Invasive Aquatic Weeds Suppress Predator–Prey Cascades: Evidence from a Mesocosm Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Statistical Analysis
3. Results
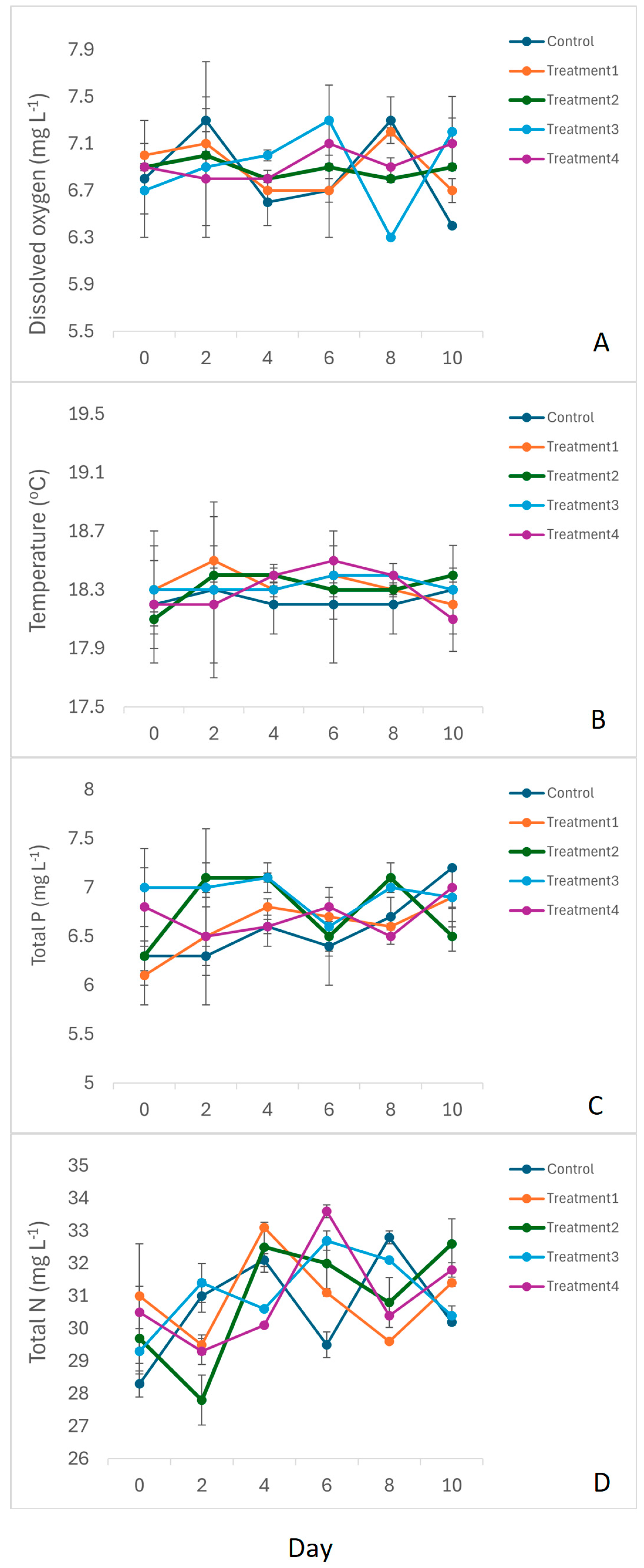
3.1. Physicochemical Variables
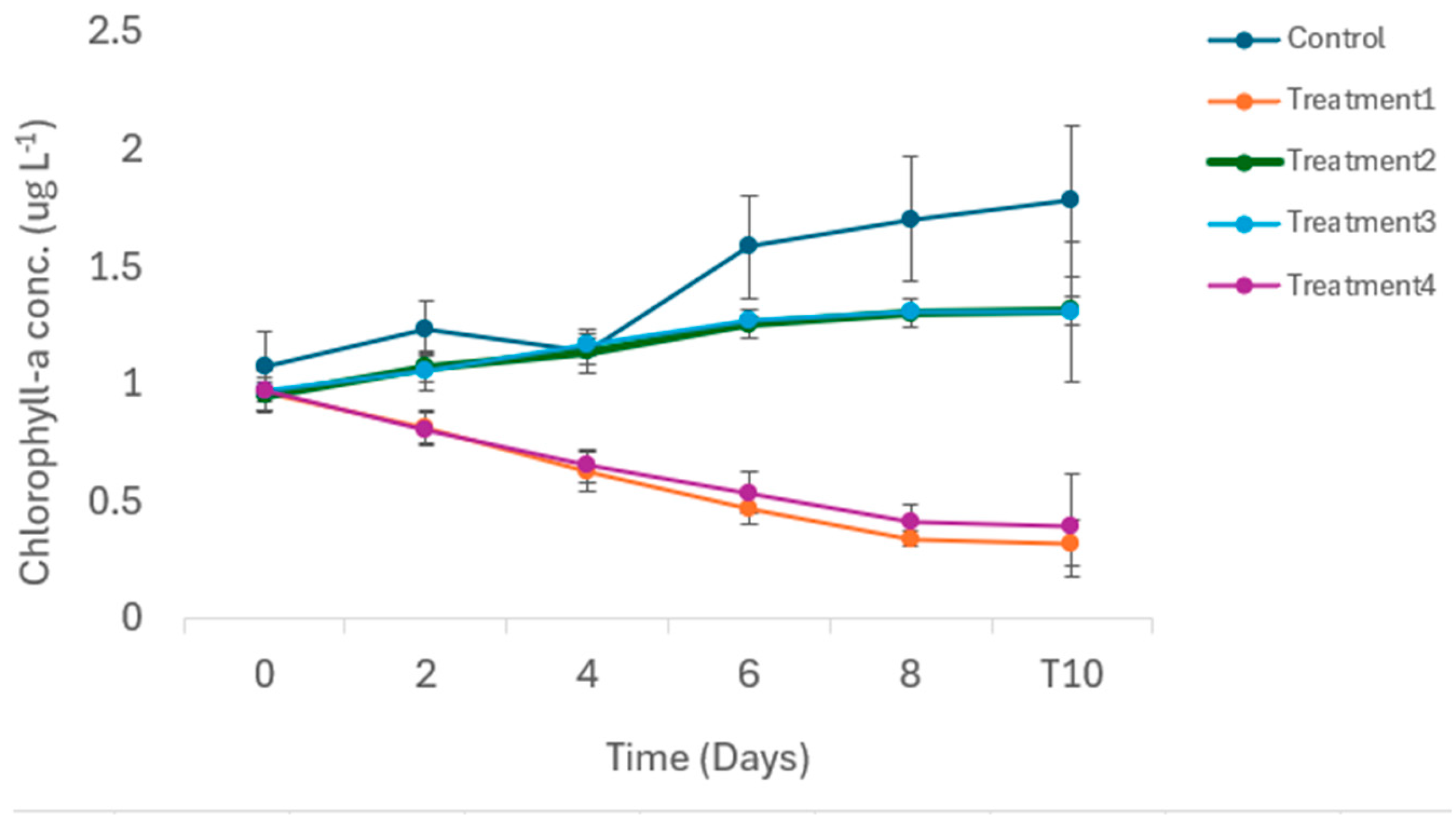
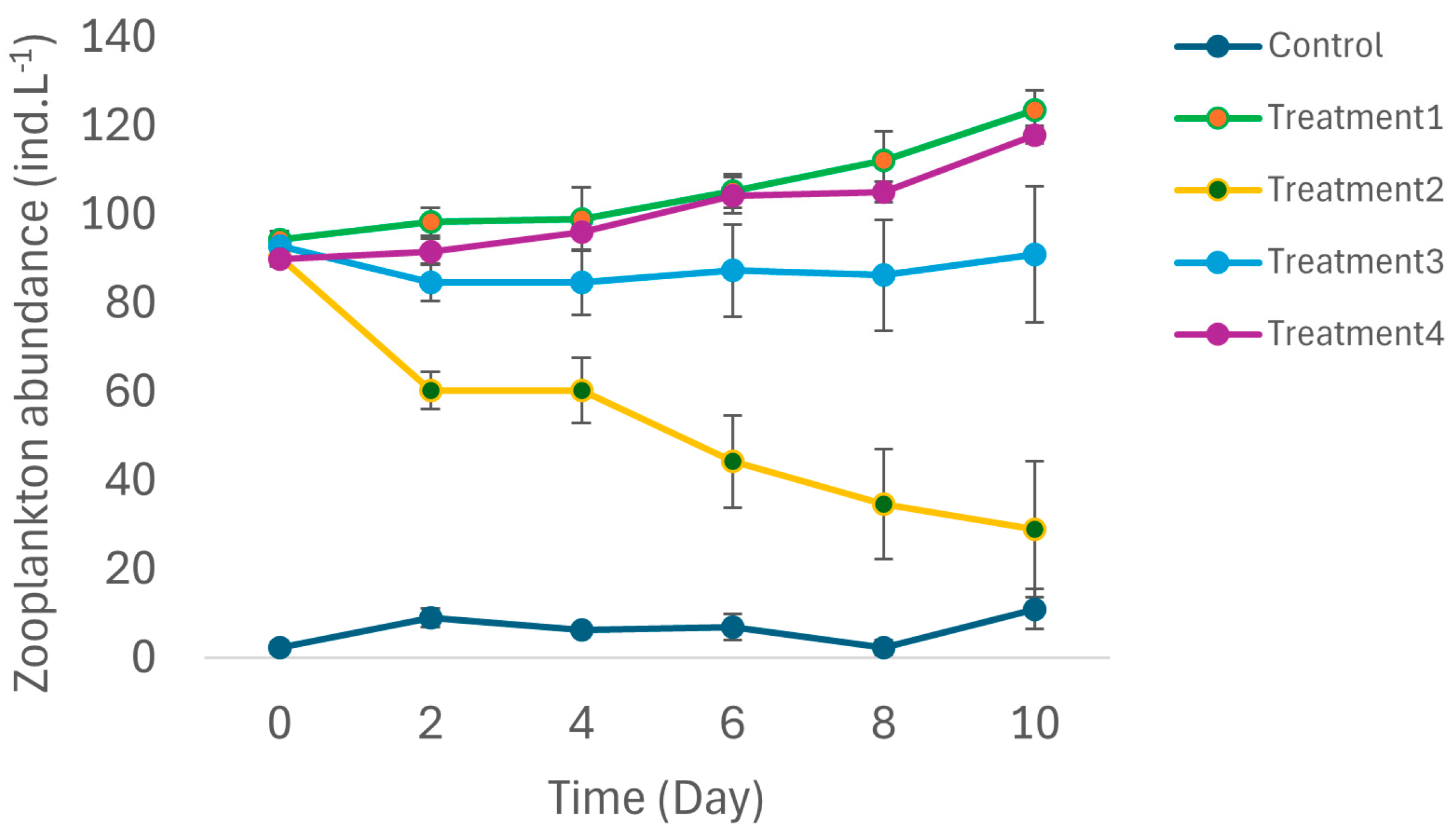
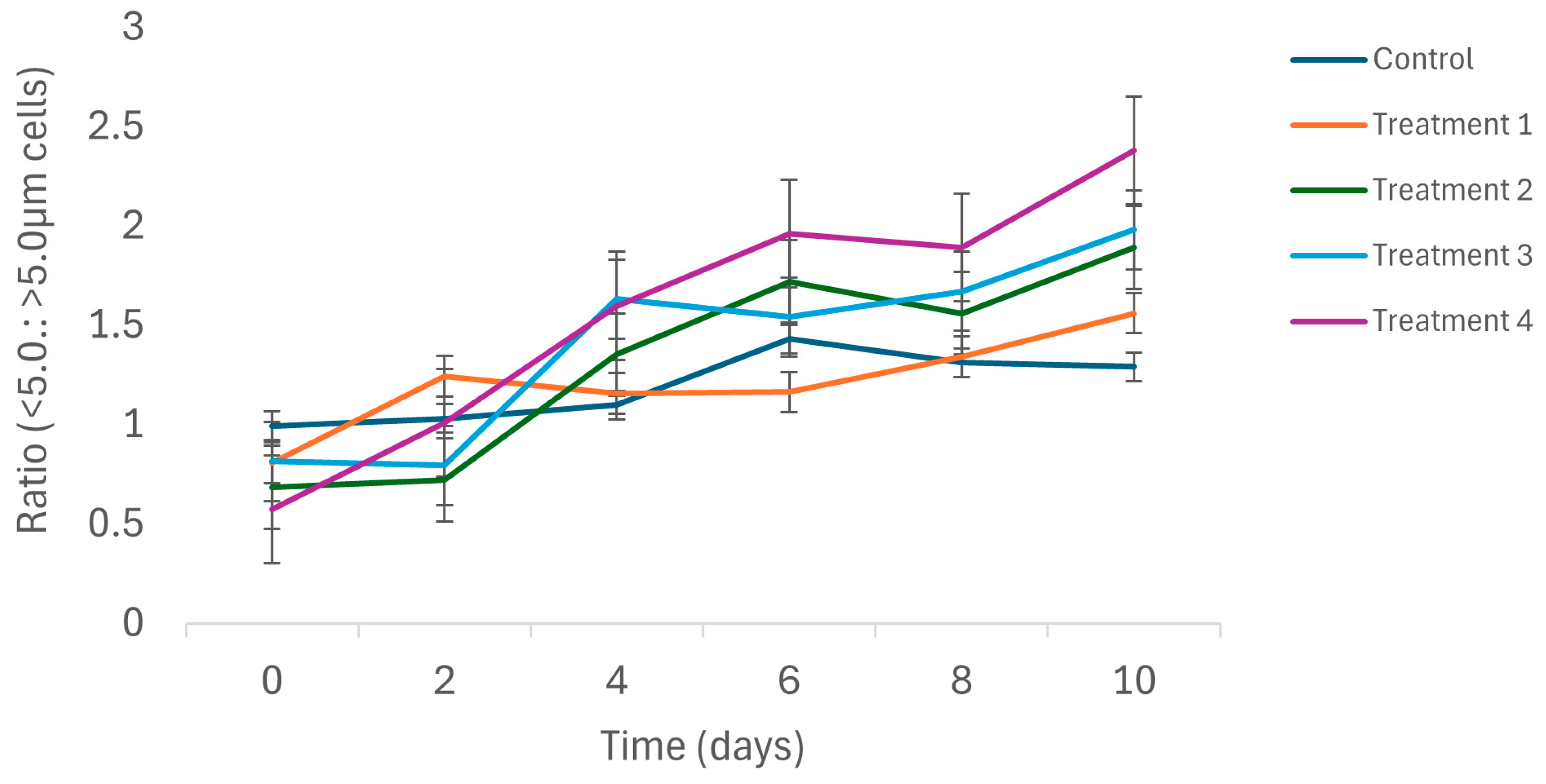
3.2. Biological Variables
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Nawchoo, I.A. Impact of Invasive Plants in Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors and prospects for future. Fresh. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Stiers, I.; Crohain, N.; Josens, G.; Triest, L. Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol. Invasions 2011, 13, 2715–2726. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterising ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Pace, M.L.; Cole, J.; Carpenter, S.R.; Kitchell, R.F. Trophic cascades revealed in diverse ecosystems. TREE Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef]
- Bruno, J.F.; Connor, M.O. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Letters 2005, 8, 1048–1056. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Vink, T.J.F.; Kramer, R.; Froneman, P.W. Hyperbenthic and pelagic predators regulate alternate key planktonic predators in shallow temperate estuaries. Mar. Freshw. Res. 2014, 65, 791–801. [Google Scholar] [CrossRef]
- Froneman, P.W.; Cuthbert, R.N. Habitat complexity alters predator-prey interactions in a shallow water ecosystem. Diversity 2022, 14, 431. [Google Scholar] [CrossRef]
- Klecka, J.; Boukal, D.S. The effect of habitat structure on prey mortality depends on predator and prey microhabitat use. Oecologia 2014, 176, 183–191. [Google Scholar] [CrossRef]
- Alexander, M.E.; Kaiser, H.; Weyl, O.L.F.; Dick, J.T.A. Habitat simplification increases the impact of a freshwater invasive fish. Environ. Biol. Fishes 2015, 98, 477–486. [Google Scholar] [CrossRef]
- Coetzee, J.A.; Hill, M.P.; Byrne, M.J.; Bownes, A. A Review of the Biological Control Programmes on Eichhornia crassipes (C.Mart.) Solms (Pontederiaceae), Salvinia molesta D.S.Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragaceae) and Azolla filiculoide. Afr.Ento. 2011, 19, 451–468. [Google Scholar] [CrossRef]
- King, A.M. The Effect of Temperature on Biological Control of Water Hyacinth, Eichhornia crassipes (Pontederiaceae) in South Africa. Master’s Thesis, University of Witwatersrand, South Africa, 2011; p. 152. [Google Scholar]
- Coetzee, J.A.; Jones, R.W.; Hill, M.P. Water hyacinth, Eichhornia crassipes (Pontederiaceae), reduces benthic macroinvertebrate diversity in a protected subtropical lake in South Africa. Biodivers. Conserv. 2014, 23, 1319–1330. [Google Scholar] [CrossRef]
- Holm-Hansen, O.; Riemann, B. Chlorophyll a Determination: Improvements in Methodology. Oikos 1978, 30, 438–447. [Google Scholar] [CrossRef]
- Venables, W.N.; Smith, D.M. The R development core team. In An Introduction to R, Version 1.0; R Core Team: Vienna, Austria, 2003. [Google Scholar]
- Dudgeon, D. Threats to Freshwater Biodiversity in a Changing World. In Global Environmental Change: Handbook of Global Environmental Pollution; Freedman, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 243–253. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chaurisia, S. Role of macrophytes: A review. Adv. Zoo. Bot. 2022, 10, 75–81. [Google Scholar] [CrossRef]
- Schultz, R.; Dibble, E. Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: The role of invasive plant traits. Hydrobiologia 2012, 684, 1–14. [Google Scholar] [CrossRef]
- Toft, J.D.; Simenstadt, C.; Cordell, J.; Grimaldo, M. The effects of introduced water hyacinth on habitat structure, invertebrate assemblages and fish diet. Est. Coasts 2003, 26, 746–758. [Google Scholar] [CrossRef]
- Midgley, J.M.; Hill, M.P.; Villet, M.H. The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr. J. Aquat. Sci. 2006, 31, 25–30. [Google Scholar] [CrossRef]
- Quirino, B.A.; Thomaz, S.M.; Jeppesen, E.; Søndergaard, M.; Dainez-Filho, M.S.; Fugi, R. Aquatic Macrophytes Shape the Foraging Efficiency, Trophic Niche Breadth, and Overlap among Small Fish in a Neotropical River. Water 2022, 14, 3543. [Google Scholar] [CrossRef]
- Cambray, J.A. Impact on indigenous species biodiversity caused by the globalisation of alien recreation freshwater fisheries. Hydrobiologia 2003, 500, 217–230. [Google Scholar] [CrossRef]
- Keller, R.P.; Masoodi, A.; Shackleton, R.T. The impact of invasive aquatic plants on ecosystem services and human well-being in Wular Lake, India. Reg. Environ. Change 2018, 18, 847–857. [Google Scholar] [CrossRef]
- Bartholomew, A. Space size relative to prey width and total cover in an area both influence the habitat choices of freshwater angelfish Pterophyllum scalare in mesocosms. Mar. Freshw. Behav. Physiol. 2012, 45, 29–43. [Google Scholar] [CrossRef]
- Auffan, M.; Tella, M.; Santaella, C.; Brousset, L.; Paillès, C.; Barakat, M.; Espinasse, B.; Artells, E.; Issartel, J.; Masion, A.; et al. An adaptable mesocosm platform for performing integrated assessments of nanomaterial risk in complex environmental systems. Sci. Rep. 2014, 4, 5608. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Caughlin, T.T.; Civitello, D.J.; Flory, S.L. Combining mesocosm and field experiments to predict invasive plant performance: A hierarchical Bayesian approach. Ecology 2015, 96, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Hodgkins, G.A.; Stoner, A.W. A mesocosm system for ecological research with marine invertebrate larvae. Mar. Ecol. Prog. Ser. 1996, 130, 97–104. [Google Scholar] [CrossRef][Green Version]
- Graney, R.L. Aquatic Mesocosms in Ecological Risk Assessment; CRC Press: Boca Raton, FL, USA, 2020; 736p. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Jarvie, H.P. Delivery and cycling of phosphorus in rivers: A review. Sci. Total Environ. 2008, 400, 379–395. [Google Scholar] [CrossRef]
- Atkinson, C.L.; Capps, K.A.; Rugenski, A.T.; Vanni, M.J. Consumer driven nutrient dynamics in freshwater ecosystems: From Individuals to ecosystems. Bio. Rev. Camb. Philos. Soc. 2017, 94, 2003–2023. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, L.; Hilt, S.; Xu, R.; Wang, B.; Li, C.; Chang, X. Root exudated algicide of Eichhornia crassipes enhances allelopathic effects of cyanobacteria Microcystis aeruginosa on green algae. Hydrobiologia 2018, 823, 67–77. [Google Scholar] [CrossRef]
- Lao, X.F.K. Isolation and identification of antialgal compounds in root system of water hyacinth. Acta. Phytophysiol. Sin. 1992, 18, 399–402. [Google Scholar]
- Wasserman, R.J.; Alexander, M.E.; Weyl, O.L.F.; Froneman, P.W.; Dalu, T. Emergent effects of structural complexity and temperrture on predator-prey interactions. Ecosphere 2016, 7, e01239. [Google Scholar] [CrossRef]
- Sarnelle, O. Daphnia as keystone predators: Effects on phytoplankton diversity and grazing resistance. J. Plankton Res. 2005, 27, 1229–1238. [Google Scholar] [CrossRef]
- Sommer, U. Trophic cascades in marine and freshwater plankton. Int. Rev. Hydrobiol. 2008, 93, 506–516. [Google Scholar] [CrossRef]
- Hoveka, L.N.; Bezeng, B.S.; Yessoufou, K.; Baotwright, J.S.; Van der Bank, M. Effects of global climate change on the future distributions of the top five freshwater invasive plants in South Africa. S. Afr. J. Bot. 2016, 102, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froneman, P.W. Invasive Aquatic Weeds Suppress Predator–Prey Cascades: Evidence from a Mesocosm Study. Diversity 2025, 17, 178. https://doi.org/10.3390/d17030178
Froneman PW. Invasive Aquatic Weeds Suppress Predator–Prey Cascades: Evidence from a Mesocosm Study. Diversity. 2025; 17(3):178. https://doi.org/10.3390/d17030178
Chicago/Turabian StyleFroneman, Pierre William. 2025. "Invasive Aquatic Weeds Suppress Predator–Prey Cascades: Evidence from a Mesocosm Study" Diversity 17, no. 3: 178. https://doi.org/10.3390/d17030178
APA StyleFroneman, P. W. (2025). Invasive Aquatic Weeds Suppress Predator–Prey Cascades: Evidence from a Mesocosm Study. Diversity, 17(3), 178. https://doi.org/10.3390/d17030178




