Urban Foraging and Plant Toxicological Risks for Rose-Ringed Parakeets (Psittacula krameri) in Athens
Abstract
1. Introduction
2. Materials and Methods
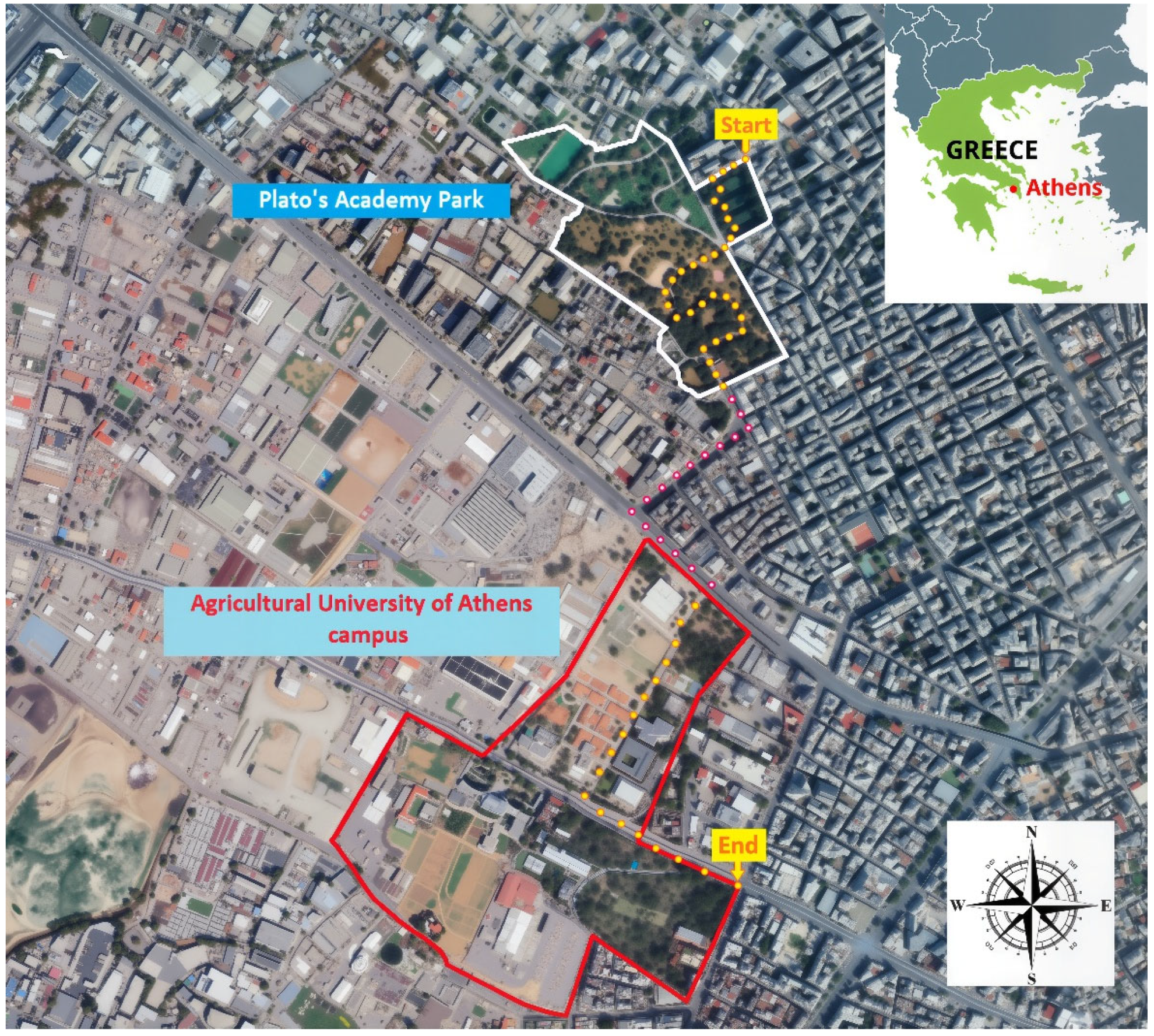
2.1. Study Site
2.2. Foraging Observations
2.3. Toxicity Testing
2.3.1. Sample Collection and Processing
2.3.2. Preparation of Plant Aqueous Extracts
2.3.3. Hatching of Artemia Cysts
2.3.4. Brine Shrimp Lethality Test (BSLT)
2.4. Statistical Analyses
Mixed Model Analyses
Model 1: Parakeet Foraging Density
Model 2: Food Consumption Patterns
3. Results
3.1. Foraging Records
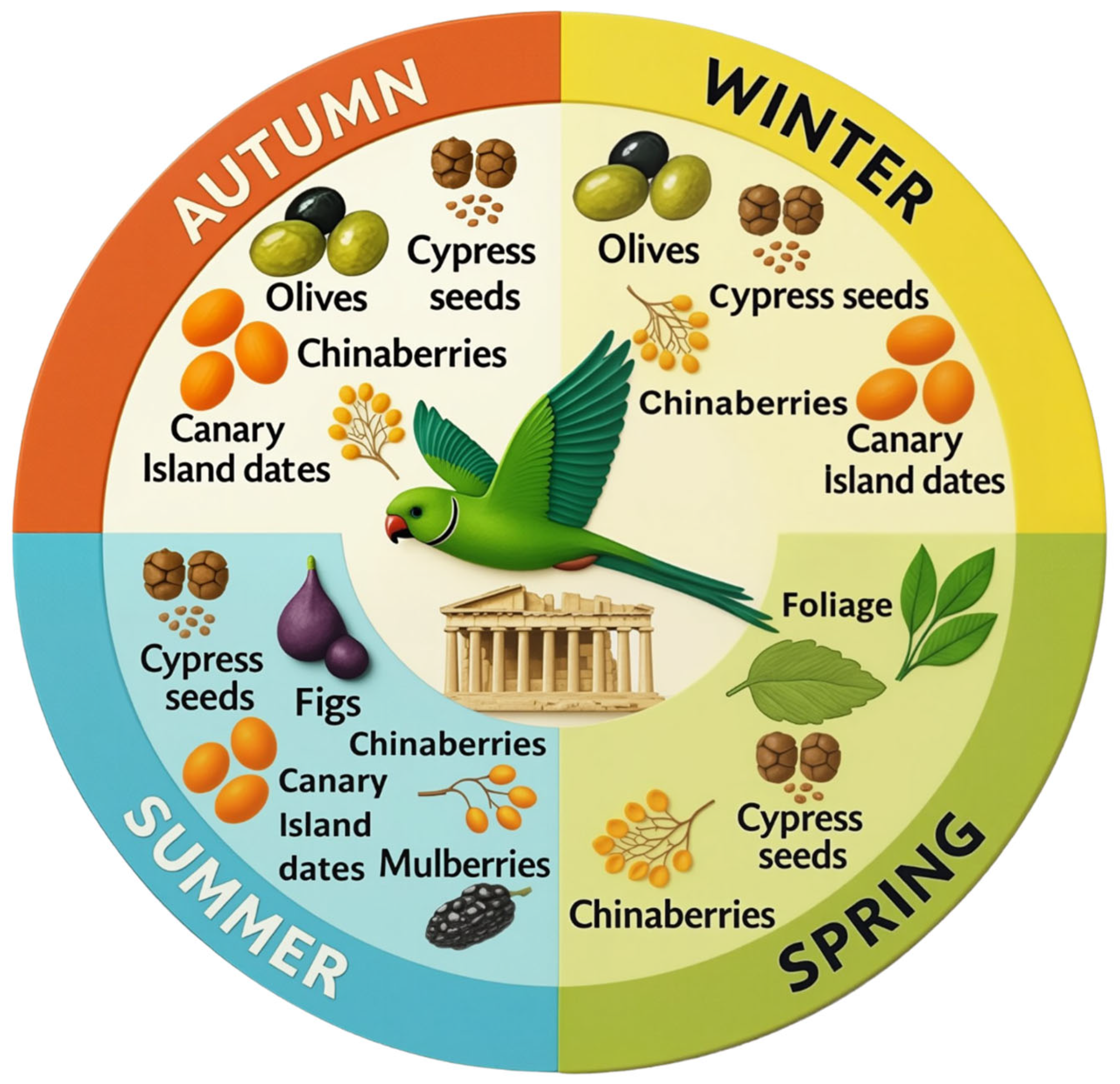
- Chinaberries (154 observations): Ripe fruits were consumed year-round. Birds held the fruit in their beaks and removed the peel with chewing movements before ingestion.
- Cypress seeds (148 observations): Seeds were taken from green cones throughout the year. Birds broke unripe, non-woody cones with their beaks to access the seeds.
- Olives (107 observations): Consumed mainly from October to February. Parakeets preferred mature olives, both green and dark-colored. After grasping an olive with their beak, they manipulated it with their feet, consumed portions of the flesh, and eventually discarded the pit (endocarp) (Figure 2).
- Canary Island dates (89 observations): Consumed from August to January at the mature stage, either eaten whole or partially, the latter in a manner similar to olives.
| Species Name | Common Name of the Tree | Item | Common Name of the Item | Native Status in Greece |
|---|---|---|---|---|
| Brachychiton populneus | Kurrajong Tree | Pod seeds | Kurrajong seeds | Non-native— locally naturalized (rare) |
| Cupressus sempervirens | Mediterranean Cypress | Cone seeds | Cypress seeds | Native |
| Ficus carica | Fig Tree | Fleshy fruits | Figs | Native |
| Ligustrum japonicum | Japanese Privet | Fleshy fruits | Privet berries | Non-native— locally naturalized |
| Laurus nobilis | Bay Laurel | Fleshy fruits | Bay laurel berries | Native |
| Melia azedarach | Chinaberry tree | Fleshy fruits | Chinaberries | Non-native— occasional naturalization (uncertain extent) |
| Morus spp. | Mulberry Tree | Fleshy fruits | Mulberries | Long naturalized (non-native) |
| Olea europaea | Olive Tree | Fleshy fruits | Olives | Native |
| Phoenix canariensis | Canary Island Date Palm | Fleshy fruits | Canary Island dates | Non-native— not naturalized * |
| Pistacia lentiscus | Mastic Tree | Fleshy fruits | Mastic berries | Native |
| Tree Name | Item | Feeding Observations (%) | |||
|---|---|---|---|---|---|
| Autumn n = 185 (100%) | Winter n = 139 (100%) | Spring n = 158 (100%) | Summer n = 119 (100%) | ||
| Kurrajong Tree | Pod seeds | 0 (0%) | 0 (0%) | 0 (0%) | 8 (6.7%) |
| Mediterranean Cypress | Cone seeds | 31 (16.8%) | 27 (19.4%) | 55 (34.8%) | 35 (29.4%) |
| Fig Tree | Fleshy fruits | 0 (0%) | 0 (0%) | 0 (0%) | 18 (15.1%) |
| Japanese Privet | Fleshy fruits | 0 (0%) | 4 (2.9%) | 0 (0%) | 0 (0%) |
| Foliage | 0 (0%) | 0 (0%) | 15 (9.5%) | 0 (0%) | |
| Bay Laurel | Fleshy fruits | 0 (0%) | 10 (7.2%) | 0 (0%) | 0 (0%) |
| Chinaberry tree | Fleshy fruits | 48 (25.9%) | 33 (23.7%) | 64 (40.5%) | 9 (7.6%) |
| Foliage | 0 (0%) | 0 (0%) | 10 (6.3%) | 0 (0%) | |
| Mulberry Tree | Fleshy fruits | 0 (0%) | 0 (0%) | 0 (0%) | 16 (13.4%) |
| Foliage | 0 (0%) | 0 (0%) | 10 (6.3%) | 0 (0%) | |
| Olive Tree | Fleshy fruits | 55 (29.7%) | 48 (34.5%) | 4 (2.5%) | 0 (0%) |
| Canary Island Date Palm | Fleshy fruits | 45 (24.3%) | 16 (11.5%) | 0 (0%) | 28 (23.5%) |
| Mastic Tree | Fleshy fruits | 1 (0.5%) | 1 (0.7%) | 0 (0%) | 2 (1.7%) |
| Shannon Index (H’) | 1.480 | 1.603 | 1.399 | 1.720 | |
3.2. Foraging Density
| Season | Birds Observed | Transects | Foraging Density | Standard Error |
|---|---|---|---|---|
| Autumn | 230 | 33 | 6.97 | 0.36 |
| Winter | 179 | 30 | 5.97 | 0.34 |
| Spring | 188 | 29 | 6.48 | 0.38 |
| Summer | 141 | 22 | 6.41 | 0.43 |
3.3. Food Consumption Patterns
3.4. Toxicity Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUA | Agricultural University of Athens |
| IMBBC-HCMR | Institute of Marine Biology, Biotechnology and Aquaculture, Hellenic Centre for Marine Research |
References
- Strubbe, D.; Matthysen, E. Establishment success of invasive ring-necked and monk parakeets in Europe. J. Biogeogr. 2009, 36, 2264–2278. [Google Scholar] [CrossRef]
- Manchester, S.J.; Bullock, J.M. The impacts of non-native species on UK biodiversity and the effectiveness of control. J. Appl. Ecol. 2000, 37, 845–864. [Google Scholar] [CrossRef]
- Spreyer, M.R.; Bucher, E.H. Monk Parakeet (Myiopsitta monachus). In The Birds of North America, No. 322; Poole, A., Gill, F., Eds.; Birds of North America, Inc.: Philadelphia, PA, USA, 1998; pp. 1–24. [Google Scholar]
- Soni, A.C. Food Habit and Morphometrics of the Roseringed Parakeet Psittacula krameri Scopoli. M.Sc. Thesis, Gujarat Agricultural University, Anand, India, 1991. Available online: https://krishikosh.egranth.ac.in/items/02d1016d-dc69-4276-a3d3-900dbfc36759 (accessed on 28 September 2024).
- Koutsos, E.A.; Matson, K.D.; Klasing, K.C. Nutrition of Birds in the Order Psittaciformes: A Review. J. Avian Med. Surg. 2001, 15, 257–275. [Google Scholar] [CrossRef]
- Shiels, A.B.; Bukoski, W.P.; Siers, S.R. Diets of Kauai’s invasive rose-ringed parakeet (Psittacula krameri): Evidence of seed predation and dispersal in a human-altered landscape. Biol. Invasions 2018, 20, 1449–1457. [Google Scholar] [CrossRef]
- Shivambu, T.C.; Shivambu, N.; Downs, C.T. Aspects of the feeding ecology of introduced Rose-ringed Parakeets Psittacula krameri in the urban landscape mosaic of Durban, KwaZulu-Natal Province, South Africa. J. Ornithol. 2021, 162, 397–407. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Invasive ring-necked parakeets Psittacula krameri in Belgium: Habitat selection and impact on native birds. Ecography 2007, 30, 578–588. [Google Scholar] [CrossRef]
- Avery, M.L.; Shiels, A.B. Monk and rose-ringed parakeets. In Ecology and Management of Terrestrial Vertebrate Invasive Species in the United States; Pitt, W.C., Beasley, J.C., Witmer, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 333–357. [Google Scholar]
- Strubbe, D. Psittacula krameri (rose-ringed parakeet). CABI Compendium. 2022. [CrossRef]
- Butler, C.J. Population Biology of the Introduced Rose-ringed Parakeet Psittacula krameri in the UK. Ph.D. Thesis, University of Oxford, Oxford, UK, 2003. [Google Scholar]
- Ważna, A.; Ciepliński, M.; Ratajczak, W.; Bojarski, J.; Cichocki, J. Parrots in the wild in Polish cities. PLoS ONE 2024, 19, e0304484. [Google Scholar] [CrossRef]
- McNeely, J.A.; Mooney, H.A.; Neville, L.E.; Schei, P.; Waage, J.K. (Eds.) A Global Strategy on Invasive Alien Species; IUCN: Gland, Switzerland; Cambridge, UK, 2001; 50p. [Google Scholar]
- Davis, M.A. Biotic globalization: Does competition from introduced species threaten biodiversity? BioScience 2003, 53, 481–489. [Google Scholar] [CrossRef]
- Gurevitch, J.; Padilla, D.K. Are invasions a major cause of extinctions? Trends Ecol. Evol. 2004, 19, 470–474. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.A.; Javed, M. An estimation of Rose-ringed Parakeet (Psittacula krameri) depredations on citrus, guava and mango in orchard fruit farm. Int. J. Agric. Biol. 2012, 14, 149–152. [Google Scholar]
- Reddy, V.R. Studies on damage to sorghum by the rose-ringed parakeet, Psittacula krameri (Scopoli), at Rajendranagar, Hyderabad, Andhra Pradesh. Pavo 1998, 36, 79–80. [Google Scholar]
- Thabethe, V.; Wilson, A.L.; Hart, L.A.; Downs, C.T. Ingestion by an invasive parakeet species reduces germination success of invasive alien plants relative to ingestion by indigenous turaco species in South Africa. Biol. Invasions 2015, 17, 3029–3039. [Google Scholar] [CrossRef]
- Tella, J.L.; Baños-Villalba, A.; Hernández-Brito, D.; Rojas, A.; Pacífico, E.; Díaz-Luque, J.A.; Carrete, M.; Blanco, G.; Hiraldo, F. Parrots as overlooked seed dispersers. Front. Ecol. Environ. 2015, 13, 338–339. [Google Scholar] [CrossRef]
- Klug, P.E.; Bukoski, W.P.; Shiels, A.B.; Kluever, B.M.; Siers, S.R. Rose-ringed parakeets. Wildl. Damage Manage. Tech. Ser. 2019, 23, 1–17. [Google Scholar]
- Hulot, M. Papagalos o Athinaios [The Athenian Parrot]. LiFO. 2021. Available online: https://www.lifo.gr/tropos-zois/urban/papagalos-o-athinaios (accessed on 28 September 2024).
- Nikolov, B.; Kralj, J.; Legakis, A.; Saveljic, D.; Velevski, M. Review of the alien bird species recorded on the Balkan Peninsula. In First ESENIAS Report: State of the Art of Invasive Alien Species in South-Eastern Europe; Rat, M., Trichkova, T., Scalera, R., Tomov, R., Uludag, A., Eds.; ESENIAS: Novi Sad, Serbia, 2016; pp. 189–201. Available online: https://www.esenias.org/files/13_Esenias_report_2016_193-205_bird.pdf (accessed on 28 September 2024).
- Gilardi, J.D.; Toft, C.A. Parrots eat nutritious foods despite toxins. PLoS ONE 2012, 7, e38293. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Solis, P.A.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phippilson, J.D. A microwell cytotoxicity using Artemia salina (Brine Shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Hamidi, M.; Jovanova, B.; Kadifkova Panovska, T. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Konan, A.M.L.; Golly, K.J.G.; Kra, A.K.M.; Adima, A.A.; Lohoues, E.E.C. Phytochemical screening and toxicity assessment of Imperata cylindria (L) P. Beauv. (Poaceae) raw extracts with brine shrimp (Artemia salina) lethality assay. J. Biosci. Med. 2022, 10, 153–171. [Google Scholar]
- Lagarto Para, A.; Silva Yhebra, R.; Guerra Sardinas, I.; Iglesias Buela, L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar] [CrossRef]
- Gosselin, R.E.; Smith, R.P.; Hodge, H.C.; Braddock, J. Clinical toxicology of commercial products, 5th ed.; Williams & Wilkins: Baltimore, MD, USA, 1984. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraju, A.V.; Rao, T.V.; Sundararaju, D.; Vanisree, M.; Tsay, H.S.; Subbaraju, G.V. Assessment of Bioactivity of Indian Medicinal Plants Using Brine Shrimp (Artemia salina) Lethality Assay. J. Ethnopharmacol. 2005, 101, 318–322. [Google Scholar]
- Mbwambo, Z.H.; Moshi, M.J.; Masimba, P.J.; Kapingu, M.C.; Nondo, R.S.O. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement. Altern. Med. 2007, 7, 9. [Google Scholar] [CrossRef]
- Karchesy, Y.M.; Kelsey, R.G.; Constantine, G.; Karchesy, J.J. Biological screening of selected Pacific Northwest forest plants using the brine shrimp (Artemia salina) toxicity bioassay. SpringerPlus 2016, 5, 510. [Google Scholar] [CrossRef]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Proposal for a short-term toxicity test with Artemia nauplii. Ecotoxicol. Environ. Saf. 1981, 5, 382–387. [Google Scholar] [CrossRef]
- Doudoroff, P.; Anderson, B.G.; Burdick, G.E.; Galtsoff, P.S.; Hart, W.B.; Patrick, R.; Strong, E.R.; Surber, E.W.; Van Horn, W.M. Bio-assay methods for the evaluation of acute toxicity of industrial wastes to fish. Sew. Ind. Wastes 1951, 23, 1380–1397. [Google Scholar]
- Hamilton, M.; Russo, R.; Thurston, R. Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. U.S. Environmental Protection Agency, Washington, DC, 1977. Available online: http://cfpub.epa.gov/si/si_public_comments.cfm?dirEntryId=43308 (accessed on 29 September 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2024. Available online: https://www.R-project.org/ (accessed on 2 October 2025).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, H.; Oksanen, M. Vegan: Community Ecology Package [R package]. R Foundation for Statistical Computing. 2010. Available online: https://CRAN.R-project.org/package=vegan (accessed on 2 October 2025).
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with Python. In Proceedings of the 9th Python in Science Conference (SciPy 2010), Austin, TX, USA, 28 June–3 July 2010; pp. 57–61. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Thabethe, V.; Thompson, L.J.; Hart, L.A.; Brown, M.; Downs, C.T. Seasonal effects on the thermoregulation of invasive rose-ringed parakeets (Psittacula krameri). J. Therm. Biol. 2013, 38, 553–559. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Carrer, M.; Gutiérrez, E.; Alla, A.Q.; Andreu-Hayles, L.; Hevia, A.; Koutavas, A.; Martínez-Sancho, E.; Nola, P.; et al. Climate extremes and predicted warming threaten Mediterranean Holocene fir forests refugia. Proc. Natl. Acad. Sci. USA 2017, 114, E10142–E10150. [Google Scholar] [CrossRef]
- Fraticelli, F. The Rose-ringed Parakeet Psittacula krameri in an urban park: Demographic trend, interspecific relationships and feeding preferences (Rome, central Italy). Avocetta 2014, 38, 23–28. [Google Scholar]
- Clergeau, P.; Vergnes, A. Bird feeders may sustain feral rose-ringed parakeets Psittacula krameri in temperate Europe. Wildl. Biol. 2011, 17, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, R.; Kotak, V.C.; Sharp, P.J.; Schmedemann, R.; Haase, E. Environmental, dietary, and hormonal factors in the regulation of seasonal breeding in free-living female Indian rose-ringed parakeets (Psittacula krameri). Horm. Behav. 1988, 22, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ohiagu, F.O.; Chikezie, P.C.; Chikezie, C.M. Toxicological significance of bioactive compounds of plant origin. Pharmacogn. Commn. 2021, 11, 67–77. [Google Scholar] [CrossRef]
- Ghica, A.; Tanase, M.L.; Niculite, C.M.; Tocila, A.; Popescu, L.; Luta, E.A.; Olaru, O.T.; Popovici, V.; Balaci, D.T.; Dutu, L.E.; et al. In vitro toxicity evaluation of some plant extracts and their potential application in Xerosis cutis. Cosmetics 2024, 11, 124. [Google Scholar] [CrossRef]
- Wu, C. An important player in brine shrimp lethality bioassay: The solvent. J. Adv. Pharm. Technol. Res. 2014, 5, 57–58. [Google Scholar] [CrossRef]
- Schneider, G.F.; Salazar, D.; Hildreth, S.B.; Helm, R.F.; Whitehead, S.R. Comparative Metabolomics of Fruits and Leaves in a Hyperdiverse Lineage Suggests Fruits Are a Key Incubator of Phytochemical Diversification. Front. Plant Sci. 2021, 12, 693739. [Google Scholar] [CrossRef]
- Hare, W.R.; Schutzman, H.; Lee, B.R.; Knight, M.W. Chinaberry poisoning in two dogs. J. Am. Vet. Med. Assoc. 1997, 210, 1638–1640. [Google Scholar] [CrossRef]
- Cooper, R.G. Poisoning in ostriches following ingestion of toxic plants—Field observations. Trop. Anim. Health Prod. 2007, 39, 439–442. [Google Scholar] [CrossRef]
- Khan, I.; Yasinzai, M.; Mehmood, Z.; Khan, J.; Khalil, A.; Saqib, S.; Rahman, W. Comparative study of green fruit extract of Melia azedarach Linn. with its ripe fruit extract for antileishmanial, larvicidal, antioxidant and cytotoxic activity. Am. J. Phytomed. Clin. Ther. 2014, 2, 442–454. [Google Scholar]
- Rjeibi, I.; Ben Saad, A.; Ncib, S.; Souid, S.; Allagui, M.S.; Hfaiedh, N. Brachychiton populneus as a novel source of bioactive ingredients with therapeutic effects: Antioxidant, enzyme inhibitory, anti-inflammatory properties and LC-ESI-MS profile. Inflammopharmacology 2020, 28, 563–574. [Google Scholar] [CrossRef]
- Eisele, T.A.; Loveland, P.M.; Kruk, D.L.; Meyers, T.R.; Sinnhuber, R.O.; Nixon, J.E. Effect of cyclopropenoid fatty acids on the hepatic microsomal mixed-function-oxidase system and aflatoxin metabolism in rabbits. Food Chem. Toxicol. 1982, 20, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Andrianaivo-Rafehivola, A.A.; Gaydou, E.M.; Rakotovao, L.H. Revue sur les effets biologiques des acides gras cyclopropéniques. Oléagineux Corps Gras Lipides 1994, 1, 177–188. [Google Scholar]
- Farag, M.A.; Abou Zeid, A.H.; Hamed, M.A.; Kandeel, Z.; El-Rafie, H.M.; El-Akad, R.H. Metabolomic fingerprint classification of Brachychiton acerifolius organs via UPLC-qTOF-PDA-MS analysis and chemometrics. Nat. Prod. Res. 2015, 29, 116–124. [Google Scholar] [CrossRef]
- Mokbli, S.; Sbihi, H.; Nehdi, I.; Romdhani-Younes, M.; Tan, C.; Al-Resayes, S. A comparative study of Brachychiton populneus seed and seed-fiber oils in Tunisia. Waste Biomass Valor. 2018, 9, 635–643. [Google Scholar] [CrossRef]
- Janzen, D.H.; Fellows, L.E.; Waterman, P.G. What Protects Lonchocarpus (Leguminosae) Seeds in a Costa Rican Dry Forest? Biotropica 1990, 22, 272–285. [Google Scholar] [CrossRef]
- Acedo, V. Ecology of the Yellow-Naped Amazon in Guatemala. AFA Watchb. 1992, 18, 31–34. [Google Scholar]
- Norconk, M.A.; Grafton, B.W.; Conklin-Brittain, N.L. Seed Dispersal by Neotropical Seed Predators. Am. J. Primatol. 1998, 45, 103–126. [Google Scholar] [CrossRef]
- Terborgh, J. Maintenance of Diversity in Tropical Forests. Biotropica 1992, 24, 283–292. [Google Scholar] [CrossRef]
- Gilardi, J.D.; Duffey, S.S.; Munn, C.A.; Izhaki, I. Biochemical Functions of Geophagy in Parrots: Detoxification of Dietary Toxins and Cytoprotective Effects. J. Chem. Ecol. 1999, 25, 897–922. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Tella, J.L.; Blanco, G.; Carrete, M. Nesting innovations allow population growth in an invasive population of rose-ringed parakeets. Curr. Zool. 2022, 68, 617–626. [Google Scholar] [CrossRef]
- Muñoz, A.R.; Real, R. Assessing the potential range expansion of the exotic monk parakeet in Spain. Divers. Distrib. 2006, 12, 656–665. [Google Scholar] [CrossRef]
- Martín-Taboada, A.; Muñoz, A.R.; Romero, D. Assessing the risk of Myiopsitta monachus (Monk Parakeet) invasion: Global perspectives and implications. Ornithol. Appl. 2025, 127, duaf035. [Google Scholar] [CrossRef]


| Effect | % Change b | Rate Ratio (95% CI) | p-Value | Interpretation |
|---|---|---|---|---|
| Food Category (vs. Fruit c) | ||||
| Seed | −6.7% | 0.93 [0.72, 1.20] | 0.592 | No significant difference |
| Foliage | −77.5% | 0.23 [0.15, 0.34] | <0.001 *** | Rarely consumed |
| Season (vs. Autumn c) | ||||
| Winter | −24.1% | 0.76 [0.69, 0.84] | <0.001 *** | Significant reduction |
| Spring | −7.8% | 0.92 [0.77, 1.10] | 0.362 | Similar to autumn |
| Summer | −17.3% | 0.83 [0.68, 1.01] | 0.058 † | Marginal reduction |
| Extract Sample | 48 h-LC50 (mg/mL) ± SD | Range (98% Conf. Int.) | Toxicity Class |
|---|---|---|---|
| Kurrajong seeds | 2.14 ± 0.01 | 1.36–3.34 | Moderately toxic |
| Cypress seeds | 84.58 ± 3.80 | 66.48–104.46 | Non-toxic |
| Bay laurel berries | 11.64 ± 0.11 | 7.92–17.14 | Slightly toxic |
| Privet berries | 14.44 ± 0.10 | 10.96–19.04 | Slightly toxic |
| Chinaberries | 0.46 ± 0.01 | 0.38–0.56 | Moderately to Very toxic |
| Olives | 51.70 ± 2.24 | 38.46–69.50 | Non-toxic |
| Canary Island dates | 4.84 ± 0.22 | 3.98–5.90 | Moderately toxic |
| Mastic berries | 5.68 ± 0.02 | 4.54–7.12 | Slightly to Moderately toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulopoulos, M.A.B.; Cotou, E.; Politakis, N.; Tsekouras, N.; Paraskeuas, V.V.; Kotzamanis, Y.; Christodoulopoulos, G.; Pappas, A.C. Urban Foraging and Plant Toxicological Risks for Rose-Ringed Parakeets (Psittacula krameri) in Athens. Diversity 2025, 17, 801. https://doi.org/10.3390/d17110801
Christodoulopoulos MAB, Cotou E, Politakis N, Tsekouras N, Paraskeuas VV, Kotzamanis Y, Christodoulopoulos G, Pappas AC. Urban Foraging and Plant Toxicological Risks for Rose-Ringed Parakeets (Psittacula krameri) in Athens. Diversity. 2025; 17(11):801. https://doi.org/10.3390/d17110801
Chicago/Turabian StyleChristodoulopoulos, Mathis A. B., Efthimia Cotou, Nektarios Politakis, Nikolaos Tsekouras, Vasileios V. Paraskeuas, Yannis Kotzamanis, Georgios Christodoulopoulos, and Athanasios C. Pappas. 2025. "Urban Foraging and Plant Toxicological Risks for Rose-Ringed Parakeets (Psittacula krameri) in Athens" Diversity 17, no. 11: 801. https://doi.org/10.3390/d17110801
APA StyleChristodoulopoulos, M. A. B., Cotou, E., Politakis, N., Tsekouras, N., Paraskeuas, V. V., Kotzamanis, Y., Christodoulopoulos, G., & Pappas, A. C. (2025). Urban Foraging and Plant Toxicological Risks for Rose-Ringed Parakeets (Psittacula krameri) in Athens. Diversity, 17(11), 801. https://doi.org/10.3390/d17110801












