Lepidopteran Biodiversity in Madagascar’s Eastern Forests: Assessing Species Distribution Across Protected and Anthropized Landscapes
Abstract
1. Introduction
2. Materials and Methods
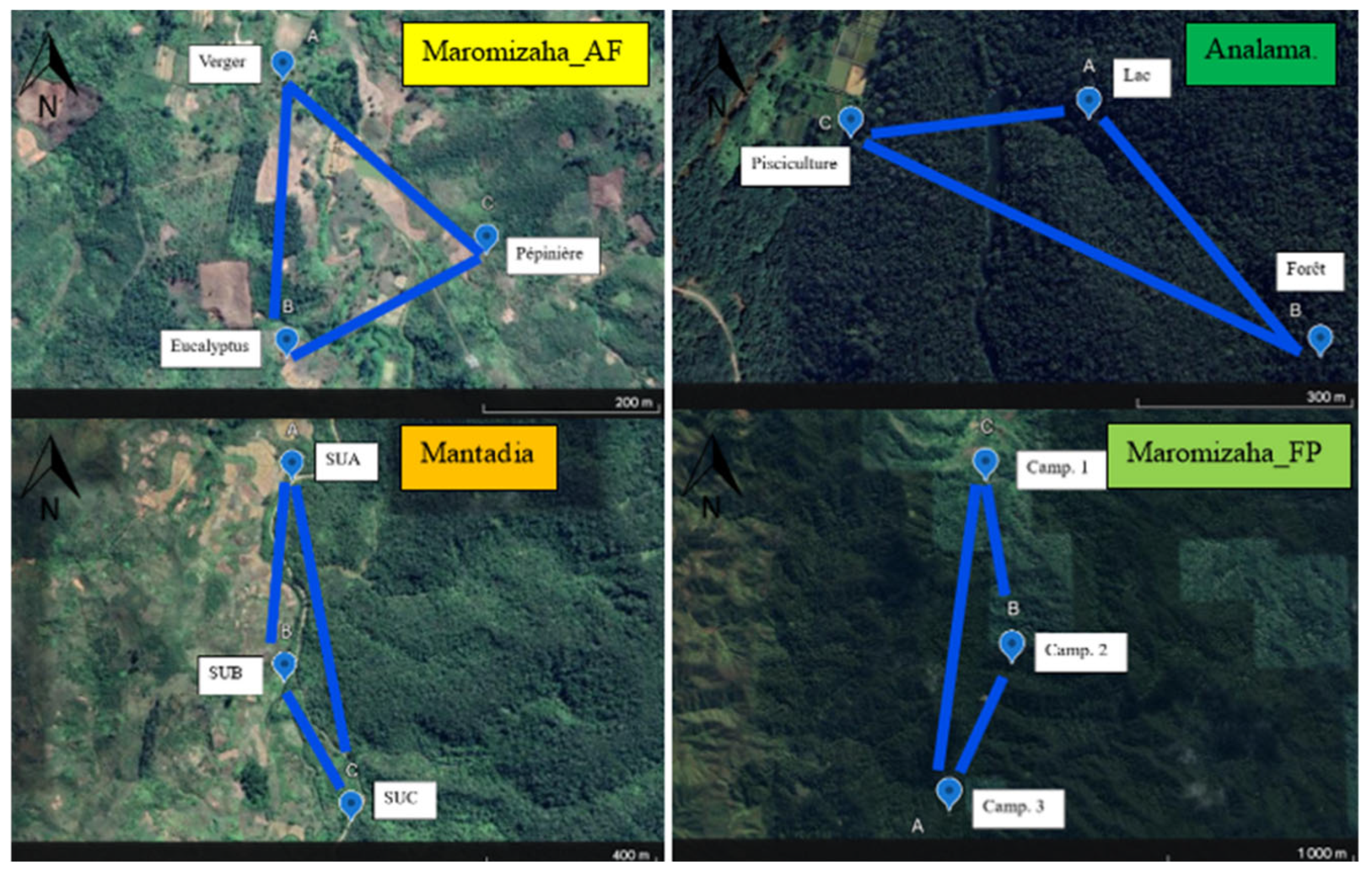
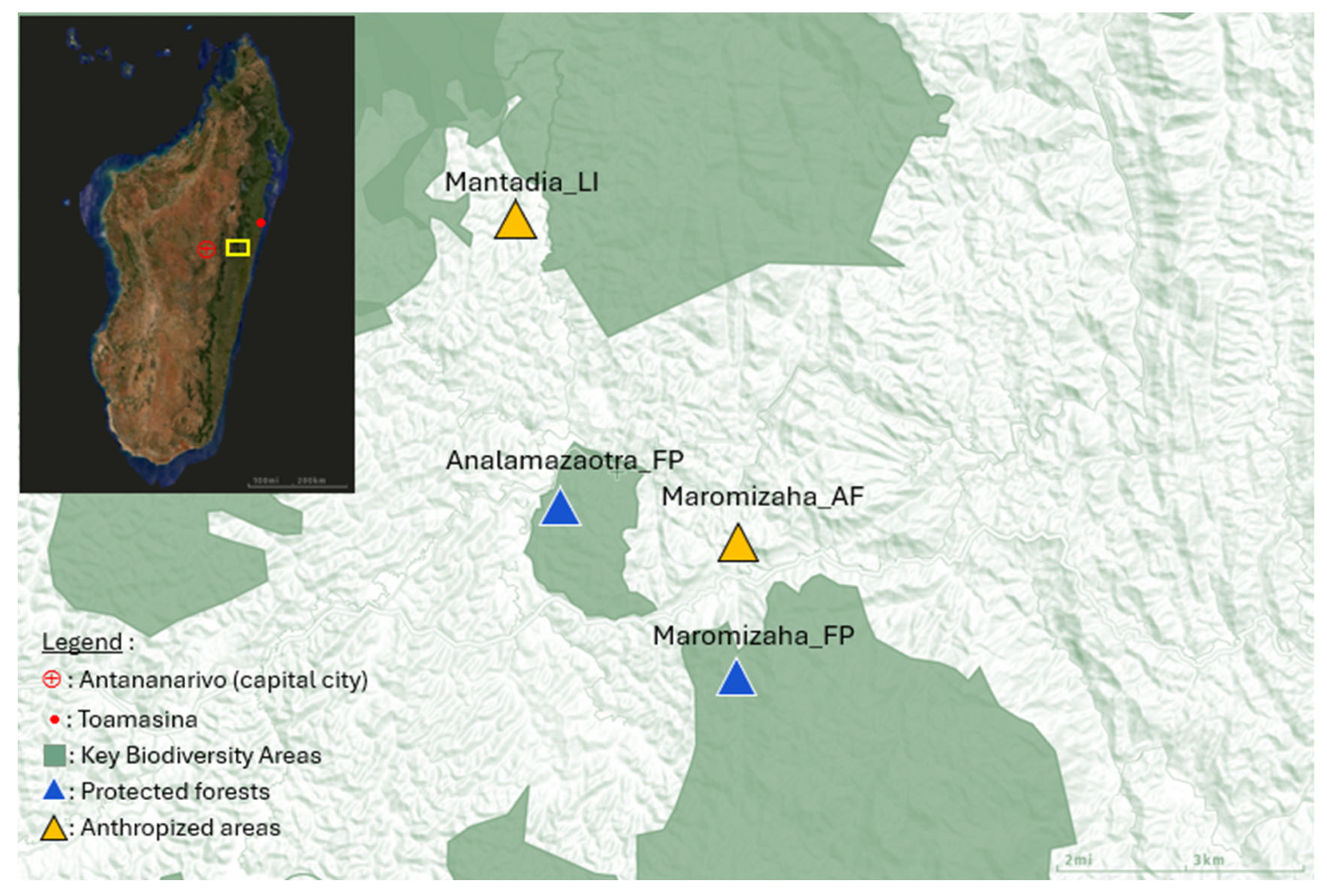
2.1. Study Area
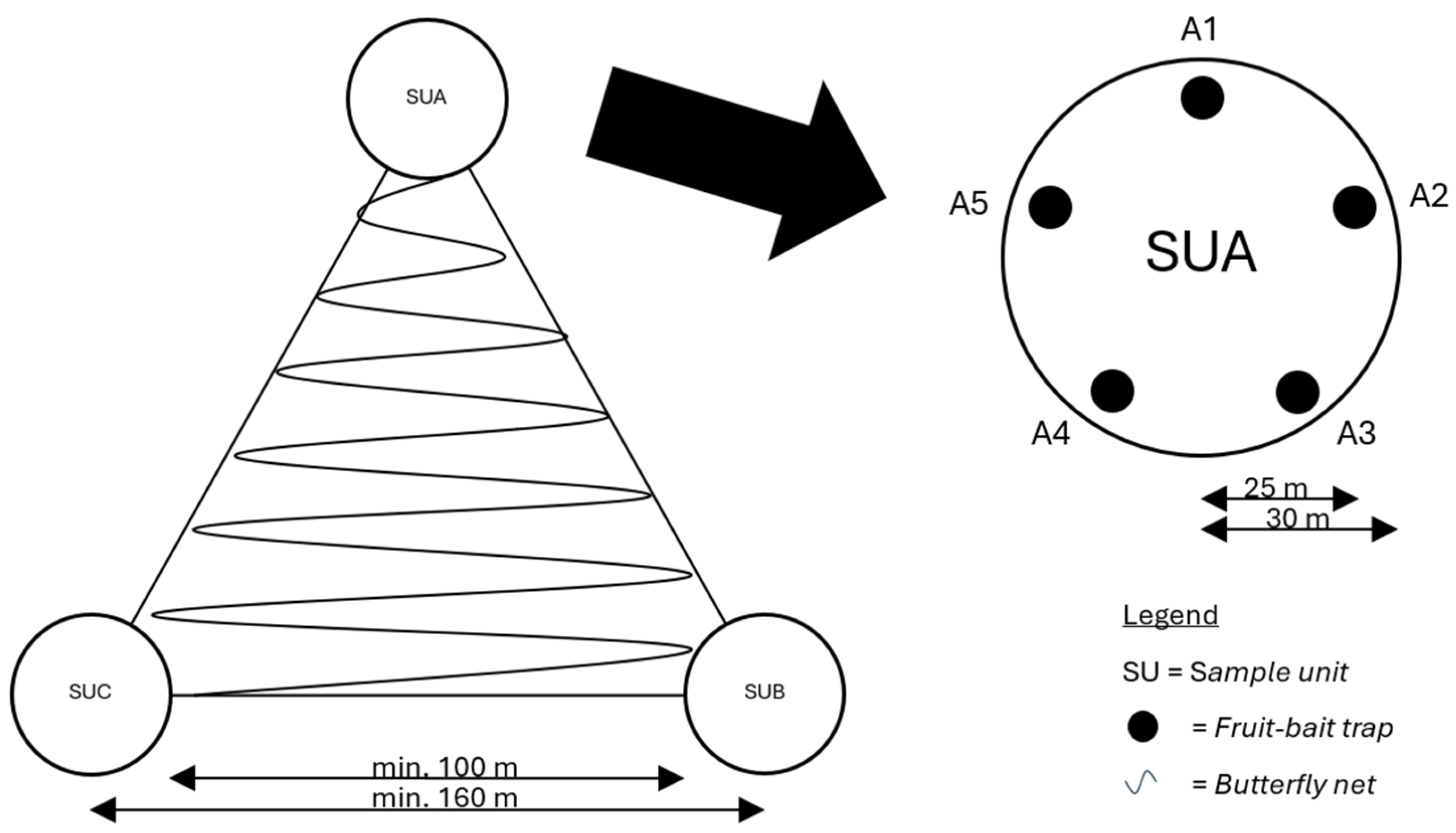
2.2. Sampling Design
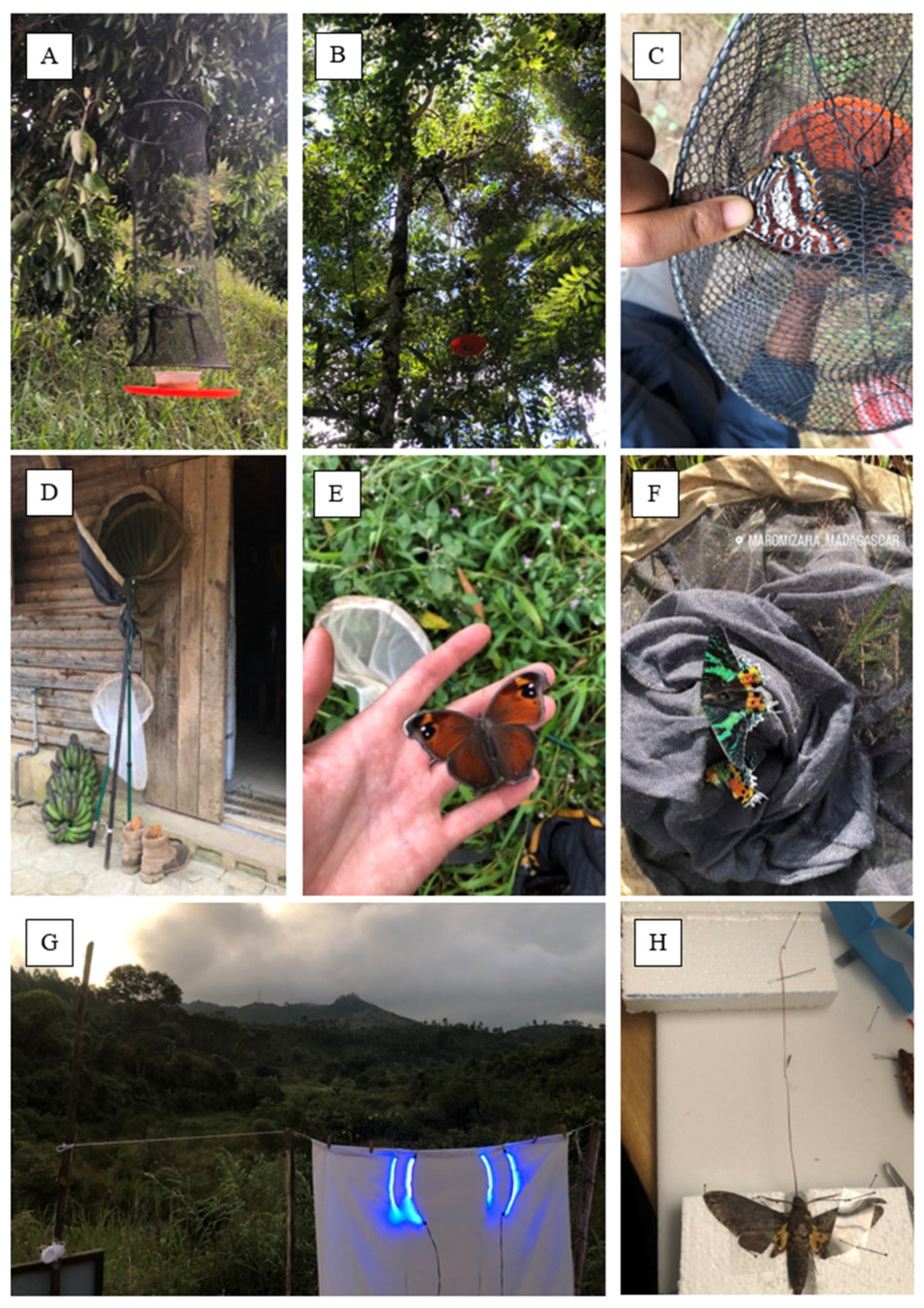
2.2.1. Fruit-Baited Traps
2.2.2. Butterfly Netting
2.2.3. Light Sheet
2.3. Specimen Preparation and Identification
- Forest species: Species depending on the woodlands (all-natural types, primary or slightly degraded), secondary formations, and forest edges (including Savona, tavy, and other types of cleared forests, typically for hillside rice).
- Generalist species: Species found in all the above habitats, marshes, grasslands, anthropogenic zones (villages, roadsides), rocky areas (rocky outcrops, ridges with rupicolous vegetation), farmlands, and beaches.
2.4. Statistical Community Analysis
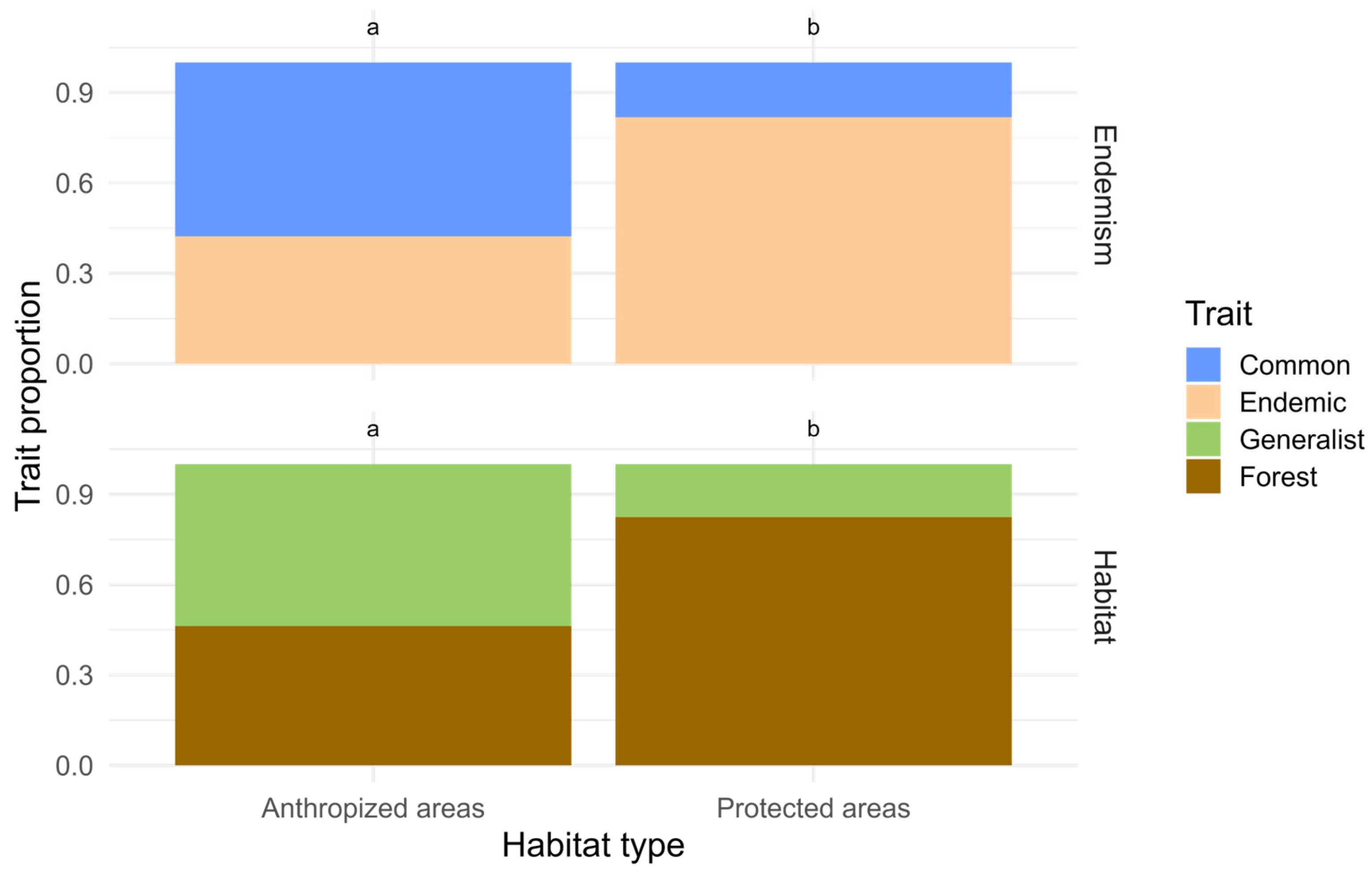
3. Results
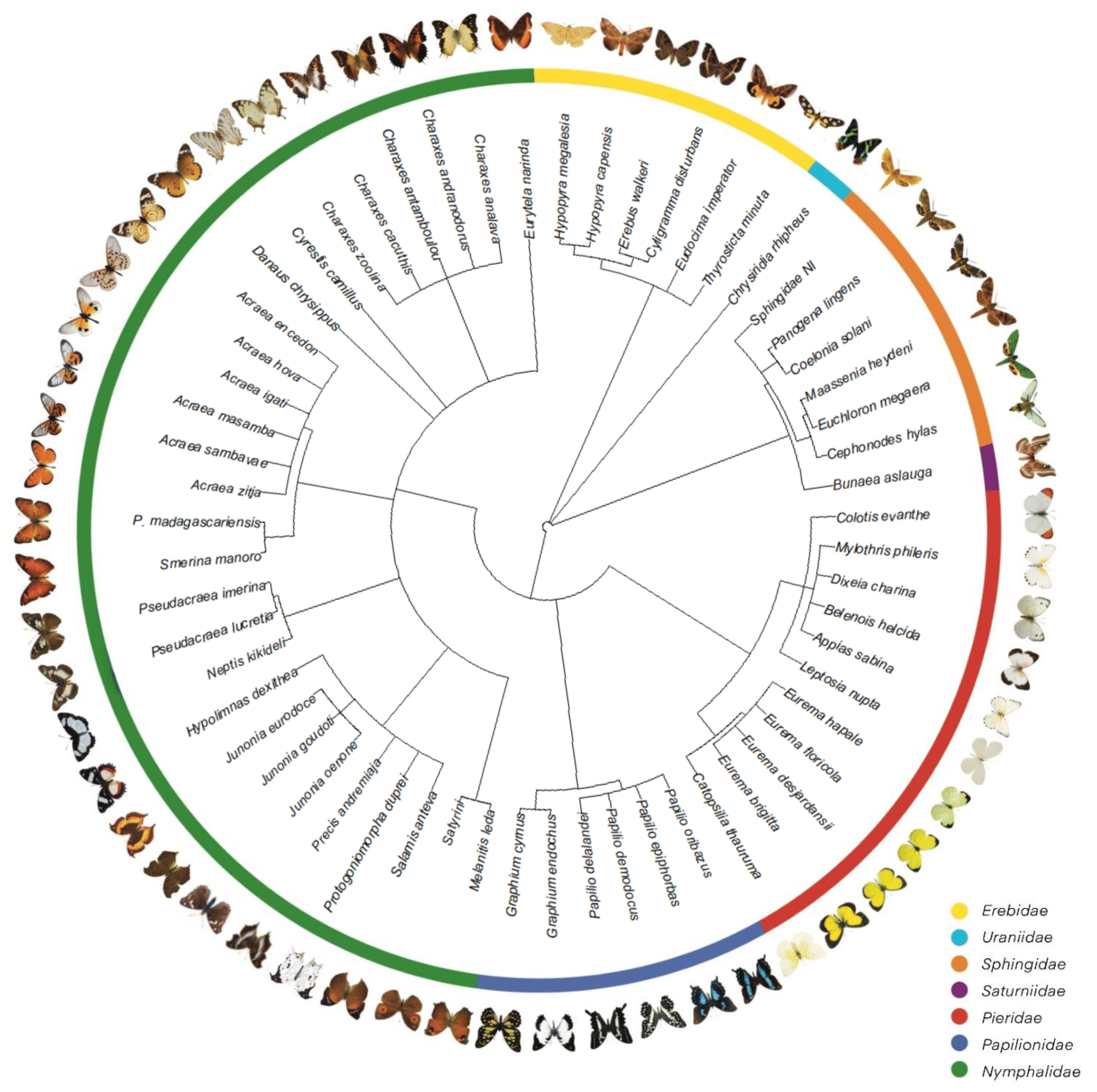
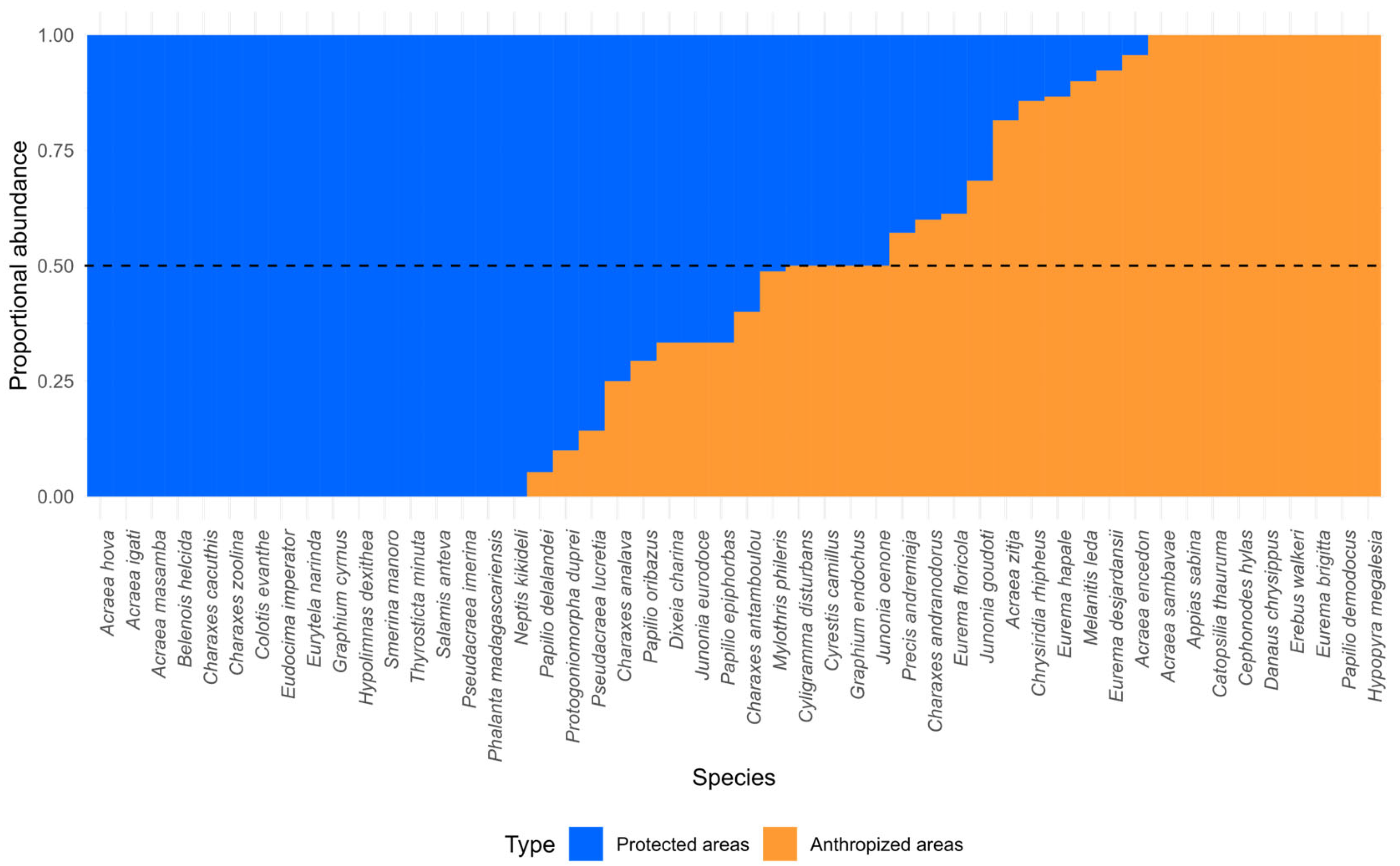
3.1. Butterfly Fauna of the Region
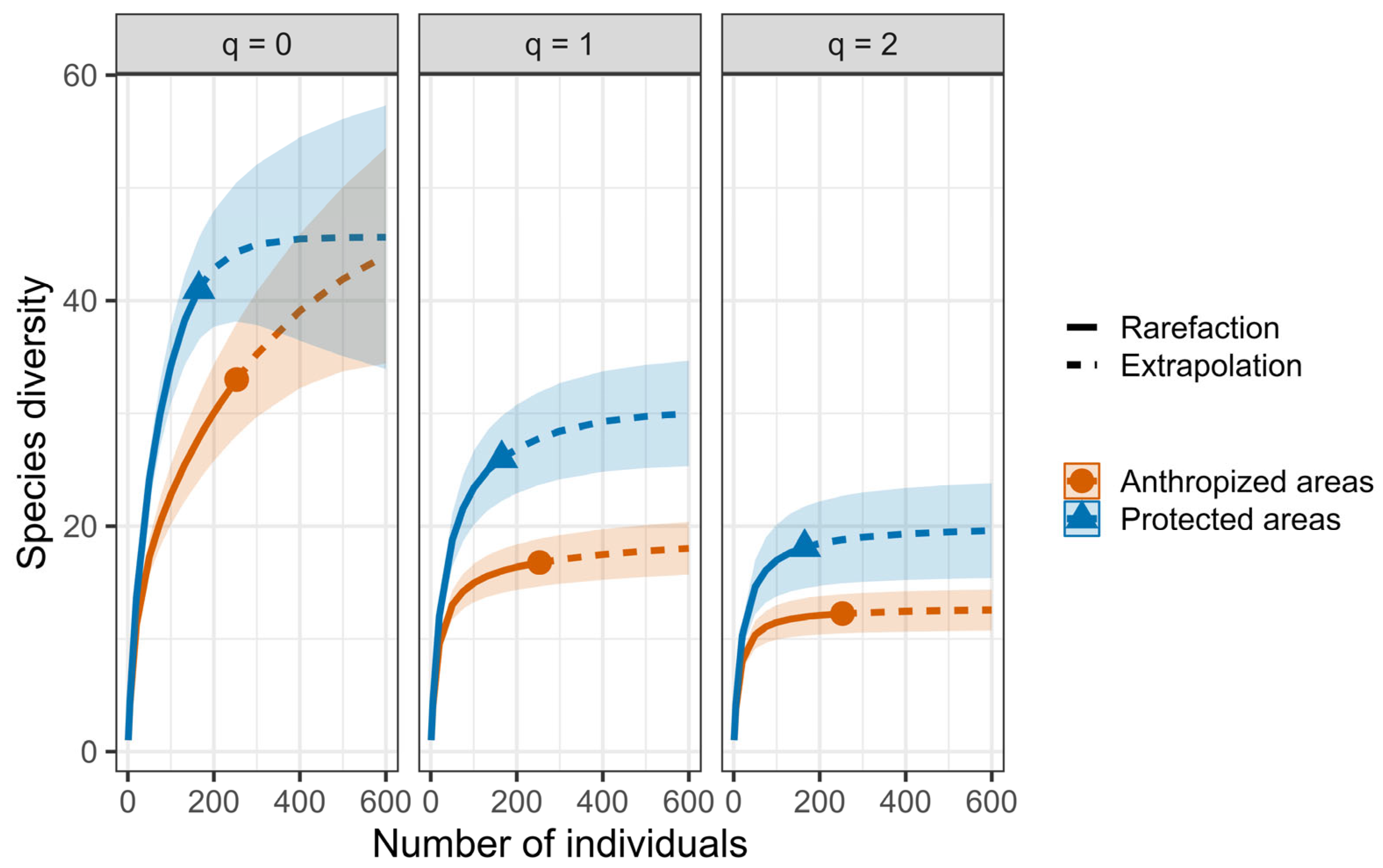
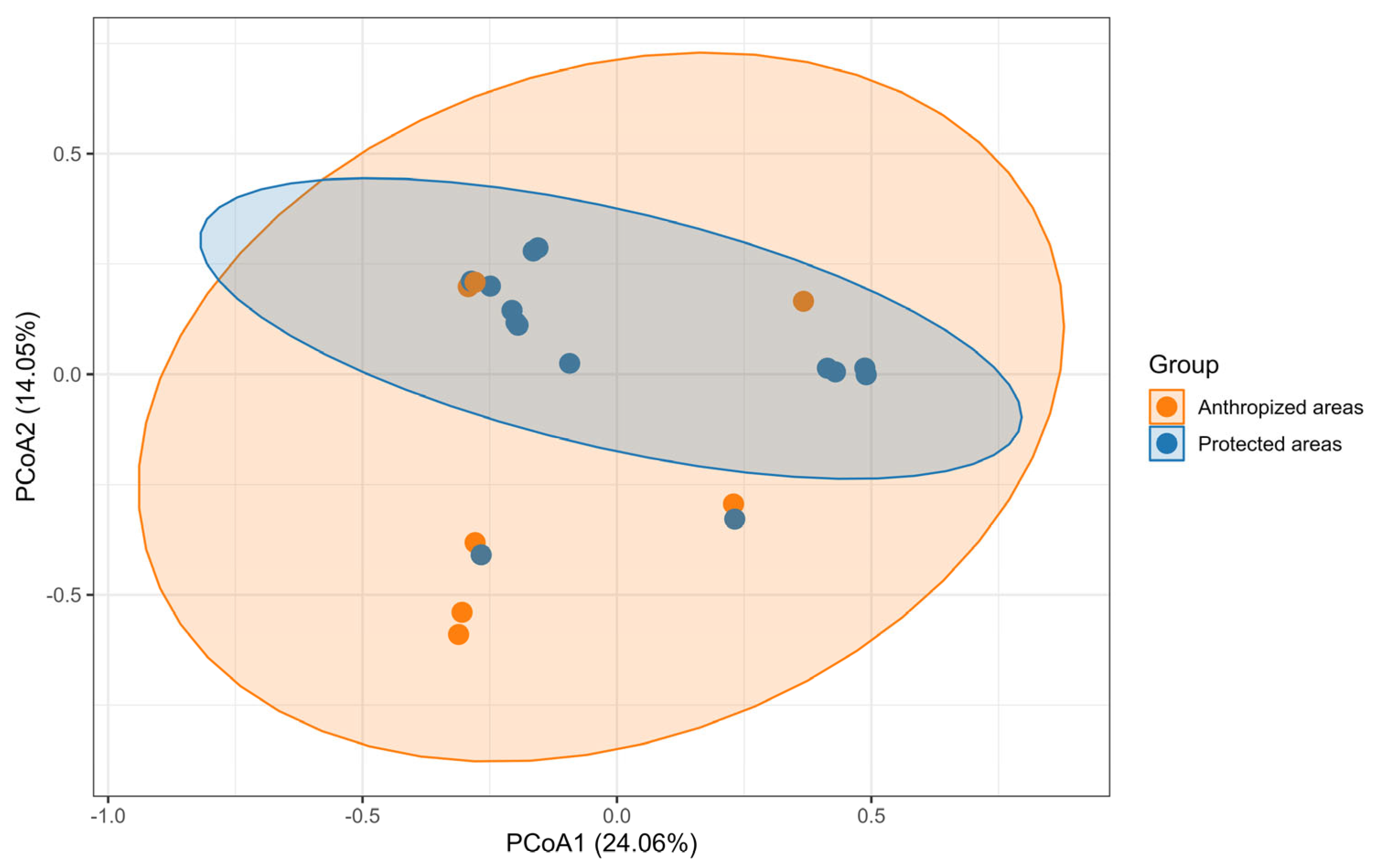
3.2. Community Structure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
| Protected Areas | Sub-Total | Antrhopozed Areas | Sub-Total | Total | |||
|---|---|---|---|---|---|---|---|
| Butterfly Species Per Family | Analamazaotra_FP | Maromizaha_FP | Mantadia_LI | Maromizaha_AF | |||
| Erebidae | 4 | 1 | 5 | 4 | 4 | 9 | |
| Cyligramma | 2 | 2 | 2 | 2 | 4 | ||
| Cyligramma disturbans (*) (Walker, 1858) | 2 | 2 | 2 | 2 | 4 | ||
| Erebus | 1 | 1 | 1 | ||||
| Erebus walkeri Butler, 1875 | 1 | 1 | 1 | ||||
| Eudocima | 1 | 1 | 1 | ||||
| Eudocima imperator (Boisduval, 1833) | 1 | 1 | 1 | ||||
| Hypopyra | 1 | 1 | 1 | ||||
| Hypopyra megalesia (*) Mabille, 1879 | 1 | 1 | 1 | ||||
| Thyrosticta | 2 | 2 | 2 | ||||
| Thyrosticta minuta (*) Boisduval, 1833 | 2 | 2 | 2 | ||||
| Nymphalidae | 43 | 40 | 83 | 96 | 24 | 120 | 203 |
| Acraea | 12 | 11 | 23 | 59 | 9 | 68 | 91 |
| Acraea encedon Linnaeus, 1758 | 1 | 1 | 2 | 37 | 7 | 44 | 46 |
| Acraea hova (*) Boisduval, 1833 | 3 | 3 | 3 | ||||
| Acraea igati Boisduval, 1833 | 3 | 3 | 3 | ||||
| Acraea masamba (*) Ward, 1872 | 9 | 1 | 10 | 10 | |||
| Acraea sambavae (*) Ward, 1873 | 2 | 2 | 2 | ||||
| Acraea zitja (*) Boisduval, 1833 | 2 | 3 | 5 | 20 | 2 | 22 | 27 |
| Charaxes | 2 | 14 | 16 | 8 | 2 | 10 | 26 |
| Charaxes analava (*) Ward, 1872 | 3 | 3 | 1 | 1 | 4 | ||
| Charaxes andranodorus (*) Mabille, 1884 | 2 | 2 | 3 | 3 | 5 | ||
| Charaxes antamboulou (*) Lucas, 1872 | 1 | 8 | 9 | 4 | 2 | 6 | 15 |
| Charaxes cacuthis (*) Hewitson, 1863 | 1 | 1 | 1 | ||||
| Charaxes zoolina (*) Lucas, 1872 | 1 | 1 | 1 | ||||
| Cyrestis | 1 | 1 | 1 | 1 | 2 | ||
| Cyrestis camillus (*) Fabricius, 1781 | 1 | 1 | 1 | 1 | 2 | ||
| Danaus | 2 | 2 | 2 | ||||
| Danaus chrysippus (Linnaeus, 1758) | 2 | 2 | 2 | ||||
| Eurytela | 1 | 1 | 1 | ||||
| Eurytela narinda (*) Ward, 1872 | 1 | 1 | 1 | ||||
| Hypolimnas | 1 | 1 | 2 | 2 | |||
| Hypolimnas dexithea (*) Hewitson, 1863 | 1 | 1 | 2 | 2 | |||
| Junonia | 7 | 2 | 9 | 7 | 8 | 15 | 24 |
| Junonia eurodoce (*) (Westwood, 1850) | 1 | 1 | 2 | 1 | 1 | 3 | |
| Junonia goudoti (Boisduval, 1833) | 5 | 1 | 6 | 7 | 6 | 13 | 19 |
| Junonia oenone (Linnaeus, 1764) | 1 | 1 | 1 | 1 | 2 | ||
| Melanitis | 1 | 1 | 2 | 16 | 2 | 18 | 20 |
| Melanitis leda Linnaeus, 1758 | 1 | 1 | 2 | 16 | 2 | 18 | 20 |
| Neptis | 2 | 2 | 2 | ||||
| Neptis kikideli (*) Boisduval, 1833 | 2 | 2 | 2 | ||||
| Phalanta | 2 | 2 | 2 | ||||
| Phalanta madagascariensis (*) (Mabille, 1887) | 2 | 2 | 2 | ||||
| Precis | 2 | 1 | 3 | 3 | 1 | 4 | 7 |
| Precis andremiaja (*) Boisduval, 1833 | 2 | 1 | 3 | 3 | 1 | 4 | 7 |
| Pseudacraea | 6 | 3 | 9 | 1 | 1 | 10 | |
| Pseudacraea imerina (*) Westwood, 1850 | 3 | 3 | 3 | ||||
| Pseudacraea lucretia (*) Cramer, 1779 | 3 | 3 | 6 | 1 | 1 | 7 | |
| Protogoniomorpha | 5 | 4 | 9 | 1 | 10 | ||
| Protogoniomorpha duprei (*) (Vinson, 1863) | 5 | 4 | 9 | 1 | 1 | 10 | |
| Salamis | 1 | 1 | 1 | ||||
| Salamis anteva (*) Ward, 1870 | 1 | 1 | 1 | ||||
| Smerina | 2 | 1 | 3 | 3 | |||
| Smerina manoro (*) (Ward, 1870) | 2 | 1 | 3 | 3 | |||
| Papilionidae | 24 | 9 | 33 | 2 | 16 | 18 | 51 |
| Graphium | 2 | 2 | 1 | 1 | 3 | ||
| Graphium cyrnus (*) (Boisduval, 1836) | 1 | 1 | 1 | ||||
| Graphium endochus (*) (Boisduval, 1836) | 1 | 1 | 1 | 1 | 2 | ||
| Papilio | 22 | 9 | 31 | 1 | 16 | 17 | 48 |
| Papilio delalandei (*) Godart, 1824 | 17 | 17 | 1 | 1 | 2 | 19 | |
| Papilio demodocus Esper, 1798 | 9 | 9 | 9 | ||||
| Papilio epiphorbas (*) Boisduval, 1833 | 2 | 2 | 1 | 1 | 3 | ||
| Papilio oribazus (*) Boisduval, 1836 | 3 | 9 | 12 | 5 | 5 | 17 | |
| Pieridae | 35 | 8 | 43 | 84 | 20 | 104 | 147 |
| Appias | 2 | 2 | 2 | ||||
| Appias sabina (*) (C. & R. Felder, 1865) | 2 | 2 | 2 | ||||
| Belenois | 3 | 3 | 3 | ||||
| Belenois helcida (*) (Boisduval, 1833) | 3 | 3 | 3 | ||||
| Catopsilia | 1 | 1 | 1 | ||||
| Catopsilia thauruma (Reakirt, 1866) | 1 | 1 | 1 | ||||
| Colotis | 1 | 1 | 1 | ||||
| Colotis evanthe (Boisduval, 1836) | 1 | 1 | 1 | ||||
| Dixeia | 2 | 2 | 1 | 1 | 3 | ||
| Dixeia charina (*) (Boisduval, 1836) | 2 | 2 | 1 | 1 | 3 | ||
| Eurema | 14 | 2 | 16 | 63 | 17 | 80 | 96 |
| Eurema brigitta (Stoll, 1780) | 19 | 5 | 24 | 24 | |||
| Eurema desjardansii (*) (Boisduval, 1833) | 2 | 2 | 17 | 7 | 24 | 26 | |
| Eurema floricola (Boisduval, 1833) | 12 | 12 | 19 | 19 | 31 | ||
| Eurema hapale (Mabille, 1882) | 2 | 2 | 8 | 5 | 13 | 15 | |
| Mylothris | 15 | 6 | 21 | 18 | 2 | 20 | 41 |
| Mylothris phileris (*) (Boisduval, 1833) | 15 | 6 | 21 | 18 | 2 | 20 | 41 |
| Sphingidae | 1 | 1 | 1 | ||||
| Cephonodes | 1 | 1 | 1 | ||||
| Cephonodes hylas Linnaeus, 1771 | 1 | 1 | 1 | ||||
| Uraniidae | 1 | 1 | 6 | 6 | 7 | ||
| Chrysiridia | 1 | 1 | 6 | 6 | 7 | ||
| Chrysiridia rhipheus (*) Drury, 1773 | 1 | 1 | 6 | 6 | 7 | ||
| Total | 107 | 58 | 165 | 182 | 71 | 253 | 418 |
References
- Goodman, S.M. Updated Estimates of Biotic Diversity and Endemism for Madagascar—Revisited after 20 Years. Oryx 2023, 57, 561–565. [Google Scholar] [CrossRef]
- Cota, G.; Sagan, V.; Maimaitijiang, M.; Freeman, K. Forest Conservation with Deep Learning: A Deeper Understanding of Human Geography around the Betampona Nature Reserve, Madagascar. Remote Sens. 2021, 13, 3495. [Google Scholar] [CrossRef]
- Vences, M.; Wollenberg, K.C.; Vieites, D.R.; Lees, D.C. Madagascar as a Model Region of Species Diversification. Trends Ecol. Evol. 2009, 24, 456–465. [Google Scholar] [CrossRef]
- Jeanne, I.; Randremanana, R.; Robert, V.; Ariey, F.; Tombo, M.L.; Wilmé, L.; Ranivoarisoa, S.; Duchemin, J.B. Biogéographie de Madagascar. In Atlas Évolutif du Paludisme à Madagascar; Institut Pasteur de Madagascar: Antananarivo, Madagascar, 2002; pp. 1–10. [Google Scholar]
- Wurz, A.; Grass, I.; Lees, D.C.; Rakotomalala, A.A.N.A.; Sáfián, S.; Martin, D.A.; Osen, K.; Loos, J.; Benasoavina, E.; Alexis, T.; et al. Land-Use Change Differentially Affects Endemic, Forest and Open-Land Butterflies in Madagascar. Insect Conserv. Divers. 2022, 15, 606–620. [Google Scholar] [CrossRef]
- Tracewski, Ł.; Butchart, S.H.M.; Di Marco, M.; Ficetola, G.F.; Rondinini, C.; Symes, A.; Wheatley, H.; Beresford, A.E.; Buchanan, G.M. Toward Quantification of the Impact of 21st-Century Deforestation on the Extinction Risk of Terrestrial Vertebrates. Conserv. Biol. 2016, 30, 1070–1079. [Google Scholar] [CrossRef]
- Lees, D.C.; Minet, J. Lepidoptera, Butterflies and Moths: Systematics and Diversity. In The New Natural History of Madagascar; Goodman, S.M., Andrianarimisa, A., Armstrong, A.H., Cooke, A., De Wit, M., Ganzhorn, J.U., Gautier, L., Goodman, S.M., Jones, J.P.G., Jungers, W.L., et al., Eds.; Princeton University Press: Princeton, NJ, USA, 2022; pp. 1141–1172. ISBN 9780691222622. [Google Scholar]
- Merckx, T.; Huertas, B.; Basset, Y.; Thomas, J.A. A Global Perspective on Conserving Butterflies and Moths and Their Habitats. In Key Topics in Conservation Biology 2; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bonebrake, T.C.; Ponisio, L.C.; Boggs, C.L.; Ehrlich, P.R. More than Just Indicators: A Review of Tropical Butterfly Ecology and Conservation. Biol. Conserv. 2010, 143, 1831–1841. [Google Scholar] [CrossRef]
- Checa, M.F.; Donoso, D.A.; Rodriguez, J.; Levy, E.; Warren, A.; Willmott, K. Combining Sampling Techniques Aids Monitoring of Tropical Butterflies. Insect Conserv. Divers. 2019, 12, 362–372. [Google Scholar] [CrossRef]
- Munyuli, T. Assessment of Indicator Species of Butterfly Assemblages in Coffee–Banana Farming System in Central Uganda. Afr. J. Ecol. 2012, 50, 77–89. [Google Scholar] [CrossRef]
- Freitas, A.V.L.; Santos, J.P.; Rosa, A.H.B.; Iserhard, C.A.; Richter, A.; Siewert, R.R.; Gueratto, P.E.; Carreira, J.Y.O.; Lourenço, G.M. Sampling Methods for Butterflies (Lepidoptera). In Measuring Arthropod Biodiversity: A Handbook of Sampling Methods; Carlos, S.J., Fernandes, G.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 101–123. ISBN 978-3-030-53226-0. [Google Scholar]
- Thomas, J.A. Monitoring Change in the Abundance and Distribution of Insects Using Butterflies and Other Indicator Groups. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Sterling, S.M.; Ducharne, A.; Polcher, J. The Impact of Global Land-Cover Change on the Terrestrial Water Cycle. Nat. Clim. Change 2013, 3, 385–390. [Google Scholar] [CrossRef]
- Jones, J.; Rakotonarivo, O.; Razafimanahaka, J. Forest Conservation in Madagascar: Past, Present, and Future. In The New Natural History of Madagascar; S. M. Goodman, Princeton University Press: Princeton, NJ, USA, 2022; ISBN 9780691222622. [Google Scholar]
- Zaehringer, J.G.; Eckert, S.; Messerli, P. Revealing Regional Deforestation Dynamics in North-Eastern Madagascar—Insights from Multi-Temporal Land Cover Change Analysis. Land 2015, 4, 454–474. [Google Scholar] [CrossRef]
- Armstrong, A.; Shugart, H.; Fatoyinbo, L. Characterization of Community Composition and Forest Structure in a Madagascar Lowland Rainforest. Trop. Conserv. Sci. 2011, 4, 428–444. [Google Scholar] [CrossRef]
- Vieilledent, G.; Grinand, C.; Rakotomalala, F.A.; Ranaivosoa, R.; Rakotoarijaona, J.R.; Allnutt, T.F.; Achard, F. Combining Global Tree Cover Loss Data with Historical National Forest Cover Maps to Look at Six Decades of Deforestation and Forest Fragmentation in Madagascar. Biol. Conserv. 2018, 222, 189–197. [Google Scholar] [CrossRef]
- Speart, J. Winged Obsession: The Pursuit of the World’s Most Notorious Butterfly Smuggler, 1st ed.; Harper Collins: New York City, NY, USA, 2011. [Google Scholar]
- Boppré, M.; Vane-Wright, R. The Butterfly House Industry: Conservation Risks and Education Opportunities. Conserv. Soc. 2012, 10, 285–303. [Google Scholar] [CrossRef]
- Wang, Z.; Chan, W.-P.; Pham, N.T.; Zeng, J.; Pierce, N.E.; Lohman, D.J.; Meng, W. One in Five Butterfly Species Sold Online across Borders. Biol. Conserv. 2023, 283, 110092. [Google Scholar] [CrossRef]
- IUCN. Red List of Lepidoptera Species from Madagascar Assessed by IUCN. Available online: https://www.iucnredlist.org/search?dl=true&permalink=e12a298a-a840-470c-bd64-96faafd9ea4c (accessed on 14 January 2025).
- Razafimanantsoa, T. Étude Bioécologique Des Papillons Séricigènes Dans Les Familles Des Lasiocampidae et Des Saturniidae Au Parc National de Ranomafana et Dans La Forêt de Tapia d’Ambohimanjaka. Master’s Thesis, Université d’Antananarivo, Antananarivo, Madagascar, 2008. [Google Scholar]
- Ravelomanana, A.; Razakanirina, T.; Ravalison, F.; Andriamandranto, R. Recolonisation des forêts dégradées par les fourmis et les papillons diurnes suite à la restauration de la forêt de Kalanoro, Moramanga. In Proceedings of the MESupReS: Forum de la Recherche: One Health; Fianarantsoa, Madagascar, 29 June 2023. [Google Scholar]
- Lees, D.; Minet, J. Lepidoptera: Systematics and Diversity. In The Natural History of Madagascar; S. M. Goodman, Princeton University Press: Princeton, NJ, USA, 2003; pp. 748–761. ISBN 0226303063. [Google Scholar]
- Li, X.; Luo, Y.; Zhang, Y.-L.; Schweiger, O.; Settele, J.; Yang, Q. On the Conservation Biology of a Chinese Population of the Birdwing Troides Aeacus (Lepidoptera: Papilionidae). J. Insect Conserv. 2010, 14, 257–268. [Google Scholar] [CrossRef]
- Cleary, D.F.R.; Mooers, A.Ø. Burning and Logging Differentially Affect Endemic vs. Widely Distributed Butterfly Species in Borneo. Divers. Distrib. 2006, 12, 409–416. [Google Scholar] [CrossRef]
- Kremen, C. Biological Inventory Using Target Taxa: A Case Study of the Butterflies of Madagascar. Ecol. Appl. 1994, 4, 407–422. [Google Scholar] [CrossRef]
- Devries, P. A Standardized Butterfly Sampling Protocol: Fruit-Feeding Butterflies (Nymphalidae). In Core Standardized Methods for Rapid Biological Field Assessment; Conversation International: Crystal City, VA, USA, 2016; pp. 139–148. ISBN 978-1-934151-96-9. [Google Scholar]
- Evans, L.C.; Oliver, T.H.; Sims, I.; Greenwell, M.P.; Melero, Y.; Watson, A.; Townsend, F.; Walters, R.J. Behavioural Modes in Butterflies: Their Implications for Movement and Searching Behaviour. Anim. Behav. 2020, 169, 23–33. [Google Scholar] [CrossRef]
- Pryke, J.S.; Vrdoljak, S.M.; Grant, P.B.C.; Samways, M.J. Butterfly Behavioural Responses to Natural Bornean Tropical Rain-Forest Canopy Gaps. J. Trop. Ecol. 2012, 28, 45–54. [Google Scholar] [CrossRef]
- Andrianambinina, F.O.D.; Rafanoharana, S.C.; Rasamuel, H.A.T.; Waeber, P.O.; Ganzhorn, J.U.; Wilmé, L. Decrease of Deforestation in Protected Areas of Madagascar during the COVID-19 Years. Madag. Conserv. Dev. 2024, 18, 15–21. [Google Scholar] [CrossRef]
- Raveloaritiana, E.; Wurz, A.; Grass, I.; Osen, K.; Soazafy, M.R.; Martin, D.A.; Faliniaina, L.; Rakotomalala, N.H.; Vorontsova, M.S.; Tscharntke, T.; et al. Land-Use Intensification Increases Richness of Native and Exotic Herbaceous Plants, but Not Endemics, in Malagasy Vanilla Landscapes. Divers. Distrib. 2021, 27, 784–798. [Google Scholar] [CrossRef]
- Segers, A. Détermination Des Visiteurs Floraux de Trois Essences Ligneuses à Haute Valeur Commerciale: Baillonella Toxisperma Pierre, Afzelia Bipindensis Harms et Erythrophleum Suaveolens (Guill. & Perr.) Brenan. Master’s Thesis, Gembloux Agro-Bio Tech, Gembloux, Belgium, 2018. [Google Scholar]
- Collins, S.; Cogdon, C. Checklist of the Butterflies of Madagascar; African Butterfly Research Institute: Nairobi, Kenya, 2017. [Google Scholar]
- Beccaloni, G.; Scoble, M.; Kitching, I.; Simonsen, T.; Robinson, G.; Pitkin, B.; Hine, A.; Lyal, C.; Ollerenshaw, J.; Wing, P.; et al. Global Lepidoptera Index (Version 1.1.24.106). Available online: https://doi.org/10.48580/dg4lg-49xk (accessed on 19 December 2024).
- Mendenhall, C.D.; Shields-Estrada, A.; Krishnaswami, A.J.; Daily, G.C. Quantifying and Sustaining Biodiversity in Tropical Agricultural Landscapes. Proc. Natl. Acad. Sci. USA 2016, 113, 14544–14551. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Magurran, A. Measuring Biologcial Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Chao, A. Estimating the Population Size for Capture-Recapture Data with Unequal Catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet Michael, F.G.F.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package, Version 2.6-8; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics, Verision 0.9-83-7; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Jose, G. Comecol—Community Ecology Analysis, Version 1.8.1; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wiorek, M.; Malik, K.; Lees, D.; Przybyłowicz, Ł. Malagasy Polka Dot Moths (Noctuoidea: Erebidae: Arctiinae: Syntomini) of Ambohitantely—Endemism in the Most Important Relict of Central Plateau Rainforest in Madagascar. PeerJ 2021, 9, e11688. [Google Scholar] [CrossRef]
- de Souza Amorim, D.; Brown, B.V.; Boscolo, D.; Ale-Rocha, R.; Alvarez-Garcia, D.M.; Balbi, M.I.P.A.; de Marco Barbosa, A.; Capellari, R.S.; de Carvalho, C.J.B.; Couri, M.S.; et al. Vertical Stratification of Insect Abundance and Species Richness in an Amazonian Tropical Forest. Sci. Rep. 2022, 12, 1734. [Google Scholar] [CrossRef]
- Rajaonarimalala, R.; Korol, Y.; Andrianarimisa, A.; Dröge, S.; Fulgence, T.; Grass, I.; Kreft, H.; Osen, K.; Rakotomalala, A.A.N.A.; Rakouth, B.; et al. Stand Structure as the Proximate Driver of Endemic Biodiversity and Ecosystem Functions in Tropical Mosaic Landscapes. bioRxiv 2024. bioRxiv:03.20.582692. [Google Scholar] [CrossRef]
- Otis, G.W.; Locke, B.; McKenzie, N.G.; Cheung, D.; MacLeod, E.; Careless, P.; Kwoon, A. Local Enhancement in Mud-Puddling Swallowtail Butterflies (Battus philenor and Papilio glaucus). J. Insect Behav. 2006, 19, 685–698. [Google Scholar] [CrossRef]
- Andriatsitohaina, R.N.N.; Laby, P.; Llopis, J.C.; Martin, D.A. Agroforestry in Madagascar: Past, Present, and Future. Agrofor. Syst. 2024, 98, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.P. Are Exotic Host Plants a Life Raft or a Trap for Butterflies? Curr. Opin. Insect Sci. 2023, 58, 101074. [Google Scholar] [CrossRef] [PubMed]
| By Land Type and Site | Abundance | Species Richness | Chao1 |
|---|---|---|---|
| Protected areas | 165 | 41 | 44.92 ± 3.37 |
| Analamazaotra_FP | 107 | 33 | 42.10 ± 6.48 |
| Maromizaha_FP | 58 | 22 | 33.25 ± 9.53 |
| Anthropized areas | 253 | 33 | 46.00 ± 9.63 |
| Mantadia_LI | 182 | 20 | 22.50 ± 3.15 |
| Maromizaha_AF | 71 | 24 | 33.17 ± 7.37 |
| Total | 418 | 50 | 55.06 ± 4.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannoteau, R.; Ravalison, F.A.T.; Randrianarivelo, B.F.; Ravelomanana, A.; Trolin, N.; Caparros Megido, R.; Segers, A.; Francis, F.; Noël, G. Lepidopteran Biodiversity in Madagascar’s Eastern Forests: Assessing Species Distribution Across Protected and Anthropized Landscapes. Diversity 2025, 17, 95. https://doi.org/10.3390/d17020095
Hannoteau R, Ravalison FAT, Randrianarivelo BF, Ravelomanana A, Trolin N, Caparros Megido R, Segers A, Francis F, Noël G. Lepidopteran Biodiversity in Madagascar’s Eastern Forests: Assessing Species Distribution Across Protected and Anthropized Landscapes. Diversity. 2025; 17(2):95. https://doi.org/10.3390/d17020095
Chicago/Turabian StyleHannoteau, Robin, Finaritra Antra Tia Ravalison, Brayan Fenontsoa Randrianarivelo, Andrianjaka Ravelomanana, Naya Trolin, Rudy Caparros Megido, Arnaud Segers, Frédéric Francis, and Grégoire Noël. 2025. "Lepidopteran Biodiversity in Madagascar’s Eastern Forests: Assessing Species Distribution Across Protected and Anthropized Landscapes" Diversity 17, no. 2: 95. https://doi.org/10.3390/d17020095
APA StyleHannoteau, R., Ravalison, F. A. T., Randrianarivelo, B. F., Ravelomanana, A., Trolin, N., Caparros Megido, R., Segers, A., Francis, F., & Noël, G. (2025). Lepidopteran Biodiversity in Madagascar’s Eastern Forests: Assessing Species Distribution Across Protected and Anthropized Landscapes. Diversity, 17(2), 95. https://doi.org/10.3390/d17020095









