New Information on the Morphology and Tooth Replacement of Xenodens calminechari (Squamata: Mosasauridae), a Unique Mosasaurid from the Maastrichtian Phosphates of Morocco
Abstract
1. Introduction
- (1)
- The assertion that the tooth arrangement is unnatural, because the tooth crowns supposedly do not exhibit a one-to-one correspondence with replacement pits (p. 2166: “However, the four “articulated” tooth crowns only show two resorption pits proximally, strongly suggesting that only two teeth should be present here”) as is typical of most, but not all mosasaurids: see, e.g., Pluridens serpentis [13], Russellosaurus coheni [16] and Dallasaurus turneri [17];
- (2)
- Supposed ‘gummy’ material on the fossil as evidence of alteration (p. 2166: “…in lateral view, the tooth crowns appear to be joined to the maxilla by a gummy, paste-like material that is smeared to the ventrolateral surface of the maxilla”);
- (3)
- The specimen was proposed to represent a juvenile of Carinodens (p. 2170: “the constituent elements are possibly juvenile and cannot be adequately distinguished from Carinodens spp.”) while providing no evidence for immaturity.
2. Materials and Methods
3. Results
3.1. Systematic Paleontology
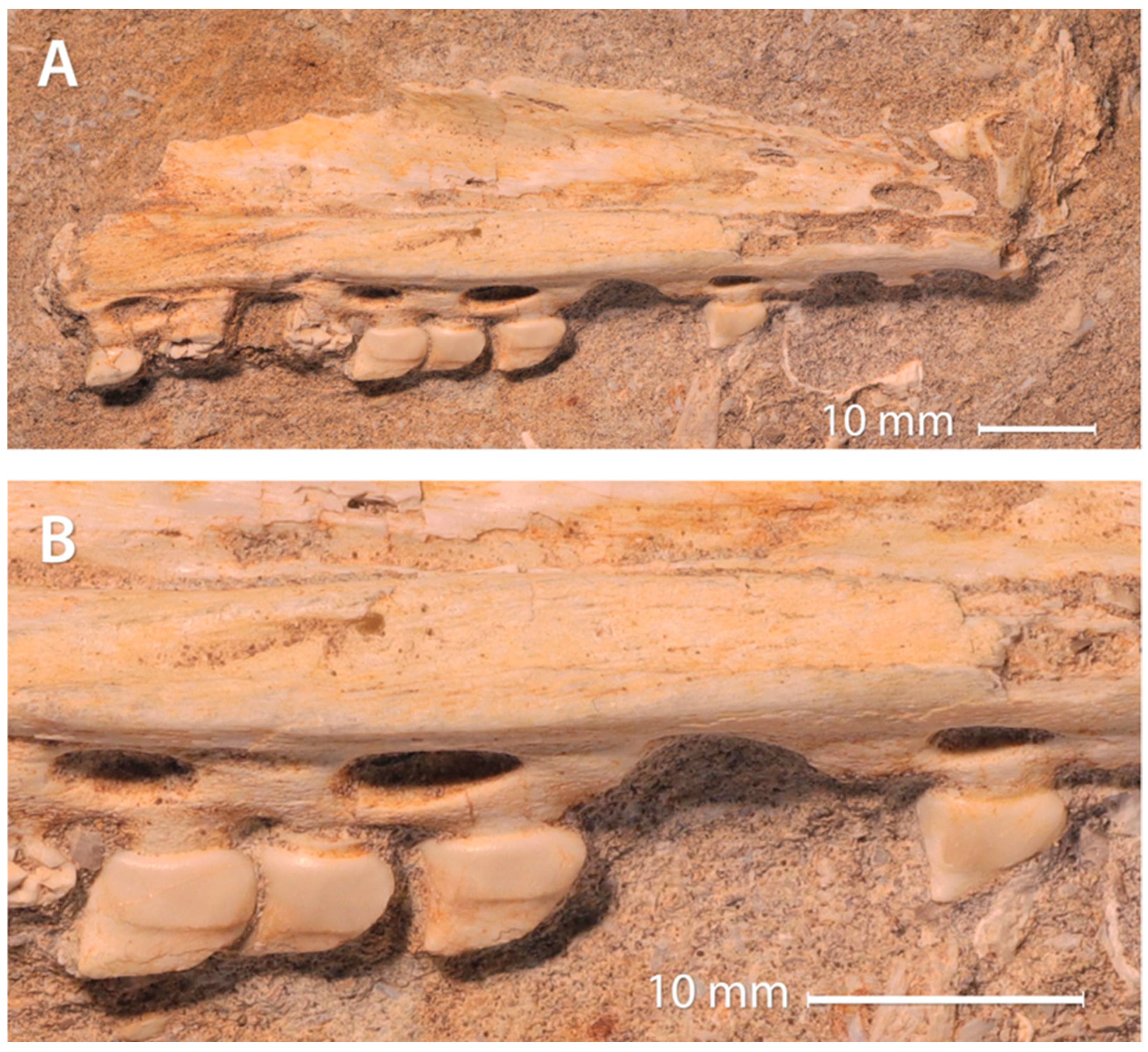
3.2. Authenticity of the Xenodens Holotype
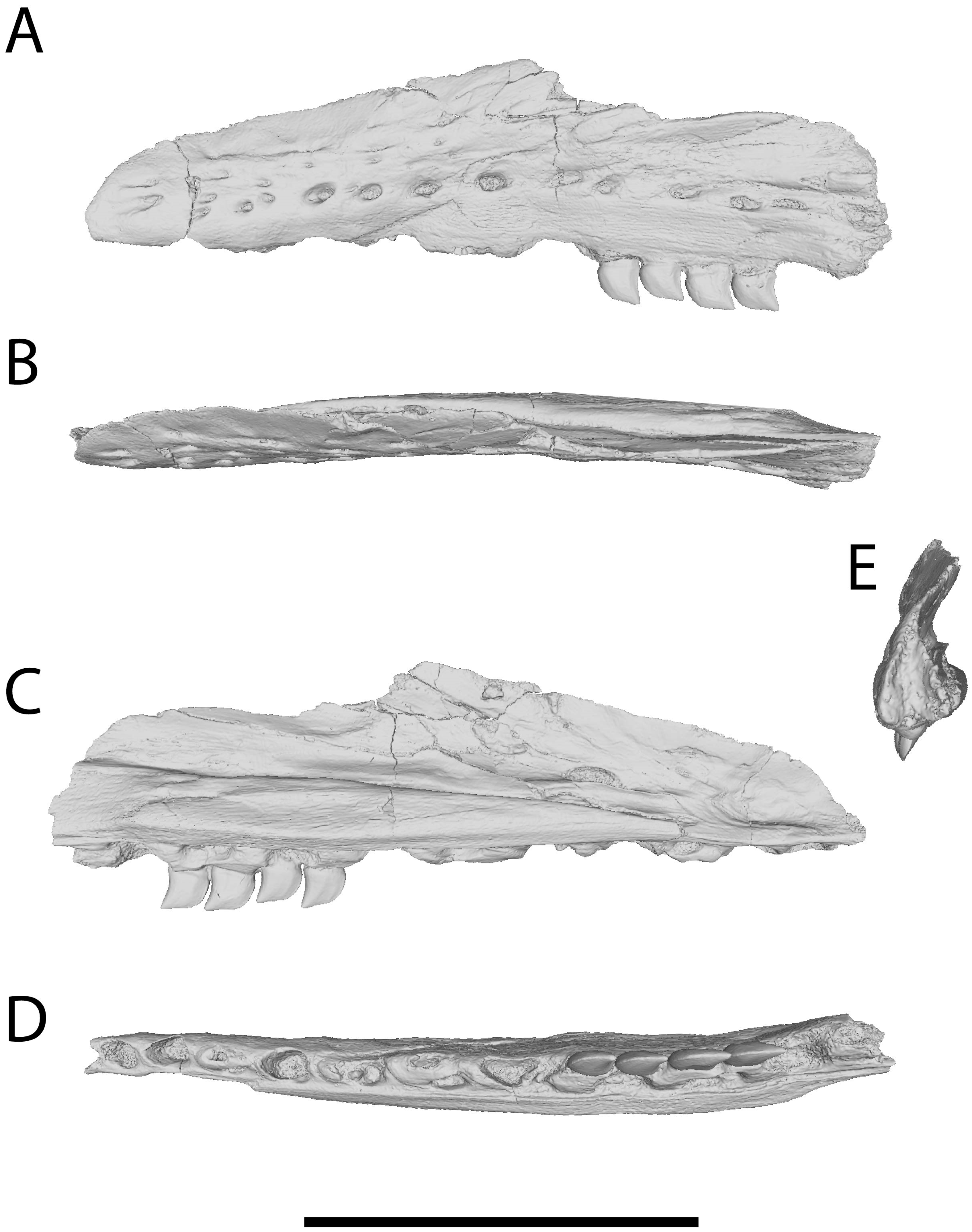
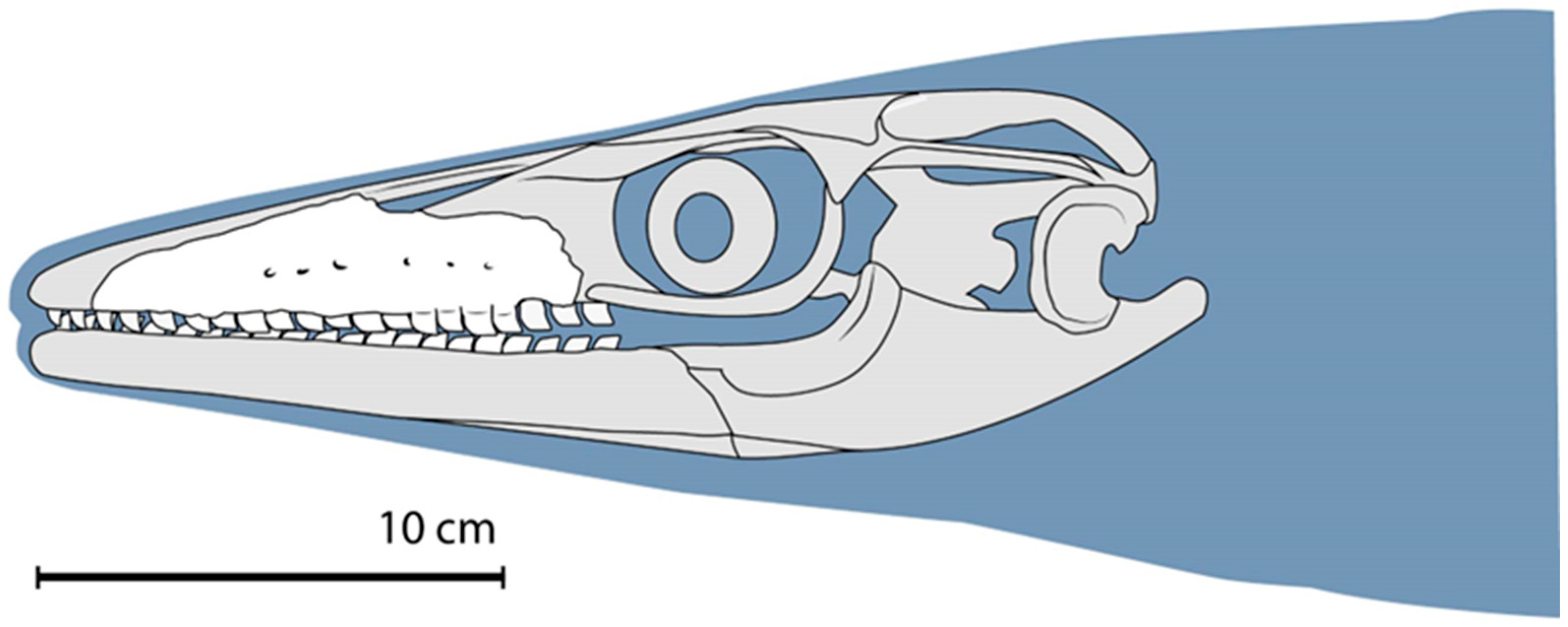
3.3. Further Description of the Xenodens Holotype
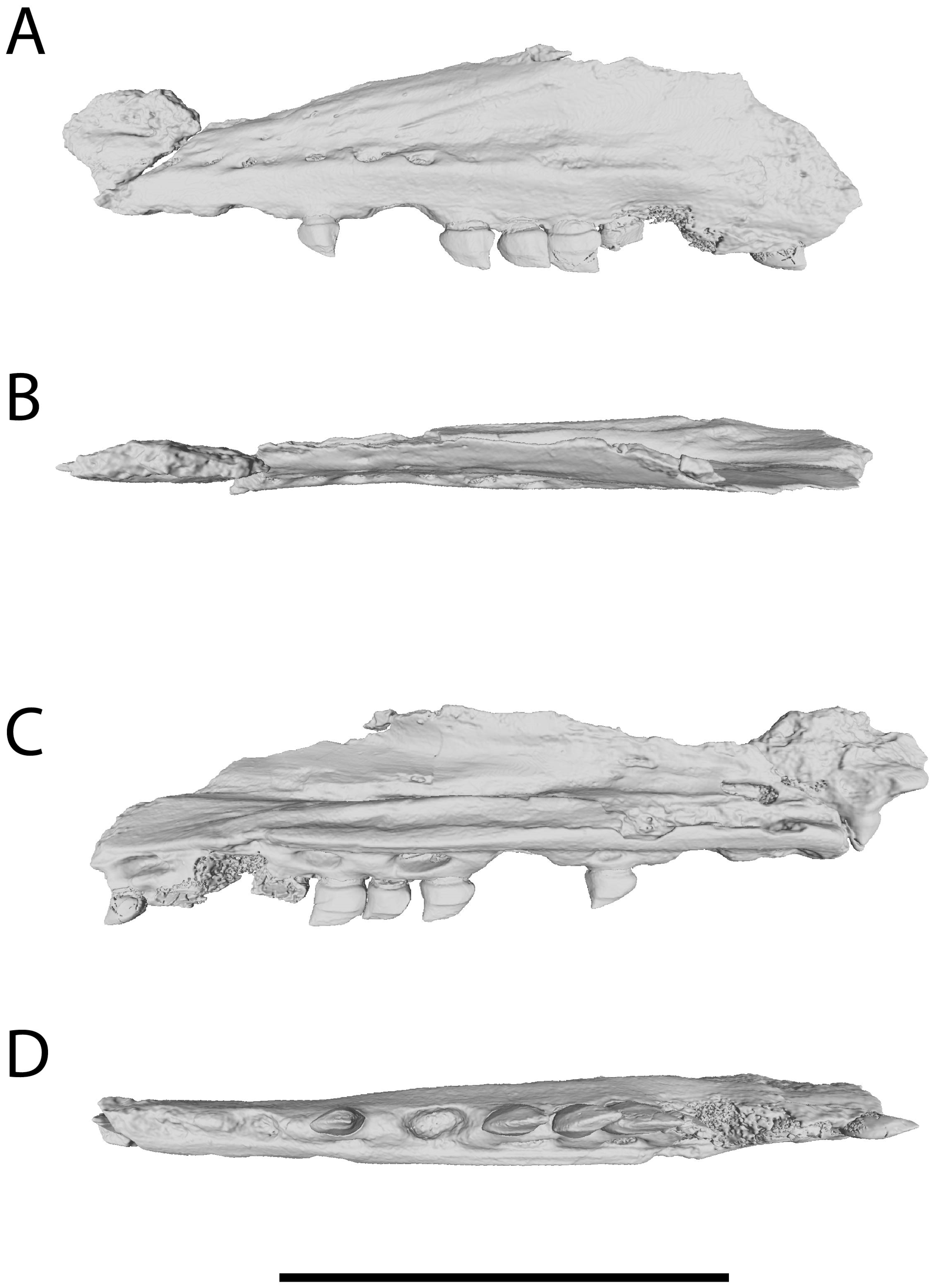
3.4. Anatomy of Xenodens Referred Specimen
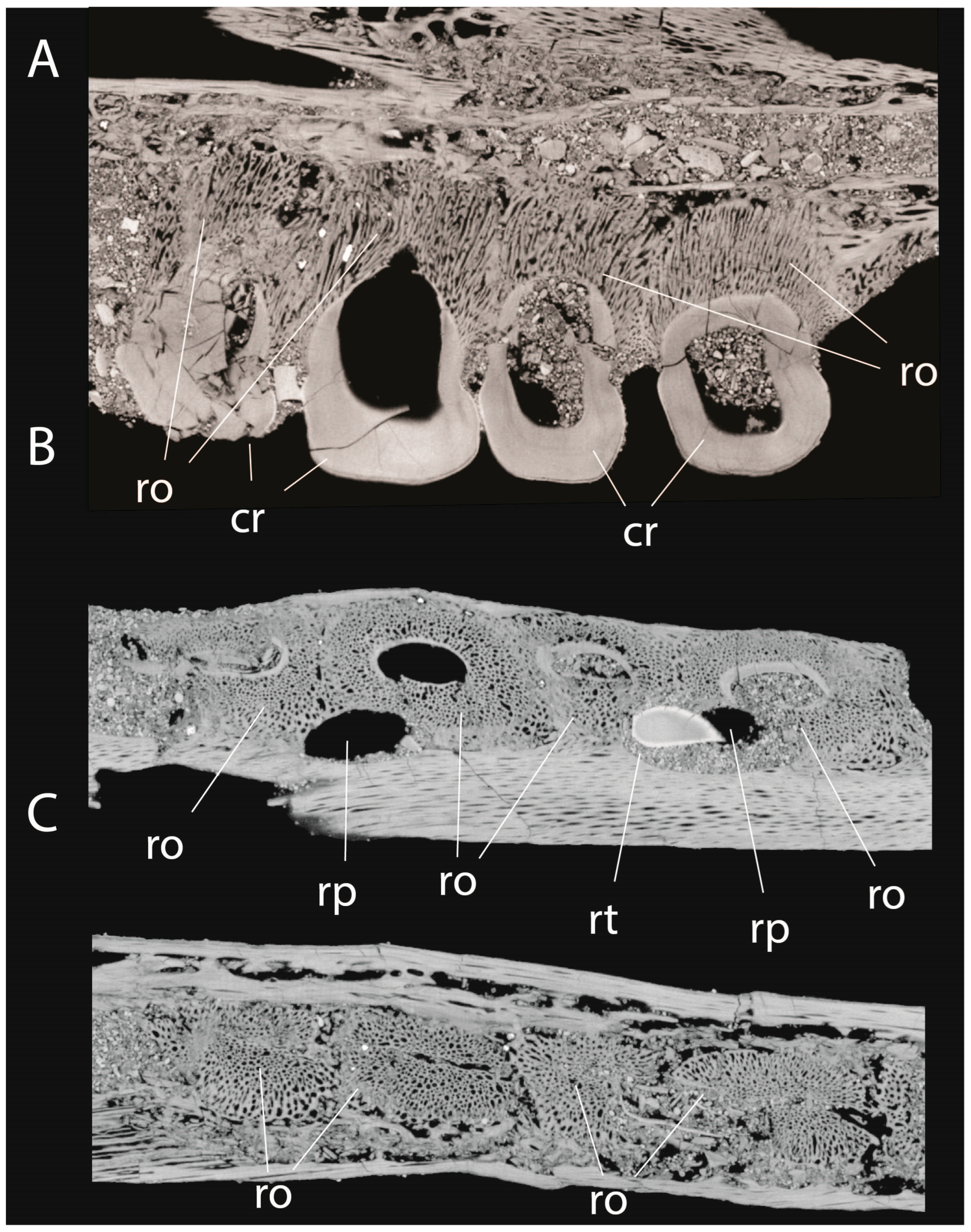
3.5. Tooth Implantation and Replacement of Xenodens
3.6. Ontogeny of Xenodens
4. Discussion
4.1. Authenticity of Xenodens
- Each tooth crown is associated with a single tooth root.
- Each tooth shows a continuous connection between the tooth crown and the tissues of the associated root.
- Each tooth root is associated with a replacement pit, as in Mosasaurinae and other derived mosasaurids.
- Original phosphate sand matrix infills the pulp cavities of teeth and replacement pits.
- No evidence of glue, filler or plaster is visible. Adjacent teeth are similar in size, shape, color, and preservation.
4.2. Synonymy with Carinodens
- Small Carinodens specimens, which overlap in size with Xenodens, lack Xenodens-like morphology. This argues against size-related changes in morphology of the jaws, teeth and tooth implantation;
- Absence of intermediate morphologies of jaws or teeth morphologies (no growth series connecting the two genera) argues against synonymy;
- Extensive differences in the maxilla structure, tooth count, tooth morphology, and tooth implantation exceed the range of intraspecific or ontogenetic variation known in any Mosasauridae.
- Distinct geographic distribution (Carinodens very broadly distributed, Xenodens only in Morocco).
4.3. Tooth Implantation and Replacement in Xenodens
5. Conclusions
- Reexamination of the holotype specimen of Xenodens calminechari, combined with the study of a referred specimen, and CT scans reveals its highly derived and unique tooth implantation and inferred replacement pattern.
- Newly identified features of Xenodens allow the recognition of new autapomorphies (tooth replacement pits extending across two teeth, paired tooth replacement in posterior teeth) which further underscore its unique morphology.
- New specimens of Carinodens show that Xenodens differs from Carinodens in terms of the maxillary morphology, maxillary tooth count, weak heterodonty, and its unique mode of tooth implantation and ornamentation. These differences, together with the absence of intermediate morphologies contradict the hypothesis that Xenodens is a juvenile of Carinodens.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardet, N.; Falconnet, J.; Fischer, V.; Houssaye, A.; Jouve, S.; Pereda-Suberbiola, X.; Perez-García, A.; Rage, J.-C.; Vincent, P. Mesozoic marine reptile palaeobiogeography in response to drifting plates. Gondwana Res. 2014, 26, 869–887. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Jacobs, L.L.; Araújo, R.; Schulp, A.S.; Mateus, O. Physical drivers of mosasaur evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 400, 17–27. [Google Scholar] [CrossRef]
- Russell, D.A. Systematics and morphology of American mosasaurs. In Bulletin of the Peabody Museum of Natural History; Yale University: New Haven, CT, USA, 1967; Volume 23, pp. 1–240. [Google Scholar]
- Bardet, N.; Fischer, V.; Jalil, N.-E.; Khaldoune, F.; Yazami, O.K.; Pereda-Suberbiola, X.; Longrich, N. Mosasaurids Bare the Teeth: An Extraordinary Ecological Disparity in the Phosphates of Morocco Just Prior to the K/Pg Crisis. Diversity 2025, 17, 114. [Google Scholar] [CrossRef]
- Bardet, N.; Pereda-Suberbiola, X.; Iarochène, M.; Amalik, M.; Bouya, B. Durophagous Mosasauridae (Squamata) from the Upper Cretaceous phosphates of Morocco, with description of a new species of Globidens. Neth. J. Geosci. 2005, 84, 167–175. [Google Scholar] [CrossRef]
- Schulp, A.S.; Bardet, N.; Bouya, B. A new species of the durophagous mosasaur Carinodens (Squamata, Mosasauridae) and additional material of Carinodens belgicus from the Maastrichtian phosphates of Morocco. Neth. J. Geosci. 2009, 88, 161–167. [Google Scholar] [CrossRef]
- LeBlanc, A.R.; Mohr, S.R.; Caldwell, M.W. Insights into the anatomy and functional morphology of durophagous mosasaurines (Squamata: Mosasauridae) from a new species of Globidens from Morocco. Zool. J. Linn. Soc. 2019, 186, 1026–1052. [Google Scholar] [CrossRef]
- Bardet, N.; Pereda-Suberbiola, X.; Iarochène, M.; Bouya, B.; Amaghzaz, M. A new species of Halisaurus from the Late Cretaceous phosphates of Morocco, and the phylogenetical relationships of the Halisaurinae (Squamata: Mosasauridae). Zool. J. Linn. Soc. 2005, 143, 447–472. [Google Scholar] [CrossRef]
- Longrich, N.R.; Bardet, N.; Schulp, A.S.; Jalil, N.-E. Xenodens calminechari gen. et sp. nov., a bizarre mosasaurid (Mosasauridae, Squamata) with shark-like cutting teeth from the upper Maastrichtian of Morocco, North Africa. Cretac. Res. 2021, 123, 104764. [Google Scholar] [CrossRef]
- Strong, C.R.; Caldwell, M.W.; Konishi, T.; Palci, A. A new species of longirostrine plioplatecarpine mosasaur (Squamata: Mosasauridae) from the Late Cretaceous of Morocco, with a re-evaluation of the problematic taxon ‘Platecarpus’ ptychodon. J. Syst. Palaeontol. 2020, 18, 1769–1804. [Google Scholar] [CrossRef]
- Longrich, N.R.; Jalil, N.-E.; Khaldoune, F.; Yazami, O.K.; Pereda-Suberbiola, X.; Bardet, N. Thalassotitan atrox, a giant predatory mosasaurid (Squamata) from the Upper Maastrichtian Phosphates of Morocco. Cretac. Res. 2022, 140, 105315. [Google Scholar] [CrossRef]
- Bardet, N.; Pereda-Suberbiola, X.; Iarochène, M.; Bouyahyaoui, F.; Bouya, B.; Amaghzaz, M. Mosasaurus beaugei Arambourg, 1952 (Squamata, Mosasauridae) from the Late Cretaceous phosphates of Morocco. Geobios 2004, 37, 315–324. [Google Scholar] [CrossRef]
- Longrich, N.R.; Bardet, N.; Khaldoune, F.; Yazami, O.K.; Jalil, N.-E. Pluridens serpentis, a new mosasaurid (Mosasauridae: Halisaurinae) from the Maastrichtian of Morocco and implications for mosasaur diversity. Cretac. Res. 2021, 126, 104882. [Google Scholar] [CrossRef]
- Longrich, N.R.; Jalil, N.-E.; Pereda-Suberbiola, X.; Bardet, N. Stelladens mysteriosus: A Strange New Mosasaurid (Squamata) from the Maastrichtian (Late Cretaceous) of Morocco. Fossils 2023, 1, 2–14. [Google Scholar] [CrossRef]
- Sharpe, H.S.; Powers, M.J.; Caldwell, M.W. Reassessment of Xenodens calminechari with a discussion of tooth morphology in mosasaurs. Anat. Rec. 2024, 308, 2160–2172. [Google Scholar] [CrossRef]
- Polcyn, M.J.; Bell, G.L. Russellosaurus coheni n. gen., n. sp., a 92 million-year-old mosasaur from Texas (USA), and the definition of the parafamily Russellosaurina. Neth. J. Geosci. 2005, 84, 321–333. [Google Scholar] [CrossRef]
- Bell, G.; Polcyn, M. Dallasaurus turneri, a new primitive mosasauroid from the Middle Turonian of Texas and comments on the phylogeny of Mosasauridae (Squamata). Neth. J. Geosci. 2005, 84, 177. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Oppel, M. Die Ordnungen, Familien und Gattungen der Reptilien als Prodrom Einer Naturgeschichte Derselben; Lindauer: München, Germany, 1811; p. 87. [Google Scholar]
- Gervais, P. Zoologie et Paléontologie Françaises (Animaux Vertébrés): Nouvelles Recherches sur les Animaux Vivants de la France; Arthus Bertrand: Paris, France, 1852. [Google Scholar]
- Cappetta, H.; Bardet, N.; Pereda-Suberbiola, X.; Adnet, S.; Akkrim, D.; Amalik, M.; Benabdallah, A. Marine vertebrate faunas from the Maastrichtian phosphates of Benguérir (Ganntour Basin, Morocco): Biostratigraphy, palaeobiogeography and palaeoecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 409, 217–238. [Google Scholar] [CrossRef]
- Bardet, N.; Gheerbrant, E.; Noubhani, A.; Cappetta, H.; Jouve, S.; Bourdon, E.; Pereda-Suberbiola, X.; Jalil, N.-E.; Vincent, P.; Houssaye, A.; et al. Les Vertébrés des phosphates crétacés-paléogènes (72, 1-47, 8 Ma) du Maroc. In Mémoires de la Société Géologique de France N.S. 180; Société géologique de France: Paris, France, 2017; pp. 351–452. [Google Scholar]
- LeBlanc, A.R.; Lamoureux, D.O.; Caldwell, M.W. Mosasaurs and snakes have a periodontal ligament: Timing and extent of calcification, not tissue complexity, determines tooth attachment mode in reptiles. J. Anat. 2017, 231, 869–885. [Google Scholar] [CrossRef]
- Longrich, N.R.; Pereda-Suberbiola, X.; Jalil, N.-E.; Bardet, N. A New Species of the Durophagous Mosasaurid carinodens from the Late Maastrichtian Phosphates of Morocco and Implications for Maastrichtian Mosasaurid Diversity. Diversity 2024, 17, 25. [Google Scholar] [CrossRef]
- Luan, X.; Walker, C.; Dangaria, S.; Ito, Y.; Druzinsky, R.; Jarosius, K.; Lesot, H.; Rieppel, O. The mosasaur tooth attachment apparatus as paradigm for the evolution of the gnathostome periodontium. Evol. Dev. 2009, 11, 247–259. [Google Scholar] [CrossRef]
- Ford, J.K.; Ellis, G.M.; Matkin, C.O.; Wetklo, M.H.; Barrett-Lennard, L.G.; Withler, R.E. Shark predation and tooth wear in a population of northeastern Pacific killer whales. Aquat. Biol. 2011, 11, 213–224. [Google Scholar] [CrossRef]
- Bardet, N.; Pereda-Suberbiola, X.; Jalil, N.-E. A new mosasauroid (Squamata) from the Late Cretaceous (Turonian) of Morocco. Comptes Rendus Palevol 2003, 2, 607–616. [Google Scholar] [CrossRef]
- Longrich, N.R. A new species of Pluridens (Mosasauridae: Halisaurinae) from the upper Campanian of Southern Nigeria. Cretac. Res. 2016, 64, 36–44. [Google Scholar] [CrossRef]
- Bell, G.L., Jr. A phylogenetic revision of North American and Adriatic Mosasauroidea. In Ancient Marine Reptiles; Elsevier: Amsterdam, The Netherlands, 1997; pp. 293–332. [Google Scholar]
- Currie, P.J. Bird like characteristics of the jaws and teeth of troodontid theropods (Dinosauria: Saurischia). J. Vertebr. Paleontol. 1987, 7, 72–81. [Google Scholar] [CrossRef]
- Estes, R.; Williams, E.E. Ontogenetic variation in the molariform teeth of lizards. J. Vertebr. Paleontol. 1984, 4, 96–107. [Google Scholar] [CrossRef]
- D’Amore, D.C. Illustrating ontogenetic change in the dentition of the Nile monitor lizard, Varanus niloticus: A case study in the application of geometric morphometric methods for the quantification of shape–size heterodonty. J. Anat. 2015, 226, 403–419. [Google Scholar] [CrossRef]
- Kaddumi, H.F. The first and most complete Carinodens (Squamata: Mosasauridae) skeleton yet with a description of a new species from the Harrana Fauna, Fossils of the Harrana Fauna and the Adjacent Areas. In Fossils of the Harrana Fauna and the Adjacent Area; Areas, A., Ed.; Publications of the Eternal River Museum of Natural History: Amman, Jordan, 2009; pp. 49–64. [Google Scholar]
- Longrich, N.R.; Saitta, E.T. Taxonomic status of Nanotyrannus lancensis (Dinosauria: Tyrannosauroidea)—A distinct taxon of small-bodied tyrannosaur. Foss. Stud. 2024, 2, 1–65. [Google Scholar] [CrossRef]
- Longrich, N.R.; Field, D. Torosaurus is not Triceratops: Ontogeny in Chasmosaurine Ceratopsids as a Case Study in Dinosaur Taxonomy. PLoS ONE 2012, 7, e32623. [Google Scholar] [CrossRef]
- Whitlock, J.A.; Richman, J.M. Biology of tooth replacement in amniotes. Int. J. Oral Sci. 2013, 5, 66–70. [Google Scholar] [CrossRef]
- Berkovitz, B. 13 Tooth replacement patterns in non-mammalian vertebrates. In Development, Function and Evolution of Teeth; Mark, F., Teaford, M.M.S., Mark, W., Ferguson, J., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 186. [Google Scholar]



| Character | Carinodens | Xenodens |
|---|---|---|
| Maxilla | Rounded and short premaxillary-nasal suture | Straight, posteriorly extended premaxillary-nasal suture |
| Heterodonty | Extreme | Weakly developed |
| Tooth morphology/ Ornamentation | Inflated/ornamented | Bladelike/smooth |
| Tooth implantation | Teeth separated by interdental ridges | Interdental ridges reduced in posterior teeth |
| Tooth replacement pits | Moderately elongate, one per tooth | Hyperelongate, extending across two teeth |
| Tooth root shape | Transversely expanded | Transversely narrow |
| Tooth root fusion | Absent | Present |
| Heterodonty | Extreme enlargement of lateral teeth | Moderate heterodonty |
| Interdental ridges | Present | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longrich, N.R.; Bardet, N.; Jalil, N.-E.; Pereda-Suberbiola, X.; Schulp, A.; Ghamizi, M. New Information on the Morphology and Tooth Replacement of Xenodens calminechari (Squamata: Mosasauridae), a Unique Mosasaurid from the Maastrichtian Phosphates of Morocco. Diversity 2025, 17, 819. https://doi.org/10.3390/d17120819
Longrich NR, Bardet N, Jalil N-E, Pereda-Suberbiola X, Schulp A, Ghamizi M. New Information on the Morphology and Tooth Replacement of Xenodens calminechari (Squamata: Mosasauridae), a Unique Mosasaurid from the Maastrichtian Phosphates of Morocco. Diversity. 2025; 17(12):819. https://doi.org/10.3390/d17120819
Chicago/Turabian StyleLongrich, Nicholas R., Nathalie Bardet, Nour-Eddine Jalil, Xabier Pereda-Suberbiola, Anne Schulp, and Mohamed Ghamizi. 2025. "New Information on the Morphology and Tooth Replacement of Xenodens calminechari (Squamata: Mosasauridae), a Unique Mosasaurid from the Maastrichtian Phosphates of Morocco" Diversity 17, no. 12: 819. https://doi.org/10.3390/d17120819
APA StyleLongrich, N. R., Bardet, N., Jalil, N.-E., Pereda-Suberbiola, X., Schulp, A., & Ghamizi, M. (2025). New Information on the Morphology and Tooth Replacement of Xenodens calminechari (Squamata: Mosasauridae), a Unique Mosasaurid from the Maastrichtian Phosphates of Morocco. Diversity, 17(12), 819. https://doi.org/10.3390/d17120819








