Activity Patterns of Native Carnivores in Central Chile: Are They Influenced by Landscape Type?
Abstract
:1. Introduction
2. Materials and Methods
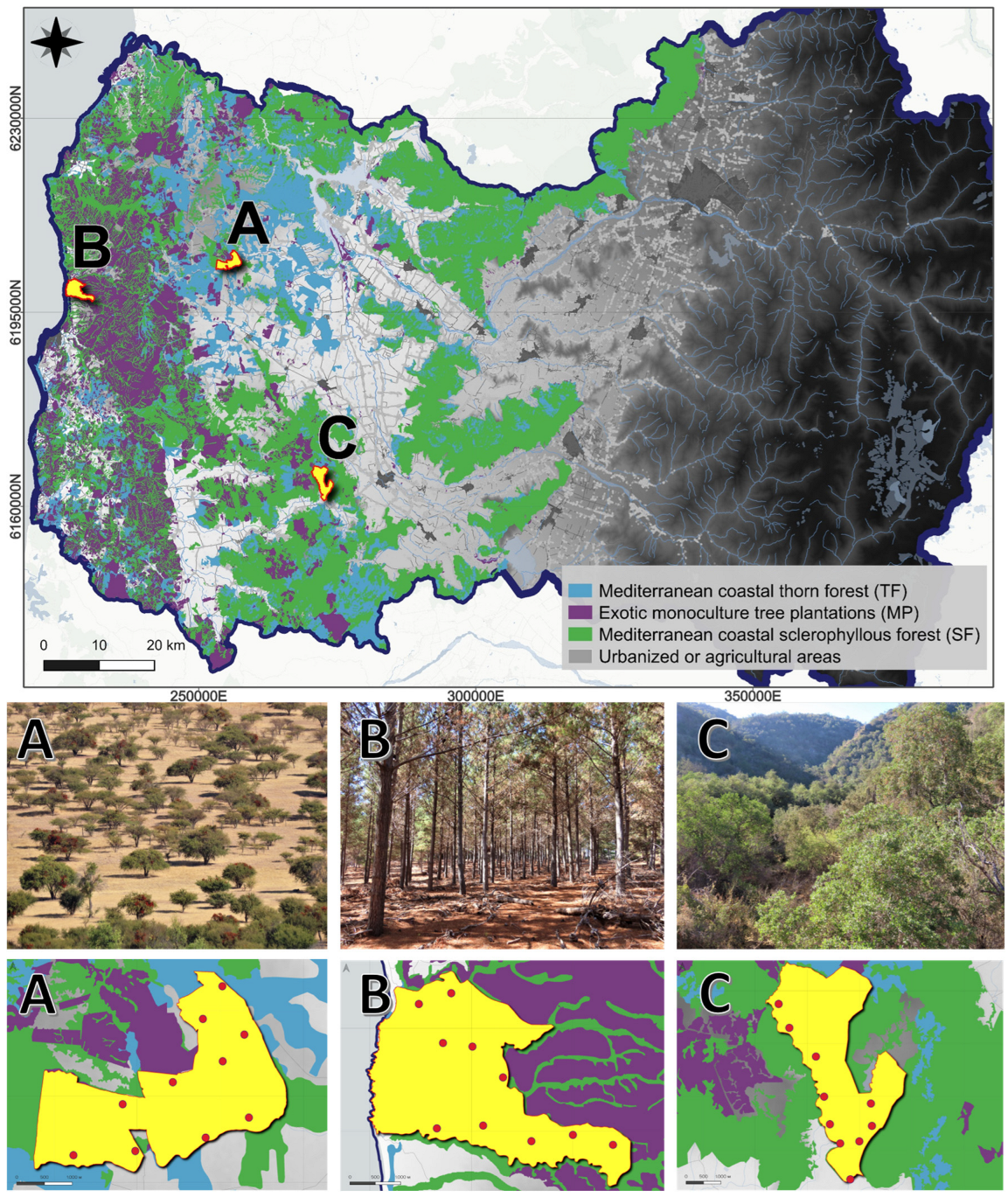
2.1. Study Area
2.2. Data Collection
2.3. Activity Patterns, Overlap, and Landscape Dependency
3. Results
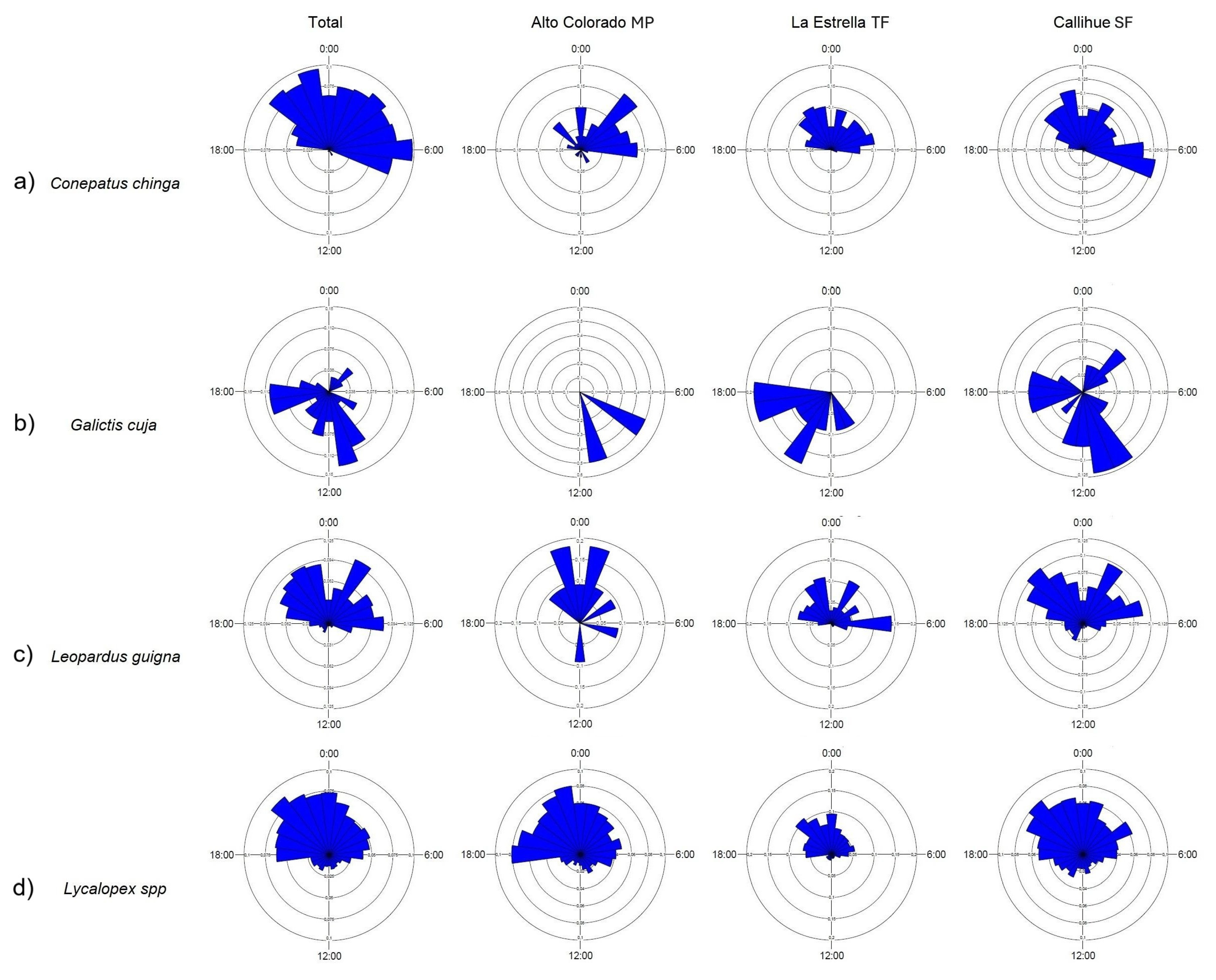
3.1. Activity Patterns
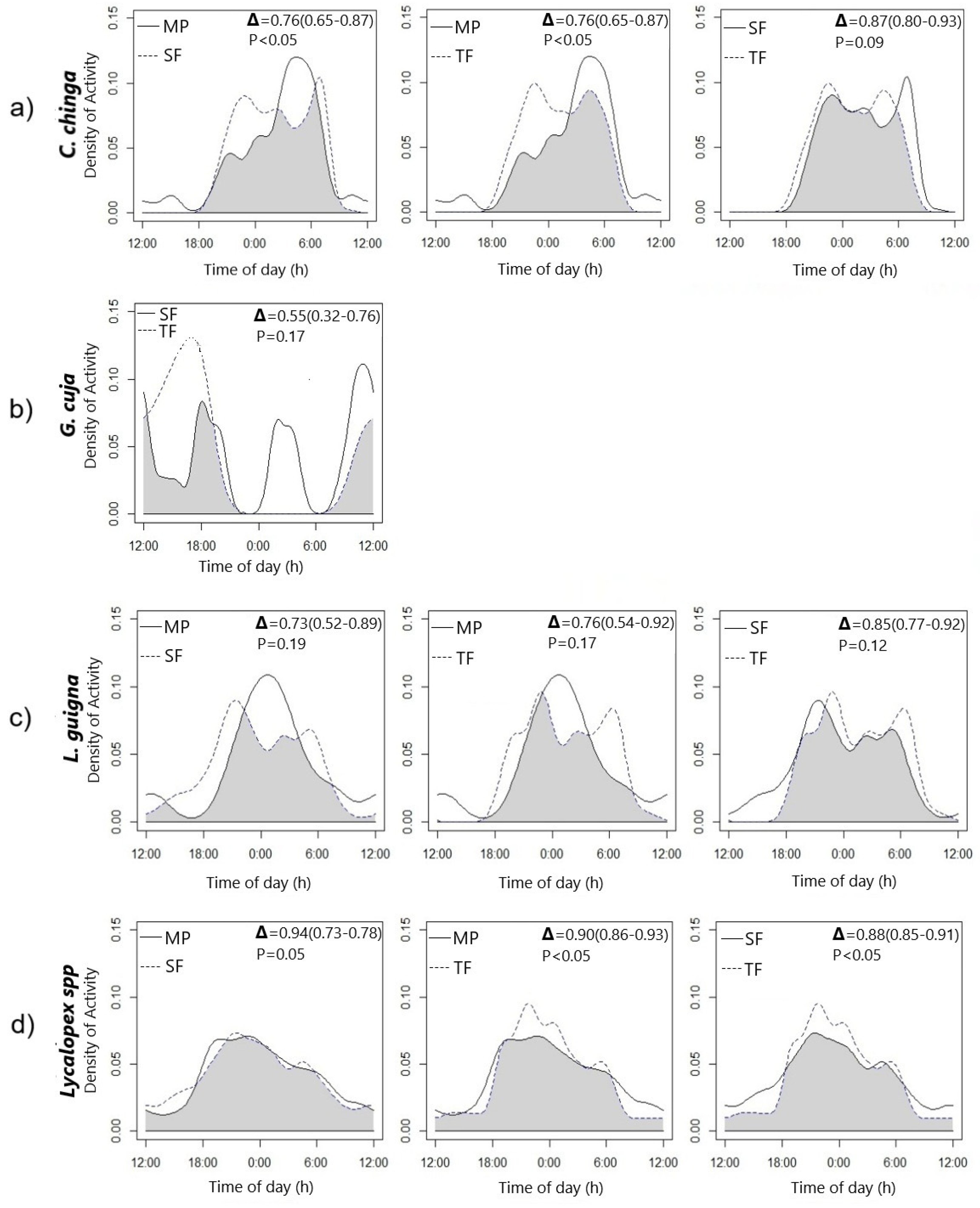
3.2. Comparisons and Overlap of Activity Patterns Among Landscape Types
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevosti, F.J.; Pereira, J.A. Community Structure of South American Carnivores in the Past and Present. J. Mamm. Evol. 2014, 21, 363–368. [Google Scholar] [CrossRef]
- Noss, R.F.; Quigley, H.B.; Hornocker, M.G.; Merrill, T.; Paquet, P.C. Conservation Biology and Carnivore Conservation in the Rocky Mountains. Conserv. Biol. 1996, 10, 949–963. [Google Scholar] [CrossRef]
- Chiaverini, L.; Macdonald, D.; Bothwell, H.; Hearn, A.; Cheyne, S.; Haidir, I.; Hunter, L.; Kaszta, Ż.; Macdonald, E.; Ross, J. Multi-scale, multivariate community models improve designation of biodiversity hotspots in the Sunda Islands. Anim. Conserv. 2022, 25, 660–679. [Google Scholar] [CrossRef]
- Finnegan, S.P.; Gantchoff, M.G.; Hill, J.E.; Silveira, L.; Tôrres, N.M.; Jácomo, A.T.; Uzal, A. “When the felid’s away, the mesocarnivores play”: Seasonal temporal segregation in a neotropical carnivore guild. Mamm. Biol. 2021, 101, 631–638. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, D. Fauna Nativa de la Región de O’Higgins Chile: Vertebrados Terrestres; El Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 2018; p. 504. [Google Scholar]
- Boitani, L.; Powell, R.A. Carnivore Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Linnell, J.D.C.; Strand, O. Interference interactions, co-existence and conservation of mammalian carnivores. Divers. Distrib. 2000, 6, 169–176. [Google Scholar] [CrossRef]
- Arias-Alzate, A.; Arroyave, F.J.; Romero Goyeneche, O.Y.; Hurtado Heredia, R.G.; González-Maya, J.F.; Arroyo-Cabrales, J.; Peterson, A.T.; Martínez-Meyer, E. Functional niche constraints on carnivore assemblages (Mammalia: Carnivora) in the Americas: What facilitates coexistence through space and time? J. Biogeogr. 2022, 49, 497–510. [Google Scholar] [CrossRef]
- Gil-Sánchez, J.M.; Mañá-Varela, B.; Herrera-Sánchez, F.J.; Urios, V. Spatio-temporal ecology of a carnivore community in middle atlas, NW of Morocco. Zoology 2021, 146, 125904. [Google Scholar] [CrossRef]
- Andrade Núñez, M.; Aide, T.M. Effects of habitat and landscape characteristics on medium and large mammal species richness and composition in northern Uruguay. Zoologia (Curitiba) 2009, 27, 909–917. [Google Scholar] [CrossRef]
- Lyra-Jorge, M.; Ribeiro, M.; Ciocheti, G.; Tambosi, L.; Pivello, V. Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna, Brazil. Eur. J. Wildl. Res. 2010, 56, 359–368. [Google Scholar] [CrossRef]
- Lantschner, V.; Rusch, V.; Hayes, J. Habitat use by carnivores at different spatial scales in a plantation forest landscape in Patagonia, Argentina. For. Ecol. Manag. 2012, 269, 271–278. [Google Scholar] [CrossRef]
- Suarez, M.; Naranjo, J.A.; Puig, A. Estratigrafía de la Cordillera de la Costa, al sur de Taltal, Chile: Etapas iniciales de la evolución Andina. Andean Geol. 1985, 24, 19–28. [Google Scholar]
- Smith-Ramírez, C.; Teillier, S.; Jiménez, J.E.; Barahona-Segovia, R.M.; Parra, L.E.; Vera, A.; Jerez, V. Plantas y animales endémicos de la Cordillera de la Costa de Chile. In Biodiversidad y Ecología de los Bosques Costeros de Chile; Smith-Ramírez, C., Squeo, F.A., Eds.; Editorial Universidad de Los Lagos: Santiago, Chile, 2019; pp. 393–416. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile, 2nd ed.; Editorial Universitaria: Santiago, Chile, 2017; p. 384. [Google Scholar]
- Heilmayr, R.; Echeverría, C.; Fuentes, R.; Lambin, E.F. A plantation-dominated forest transition in Chile. Appl. Geogr. 2016, 75, 71–82. [Google Scholar] [CrossRef]
- Muñoz-Pedreros, A.; Yáñez, J.; Norambuena, H.V.; Zúñiga, A. Diet, dietary selectivity and density of South American grey fox, Lycalopex griseus, in Central Chile. Integr. Zool. 2018, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Trolliet, F.; Vermeulen, C.; Huynen, M.-C.; Hambuckers, A. Use of camera traps for wildlife studies: A review. Biotechnol. Agron. Soc. Environ. 2014, 18, 446–454. [Google Scholar]
- Sanderson, J.; Harris, G. Automatic data organization, storage, and analysis of camera trap pictures. J. Indon. Nat. Hist. 2013, 1, 11–19. [Google Scholar]
- Vásquez-Ibarra, V.; Cortés, E.; Silva-Rodriguez, E. Tutorial para la Clasificación de Imágenes Obtenidas Mediante Cámaras Trampa; Universidad Austral de Chile: Valdivia, Chile, 2021; p. 31. [Google Scholar]
- Foster, R.J.; Harmsen, B.J. A critique of density estimation from camera-trap data. J. Wildl. Manag. 2012, 76, 224–236. [Google Scholar] [CrossRef]
- Johansson, Ö.; Samelius, G.; Wikberg, E.; Chapron, G.; Mishra, C.; Low, M. Identification errors in camera-trap studies result in systematic population overestimation. Sci. Rep. 2020, 10, 6393. [Google Scholar] [CrossRef]
- Yoshizaki, J.; Pollock, K.H.; Brownie, C.; Webster, R.A. Modeling misidentification errors in capture–recapture studies using photographic identification of evolving marks. Ecology 2009, 90, 3–9. [Google Scholar] [CrossRef]
- Pizarro, E.J.; Julio-Kalajžić, B.; Sallaberry-Pincheira, N.; Muñoz, V.; González-Acuña, D.; Cabello, J.; Acosta-Jamett, G.; Bonacic, C.; Iriarte, A.; Rodríguez, A.; et al. Species delimitation and intraspecific diversification in recently diverged South American foxes. Mammal. Res. 2024, 69, 71–87. [Google Scholar] [CrossRef]
- Tchaicka, L.; Freitas, T.R.; Bager, A.; Vidal, S.L.; Lucherini, M.; Iriarte, A.; Novaro, A.; Geffen, E.; Garcez, F.S.; Johnson, W.E.; et al. Molecular assessment of the phylogeny and biogeography of a recently diversified endemic group of South American canids (Mammalia: Carnivora: Canidae). Genet. Mol. Biol. 2016, 39, 442–451. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: An R package for efficient camera trap data management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Envir. St. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overview of the Overlap Package. Available online: https://kar.kent.ac.uk/id/eprint/41474 (accessed on 7 April 2024).
- Rowcliffe, M. Package ‘Activity’. Animal Activity Statistics R Package, Version 1.3.4; R Foundation: Vienna, Austria. Available online: https://cran.r-project.org/web/packages/activity/index.html (accessed on 7 April 2024).
- Allen, M.L.; Peterson, B.; Krofel, M. No respect for apex carnivores: Distribution and activity patterns of honey badgers in the Serengeti. Mamm. Biol. 2018, 89, 90–94. [Google Scholar] [CrossRef]
- Milanesi, P.; Puopolo, F.; Zellweger, F. Landscape Features, Human Disturbance or Prey Availability? What Shapes the Distribution of Large Carnivores in Europe? Land 2022, 11, 1807. [Google Scholar] [CrossRef]
- Twining, J.P.; Montgomery, W.I.; Reid, N.; Marks, N.; Tosh, D.G.; Scantlebury, D.M. All forests are not equal: Population demographics and denning behaviour of a recovering small carnivore in human modified landscapes. Wildl. Biol. 2020, 2020, wlb.00760. [Google Scholar] [CrossRef]
- Smith, J.A.; Duane, T.P.; Wilmers, C.C. Moving through the matrix: Promoting permeability for large carnivores in a human-dominated landscape. Landsc. Urban. Plann. 2019, 183, 50–58. [Google Scholar] [CrossRef]
- Iriarte, A.; Jaksic, F. Los Carnívoros de Chile; Ediciones Flora & Fauna Chile Limitada: Santiago, Chile, 2012; p. 235. [Google Scholar]
- Lewis, J.S.; Bailey, L.L.; VandeWoude, S.; Crooks, K.R. Interspecific interactions between wild felids vary across scales and levels of urbanization. Ecol. Evol. 2015, 5, 5946–5961. [Google Scholar] [CrossRef]
- Guntiñas, M.; Lozano, J.; Cisneros, R.; LLorente, E.; Malo, A.F. Ecology of the culpeo (Lycalopex culpaeus): A synthesis of existing knowledge. Hystrix 2021, 32, 5–17. [Google Scholar] [CrossRef]
- Chávez-Villavicencio, C.; Tabilo-Valdivieso, E. Estado de conservación del mustélido Galictis cuja (Molina, 1782): Análisis sobre las áreas de extensión de presencia y ocupación en Chile. An. Inst. Patagon. 2023, 51. [Google Scholar] [CrossRef]
- Delibes-Mateos, M.; Díaz-Ruiz, F.; Caro, J.; Ferreras, P. Activity patterns of the vulnerable guiña (Leopardus guigna) and its main prey in the Valdivian rainforest of southern Chile. Mamm. Biol. 2014, 79, 393–397. [Google Scholar] [CrossRef]
- Hernández, F.; Gálvez Robinson, N.C.; Gimona, A.; Laker, J.; Bonacic Salas, C. Activity patterns by two colour morphs of the vulnerable guiña, Leopardus guigna (Molina 1782), in temperate forests of southern Chile. Gayana 2015, 79, 102–105. [Google Scholar] [CrossRef]
- Napolitano, C.; Johnson, W.E.; Sanderson, J.; O’Brien, S.J.; Rus Hoelzel, A.; Freer, R.; Dunstone, N.; Ritland, K.; Ritland, C.E.; Poulin, E. Phylogeography and population history of Leopardus guigna, the smallest American felid. Conserv. Genet. 2014, 15, 631–653. [Google Scholar] [CrossRef]
- Napolitano, C.; Larraguibel-González, C.; Cepeda-Mercado, A.A.; Vial, P.; Sanderson, J. New records of Leopardus guigna in its northern-most distribution in Chile: Implications for conservation. Rev. Chil. Hist. Nat. 2020, 93, 7. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, D.; Napolitano, C.; Arriagada, G.; Salgado, I.; Cox, S.; Céspedes-Parada, B. Native Carnivore Diversity and Relative Abundance in Landscapes of the Coast Range in Central Chile: Insights for Conservation Decision-Making. Conservation 2023, 3, 379–393. [Google Scholar] [CrossRef]
- Laner, P.; Rossi, C.; Luethi, R.; Favilli, F.; Bertoncelj, I.; Plassmann, G.; Haller, R.M. Landscape permeability for ecological connectivity at the macro-regional level: The Continuum Suitability Index and its practical implications. Ecol. Indic. 2024, 164, 112145. [Google Scholar] [CrossRef]
- Iannella, M.; Biondi, M.; Serva, D. Functional connectivity and the current arrangement of protected areas show multiple, poorly protected dispersal corridors for the Eurasian lynx. Biol. Conserv. 2024, 291, 110498. [Google Scholar] [CrossRef]
- Rytwinski, T.; Fahrig, L. Do species life history traits explain population responses to roads? A meta-analysis. Biol. Conserv. 2012, 147, 87–98. [Google Scholar] [CrossRef]
- Steiner, W.; Schöll, E.; Leisch, F.; Hackländer, K. Temporal patterns of roe deer traffic accidents: Effects of season, daytime and lunar phase. PLoS ONE 2021, 16, e0249082. [Google Scholar] [CrossRef]
- Visintin, C.; Golding, N.; van der Ree, R.; McCarthy, M. Managing the timing and speed of vehicles reduces wildlife-transport collision risk. Transp. Res. D Transp. Environ. 2018, 59, 86–95. [Google Scholar] [CrossRef]
- Johnson, W.E.; Franklin, W.L. Spatial resource partitioning by sympatric grey fox (Dusicyon griseus) and culpeo fox (Dusicyon culpaeus) in southern Chile. Can. J. Zool. 1994, 72, 1788–1793. [Google Scholar] [CrossRef]
- Nascimento, F.O.D.; Cheng, J.; Feijó, A. Taxonomic revision of the pampas cat Leopardus colocola complex (Carnivora: Felidae): An integrative approach. Zool. J. Linn. Soc. 2021, 191, 575–611. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, D.; Napolitano, C.; Salgado, I. Puma (Puma concolor) in the Neighborhood? Records Near Human Settlements and Insights into Human-Carnivore Coexistence in Central Chile. Animals 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- García, C.B.; Svensson, G.L.; Bravo, C.; Undurraga, M.I.; Díaz-Forestier, J.; Godoy, K.; Neaman, A.; Barbosa, O.; Abades, S.; Celis-Diez, J.L. Remnants of native forests support carnivore diversity in the vineyard landscapes of central Chile. Oryx 2021, 55, 227–234. [Google Scholar] [CrossRef]
- Teixeira, D.F.; Guillera-Arroita, G.; Hilário, R.R.; Fonseca, C.; Rosalino, L.M. Influence of life-history traits on the occurrence of carnivores within exotic Eucalyptus plantations. Divers. Distrib. 2020, 26, 1071–1082. [Google Scholar] [CrossRef]
- Amici, V. Selecting Focal Species in Ecological Network Planning following an Expert-Based Approach: A Case Study and a Conceptual Framework. Landsc. Res. 2009, 34, 545–561. [Google Scholar] [CrossRef]
- Bennett, A. Linkages in the Landscape; The Role of Corridors and Connectivity in Wildlife Conservation; IUCN: Gland, Switzerland; Cambridge, UK, 2003. [Google Scholar]
- Miranda, A.; Altamirano, A.; Cayuela, L.; Lara, A.; González, M. Native forest loss in the Chilean biodiversity hotspot: Revealing the evidence. Reg. Environ. Change 2017, 17, 285–297. [Google Scholar] [CrossRef]
- Schulz, J.; Cayuela, L.; Echeverria, C.; Salas, J.; Benayas, J. Monitoring land cover change of the dryland forest landscape of Central Chile (1975–2008). Appl. Geogr. 2010, 30, 436–447. [Google Scholar] [CrossRef]
- Figueiredo, L.; Krauss, J.; Steffan-Dewenter, I.; Sarmento Cabral, J. Understanding extinction debts: Spatio–temporal scales, mechanisms and a roadmap for future research. Ecography 2019, 42, 1973–1990. [Google Scholar] [CrossRef]
- Redford, K. The Empty Forest. Bioscience 1992, 42, 412–422. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Aizen, M.A.; Alcántara, J.M.; Arroyo, J.; Cocucci, A.; Galetti, M.; García, M.B.; García, D.; Gómez, J.M.; Jordano, P.; et al. Beyond species loss: The extinction of ecological interactions in a changing world. Funct. Ecol. 2015, 29, 299–307. [Google Scholar] [CrossRef]
- Galetti, M.; Guevara, R.; Corrêa Côrtes, M.; Fadini, R.; Matter, S.; Leite, A.D.; Labecca, F.; Ribeiro, T.; Carvalho, C.; Collevatti, R.; et al. Functional Extinction of Birds Drives Rapid Evolutionary Changes in Seed Size. Science 2013, 340, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Ockinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Battisti, C. Unifying the trans-disciplinary arsenal of project management tools in a single logical framework: Further suggestion for IUCN project cycle development. J. Nat. Conserv. 2018, 41, 63–72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Alvarez, D.; Arenas-Rodríguez, K.; Kaiser, M.; Napolitano, C. Activity Patterns of Native Carnivores in Central Chile: Are They Influenced by Landscape Type? Diversity 2025, 17, 156. https://doi.org/10.3390/d17030156
Ramírez-Alvarez D, Arenas-Rodríguez K, Kaiser M, Napolitano C. Activity Patterns of Native Carnivores in Central Chile: Are They Influenced by Landscape Type? Diversity. 2025; 17(3):156. https://doi.org/10.3390/d17030156
Chicago/Turabian StyleRamírez-Alvarez, Diego, Kathia Arenas-Rodríguez, Melanie Kaiser, and Constanza Napolitano. 2025. "Activity Patterns of Native Carnivores in Central Chile: Are They Influenced by Landscape Type?" Diversity 17, no. 3: 156. https://doi.org/10.3390/d17030156
APA StyleRamírez-Alvarez, D., Arenas-Rodríguez, K., Kaiser, M., & Napolitano, C. (2025). Activity Patterns of Native Carnivores in Central Chile: Are They Influenced by Landscape Type? Diversity, 17(3), 156. https://doi.org/10.3390/d17030156






