A Complete Reference DNA Barcode Library for Austrian Bumblebees
Abstract
1. Introduction
2. Materials and Methods
3. Results
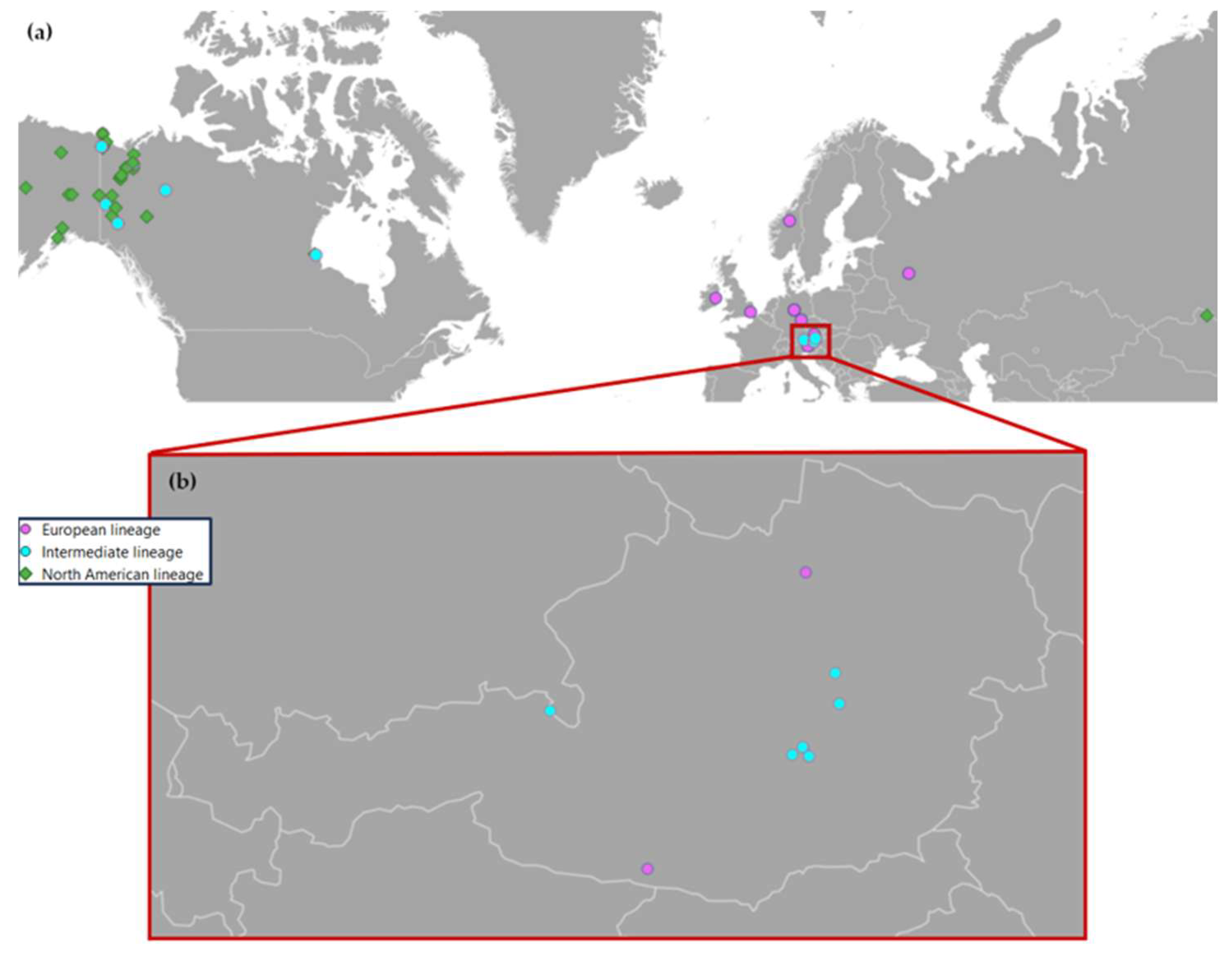
3.1. Phylogeny and Species Coverage
3.2. Intraspecific Structure in B. jonellus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fijen, T.P.; Scheper, J.A.; Boom, T.M.; Janssen, N.; Raemakers, I.; Kleijn, D. Insect pollination is at least as important for marketable crop yield as plant quality in a seed crop. Ecol. Lett. 2018, 21, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Hines, H.M. Historical biogeography, divergence times, and diversification patterns of bumble bees (Hymenoptera: Apidae: Bombus). Syst. Biol. 2008, 57, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Colla, S.; Xie, Z. Bumblebee vulnerability: Common correlates of winners and losers across three continents. Conserv. Biol. 2009, 23, 931–940. [Google Scholar] [CrossRef]
- Rasmont, P.; Franzen, M.; Lecocq, T.; Harpke, A.; Roberts, S.P.; Biesmeijer, J.C.; Castro, L.; Cederber, B.; Dvorak, L.; Fitzpatrick, Ú.; et al. Climatic Risk and Distribution Atlas of European Bumblebees; Pensoft Publishers: Sofia, Bulgaria, 2015. [Google Scholar] [CrossRef]
- Neumayer, J.; Leiner, O.; Schied, J.; Wallner, W. Rote Liste der Hummeln (Bombus spp.) Österreichs. In Rote Listen Gefährdeter Tiere Österreichs; Zulka, K.P., Ed.; Umweltbundesamt: Vienna, Austria, 2024; Available online: https://www.umweltbundesamt.at/fileadmin/site/publikationen/rep0894.pdf (accessed on 22 September 2025).
- Streinzer, M. Erstnachweis von Bombus semenoviellus Skorikov, 1910 (Hymenoptera, Apidae) für Österreich. Entomofauna 2010, 31, 265–268. [Google Scholar]
- Neumayer, J. Erstfund von Bombus haematurus Kriechbaumer, 1870 (Hymenoptera, Apidae) in Österreich. Beitr. Entomofaun. 2004, 5, 134. [Google Scholar]
- Duennes, M.A.; Lozier, J.D.; Hines, H.M.; Cameron, S.A. Geographical patterns of genetic divergence in the widespread Mesoamerican bumble bee Bombus ephippiatus (Hymenoptera: Apidae). Mol. Phylogenet. Evol. 2012, 64, 219–231. [Google Scholar] [CrossRef]
- Lecocq, T.; Dellicour, S.; Michez, D.; Lhomme, P.; Vanderplanck, M.; Valterová, I.; Rasplus, J.-Y.; Rasmont, P. Scent of a break-up: Phylogeography and reproductive trait divergences in the red-tailed bumblebee (Bombus lapidarius). BMC Evol. Biol. 2013, 13, 263. [Google Scholar] [CrossRef]
- Lecocq, T.; Vereecken, N.J.; Michez, D.; Dellicour, S.; Lhomme, P.; Valterova, I.; Rasplus, J.-Y.; Rasmont, P. Patterns of genetic and reproductive traits differentiation in mainland vs. Corsican populations of bumblebees. PLoS ONE 2013, 8, e65642. [Google Scholar] [CrossRef]
- Lecocq, T.; Brasero, N.; Martinet, B.; Valterova, I.; Rasmont, P. Highly polytypic taxon complex: Interspecific and intraspecific integrative taxonomic assessment of the widespread pollinator Bombus pascuorum Scopoli 1763 (Hymenoptera: Apidae). Syst. Entomol. 2015, 40, 881–890. [Google Scholar] [CrossRef]
- Françoso, E.; de Oliveira, F.F.; Arias, M.C. An integrative approach identifies a new species of bumblebee (Hymenoptera: Apidae: Bombini) from northeastern Brazil. Apidologie 2016, 47, 171–185. [Google Scholar] [CrossRef]
- Bossert, S.; Gereben-Krenn, B.A.; Neumayer, J.; Schneller, B.; Krenn, H.W. The cryptic Bombus lucorum complex (Hymenoptera: Apidae) in Austria: Phylogeny, distribution, habitat usage and a climatic characterization based on COI sequence data. Zool. Stud. 2016, 55, e13. [Google Scholar] [CrossRef]
- Martinet, B.; Ghisbain, G.; Przybyla, K.; Zambra, E.; Brasero, N.; Kondakov, A.V.; Tomilova, A.A.; Kolosova, Y.S.; Bolotov, I.N.; Rasmont, P.; et al. Distant but related: Genetic structure in the circum-boreal bumblebee Bombus jonellus (Kirby, 1802). Polar Biol. 2021, 44, 2039–2047. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; DeWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef]
- Hawlitschek, O.; Morinière, J.; Dunz, A.; Franzen, M.; Rödder, D.; Glaw, F.; Haszprunar, G. Comprehensive DNA barcoding of the herpetofauna of Germany. Mol. Eclo. Resour. 2016, 16, 242–253. [Google Scholar] [CrossRef]
- Zangl, L.; Daill, D.; Schweiger, S.; Gassner, G.; Koblmueller, S. A reference DNA barcode library for Austrian amphibians and reptiles. PLoS ONE 2020, 15, e0229353. [Google Scholar] [CrossRef]
- Geiger, M.; Koblmüller, S.; Assandri, G.; Chovanec, A.; Ekrem, T.; Fischer, I.; Galimberti, A.; Grabowski, M.; Haring, E.; Hausmann, A.; et al. Coverage and quality of DNA barcode references for Central and Northern European Odonata. PeerJ 2021, 9, e11192. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Bossert, S. The high alpine bee fauna (Hymenoptera: Apoidea) of the Zillertal Alps, Austria. Biodivers. Data J. 2014, 2, e1115. [Google Scholar] [CrossRef]
- Sonnleitner, M.; Schoder, S.; Macek, O.; Leeb, C.; Bräuchler, C.; Haring, E.; Dötterl, S.; Eckelt, A.; Fauster, R.; Glatzhofer, E.; et al. Beitrag der ABOL-BioBlitze zur österreichischen Biodiversitäts-Erfassung: DNA-Barcodes aus 2019 und 2020. Acta ZooBot Austria 2022, 158, 81–95. [Google Scholar]
- Gokcezade, J.F.; Gereben-Krenn, B.A.; Neumayer, J.; Krenn, H.W. Feldbestimmungsschlüssel Für Die Hummeln Österreichs, Deutschlands und der Schweiz (Hymenoptera, Apidae), 2nd ed.; Quelle & Meyer Verlag GmbH & Co.: Wiebelsheim, Germany, 2018. [Google Scholar]
- Amiet, F. Insecta Helvetica 12: Hymenoptera, Apidae, 1. Teil. Allgemeiner Teil, Gattungsschlüssel Apis, Bombus und Psithyrus; Schweizerische Entomologische Gesellschaft: Neuchâtel, Switzerland, 1996. [Google Scholar]
- Casquet, J.; Thebaut, C.; Gillespie, R.G. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Koblmüller, S.; Resl, P.; Klar, N.; Bauer, H.; Zangl, L.; Hahn, C. DNA barcoding for species identification of moss-dwelling invertebrates: Performance of nanopore sequencing and coverage in reference database. Diversity 2024, 16, 196. [Google Scholar] [CrossRef]
- Geci, D.; Ibrahimi, H.; Strohmeier, T.; Bilalli, A.; Musliu, M.; Koblmüller, S.; Sherwood, D. Trapdoor spiders of the family Nemesiidae Simon, 1889 (Araneae: Mygalomorphae) from Kosovo. Arachnology 2025, 20, 281–286. [Google Scholar] [CrossRef]
- Astrin, J.J.; Höfer, H.; Spelda, J.; Holstein, J.; Bayer, S.; Hendrich, L.; Huber, B.A.; Kielhorn, K.-H.; Krammer, H.-J.; Lemke, M.; et al. Towards a DNA barcode reference database for spiders and harvestmen of Germany. PLoS ONE 2016, 11, e0162624. [Google Scholar] [CrossRef]
- Srivathsan, A.; Hartop, E.; Puniamoorthy, J.; Lee, W.T.; Kutty, S.N.; Kurina, O.; Meier, R. Rapid, large-scale species discovery in hyperdiverse taxa using 1D MinION sequencing. BMC Biol. 2019, 17, 96. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Prosser, S.W.; Rodríguez-Perez, M.A.; Chaverri, L.G.; Hebert, P.D.N.; Gregory, T.R. Recovery of DNA barcodes from blackfly museum specimens (Diptera: Simuliidae) using primer sets that target a variety of sequence lengths. Mol. Ecol. Res. 2014, 14, 508–518. [Google Scholar] [CrossRef]
- Srivathsan, A.; Fend, V.; Suárez, D.; Meier, R. ONTbarcoder 2.0: Rapid species discovery and identification with real-time barcoding facilitated by Oxford Nanopore R10.4. Cladistics 2024, 40, 192–203. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Schmidt, S.; Schmid-Egger, C.; Morinière, J.; Haszprunar, G.; Hebert, P.D.N. DNA barcoding largely supports 250 years of classical taxonomy: Identifications for Central European bees (Hymenoptera, Apoidea partim). Mol. Ecol. Resour. 2015, 15, 985–1000. [Google Scholar] [CrossRef]
- Potapov, G.S.; Kondakov, A.V.; Kolosova, Y.S.; Tomilova, A.A.; Filippov, B.Y.; Gofarov, M.Y.; Bolotov, I.N. Widespread continental mtDNA lineages prevail in the bumblebee fauna of Iceland. ZooKeys 2018, 774, 141. [Google Scholar] [CrossRef]
- Magnacca, K.N.; Brown, M.J.F. DNA barcoding of a regional fauna: Irish solitary bees. Mol. Ecol. Resour. 2012, 12, 990–998. [Google Scholar] [CrossRef]
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. 3. Cladogram estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D.; Nakagawa, S. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Williams, P.H.; Brown, M.J.; Carolan, J.C.; An, J.; Goulson, D.; Aytekin, A.M.; Best, L.R.; Byvaltsev, A.M.; Cederberg, B.; Dawson, R.; et al. Unveiling cryptic species of the bumblebee subgenus Bombus s. str. worldwide with COI barcodes (Hymenoptera: Apidae). Syst. Biodiv. 2012, 10, 21–56. [Google Scholar] [CrossRef]
- Wolf, S.; Rohde, M.; Moritz, R.F.A. The reliability of morphological traits in the differentiation of Bombus terrestris and B. lucorum (Hymenoptera: Apidae). Apidologie 2010, 41, 45–53. [Google Scholar] [CrossRef]
- Carolan, J.C.; Murray, T.E.; Fitzpatrick, Ú.; Crossley, J.; Schmidt, H.; Cederberg, B.; McNally, L.; Paxton, R.J.; Williams, P.H.; Brown, M.J.F. Colour patterns do not diagnose species: Quantitative evaluation of a DNA barcoded cryptic bumble complex. PLoS ONE 2012, 7, e29251. [Google Scholar] [CrossRef]
- Chiovitti, A.; Thorpe, F.; Gorman, C.; Cuxson, J.L.; Robevska, G.; Szwed, C.; Duncan, J.C.; Vanyai, H.K.; Cross, J.; Siemering, K.R.; et al. A citizen science model for implementing statewide educational DNA barcoding. PLoS ONE 2019, 14, e0208604. [Google Scholar] [CrossRef]
- Chua, P.Y.S.; Bourlat, S.J.; Ferguson, C.; Korlevic, P.; Zhao, L.; Ekrem, T.; Meier, R.; Lawniczak, M.K.N. Future of DNA-based insect monitoring. Trends Genet. 2023, 39, 531–544. [Google Scholar] [CrossRef]
- Jones, K.S.; Pilliod, D.S.; Aunis, A.W. Metabarcoding analysis of arthropod pollinator diversity: A methodological comparison of eDNA derived from flowers and DNA derived from bulk samples of insects. Mol. Ecol. 2025, 34, e70003. [Google Scholar] [CrossRef]
- Slater-Baker, M.R.; Fagan-Jeffries, E.P.; Oestmann, K.J.; Portmann, O.G.; Bament, T.M.; Howe, A.G.; Guzik, M.T.; Bradford, T.M.; McClelland, A.R.; Woodward, A.; et al. DNA barcoding, integrative taxonomy, citizen science, and Bush Blitz surveys combine to reveal 34 new species of Apantales (Hymenoptera, Braconidae, Microgastrinae) in Australia. Zookeys 2025, 1227, 1–128. [Google Scholar] [CrossRef]
- Bertsch, A. Barcoding cryptic bumblebee taxa: B. lucorum, B. crytarum and B. magnus, a case study (Hymenoptera: Apidae: Bombus). Contrib. Entomol. 2009, 59, 287–310. [Google Scholar] [CrossRef]
- Török, P.; Ambarlı, D.; Kramp, H.; Wesche, K.; Dengler, J. Step(pe) up! Raising the profile of the Palaearctic natural grasslands. Biodiv. Conserv. 2016, 25, 2187–2195. [Google Scholar] [CrossRef]
- Douglas, D.J.; Waldinger, J.; Buckmire, Z.; Gibb, K.; Medina, J.P.; Sutcliffe, L.; Beckmann, C.; Collar, N.J.; Jansen, R.; Kamp, J.; et al. A global review identifies agriculture as the main threat to declining grassland birds. Ibis 2023, 165, 1107–1128. [Google Scholar] [CrossRef]



| Species | BIN | N | Imax | Nearest Neighbor | DNN |
|---|---|---|---|---|---|
| Bombus alpinus | BOLD:AAN9551 | 2 | 0 | Bombus humilis | 8.08 |
| Bombus argillaceus | BOLD:AGH5555 | 5 | 0.31 | Bombus ruderatus | 2.97 |
| Bombus barbutellus | BOLD:AAF7051 | 11 | 0.31 | Bombus quadricolor | 9.83 |
| Bombus bohemicus | BOLD:AAD2530 | 25 | 0.62 | Bombus norvegicus | 8.77 |
| Bombus campestris | BOLD:AAD8221 | 22 | 1.08 | Bombus quadricolor | 7.94 |
| Bombus confusus | BOLD:AAJ7886 | 7 | 0.31 | Bombus subterraneus | 6.56 |
| Bombus cryptarum | BOLD:ACE4135 | 16 | 1.01 | Bombus lucorum | 3.61 |
| Bombus distinguendus | BOLD:ACF4670 | 1 | na | Bombus subterraneus | 3.29 |
| Bombus flavidus | BOLD:AEI1064 | 2 | 0 | Bombus norvegicus | 5.27 |
| Bombus gerstaeckeri | BOLD:AAF0186 | 6 | 0.15 | Bombus hortorum | 6.25 |
| Bombus haematurus | BOLD:ADK0974 | 7 | 0.15 | Bombus pratorum | 6.76 |
| Bombus hortorum | BOLD:AAD2566 | 39 | 0.15 | Bombus ruderatus | 5.74 |
| Bombus humilis | BOLD:ABY7210 | 21 | 0.48 | Bombus ruderarius | 2.33 |
| Bombus hypnorum | BOLD:AAB3227 | 13 | 0.48 | Bombus pratorum | 6.49 |
| Bombus inexspectatus | BOLD:AEI3825 | 4 | 0.15 | Bombus veteranus | 3.3 |
| Bombus jonellus | BOLD:AAD4941 | 10 | 1.24 | Bombus pratorum | 5.9 |
| Bombus lapidarius | BOLD:AAC6016 | 34 | 0.16 | Bombus sichelii | 9.65 |
| Bombus lucorum | BOLD:AAB1060 | 51 | 1.01 | Bombus cryptarum | 3.61 |
| Bombus mastrucatus | BOLD:AAD8243 | 31 | 0.31 | Bombus semenoviellus | 8.93 |
| Bombus mendax | BOLD:AAF5994 | 4 | 0.15 | Bombus confusus | 7.72 |
| Bombus mesomelas | BOLD:AAD9869 | 1 | na | Bombus pomorum | 4.11 |
| Bombus monticola | BOLD:AAA8078 | 17 | 0.31 | Bombus pratorum | 6.75 |
| Bombus mucidus | BOLD:ABW9027 | 16 | 0.46 | Bombus veteranus | 4.6 |
| Bombus muscorum | BOLD:AAD8159 | 3 | 0 | Bombus pascuorum | 3.12 |
| Bombus norvegicus | BOLD:AAD4756 | 3 | 0.31 | Bombus quadricolor | 4.45 |
| Bombus pascuorum | BOLD:AAC4378 | 22 | 0.62 | Bombus ruderarius | 2.49 |
| Bombus pomorum | BOLD:AEI7391 | 2 | 0 | Bombus mesomelas | 4.11 |
| Bombus pratorum | BOLD:AAD4735 | 21 | 0.31 | Bombus pyrenaeus | 5.22 |
| Bombus pyrenaeus | BOLD:AAF0421 | 17 | 0.15 | Bombus pratorum | 5.22 |
| Bombus quadricolor | BOLD:AAF6417 | 3 | 0.62 | Bombus norvegicus | 4.45 |
| Bombus ruderarius | BOLD:ABY7209 | 13 | 0.16 | Bombus veteranus | 1.87 |
| Bombus ruderatus | BOLD:AAJ7737 | 4 | 0.15 | Bombus argillaceus | 2.97 |
| Bombus rupestris | BOLD:AAD8184 | 16 | 0.92 | Bombus quadricolor | 8.52 |
| Bombus semenoviellus | BOLD:AAF6479 | 1 | na | Bombus mastrucatus | 8.93 |
| Bombus sichelii | BOLD:ACF1761 | 17 | 0 | Bombus semenoviellus | 9.27 |
| Bombus soroeensis | BOLD:AAB1065 | 46 | 0.31 | Bombus mastrucatus | 12.35 |
| Bombus subterraneus | BOLD:AAA8260 | 2 | 0.31 | Bombus distinguendus | 3.29 |
| Bombus sylvarum | BOLD:AAD2551 | 13 | 0.62 | Bombus veteranus | 1.87 |
| Bombus sylvestris | BOLD:AAB4516 | 20 | 0.31 | Bombus quadricolor | 4.45 |
| Bombus terrestris | BOLD:AAB1062 | 27 | 1.35 | Bombus cryptarum | 6.39 |
| Bombus vestalis | BOLD:AAI8745 | 8 | 0.46 | Bombus norvegicus | 9.29 |
| Bombus veteranus | BOLD:AAM3090 | 3 | 0.46 | Bombus sylvarum | 1.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohmeier, T.; Schoder, S.; Schäffer, S.; Grimm, J.; Sturmbauer, C.; Koblmüller, S. A Complete Reference DNA Barcode Library for Austrian Bumblebees. Diversity 2025, 17, 746. https://doi.org/10.3390/d17110746
Strohmeier T, Schoder S, Schäffer S, Grimm J, Sturmbauer C, Koblmüller S. A Complete Reference DNA Barcode Library for Austrian Bumblebees. Diversity. 2025; 17(11):746. https://doi.org/10.3390/d17110746
Chicago/Turabian StyleStrohmeier, Thomas, Sabine Schoder, Sylvia Schäffer, Jacqueline Grimm, Christian Sturmbauer, and Stephan Koblmüller. 2025. "A Complete Reference DNA Barcode Library for Austrian Bumblebees" Diversity 17, no. 11: 746. https://doi.org/10.3390/d17110746
APA StyleStrohmeier, T., Schoder, S., Schäffer, S., Grimm, J., Sturmbauer, C., & Koblmüller, S. (2025). A Complete Reference DNA Barcode Library for Austrian Bumblebees. Diversity, 17(11), 746. https://doi.org/10.3390/d17110746







