.99 | 0.89 | 0.96 | 0.04 |
| P5.2R1 | 46 | 500 | 4.95 | 0.90 | 0.96 | 0.04 |
| P5.2R2 | 49 | 500 | 5.01 | 0.89 | 0.96 | 0.04 |
| P5.2R3 | 45 | 500 | 5.05 | 0.92 | 0.96 | 0.04 |
| P5.3R1 | 53 | 500 | 5.13 | 0.90 | 0.97 | 0.03 |
| P5.3R2 | 49 | 500 | 5.04 | 0.90 | 0.96 | 0.04 |
| P5.3R3 | 45 | 500 | 4.96 | 0.90 | 0.96 | 0.04 |
| P6.1R1 | 49 | 500 | 5.00 | 0.89 | 0.96 | 0.04 |
| P6.1R2 | 43 | 500 | 4.89 | 0.90 | 0.96 | 0.04 |
| P6.1R3 | 41 | 500 | 4.88 | 0.91 | 0.96 | 0.04 |
| P6.2R1 | 52 | 500 | 5.10 | 0.89 | 0.96 | 0.04 |
| P6.2R2 | 47 | 500 | 4.90 | 0.88 | 0.96 | 0.04 |
| P6.2R3 | 40 | 500 | 4.90 | 0.92 | 0.96 | 0.04 |
| P6.3R1 | 47 | 500 | 4.96 | 0.89 | 0.96 | 0.04 |
| P6.3R2 | 43 | 500 | 4.93 | 0.91 | 0.96 | 0.04 |
| P6.3R3 | 45 | 500 | 5.04 | 0.92 | 0.96 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siqueiros Beltrones, D.A.; Echevarría Herrera, E.; López-Fuerte, F.O.; Martínez, Y.J. Species Diversity of Benthic Marine Diatoms from a Natural Protected Area in Cuba. Diversity 2025, 17, 181. https://doi.org/10.3390/d17030181
Siqueiros Beltrones DA, Echevarría Herrera E, López-Fuerte FO, Martínez YJ. Species Diversity of Benthic Marine Diatoms from a Natural Protected Area in Cuba. Diversity. 2025; 17(3):181. https://doi.org/10.3390/d17030181
Chicago/Turabian StyleSiqueiros Beltrones, David Alfaro, Erisbel Echevarría Herrera, Francisco Omar López-Fuerte, and Yuriko Jocselin Martínez. 2025. "Species Diversity of Benthic Marine Diatoms from a Natural Protected Area in Cuba" Diversity 17, no. 3: 181. https://doi.org/10.3390/d17030181
APA StyleSiqueiros Beltrones, D. A., Echevarría Herrera, E., López-Fuerte, F. O., & Martínez, Y. J. (2025). Species Diversity of Benthic Marine Diatoms from a Natural Protected Area in Cuba. Diversity, 17(3), 181. https://doi.org/10.3390/d17030181






