Evaluation of Soil Fertility in Alpine Shrub Communities of the Qilian Mountains, Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Soil Sample Collection and Analysis
2.4. Statistical Analyses
3. Results
3.1. Statistical Analysis of Soil Physicochemical Properties
3.2. Spatial Variability in Soil Physicochemical Properties
3.2.1. Spatial Variability in BD and SWC
3.2.2. Spatial Variability in SOM and pH
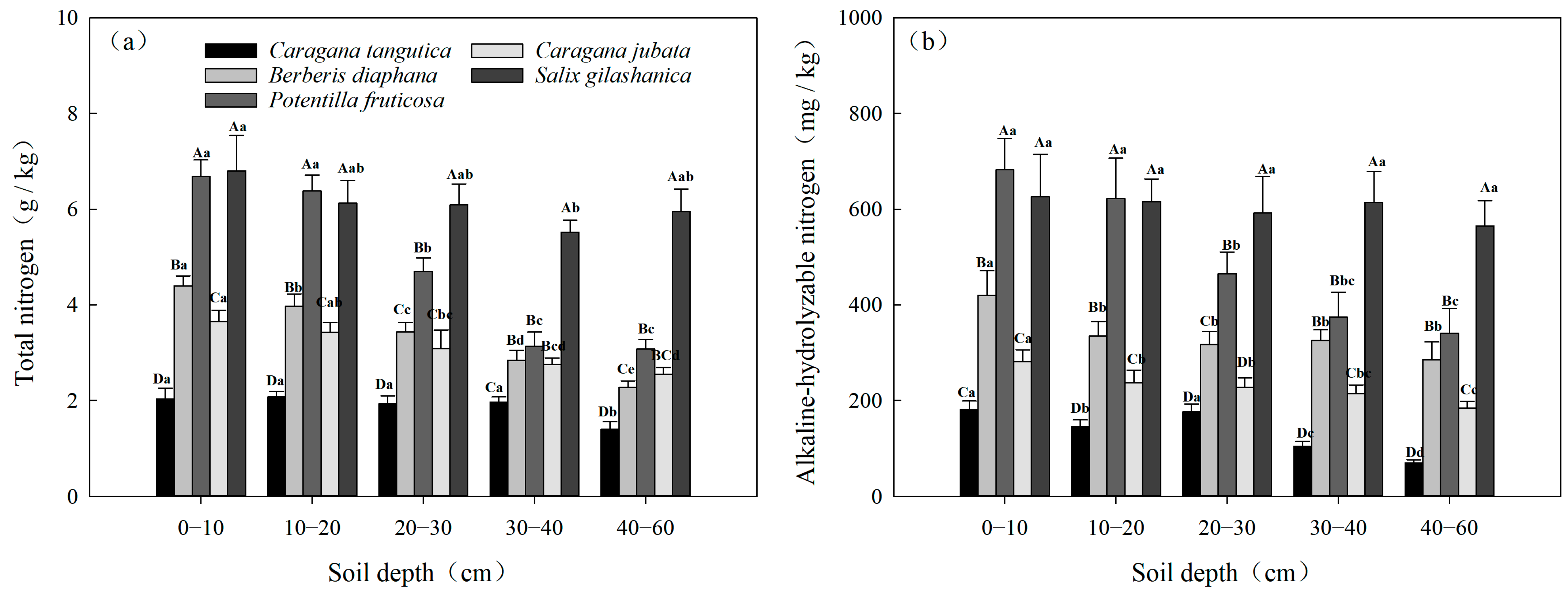
3.2.3. Spatial Variability in TN and AN
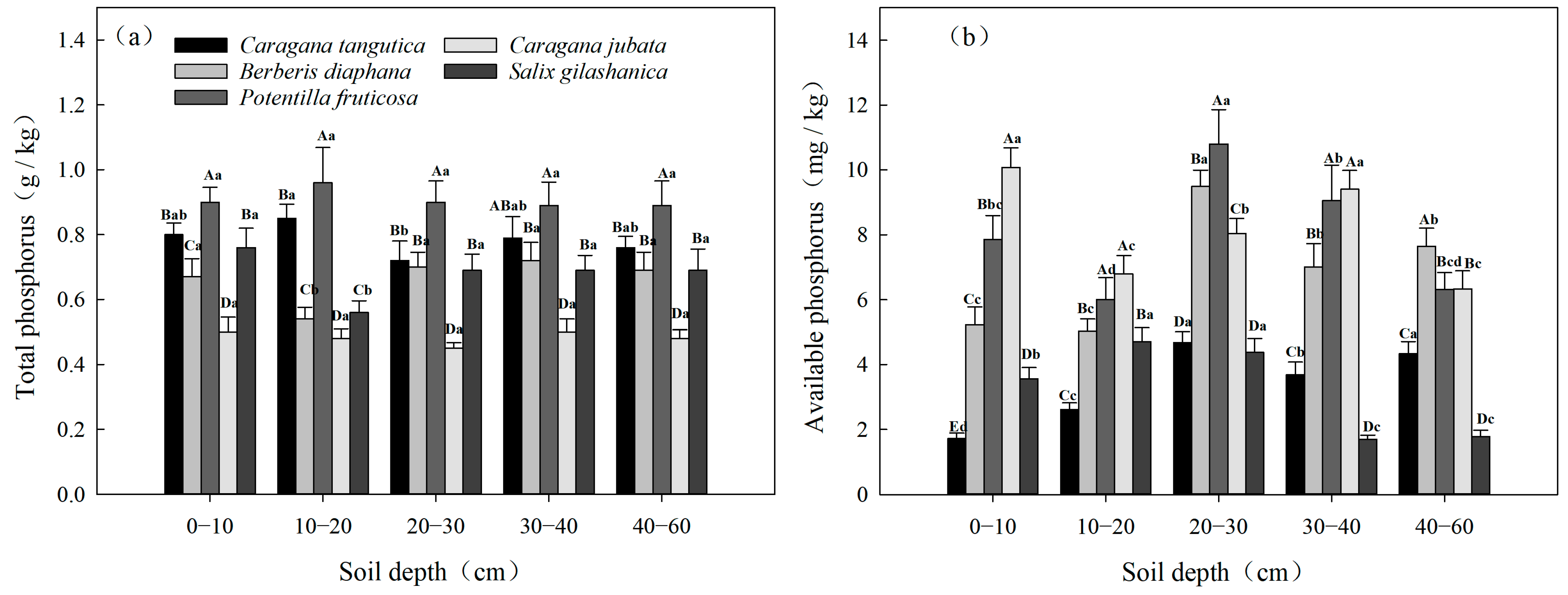
3.2.4. Spatial Variability in TP and AP
3.2.5. Spatial Variability in TK and AK
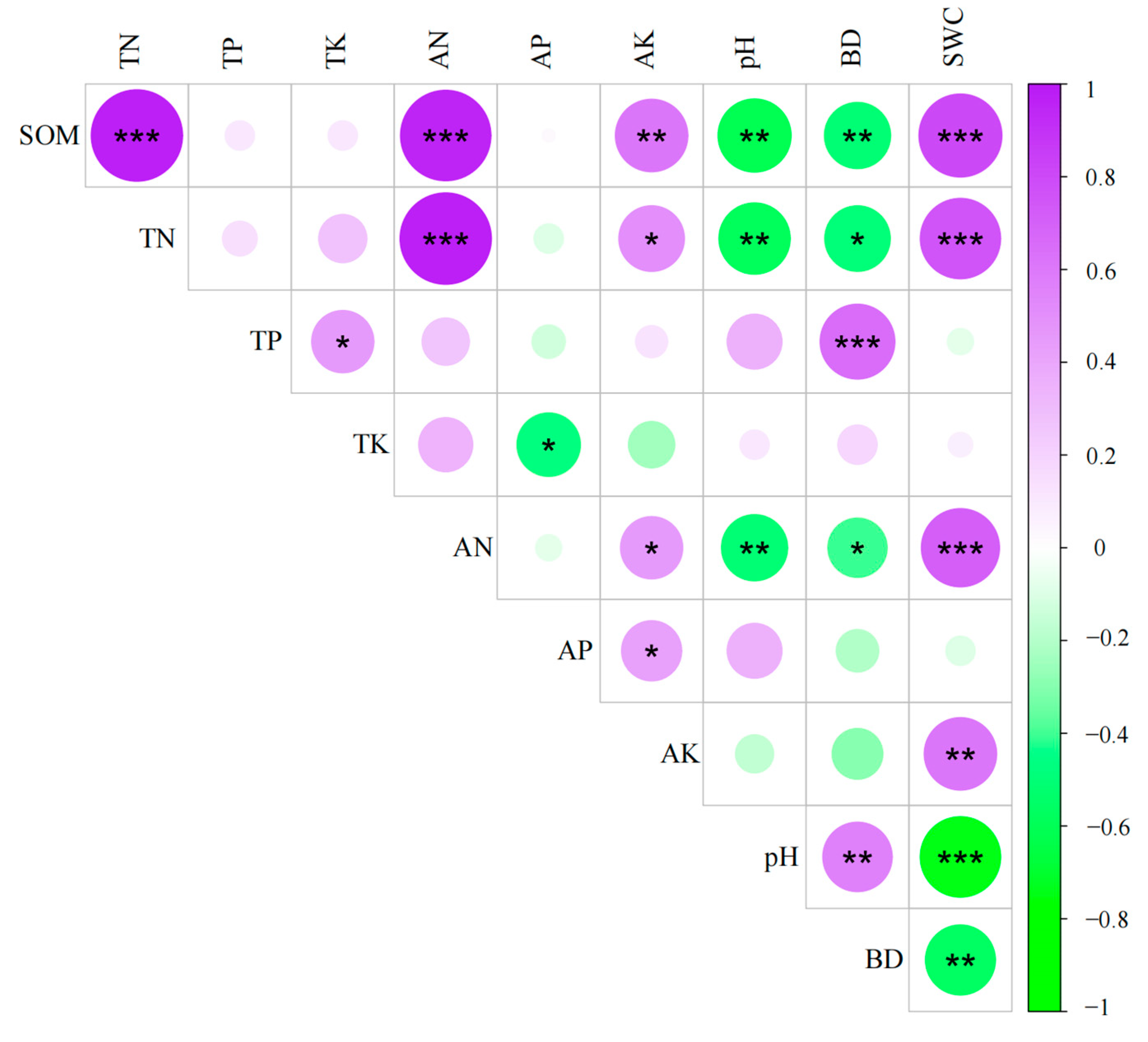
3.3. Correlation Analysis of Soil Physicochemical Properties
3.4. Principal Component Analysis of Soil Fertility Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.G.; Chen, Y.W.; Padilla, F.M.; Zhang, Y.M.; Huang, G. The influence of biocrusts on the spatial pattern of soil bacterial communities: A case study at landscape and slope scales. Soil Biol. Biochem. 2020, 142, 107721. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Crawford, K.M.; Bauer, J.T.; Comita, L.S.; Eppinga, M.B.; Johnson, D.J.; Mangan, S.A.; Queenborough, S.A.; Strand, A.E.; Suding, K.N.; Umbanhowar, J.; et al. When and where plant-soil feedback may promote plant coexistence: A meta-analysis. Ecol. Lett. 2019, 22, 1274–1284. [Google Scholar] [CrossRef]
- Qiao, L.L.; Li, Y.Z.; Song, Y.H.; Zhai, J.Y.; Wu, Y.; Chen, W.J.; Liu, G.B.; Xue, S. Effects of vegetation restoration on the distribution of nutrients, glomalin-related soil protein, and enzyme activity in soil aggregates on the Loess Plateau, China. Forests 2019, 10, 796. [Google Scholar] [CrossRef]
- Wellbrock, N.; Cools, N.; De Vos, B.; Jandl, R.; Lehtonen, A.; Leitgeb, E.; Mäkipää, R.; Pavlenda, P.; Schwärtzel, K.; Šrámek, V. There is a need to better take into account forest soils in the planned soil monitoring law of the european union. Ann. For. Sci. 2024, 81, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, C.; Li, W.; Lin, L.; Liao, T.L.; Fan, X.P.; Peng, H.Y.; An, Q.; Liang, Y.C. Phosphorus-, potassium-, and silicon-solubilizing bacteria from forest soils can mobilize soil minerals to promote the growth of rice (Oryza sativa L.). Chem. Biol. Technol. Agric. 2024, 11, 103. [Google Scholar] [CrossRef]
- Yu, S.Y.; Liao, Z.H.; Yang, M.W.; Hu, R.H.; Shi, Y.Y.; Zhao, Y.Y. Evaluation of soil fertility quality and environmental driving factors in different soil types of artificial forests. Meteorol. Environ. Res. 2024, 15, 64–70. [Google Scholar]
- Nabiollahi, K.; Golmohamadi, F.; Taghizadeh-mehrjardi, R.; Kerry, R.; Davari, M. Assessing the effects of slope gradient and land use change on soil quality degradation through digital mapping of soil quality indices and soil loss rate. Geoderma 2018, 318, 16–28. [Google Scholar] [CrossRef]
- Zhu, M.; Feng, Q.; Zhang, M.; Liu, W.; Qin, Y.; Deo, R.C.; Zhang, C. Effects of topography on soil organic carbon stocks in grasslands of a semiarid alpine region, northwestern China. J. Soils Sediments 2019, 19, 640–1650. [Google Scholar] [CrossRef]
- Guo, Y.; Abdalla, M.; Espenberg, M.; Hastings, A.; Hallett, P.; Smith, P. A systematic analysis and review of the impacts of afforestation on soil quality indicators as modified by climate zone, forest type and age. Sci. Total Environ. 2020, 757, 143824. [Google Scholar] [CrossRef]
- Qian, D.W.; Cao, G.M.; Du, Y.G.; Li, Q.; Guo, X.W. Impacts of climate change and human factors on land cover change in inland mountain protected areas: A case study of the Qilian Mountain National Nature Reserve in China. Environ. Monit. Assess. 2019, 191, 486. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, S.W.; Ran, Y.H.; Wang, H.W.; Wu, J.C.; Lian, X.H.; Luo, D.L. Mapping frozen ground in the Qilian Mountains in 2004–2019 using Google earth engine cloud computing. Remote. Sens. 2021, 13, 149. [Google Scholar] [CrossRef]
- Sun, F.X.; Lyu, Y.H.; Fu, B.J.; Hu, J. Hydrological services by mountain ecosystems in Qilian Mountain of China: A review. Chin. Geogr. Sci. 2016, 26, 174–187. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.H.; Yang, G.J.; Guo, R.; Xia, C.Z.; Liu, Y. Performance and obstacle tracking to Natural Forest Resource Protection Project: A rangers’ case of Qilian Mountain, China. Int. J. Environ. Res. Public Health 2020, 17, 5672. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; He, Z.B.; Chen, L.F.; Lin, P.F.; Zhu, X.; Tian, Q.Y. Impact of climate change on alpine plant community in Qilian Mountains of China. Int. J. Biometeorol. 2021, 65, 1849–1858. [Google Scholar] [CrossRef]
- He, X.L.; Ma, J.; Jin, M.; Li, Z. Characteristics and controls of ecological stoichiometry of shrub leaf in the alpine region of northwest China. Catena 2023, 224, 107005. [Google Scholar] [CrossRef]
- Zhao, J.; Adu, B.; Wang, J.; Fan, Y. Assessing Shrub Patch Characteristics and Soil Nutrient Distribution Patterns of Four Typical Alpine Shrub Plants in the Eastern Qilian Mountains. Sustainability 2024, 16, 1547. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, Y.; Geng, Y.; Wu, Y.; Niu, Z. Biochemical composition and function of subalpine shrubland and meadow soil microbiomes in the Qilian Mountains, Qinghai–Tibetan plateau, China. PeerJ 2022, 10, e13188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, W.; Yang, L.; Zhu, G.; Lan, X.; Luo, H.; Yu, Z. Change Characteristics of Soil Organic Carbon and Soil Available Nutrients and Their Relationship in the Subalpine Shrub Zone of Qilian Mountains in China. Sustainability 2023, 15, 13028. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014. [Google Scholar]
- Lu, R.K. Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Soil Society of China. Soil Argrochemistry Analysis Protocoes; China Agriculture Science Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Xu, H.; Qu, Q.; Li, P.; Guo, Z.Q.; Wulan, E.; Xue, S. Stocks and stoichiometry of soil organic carbon, total nitrogen, and total phosphorus after vegetation restoration in the Loess Hilly Region, China. Forests 2019, 10, 27. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.G. Response of forest growth to C: N: P stoichiometry in plants and soils during Robinia pseudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C: N: P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Cheng, M.; An, S.S. Responses of soil nitrogen, phosphorous and organic matter to vegetation succession on the Loess Plateau of China. J. Arid. Land 2015, 7, 216–223. [Google Scholar] [CrossRef]
- Tao, W.; Yuanhe, Y.; Wenhong, M. Storage, Patterns and Environmental Controls of Soil Phosphorus in China. Acta Sci. Nat. Univ. Pekin. 2008, 44, 945–952. (In Chinese) [Google Scholar]
- Bui, E.-N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Wilding, L.P. Spatial variability: Its documentation, accomodation and implication to soil surveys. Spatial Variations, 1985.
- Herbert, S.J. Spatial variability of nutrient properties in black soil of northeast China. Pedosphere 2007, 17, 19–29. [Google Scholar]
- Chapin, F.S.; Matson, P.A.; Mooney, H.A.; Viyousek, P.M. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002; pp. 1–447. [Google Scholar]
- Gaston, L.; Locke, M.; Zablotowicz, R.; Reddy, K. Spatial variability of soil properties and weed populations in the Mississippi Delta. Soil Sci. Soc. Am. J. 2001, 65, 449–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Wang, M. Linkages of C: N: P stoichiometry between soil and leaf and their response to climatic factors along altitudinal gradients. J. Soils Sediments 2019, 19, 1820–1829. [Google Scholar] [CrossRef]
- Ren, C.J.; Zhang, W.; Zhong, Z.K.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610–611, 750–758. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Tan, H.; Hu, R.; Su, J.Q.; Wang, J.; Huang, L.; Zhang, Y.F.; Li, X.R. Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef]
- Su, Y.Q.; Wu, Z.L.; Xie, P.Y.; Zhang, L.; Chen, H. Warming effects on topsoil organic carbon and C:N:P stoichiometry in a subtropical forested landscape. Forests 2020, 11, 66. [Google Scholar] [CrossRef]
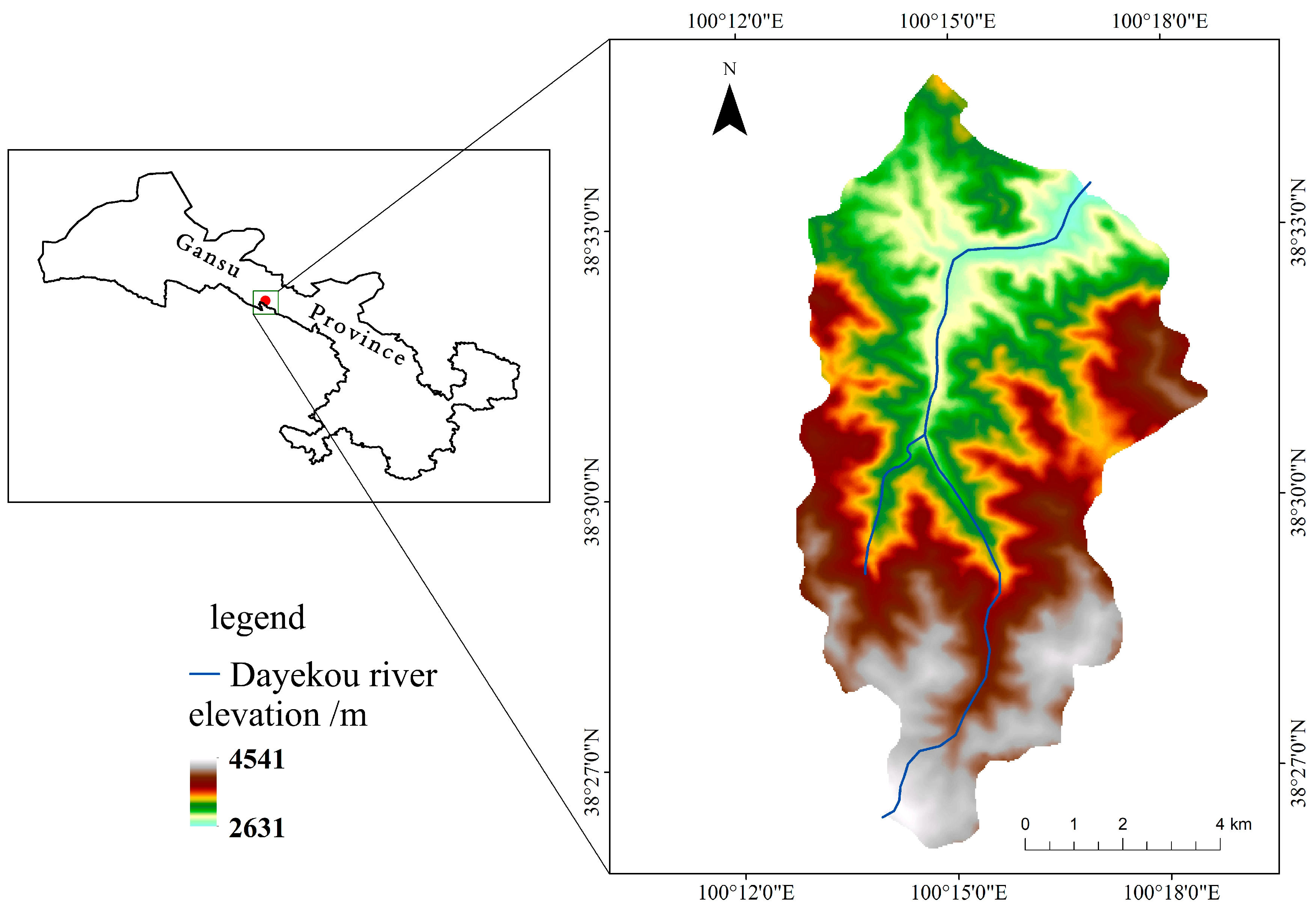
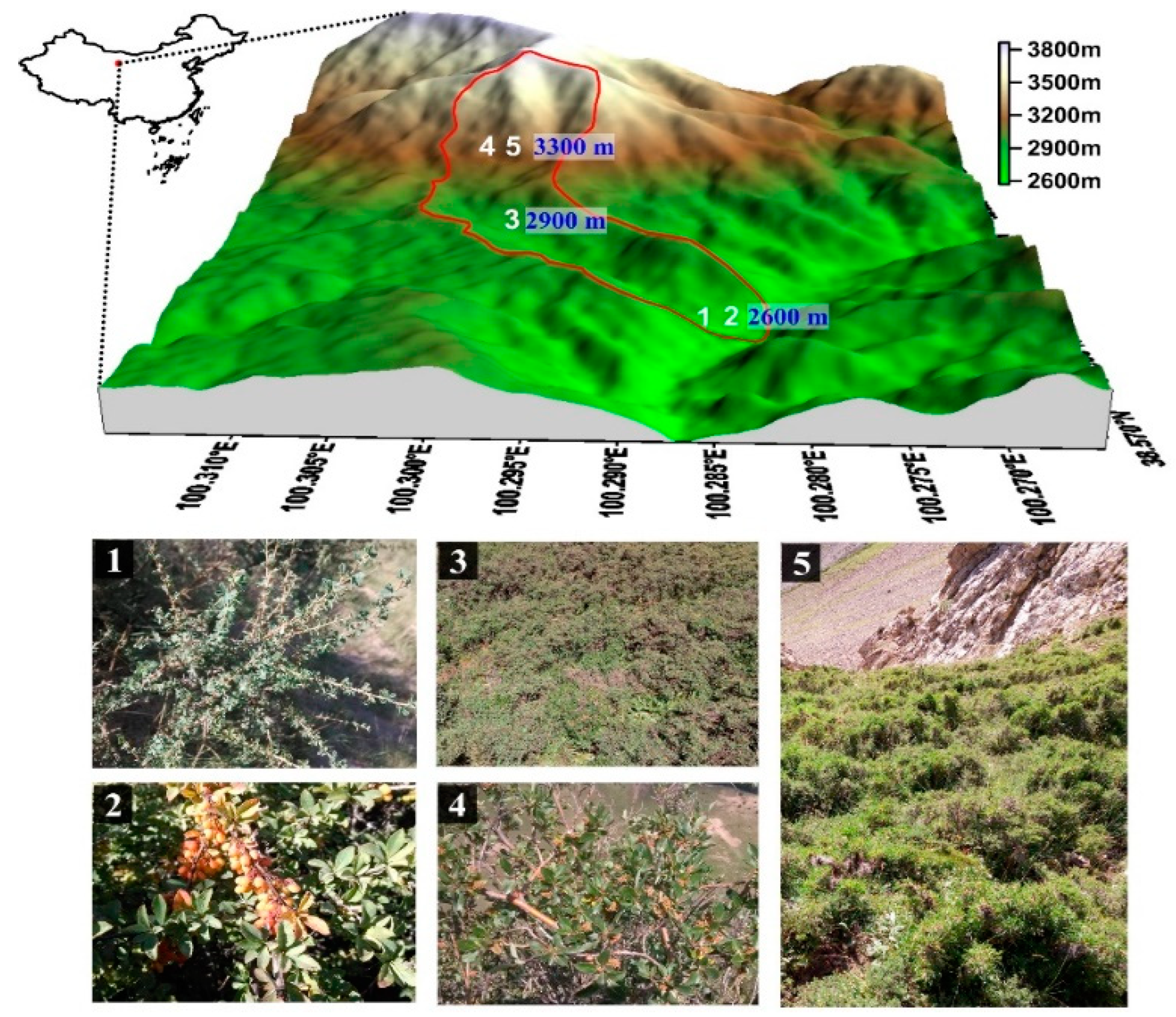
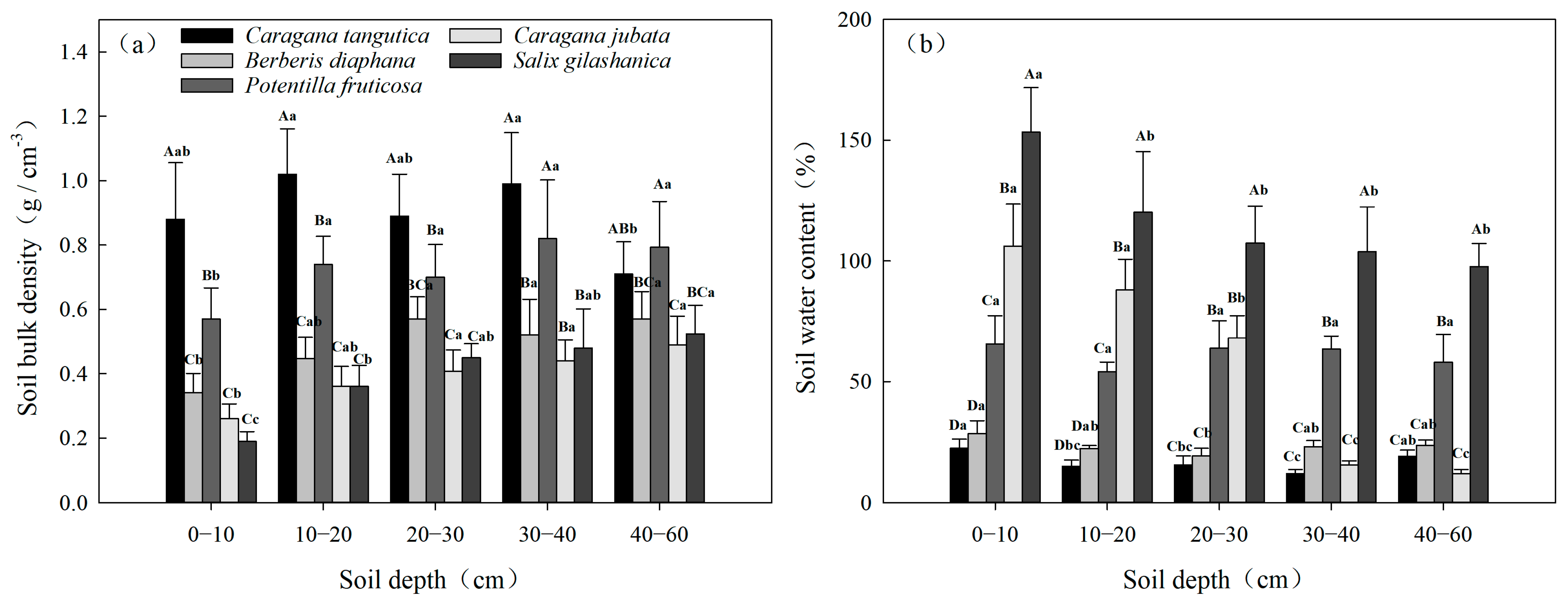
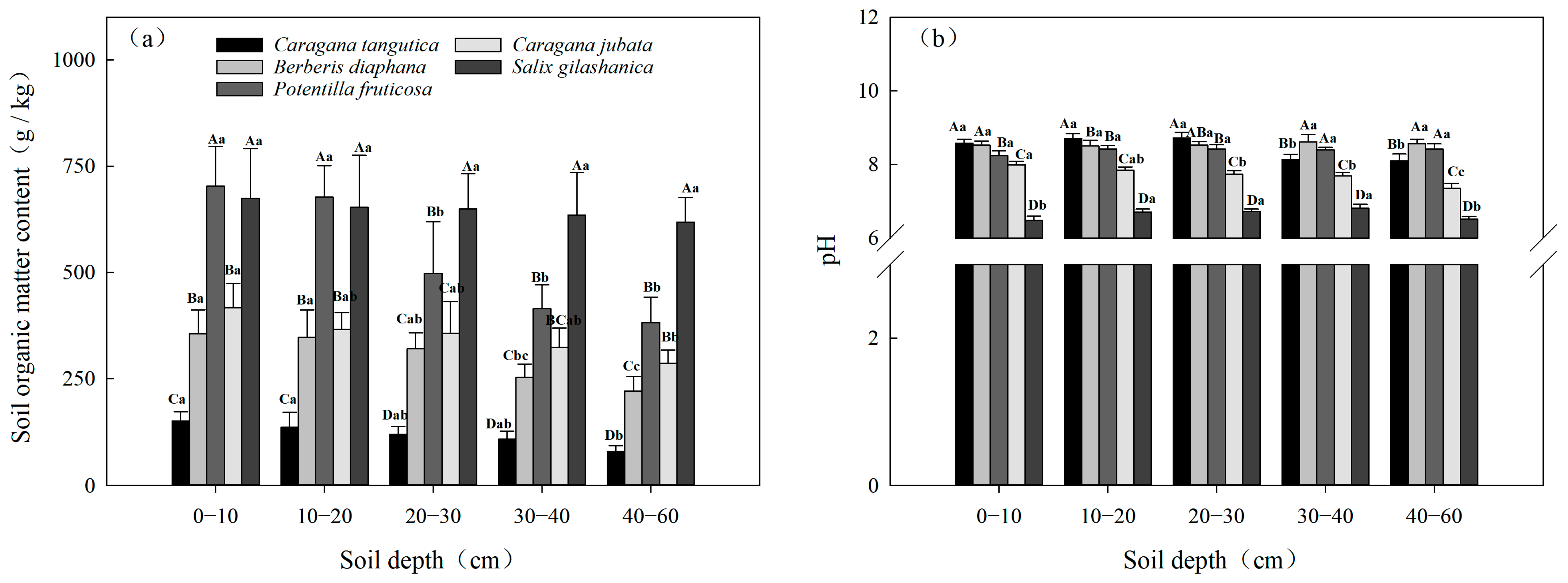

| Vegetation Type | Soil Depth (cm) | Soil Type | Elevation (m) | Slope Gradient (°) | Slope Aspect (°) | Growth Status | Basal Diameter (mm) | Coverage (%) | Average Height (m) |
|---|---|---|---|---|---|---|---|---|---|
| Caragana jubata | 60 | Alpine meadow soil | 3300 | 40 | NE | Average growth | 20 | 60 | 0.60 |
| Salix gilashanica | 60 | Alpine meadow soil | 3300 | 32 | NE | Good growth | 26 | 55 | 1.40 |
| Potentilla fruticosa | 60 | Alpine meadow soil | 2900 | 33 | E | Optimal growth | 16 | 90 | 0.90 |
| Berberis diaphana | 60 | Chestnut soil | 2600 | 30 | W | Average growth | 20 | 70 | 1.80 |
| Caragana tangutica | 60 | Chestnut soil | 2600 | 22 | SW | Average growth | 25 | 50 | 1.40 |
| Vegetation Type | SWC (%) | BD (g·cm−3) | pH | SOM (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Caragana tangutica | 16.79 c | 0.90 a | 8.45 a | 23.69 c | 1.88 d | 0.78 b | 14.15 ab | 135.00 c | 3.41 b | 52.74 c |
| Berberis diaphana | 23.27 c | 0.49 c | 8.54 a | 59.85 b | 3.38 bc | 0.66 c | 14.78 a | 336.40 b | 6.88 a | 59.72 c |
| Potentilla fruticosa | 61.00 b | 0.72 b | 8.38 a | 106.97 a | 4.79 ab | 0.91 a | 13.56 b | 496.60 a | 8.01 a | 192.96 a |
| Caragana jubata | 57.92 b | 0.39 c | 7.72 b | 70.01 b | 3.09 cd | 0.48 d | 11.40 c | 228.60 bc | 8.13 a | 151.60 ab |
| Salix gilashanica | 116.49 a | 0.40 c | 6.64 c | 129.18 a | 6.10 a | 0.68 c | 14.48 ab | 602.60 a | 3.22 b | 122.20 b |
| Mean value | 55.09 | 0.58 | 7.95 | 77.94 | 3.85 | 0.70 | 13.67 | 359.84 | 5.93 | 115.84 |
| Standard deviation | 41.27 | 0.23 | 0.75 | 40.13 | 1.71 | 0.15 | 1.42 | 187.89 | 2.68 | 65.48 |
| Variable coefficient % | 0.75 | 0.39 | 0.09 | 0.51 | 0.44 | 0.22 | 0.10 | 0.52 | 0.45 | 0.57 |
| Principal Components | Initial Eigenvalue | Sum of Square Loadings | ||||
|---|---|---|---|---|---|---|
| Eigenvalue | Contribution Rate % | Cumulative Contribution Rate % | Eigenvalue | Contribution Rate % | Cumulative Contribution Rate % | |
| 1 | 2.190 | 48.000 | 48.000 | 2.190 | 48.000 | 48.000 |
| 2 | 1.480 | 22.000 | 70.000 | 1.480 | 22.000 | 70.000 |
| 3 | 1.260 | 16.000 | 86.000 | 1.260 | 16.000 | 86.000 |
| 4 | 0.827 | 6.800 | 92.600 | |||
| 5 | 0.624 | 3.900 | 96.500 | |||
| 6 | 0.423 | 1.800 | 98.300 | |||
| 7 | 0.307 | 0.940 | 99.200 | |||
| 8 | 0.237 | 0.560 | 99.760 | |||
| 9 | 0.142 | 0.200 | 99.960 | |||
| 10 | 0.060 | 0.035 | 100.000 | |||
| Indicators | Principal Component | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| SOM (X1) | 0.441 | 0.064 | 0.113 |
| TN (X2) | 0.431 | 0.153 | 0.040 |
| TP (X3) | −0.019 | 0.547 | 0.397 |
| TK (X4) | 0.045 | 0.551 | −0.156 |
| AN (X5) | 0.412 | 0.218 | 0.088 |
| AP (X6) | −0.017 | −0.378 | 0.573 |
| AK (X7) | 0.277 | −0.140 | 0.504 |
| pH (X8) | −0.334 | 0.114 | 0.417 |
| BD (X9) | −0.296 | 0.383 | 0.192 |
| SWC (X10) | 0.413 | −0.034 | −0.054 |
| Shrub Community | F1 | F2 | F3 | Comprehensive Scores | Average Scores | Rank |
|---|---|---|---|---|---|---|
| Salix gilashanica | 4.480 | 0.819 | −1.096 | 2.505 | 1.709 | 1 |
| 3.376 | 0.096 | −1.401 | 1.647 | |||
| 2.850 | 0.232 | −1.102 | 1.444 | |||
| 2.575 | 0.863 | −1.773 | 1.328 | |||
| 2.899 | 1.042 | −1.424 | 1.620 | |||
| Potentilla fruticosa | 2.514 | 1.113 | 2.190 | 2.094 | 1.082 | 2 |
| 1.733 | 1.636 | 2.100 | 1.776 | |||
| 0.651 | 0.357 | 2.630 | 0.943 | |||
| −0.280 | 0.387 | 2.231 | 0.357 | |||
| −0.441 | 0.731 | 1.615 | 0.241 | |||
| Berberis diaphana | −0.019 | 0.628 | −0.440 | 0.069 | −0.597 | 3 |
| −0.729 | −0.024 | −1.033 | −0.604 | |||
| −1.236 | −0.010 | 0.460 | −0.606 | |||
| −1.467 | 0.183 | −0.074 | −0.785 | |||
| −1.839 | −0.062 | −0.087 | −1.057 | |||
| Caragana jubata | 1.372 | −3.116 | 1.444 | 0.232 | −0.747 | 4 |
| 0.468 | −2.190 | −0.196 | −0.338 | |||
| −0.094 | −2.834 | −0.195 | −0.817 | |||
| −1.018 | −2.932 | 0.074 | −1.307 | |||
| −1.221 | −2.473 | −1.006 | −1.503 | |||
| Caragana tangutica | −2.607 | 1.630 | −0.774 | −1.178 | −1.448 | 5 |
| −3.058 | 2.199 | −0.402 | −1.214 | |||
| −2.935 | 0.855 | −0.339 | −1.480 | |||
| −0.035 | 1.302 | −0.700 | −1.488 | |||
| −2.938 | −0.432 | −0.703 | −1.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Feng, Q.; Li, G.; Liu, W.; Chen, P.; Li, N.; Qian, W.; Teng, Y.; Li, X.; Li, J. Evaluation of Soil Fertility in Alpine Shrub Communities of the Qilian Mountains, Northwest China. Diversity 2025, 17, 175. https://doi.org/10.3390/d17030175
Ma J, Feng Q, Li G, Liu W, Chen P, Li N, Qian W, Teng Y, Li X, Li J. Evaluation of Soil Fertility in Alpine Shrub Communities of the Qilian Mountains, Northwest China. Diversity. 2025; 17(3):175. https://doi.org/10.3390/d17030175
Chicago/Turabian StyleMa, Jian, Qi Feng, Guang Li, Wei Liu, Peng Chen, Ning Li, Wanjian Qian, Yufeng Teng, Xiaopeng Li, and Jing Li. 2025. "Evaluation of Soil Fertility in Alpine Shrub Communities of the Qilian Mountains, Northwest China" Diversity 17, no. 3: 175. https://doi.org/10.3390/d17030175
APA StyleMa, J., Feng, Q., Li, G., Liu, W., Chen, P., Li, N., Qian, W., Teng, Y., Li, X., & Li, J. (2025). Evaluation of Soil Fertility in Alpine Shrub Communities of the Qilian Mountains, Northwest China. Diversity, 17(3), 175. https://doi.org/10.3390/d17030175







