Origin and Diversification of the Genera Aratinga, Eupsittula, and Psittacara (Aves: Psittacidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Samples, DNA Extraction, PCR Amplification, and Sequencing
2.2. Sequence Samples
2.3. Phylogenetic Analysis
2.4. Inference of Divergence Time
2.5. Reconstruction of Ancestral Areas
3. Results
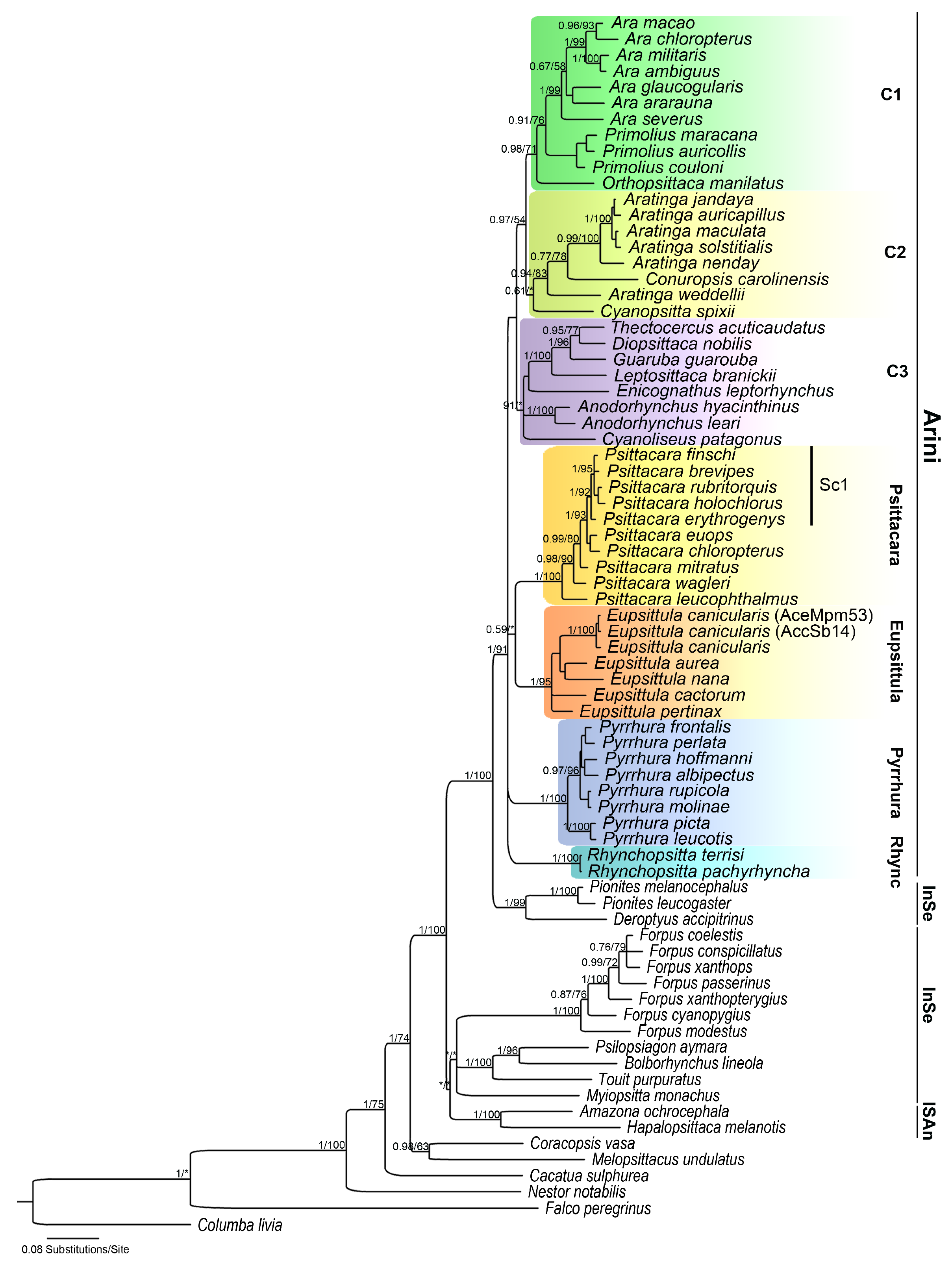
3.1. Phylogenetic Analyses
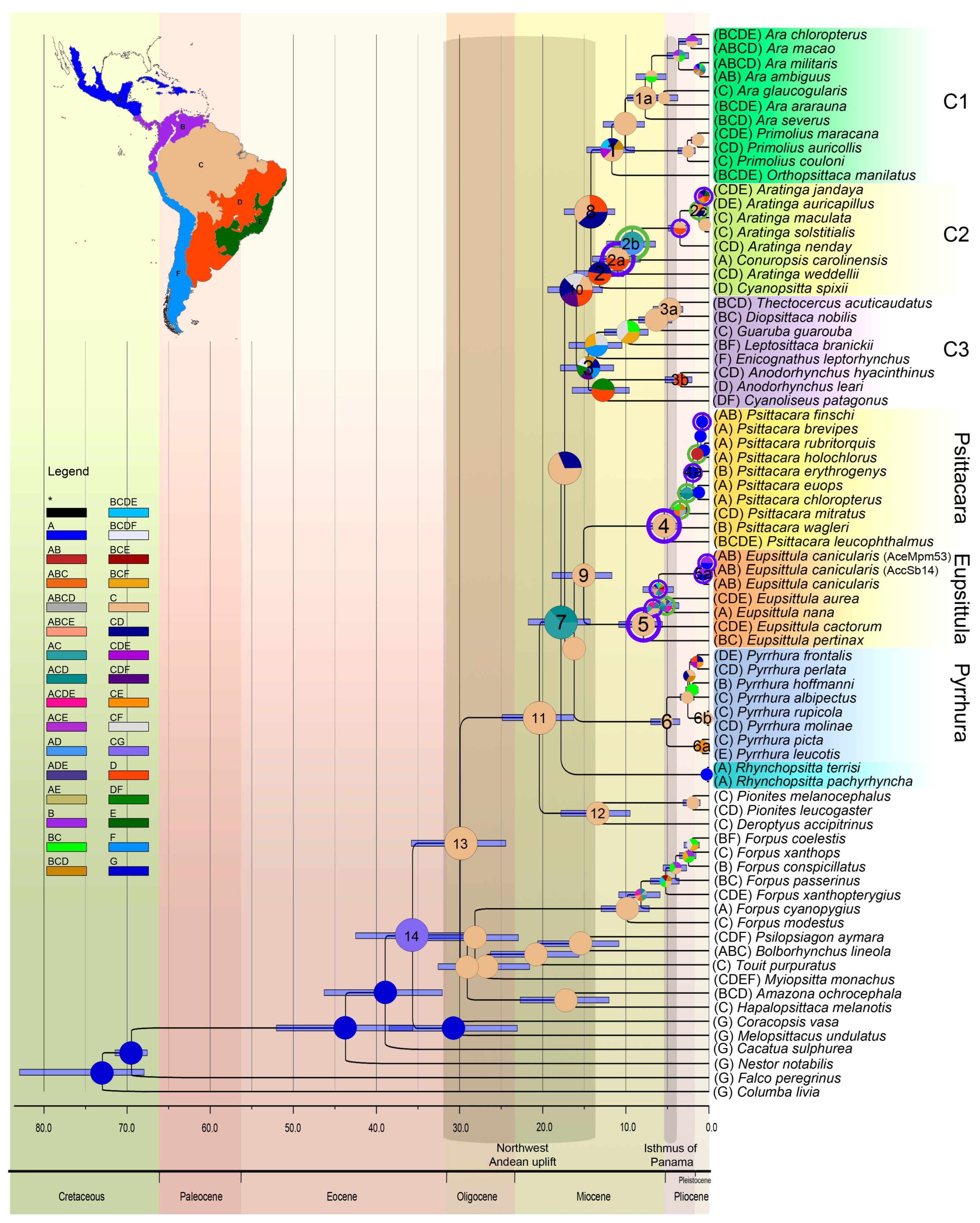
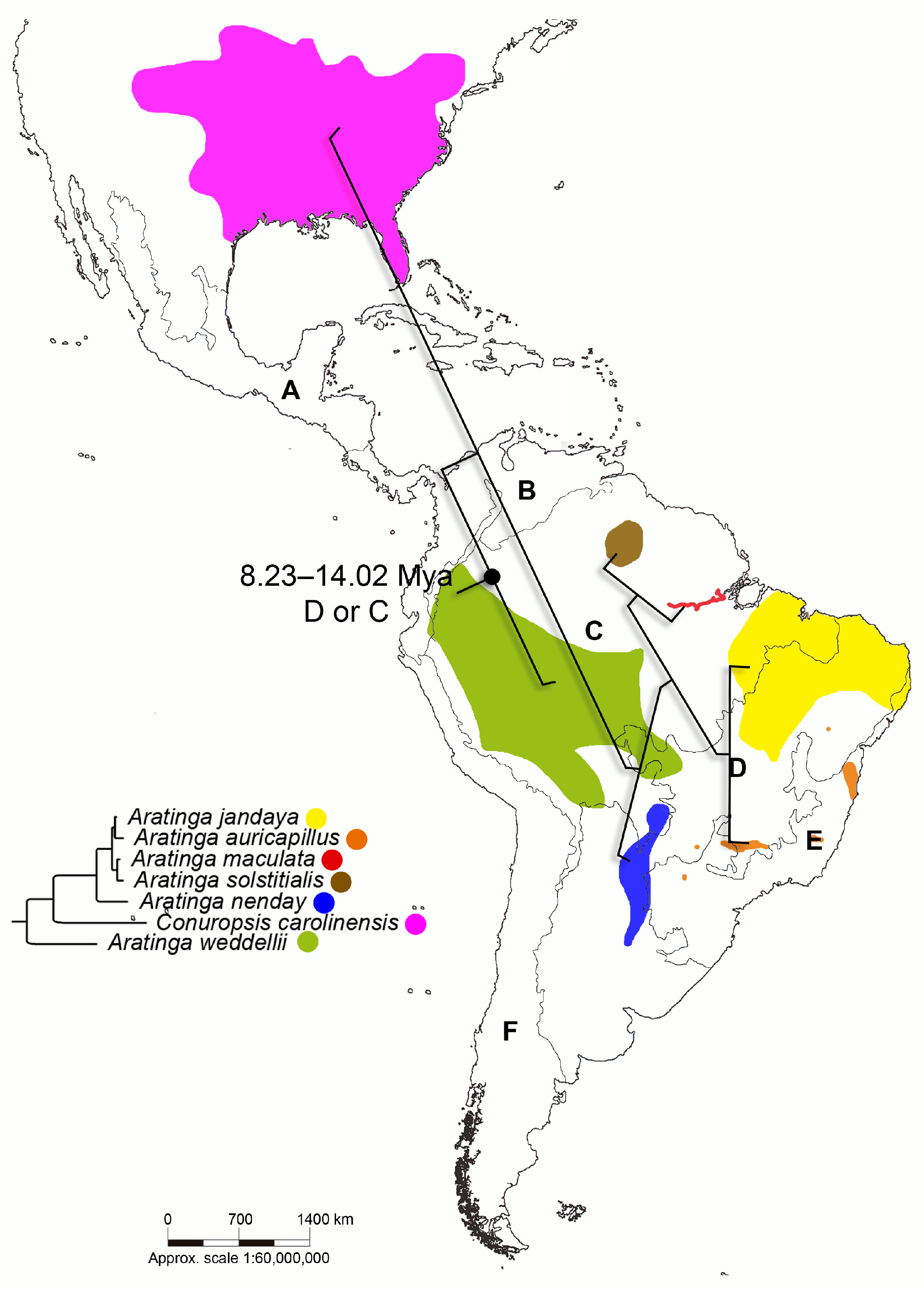
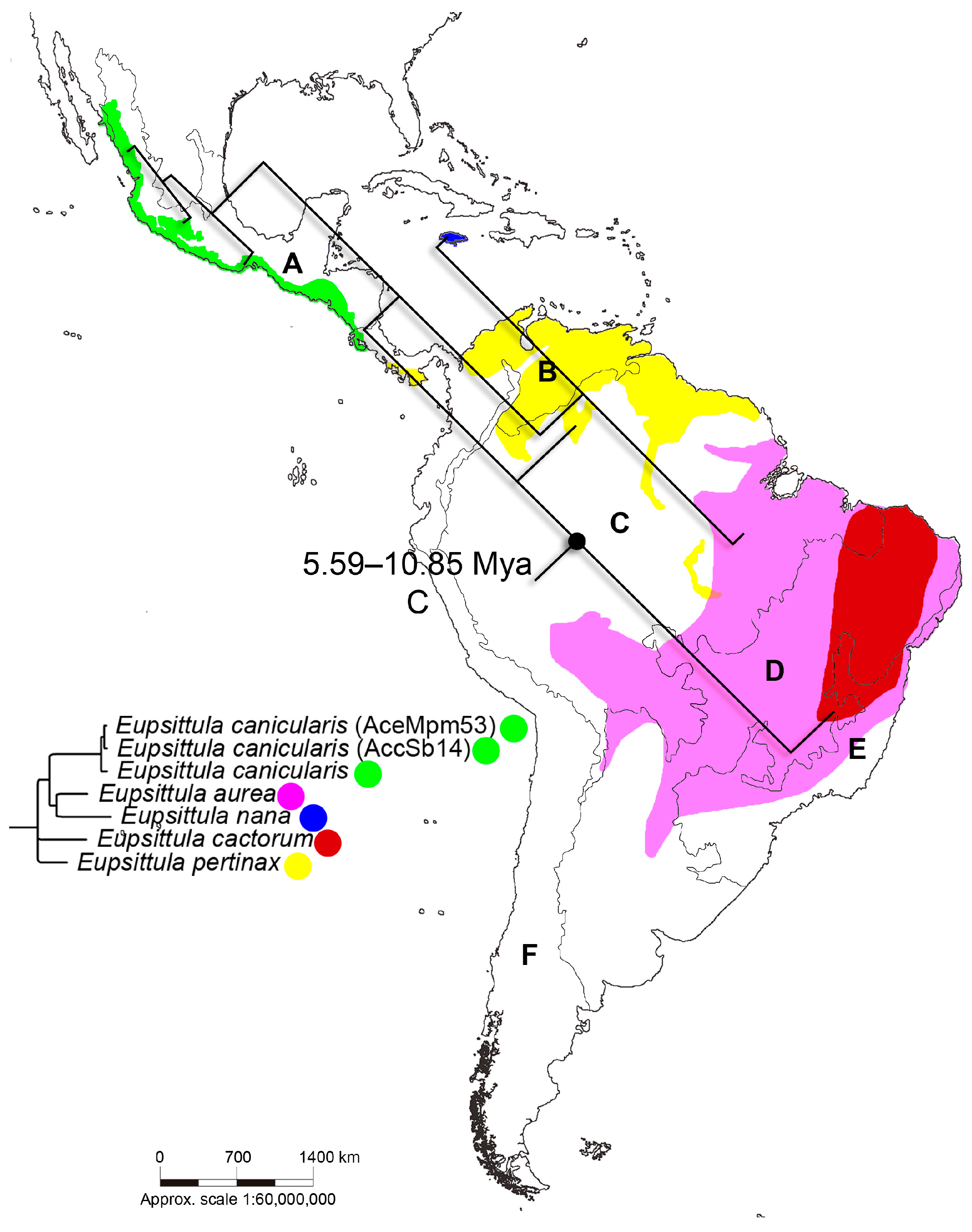
3.2. Inference of Divergence Times and Reconstruction of Ancestral Areas
4. Discussion
4.1. Phylogenetic Analyses
4.2. Divergence Times and Reconstruction of Ancestral Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantú-Guzmán, J.C.; Sánchez-Saldaña, M.; Grosselet, M.; Silva-Gámez, J. Tráfico Ilegal de Pericos en México: Una Evaluación Detallada; Defenders of Wildlife: Washington, DC, USA, 2007; p. 75. [Google Scholar]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.; Aguilar, J.; Alemán-Zelaya, U.; Aramburú, R.M.; Arias, A.A.; McNab, R.B.; Balsby, T.J. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- CITES. The Convention on International Trade in Endangered Species of Wild Fauna and Flora Appendices. Available online: https://cites.org/eng/app/appendices.php (accessed on 21 January 2024).
- Forshaw, J.M. Parrots of the World; Lansdowne Editions: Sydney, Australia, 1989; p. 672. [Google Scholar]
- Clements, J.; Rasmussen, P.; Schulenberg, T.; Iliff, M.; Fredericks, T.; Gerbracht, J.; Lepage, D.; Spencer, A.; Billerman, S.; Sullivan, B.; et al. The eBird/Clements Checklist of Birds of the World: v2024. Available online: http://www.birds.cornell.edu/clementschecklist/download/ (accessed on 6 December 2024).
- Darlington, P.J., Jr. Zoogeography: The Geographical Distribution of Animals; John Wiley & Sons: Malabar, FL, USA, 1957; p. 675. [Google Scholar]
- Collar, N.J. Family Psittacidae (Parrots). In Handbook of the Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Editions: Barcelona, Spain, 1997; Volume 4, pp. 280–477. [Google Scholar]
- Schweizer, M.; Seehausen, O.; Hertwig, S.T. Macroevolutionary patterns in the diversification of parrots: Effects of climate change, geological events and key innovations. J. Biogeogr. 2011, 38, 2176–2194. [Google Scholar] [CrossRef]
- Joseph, L.; Toon, A.; Schirtzinger, E.E.; Wright, T.F.; Schodde, R. A revised nomenclature and classification for family-group taxa of parrots (Psittaciformes). Zootaxa 2012, 3205, 26–49. [Google Scholar] [CrossRef]
- Wetmore, A. The Thick-Billed Parrot in Southern Arizona. Condor 1935, 37, 18–21. [Google Scholar] [CrossRef]
- Howell, S.N.; Webb, S. A Guide to the Birds of Mexico and Northern Central America; Oxford University Press: Oxford, UK, 1995; pp. 333–345. [Google Scholar]
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; de Dios Valdez-Leal, J.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruz, C.; Rubio-Rocha, Y. Distribución potencial histórica y contemporánea de la familia Psittacidae en México. Rev. Mex. Biodivers. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- Padilla-Jacobo, G.; Monterrubio-Rico, T.C.; Cano-Camacho, H.; Zavala-Páramo, M.G. Genealogical relationship inference to identify areas of intensive poaching of the Orange-fronted Parakeet (Eupsittula canicularis). BMC Zool. 2021, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.R.; Bermingham, E.; Zink, R. Phylogeny and biogeography of the Amazona ochrocephala (Aves: Psittacidae) complex. Auk 2004, 121, 318–332. [Google Scholar] [CrossRef]
- Ribas, C.C.; Miyaki, C.Y. Molecular systematics in Aratinga parakeets: Species limits and historical biogeography in the ‘solstitialis’ group, and the systematic position of Nandayus nenday. Mol. Phylogenet. Evol. 2004, 30, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.R.; Bermingham, E. Phylogeny and comparative biogeography of Pionopsitta parrots and Pteroglossus toucans. Mol. Phylogenet. Evol. 2005, 36, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Ribas, C.C.; Gaban-Lima, R.; Miyaki, C.Y.; Cracraft, J. Historical biogeography and diversification within the Neotropical parrot genus Pionopsitta (Aves: Psittacidae). J. Biogeogr. 2005, 32, 1409–1427. [Google Scholar] [CrossRef]
- Ribas, C.C.; Joseph, L.; Miyaki, C.Y. Molecular systematics and patterns of diversification in Pyrrhura (Psittacidae), with special reference to the Picta-Leucotis complex. Auk 2006, 123, 660–680. [Google Scholar] [CrossRef]
- Tavares, E.S.; Baker, A.J.; Pereira, S.L.; Miyaki, C.Y. Phylogenetic relationships and historical biogeography of neotropical parrots (Psittaciformes: Psittacidae: Arini) inferred from mitochondrial and nuclear DNA sequences. Syst. Biol. 2006, 55, 454–470. [Google Scholar] [CrossRef] [PubMed]
- Escalante-Pliego, P.; Navarro, A.; Peterson, A.T. A geographic, ecological and historical analysis of land bird diversity in Mexico. In Biological Diversity of Mexico: Origins and Distribution; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 281–307. [Google Scholar]
- Weir, J.T.; Bermingham, E.; Schluter, D. The great American biotic interchange in birds. Proc. Natl Acad. Sci. USA 2009, 106, 21737–21742. [Google Scholar] [CrossRef] [PubMed]
- Selvatti, A.P.; Galvão, A.; Mayr, G.; Miyaki, C.Y.; Russo, C.A.M. Southern hemisphere tectonics in the Cenozoic shaped the pantropical distribution of parrots and passerines. J. Biogeogr. 2022, 49, 1753–1766. [Google Scholar] [CrossRef]
- Forshaw, J.M. Parrots of the World; Princeton University Press: Sydney, Australia, 2010; p. 328. [Google Scholar]
- Vigors, N.A. Sketches in ornithology; or observations on the leading affinities of some of the more extensive groups of birds. Zool. J. 1825, 2, 37–69. [Google Scholar]
- Salvadori, T. Catalogue of the Psittaci, or Parrots, in the collection of the British Museum; Longmans and Co.: London, UK, 1891; pp. 147–267. [Google Scholar]
- Peters, J.L. Check-List of Birds of the World; Harvard University Press: Cambridge, UK, 1937; pp. 179–246. [Google Scholar]
- Remsen, J.J.V.; Schirtzinger, E.E.; Ferraroni, A.; Silveira, L.F.; Wright, T.F. DNA-sequence data require revision of the parrot genus Aratinga (Aves: Psittacidae). Zootaxa 2013, 3641, 296–300. [Google Scholar] [CrossRef]
- Chesser, R.T.; Banks, R.C.; Cicero, C.; Dunn, J.L.; Kratter, A.W.; Lovette, I.J.; Navarro-Sigüenza, A.G.; Rasmussen, P.C.; Remsen, J.V.; Rising, J.D.; et al. Fifty-Fifth Supplement to the American Ornithologists’ UnionCheck-list of North American Birds. Auk 2014, 131, CSi–CSxv. [Google Scholar] [CrossRef]
- del Hoyo, J.; Elliott, A.; Sargatal, J.; Christie, D.A.; de Juana, E. (Eds.) Handbook of the Birds of the World Alive; Lynx Editions: Barcelona, Spain, 1997; Volume 4, pp. 280–477. [Google Scholar]
- HBW-BLI. Handbook of the Birds of the World and BirdLife International Digital Checklist of the Birds of the World. Version 9. Available online: https://datazone.birdlife.org/species/taxonomy (accessed on 4 December 2024).
- Tavares, E.S.; Yamashita, C.; Miyaki, C.Y. Phylogenetic relationships among some Neotropical parrot genera (Psittacidae) based on mitochondrial sequences. Auk 2004, 121, 230–242. [Google Scholar] [CrossRef]
- Kirchman, J.J.; Schirtzinger, E.E.; Wright, T.F. Phylogenetic relationships of the extinct Carolina Parakeet (Conuropsis carolinensis) inferred from DNA sequence data. Auk 2012, 129, 197–204. [Google Scholar] [CrossRef]
- Padilla-Jacobo, G.; Monterrubio-Rico, T.C.; Camacho, H.C.; Zavala-Páramo, M.G. Use of phylogenetic analysis to identify evolutionarily significant units for the Orange-fronted Parakeet (Eupsittula canicularis) in Mexico. Ornitol. Neotrop. 2016, 26, 325–335. [Google Scholar] [CrossRef]
- FitzSimmons, N.N. Male Marine Turtles: Gene Flow, Philopatry and Mating Systems of the Green Turtle (Chelonia mydas). Ph.D. Thesis, University of Queensland, Brisbane, Queensland, Australia, 1997. [Google Scholar]
- Palumbi, S.; Martin, A.; Romano, S.; McMillan, W.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR; Version 2.0; University of Hawaii: Honolulu, HI, USA, 1991; p. 45. [Google Scholar]
- Hackett, S.J. Molecular phylogenetics and biogeography of tanagers in the genus Ramphocelus (Aves). Mol. Phylogenet. Evol. 1996, 5, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Gene Codes Corporation. Sequencher Version 5.4.6 DNA Sequence Analysis Software. Available online: http://www.genecodes.com/sequencher (accessed on 6 December 2024).
- Müller, J.; Müller, K.; Neinhuis, C.; Quandt, D. PhyDE-Phylogenetic Data Editor. Available online: http://www.phyde.de (accessed on 6 December 2024).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Alfaro, M.E.; Huelsenbeck, J.P. Comparative performance of Bayesian and AIC-based measures of phylogenetic model uncertainty. Syst. Biol. 2006, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T.-A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Molec. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. In Lectures on Mathematics in the Life Sciences; Miura, R.M., Ed.; American Mathematical Society: Providence, RI, USA, 1986; Volume 17, pp. 57–86. [Google Scholar]
- Zwickl, D.J. Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets Under the Maximum Likelihood Criterion. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2006. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 8 December 2024).
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.; Faircloth, B.C.; Nabholz, B.; Howard, J.T. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed]
- Yule, G.U. A mathematical theory of evolution, based on the conclusions of Dr. JC Willis, FRS. Phil. Trans. R. Soc. Lon. B 1925, 213, 21–87. [Google Scholar] [CrossRef]
- Aldous, D.J. Stochastic Models and Descriptive Statistics for Phylogenetic Trees, from Yule to Today. Stat. Sci. 2001, 16, 23–34. Available online: https://www.jstor.org/stable/2676778 (accessed on 26 November 2024). [CrossRef]
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral State Reconstruction Tool for Multiple Genes and Characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Harris, A.; He, X. S-DIVA (Statistical Dispersal-Vicariance Analysis): A tool for inferring biogeographic histories. Mol. Phylogenet. Evol. 2010, 56, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Biogeografıa de América Latina y el Caribe; Manuales & Tesis SEA: Zaragoza, Spain, 2001; Volume 3, p. 148. [Google Scholar]
- Morrone, J.J. Biogeographical Regionalisation of the Neotropical Region; Magnolia Press: Auckland, NZ, USA, 2014; p. 110. [Google Scholar]
- Kass, R.E.; Carlin, B.P.; Gelman, A.; Neal, R.M. Markov Chain Monte Carlo in practice: A roundtable discussion. Am. Stat. 1998, 52, 93–100. [Google Scholar] [CrossRef]
- Smith, B.T.; Merwin, J.; Provost, K.L.; Thom, G.; Brumfield, R.T.; Ferreira, M.; Mauck, W.M., III; Moyle, R.G.; Wright, T.F.; Joseph, L. Phylogenomic analysis of the parrots of the world distinguishes artifactual from biological sources of gene tree discordance. Syst. Biol. 2023, 72, 228–241. [Google Scholar] [CrossRef]
- Snyder, N.F.; Russell, K. Carolina Parakeet (Conuropsis carolinensis), version 1.0. In Birds of the World; Poole, F., Gill, F.B., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Collar, N.J.; Boesman, P.; Sharpe, C.J. White-eyed Parakeet (Psittacara leucophthalmus). In Handbook of the Birds of the World Alive; Lynx Edicións: Barcelona, Spain, 1997; Volume 4, pp. 430–431. [Google Scholar]
- Collar, N.J.; Sharpe, C.J. Red-throated Parakeet (Psittacara rubritorquis). In Handbook of the Birds of the World Alive; Lynx Edicións: Barcelona, Spain, 1997; Volume 4, pp. 429–430. [Google Scholar]
- García-Moreno, J.; Fjeldså, J. Chronology and mode of speciation in the Andean avifauna. Bonn. Zool. Monogr. 2000, 46, 25–46. [Google Scholar]
- Cortés-Ortiz, L.; Bermingham, E.; Rico, C.; Rodrıguez-Luna, E.; Sampaio, I.; Ruiz-Garcıa, M. Molecular systematics and biogeography of the Neotropical monkey genus, Alouatta. Mol. Phylogenet. Evol. 2003, 26, 64–81. [Google Scholar] [CrossRef]
- Burns, K.J.; Naoki, K. Molecular phylogenetics and biogeography of Neotropical tanagers in the genus Tangara. Mol. Phylogenet. Evol. 2004, 32, 838–854. [Google Scholar] [CrossRef]
- Lovette, I.J. Molecular phylogeny and plumage signal evolution in a trans Andean and circum Amazonian avian species complex. Mol. Phylogenet. Evol. 2004, 32, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, Z.; Hackett, S.J.; Capparella, A.P. Complex evolutionary history of a Neotropical lowland forest bird (Lepidothrix coronata) and its implications for historical hypotheses of the origin of Neotropical avian diversity. Mol. Phylogenet. Evol. 2005, 36, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.K. Avifaunal interchange across the Panamanian isthmus: Insights from Campylorhynchus wrens. Biol. J. Linn. Soc. Lond. 2007, 90, 687–702. [Google Scholar] [CrossRef]
- Brumfield, R.T.; Edwards, S.V. Evolution into and out of the Andes: A Bayesian analysis of historical diversification in Thamnophilus antshrikes. Evolution 2007, 61, 346–367. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, R.T.; Tello, J.G.; Cheviron, Z.; Carling, M.D.; Crochet, N.; Rosenberg, K.V. Phylogenetic conservatism and antiquity of a tropical specialization: Army-ant-following in the typical antbirds (Thamnophilidae). Mol. Phylogenet. Evol. 2007, 45, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.K. Divergence times and origin of neotropical sheath-tailed bats (Tribe Diclidurini) in South America. Mol. Phylogenet. Evol. 2007, 45, 777–791. [Google Scholar] [CrossRef]
- Miller, M.J.; Bermingham, E.; Klicka, J.; Escalante, P.; do Amaral, F.S.R.; Weir, J.T.; Winker, K. Out of Amazonia again and again: Episodic crossing of the Andes promotes diversification in a lowland forest flycatcher. Proc. Biol. Sci. 2008, 275, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Nylander, J.A.; Persson, C.; Sanmartín, I. Tracing the impact of the Andean uplift on Neotropical plant evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 9749–9754. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.C.; Coloma, L.A.; Summers, K.; Caldwell, J.P.; Ree, R.; Cannatella, D.C. Amazonian amphibian diversity is primarily derived from late Miocene Andean lineages. PLoS Biol. 2009, 7, e1000056. [Google Scholar] [CrossRef]
- Richardson, J.E.; Pennington, R.T.; Pennington, T.D.; Hollingsworth, P.M. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science 2001, 293, 2242–2245. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Eastwood, R. Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. USA 2006, 103, 10334–10339. [Google Scholar] [CrossRef]
- Madriñán, S.; Cortés, A.J.; Richardson, J.E. Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front. Genet. 2013, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Orduña, I.J.; Benetti-Longhini, J.E.; Brower, A.V. Timing the diversification of the Amazonian biota: Butterfly divergences are consistent with Pleistocene refugia. J. Biogeogr. 2014, 41, 1631–1638. [Google Scholar] [CrossRef]
- Koenen, E.J.; Clarkson, J.J.; Pennington, T.D.; Chatrou, L.W. Recently evolved diversity and convergent radiations of rainforest mahoganies (Meliaceae) shed new light on the origins of rainforest hyperdiversity. New Phytol. 2015, 207, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Byrne, H.; Rylands, A.B.; Carneiro, J.C.; Alfaro, J.W.L.; Bertuol, F.; da Silva, M.N.; Messias, M.; Groves, C.P.; Mittermeier, R.A.; Farias, I. Phylogenetic relationships of the New World titi monkeys (Callicebus): First appraisal of taxonomy based on molecular evidence. Front. Zool. 2016, 13, 1–26. [Google Scholar] [CrossRef]
- Woodburne, M.O. The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. J. Mamm. Evol. 2010, 17, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Leigh, E.G.; O’Dea, A.; Vermeij, G.J. Historical biogeography of the Isthmus of Panama. Biol. Rev. Camb. Philos. Soc. 2013, 89, 148–172. [Google Scholar] [CrossRef]
- O’Dea, A.; Lessios, H.A.; Coates, A.G.; Eytan, R.I.; Restrepo-Moreno, S.A.; Cione, A.L.; Collins, L.S.; de Queiroz, A.; Farris, D.W.; Norris, R.D. Formation of the Isthmus of Panama. Sci. Adv. 2016, 2, e1600883. [Google Scholar] [CrossRef]
- Hoorn, C.; Wesselingh, F.P.; ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartin, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 2010, 330, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.T. Implications of genetic differentiation in neotropical montane forest birds. Ann. Mo. Bot. Gard. 2009, 96, 410–433. [Google Scholar] [CrossRef]
- Gregory-Wodzicki, K.M. Uplift history of the Central and Northern Andes: A review. Geol. Soc. Am. Bull. 2000, 112, 1091–1105. [Google Scholar] [CrossRef]
- Audemard, M.F. Geomorphic and geologic evidence of ongoing uplift and deformation in the Mérida Andes, Venezuela. Quat. Int. 2003, 101, 43–65. [Google Scholar] [CrossRef]
- Dhont, D.; Backé, G.; Hervouët, Y. Plio-Quaternary extension in the Venezuelan Andes: Mapping from SAR JERS imagery. Tectonophysics 2005, 399, 293–312. [Google Scholar] [CrossRef]
- Coates, A.G.; Collins, L.S.; Aubry, M.-P.; Berggren, W.A. The geology of the Darien, Panama, and the late Miocene-Pliocene collision of the Panama arc with northwestern South America. Geol. Soc. Am. Bull. 2004, 116, 1327–1344. [Google Scholar] [CrossRef]
- Weir, J.T.; Bermingham, E.; Miller, M.J.; Klicka, J.; González, M.A. Phylogeography of a morphologically diverse Neotropical montane species, the Common Bush-Tanager (Chlorospingus ophthalmicus). Mol. Phylogenet. Evol. 2008, 47, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Fritz, U.; Stuckas, H.; Vargas-Ramírez, M.; Hundsdörfer, A.K.; Maran, J.; Päckert, M. Molecular phylogeny of Central and South American slider turtles: Implications for biogeography and systematics (Testudines: Emydidae: Trachemys). J. Zool. Syst. Evol. Res. 2012, 50, 125–136. [Google Scholar] [CrossRef]
- Parada, A.; Pardiñas, U.F.; Salazar-Bravo, J.; D’Elía, G.; Palma, R.E. Dating an impressive Neotropical radiation: Molecular time estimates for the Sigmodontinae (Rodentia) provide insights into its historical biogeography. Mol. Phylogenet. Evol. 2013, 66, 960–968. [Google Scholar] [CrossRef]
- Prothero, D.R.; Campbell, K.E., Jr.; Beatty, B.L.; Frailey, C.D. New late Miocene dromomerycine artiodactyl from the Amazon Basin: Implications for interchange dynamics. J. Paleont. 2014, 88, 434–443. [Google Scholar] [CrossRef]

| Species | Distribution | Area Code |
|---|---|---|
| Amazona ochrocephala | Panama, Colombia, Venezuela, Guianas, Brazil, Ecuador, Peru, Bolivia | BCD |
| Anodorhynchus hyacinthinus | Brazil, Bolivia, Paraguay | CD |
| Anodorhynchus leari | Brazil | D |
| Ara ambiguous | Honduras, Nicaragua, Costa Rica, Panama, Colombia Ecuador | AB |
| Ara ararauna | Panama, Colombia, Venezuela, Guianas, Ecuador, Peru, Bolivia Brazil, Paraguay, Argentina, Ecuador | BCDE |
| Ara chloropterus | Panama, Colombia, Venezuela, Guianas, Brazil, Paraguay, Ecuador, Peru, Bolivia, Argentina. | BCDE |
| Ara glaucogularis | Bolivia | C |
| Ara macao | Mexico, Central America, Colombia, Venezuela, Guianas, Brazil, Ecuador, Peru, Bolivia | ABCD |
| Ara militaris | Mexico, Venezuela, Colombia, Ecuador, Peru, Bolivia, Argentina | ABCD |
| Ara severus | Panama, Colombia, Ecuador, Venezuela, Guianas, Peru, Bolivia, Brazil | BCD |
| Aratinga auricapillus | Brazil | DE |
| Aratinga jandaya | Brazil | CDE |
| Aratinga maculata | Brazil | C |
| Aratinga nenday | Bolivia, Brazil, Paraguay, Argentina | CD |
| Aratinga solstitialis | Brazil, Guiana | C |
| Aratinga weddellii | Colombia, Ecuador, Peru, Brazil, Bolivia | CD |
| Bolborhynchus lineola | Mexico, Panama, Venezuela, Colombia, Bolivia | ABC |
| Cacatua sulphurea | - | G |
| Columba livia | - | G |
| Conuropsis carolinensis | United States of America | A |
| Coracopsis vasa | - | G |
| Cyanoliseus patagonus | Argentina, Uruguay, Chile | DF |
| Cyanopsitta spixii | Brazil | D |
| Deroptyus accipitrinus | Colombia, Ecuador, Peru, Venezuela, Guianas, Brazil, Bolivia | C |
| Diopsittaca nobilis | Venezuela, Guianas, Brazil | BC |
| Enicognathus leptorhynchus | Chile | F |
| Eupsittula aurea | Suriname, Brazil, Peru, Bolivia, Paraguay, Argentina | CDE |
| Eupsittula cactorum | Brazil | CDE |
| Eupsittula canicularis | Mexico, Costa Rica | AB |
| Eupsittula canicularis | Mexico, Costa Rica | AB |
| Eupsittula canicularis | Mexico, Costa Rica | AB |
| Eupsittula nana | Mexico, Jamaica | A |
| Eupsittula pertinax | Panama, Colombia, Venezuela, Antillas, Guiana, Brazil | BC |
| Falco peregrinus | - | G |
| Forpus coelestis | Ecuador, Peru, Colombia | BF |
| Forpus conspicillatus | Panama, Colombia, Venezuela | B |
| Forpus cyanopygius | Mexico | A |
| Forpus modestus | Colombia, Venezuela, Brazil, Ecuador, Peru, Bolivia | C |
| Forpus passerinus | Colombia, Venezuela, Trinidad, Guianas, Brazil | BC |
| Forpus xanthops | Peru | C |
| Forpus xanthopterygius | Colombia, Ecuador, Peru, Brazil, Bolivia, Paraguay, Argentina | CDE |
| Guaruba guarouba | Brazil | C |
| Hapalopsittaca melanotis | Peru, Bolivia | C |
| Leptosittaca branickii | Colombia, Ecuador, Peru | BF |
| Melopsittacus undulatus | - | G |
| Myiopsitta monachus | Bolivia, Paraguay, Brazil, Argentina, Uruguay | CDEF |
| Nestor notabilis | - | G |
| Orthopsittaca manilatus | Colombia, Ecuador, Peru, Bolivia, Venezuela, Trinidad, Guianas, Brazil | BCDE |
| Pionites leucogaster | Brazil | CD |
| Pionites melanocephalus | Colombia, Venezuela, Guianas, Brazil, Ecuador, Peru | C |
| Primolius auricollis | Bolivia, Brazil, Paraguay, Argentina | CD |
| Primolius couloni | Peru, Brazil, Bolivia | C |
| Primolius maracana | Brazil, Parana, Paraguay, Argentina | CDE |
| Psilopsiagon aymara | Bolivia, Argentina | CDF |
| Psittacara brevipes | Mexico | A |
| Psittacara chloropterus | Hispaniola Island | A |
| Psittacara erythrogenys | Ecuador, Peru | B |
| Psittacara euops | Cuba | A |
| Psittacara finschi | Nicaragua, Costa Rica, Panama | AB |
| Psittacara holochlorus | Mexico, Nicaragua | A |
| Psittacara h. rubritorquis | Guatemala, El Salvador, Honduras, Nicaragua | A |
| Psittacara leucophthalmus | Colombia, Ecuador, Peru, Brazil, Guianas, Venezuela, Bolivia, Paraguay, Argentina, Uruguay | BCDE |
| Psittacara mitratus | Peru, Bolivia, Argentina | CD |
| Psittacara wagleri | Colombia, Venezuela | B |
| Pyrrhura albipectus | Ecuador, Peru | C |
| Pyrrhura frontalis | Brazil, Paraguay, Argentina, Uruguay | DE |
| Pyrrhura hoffmanni | Costa Rica, Panama | B |
| Pyrrhura leucotis | Brazil | E |
| Pyrrhura molinae | Bolivia, Brazil, Paraguay, Argentina | CD |
| Pyrrhura perlata | Brazil, Bolivia | CD |
| Pyrrhura picta | Venezuela, Brazil | C |
| Pyrrhura rupicola | Peru, Brazil, Bolivia | C |
| Rhynchopsitta pachyrhyncha | Mexico | A |
| Rhynchopsitta terrisi | Mexico | A |
| Thectocercus acuticaudatus | Colombia, Venezuela, Brazil, Bolivia, Paraguay, Argentina | BCD |
| Touit purpuratus | Venezuela, Brazil, Colombia, Ecuador, Peru | C |
| Pleu | Pwag | Pmit | Peuo | Pchl | Pery | Phru | Phol | Pfin | Pbre | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pleu | 0.051 | 0.061 | 0.060 | 0.066 | 0.067 | 0.071 | 0.070 | 0.070 | 0.063 | |
| Pwag | 0.053 | 0.030 | 0.044 | 0.045 | 0.039 | 0.043 | 0.047 | 0.041 | 0.045 | |
| Pmit | 0.043 | 0.026 | 0.029 | 0.028 | 0.027 | 0.032 | 0.040 | 0.029 | 0.034 | |
| Peuo | 0.053 | 0.033 | 0.029 | 0.022 | 0.025 | 0.033 | 0.041 | 0.025 | 0.029 | |
| Pchl | 0.058 | 0.036 | 0.031 | 0.015 | 0.027 | 0.034 | 0.042 | 0.027 | 0.030 | |
| Pery | - | - | - | - | - | 0.018 | 0.025 | 0.015 | 0.020 | |
| Phru | 0.063 | 0.041 | 0.036 | 0.019 | 0.022 | - | 0.016 | 0.013 | 0.019 | |
| Phol | 0.066 | 0.043 | 0.038 | 0.022 | 0.024 | - | 0.006 | 0.021 | 0.024 | |
| Pfin | 0.064 | 0.041 | 0.036 | 0.019 | 0.022 | - | 0.013 | 0.011 | 0.011 | |
| Pbre | 0.061 | 0.038 | 0.029 | 0.017 | 0.020 | - | 0.011 | 0.008 | 0.006 |
| Node | Mean | HPD 95% | Origin | Probability of Area |
|---|---|---|---|---|
| 1 | 11.69 | 8.96–14.71 | Indeterminate (most probable C) | 0.37 |
| 1a | 7.66 | 5.76–9.82 | Amazonian (C) | 1 |
| 2 | 13.15 | 10.25–16.24 | Most probable D/or CD | 0.52/0.47 |
| 2a | 10.98 | 8.23–14.02 | Most probable D/or C | 0.52/0.47 |
| 2b | 9.24 | 6.43–12.32 | Most probable AD/or AC | 0.53/0.46 |
| 2c | 1.2 | 0.74–1.78 | Indeterminate (most probable CD) | 0.35 |
| 3 | 14.5 | 11.46–17.86 | Indeterminate (most probable F) | 0.18 |
| 3a | 4.77 | 3.13–6.72 | Amazonian (C) | 1 |
| 3b | 3.45 | 2.03–5.28 | Chaco (D) | 1 |
| 4 | 5.38 | 3.76–7.36 | Amazonian (C) | 1 |
| 4a | 1.92 | 1.36–2.58 | North American (A) | 1 |
| 5 | 7.88 | 5.59–10.85 | Amazonian (C) | 1 |
| 5a | 0.66 | 0.34–1.11 | Most probable A/B | 0.51/0.49 |
| 6 | 5.09 | 3.5–7.01 | Amazonian (C) | 1 |
| 6a | 0.82 | 0.33–1.48 | Indeterminate (CE) | 1 |
| 6b | 0.2 | 0.0–0.84 | Amazonian (C) | 1 |
| 7 | 17.8 | 14.28–21.75 | Indeterminate (most probable AC/ACD) | 0.63/0.37 |
| 8 | 14.21 | 11.32–17.41 | Indeterminate (most probable CD) | 0.38 |
| 9 | 15.08 | 11.66–18.87 | Amazonian (C) | 1 |
| 10 | 15.95 | 12.81–19.39 | Indeterminate (most probable D) | 0.22 |
| 11 | 20.41 | 16.25–24.86 | Amazonian (C) | 1 |
| 12 | 13.4 | 9.47–17.82 | Amazonian (C) | 1 |
| 13 | 29.93 | 24.45–35.81 | Amazonian (C) | 1 |
| 14 | 35.65 | 29.21–42.52 | Indeterminate (CG) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Jacobo, G.; Monterrubio-Rico, T.C.; Cano-Camacho, H.; Zavala-Páramo, M.G. Origin and Diversification of the Genera Aratinga, Eupsittula, and Psittacara (Aves: Psittacidae). Diversity 2025, 17, 155. https://doi.org/10.3390/d17030155
Padilla-Jacobo G, Monterrubio-Rico TC, Cano-Camacho H, Zavala-Páramo MG. Origin and Diversification of the Genera Aratinga, Eupsittula, and Psittacara (Aves: Psittacidae). Diversity. 2025; 17(3):155. https://doi.org/10.3390/d17030155
Chicago/Turabian StylePadilla-Jacobo, Gabriela, Tiberio Cesar Monterrubio-Rico, Horacio Cano-Camacho, and María Guadalupe Zavala-Páramo. 2025. "Origin and Diversification of the Genera Aratinga, Eupsittula, and Psittacara (Aves: Psittacidae)" Diversity 17, no. 3: 155. https://doi.org/10.3390/d17030155
APA StylePadilla-Jacobo, G., Monterrubio-Rico, T. C., Cano-Camacho, H., & Zavala-Páramo, M. G. (2025). Origin and Diversification of the Genera Aratinga, Eupsittula, and Psittacara (Aves: Psittacidae). Diversity, 17(3), 155. https://doi.org/10.3390/d17030155







