What Are the Effects of Cattle Grazing on Conservation and Forage Value Across Grazing Pressure Gradients in Alkali Grasslands?
Abstract
1. Introduction
2. Materials and Methods
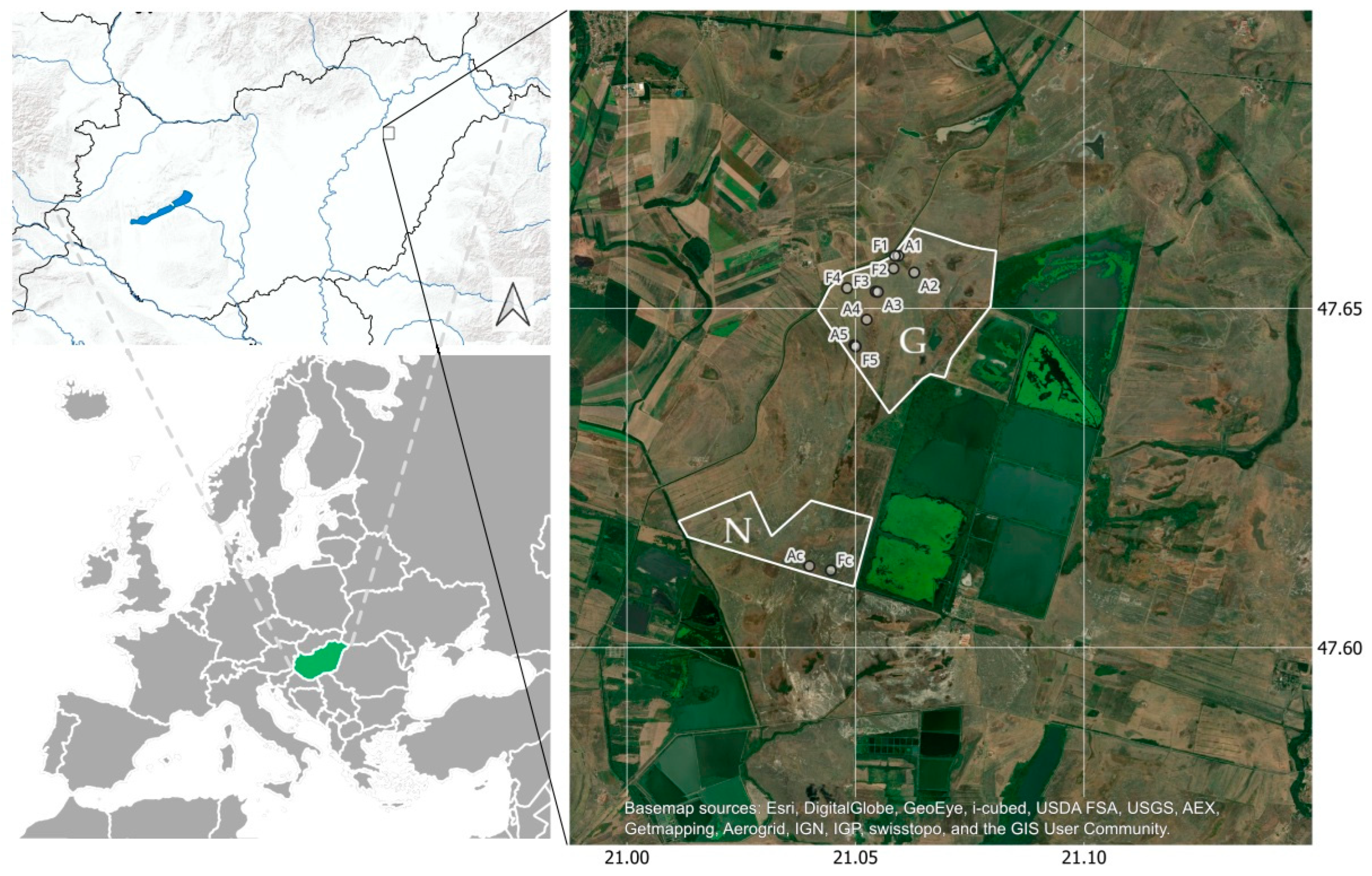
2.1. Sample Areas
2.2. Coenological Sampling
- K 50: plots located at a nominal distance of 50 m from the livestock enclosure
- K 250: plots located at a nominal distance of 250 m from the livestock enclosure
- K 500: plots located at a nominal distance of 500 m from the livestock enclosure
- K 1000: plots located at a nominal distance of 1000 m from the livestock enclosure
- K 1700: plots located at a nominal distance of 1700 m from the livestock enclosure
2.3. Yield and Quality Estimation
2.4. Nature Conservational Value
3. Results
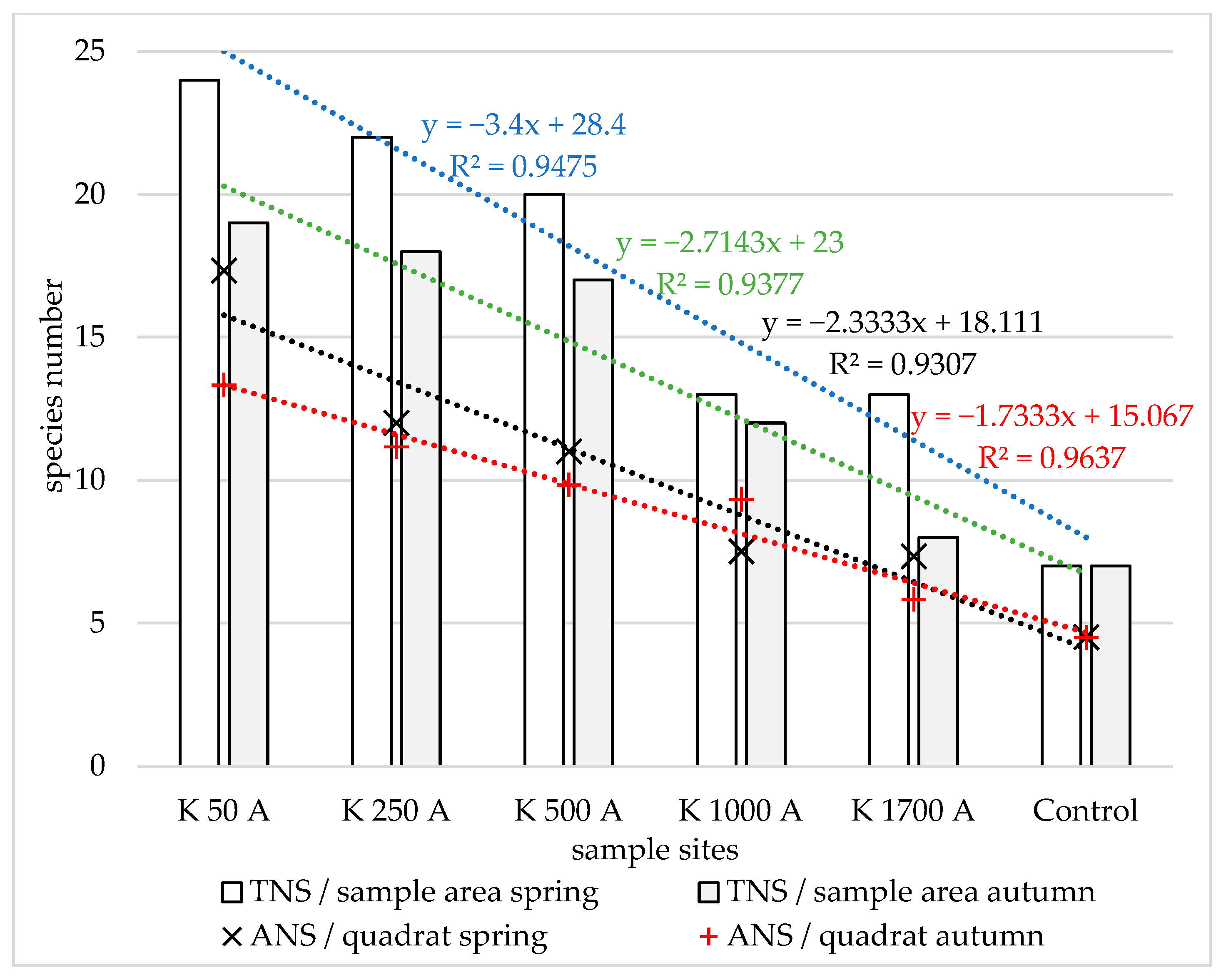
3.1. Compositional Homogeneity, Species Numbers per Quadrat and per Habitat
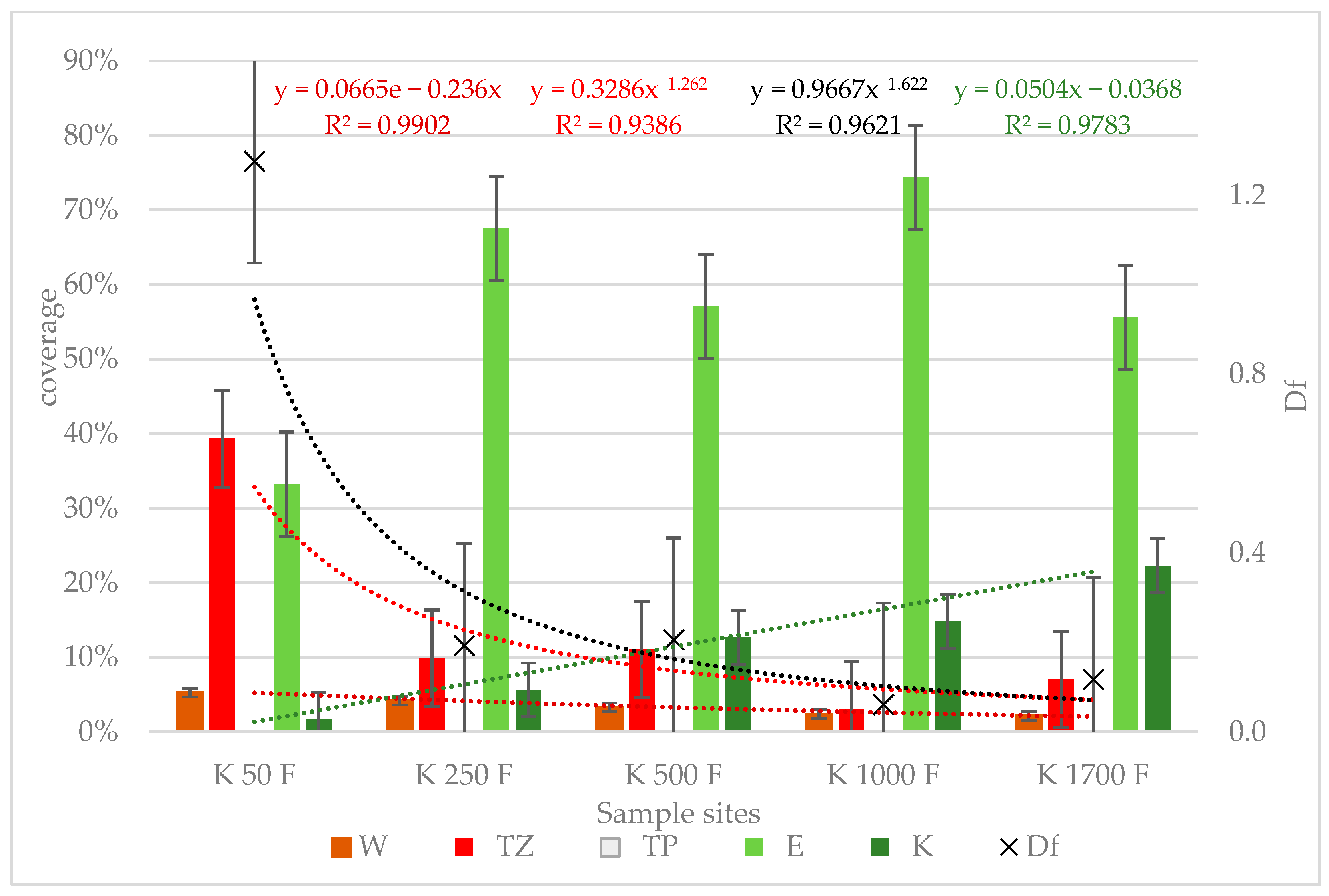
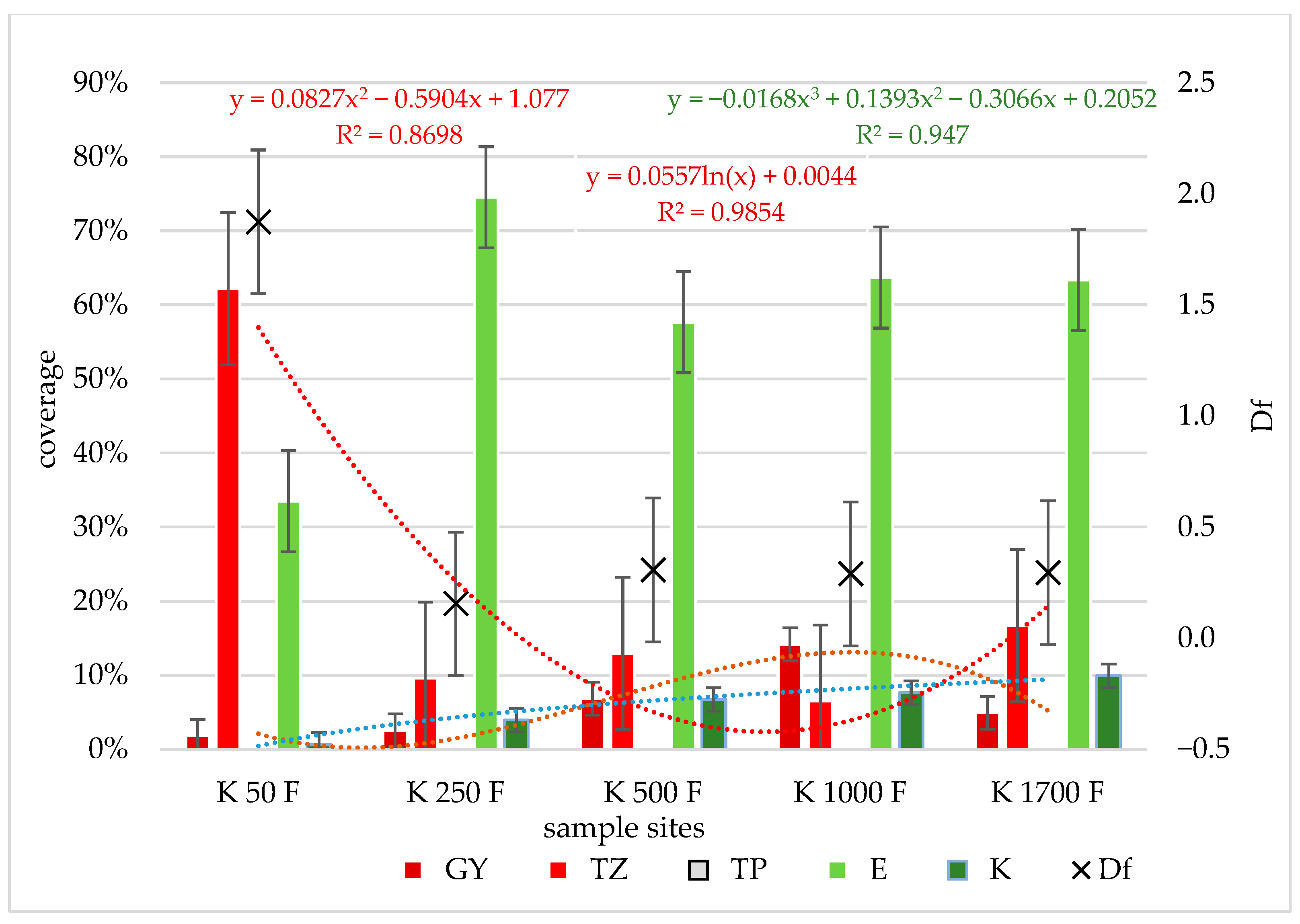
3.2. Distribution of Species by Conservation Value Category
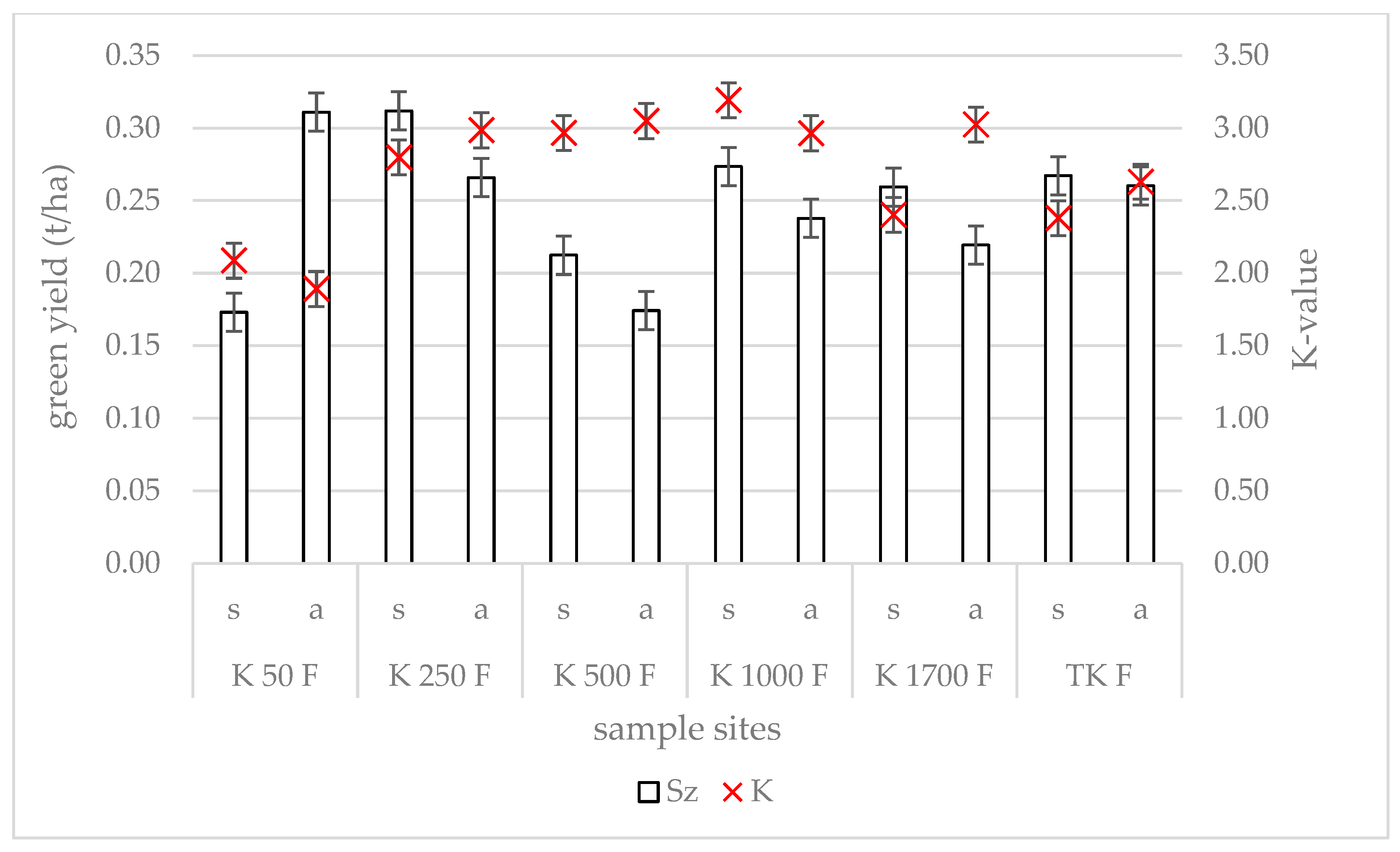
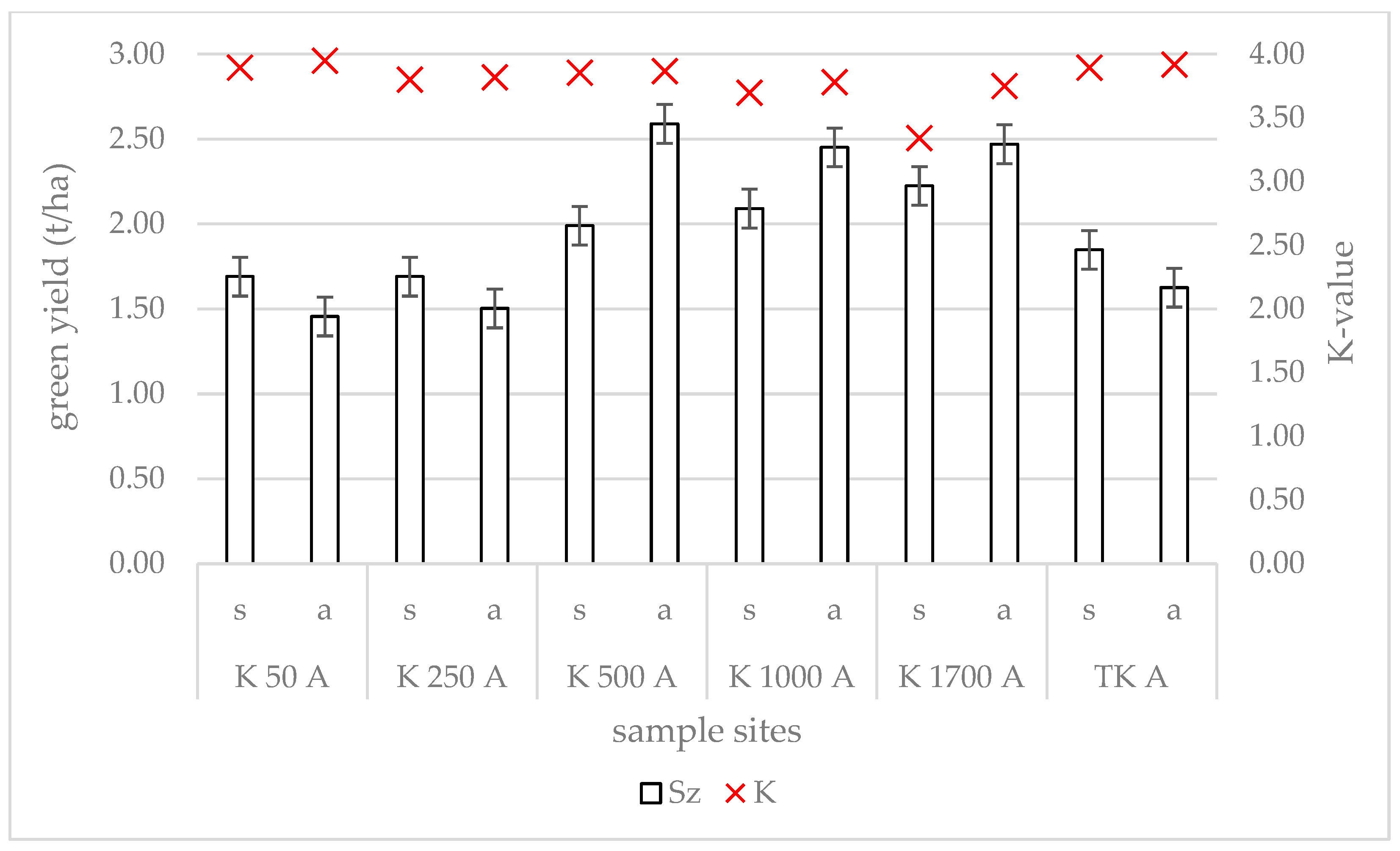
3.3. Changes in Yield and Forage Quality
3.4. Results of Diversity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Available online: https://whc.unesco.org/en/list/474 (accessed on 3 March 2025).
- Török, P.; Valkó, O.; Deák, B.; Kelemen, A.; Tóthmérész, B. Traditional Cattle Grazing in a Mosaic Alkali Landscape: Effects on Grassland Biodiversity along a Moisture Gradient. PLoS ONE 2014, 9, e97095. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hu, Z.-M.; Lai, D.Y.F.; Han, D.-R.; Wang, M.; Liu, M.; Zhang, M.; Guo, M.-Y. Light Grazing Facilitates Carbon Accumulation in Subsoil in Chinese Grasslands: A Meta-Analysis. Glob. Change Biol. 2020, 26, 7186–7197. [Google Scholar] [CrossRef]
- Múgica, L.; Canals, R.M.; San Emeterio, L.; Peralta, J. Decoupling of Traditional Burnings and Grazing Regimes Alters Plant Diversity and Dominant Species Competition in High-Mountain Grasslands. Sci. Total Environ. 2021, 790, 147917. [Google Scholar] [CrossRef]
- Gordon, I.J.; Gregorini, P.; Evans, M.J. Herding the Literature: Trends in Large Mammalian Herbivore Grazing and Foraging Ecology Research over the Past Three Decades. Rangel. Ecol. Manag. 2023, 90, 256–270. [Google Scholar] [CrossRef]
- Bahar, A.; Tavşanoğlu, Ç. The Effect of Grazing on Central Anatolian Grassland Vegetation: A Modeling Approach Using Functional Traits. Ecol. Evol. 2024, 14, e70499. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Jia, A.; Liu, D.; Zhang, C.; Wang, M. How Seasonal Grazing Exclusion Affects Grassland Productivity and Plant Community Diversity. Grasses 2022, 1, 12–29. [Google Scholar] [CrossRef]
- Hou, L.; Xin, X.; Shen, B.; Qin, Q.; Altome, A.I.A.; Hamed, Y.M.Z.; Yan, R.; Nurlan, S.; Adilbek, N.; Balzhan, A.; et al. Effects of Long-Term Grazing on Feed Intake and Digestibility of Cattle in Grassland. Agronomy 2023, 13, 1760. [Google Scholar] [CrossRef]
- Insua, J.R.; Utsumi, S.A.; Basso, B. Estimation of Spatial and Temporal Variability of Pasture Growth and Digestibility in Grazing Rotations Coupling Unmanned Aerial Vehicle (UAV) with Crop Simulation Models. PLoS ONE 2019, 14, e0212773. [Google Scholar] [CrossRef]
- Döpper, V.; Rocha, A.D.; Berger, K.; Gränzig, T.; Verrelst, J.; Kleinschmit, B.; Förster, M. Estimating Soil Moisture Content under Grassland with Hyperspectral Data Using Radiative Transfer Modelling and Machine Learning. Int. J. Appl. Earth Obs. Geoinf. 2022, 110, 102817. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J.; Paixão, L.; Calado, J.; Carvalho, M.D. Integration of Soil Electrical Conductivity and Indices Obtained through Satellite Imagery for Differential Management of Pasture Fertilization. AgriEngineering 2019, 1, 567–585. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, O.J.; Luo, Z.; Jin, H.; Chen, Q.; Han, X. Variation in Small-Scale Spatial Heterogeneity of Soil Properties and Vegetation with Different Land Use in Semiarid Grassland Ecosystem. Plant Soil 2008, 310, 103–112. [Google Scholar] [CrossRef]
- Collins, S.; Smith, M. Scale-dependent interaction of fire and grazing on community heterogeneity in tallgrass prairie. Ecology 2006, 87, 2058–2067. [Google Scholar] [CrossRef]
- Tonn, B.; Densing, E.M.; Gabler, J.; Isselstein, J. Grazing-Induced Patchiness, Not Grazing Intensity, Drives Plant Diversity in European Low-Input Pastures. J. Appl. Ecol. 2019, 56, 1624–1636. [Google Scholar] [CrossRef]
- Marion, B.; Bonis, A.; Bouzillé, J.-B. How Much Does Grazing-Induced Heterogeneity Impact Plant Diversity in Wet Grasslands? Écoscience 2010, 17, 229–239. [Google Scholar] [CrossRef]
- Liu, J.; Feng, C.; Wang, D.; Wang, L.; Wilsey, B.J.; Zhong, Z. Impacts of Grazing by Different Large Herbivores in Grassland Depend on Plant Species Diversity. J. Appl. Ecol. 2015, 52, 1053–1062. [Google Scholar] [CrossRef]
- Remington, K.K.; Bonham, C.D.; Reich, R.M. Blue Grama-Buffalograss Responses to Grazing: A Weibull Distribution. J. Range Manag. 1992, 45, 272–276. [Google Scholar] [CrossRef]
- Bloor, J.M.G.; Tardif, A.; Pottier, J. Spatial Heterogeneity of Vegetation Structure, Plant N Pools and Soil N Content in Relation to Grassland Management. Agronomy 2020, 10, 716. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Zhao, R.; Du, Y.; Yang, D.; Ding, H.; Yang, G.; Gai, S.; He, F.; Yang, P. Engineering Oxygen Vacancy of MoOx Nanoenzyme by Mn Doping for Dual-Route Cascaded Catalysis Mediated High Tumor Eradication. J. Colloid Interface Sci. 2022, 623, 155–167. [Google Scholar] [CrossRef]
- López-Mársico, L.; Altesor, A.; Oyarzabal, M.; Baldassini, P.; Paruelo, J.M. Grazing Increases Below-Ground Biomass and Net Primary Production in a Temperate Grassland. Plant Soil 2015, 392, 155–162. [Google Scholar] [CrossRef]
- Bakker, E.S.; Ritchie, M.E.; Olff, H.; Milchunas, D.G.; Knops, J.M.H. Herbivore Impact on Grassland Plant Diversity Depends on Habitat Productivity and Herbivore Size. Ecol. Lett. 2006, 9, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Alados, C.L.; Saiz, H.; Nuche, P.; Gartzia, M.; Komac, B.; de Frutos, Á.; Pueyo, Y. Clearing vs. Burning for Restoring Pyrenean Grasslands after Shrub Encroachment. Cuad. Investig. Geográfica 2019, 45, 441–468. [Google Scholar] [CrossRef]
- Baur, B.; Cremene, C.; Groza, G.; Schileyko, A.A.; Baur, A.; Erhardt, A. Intensified Grazing Affects Endemic Plant and Gastropod Diversity in Alpine Grasslands of the Southern Carpathian Mountains (Romania). Biologia 2007, 62, 438–445. [Google Scholar] [CrossRef]
- Török, P.; Valkó, O.; Deák, B.; Kelemen, A.; Tóth, E.; Tóthmérész, B. Managing for species composition or diversity? pastoral and free grazing systems in alkali grasslands. Agric. Ecosyst. Environ. 2016, 234, 23–30. [Google Scholar] [CrossRef]
- Török, P.; Penksza, K.; Tóth, E.; Kelemen, A.; Sonkoly, J.; Tóthmérész, B. Vegetation Type and Grazing Intensity Jointly Shape Grazing Effects on Grassland Biodiversity. Ecol. Evol. 2018, 8, 10326–10335. [Google Scholar] [CrossRef]
- Li, W.; Gan, X.; Ye, X.; Yiang, Y.; Zhao, C. Contrasting Effects of Nitrogen Enrichment on Ecosystem Temporal Stability before and after Nitrogen Saturation in a Subalpine Grassland. Sci. Total Environ. 2023, 893, 164535. [Google Scholar] [CrossRef]
- Wu, G.-L.; Du, G.-Z.; Liu, Z.-H.; Thirgood, S. Effect of Fencing and Grazing on a Kobresia-Dominated Grassland in the Qinghai-Tibetan Plateau. Plant Soil 2009, 319, 115–126. [Google Scholar] [CrossRef]
- Wan, H.; Bai, Y.; Hooper, D.U.; Schönbach, P.; Gierus, M.; Schiborra, A.; Taube, F. Selective Grazing and Seasonal Precipitation Play Key Roles in Shaping Plant Community Structure of Semi-Arid Grasslands. Landsc. Ecol. 2015, 30, 1767–1782. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y. A Hybrid Physics-Data-Driven Optimization Model for Grassland Grazing Management. Appl. Math. Nonlinear Sci. 2024, 8, 3215–3228. [Google Scholar] [CrossRef]
- Deng, L.; Sweeney, S.; Shangguan, Z.-P. Grassland Responses to Grazing Disturbance: Plant Diversity Changes with Grazing Intensity in a Desert Grassland. Grass Forage Sci. 2014, 69, 524–533. [Google Scholar] [CrossRef]
- Hao, Y.; He, Z. Effects of Grazing Patterns on Grassland Biomass and Soil Environments in China: A Meta-Analysis. PLoS ONE 2019, 14, e0215223. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M.; de Deus Junior, J.C.; Borges Junior, N.; Cavalcante, R.B.L. Experimental Catchments in the Pampa Biome: Database on Hydrology in Grasslands and Eucalyptus Plantations in Subtropical Brazil. Hydrol. Process. 2021, 35, e14285. [Google Scholar] [CrossRef]
- Pulido, M.; Schnabel, S.; Lavado Contador, J.F.; Lozano-Parra, J.; González, F. The Impact of Heavy Grazing on Soil Quality and Pasture Production in Rangelands of SW Spain. Land Degrad. Dev. 2018, 29, 219–230. [Google Scholar] [CrossRef]
- Donovan, M.; Monaghan, R. Impacts of Grazing on Ground Cover, Soil Physical Properties and Soil Loss via Surface Erosion: A Novel Geospatial Modelling Approach. J. Environ. Manag. 2021, 287, 112206. [Google Scholar] [CrossRef]
- Liang, M.; Liang, C.; Hautier, Y.; Wilcox, K.R.; Wang, S. Grazing-Induced Biodiversity Loss Impairs Grassland Ecosystem Stability at Multiple Scales. Ecol. Lett. 2021, 24, 2054–2064. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Zhou, H.; Hosseinibai, S. Grazing Intensity Significantly Affects Belowground Carbon and Nitrogen Cycling in Grassland Ecosystems: A Meta-Analysis. Glob. Change Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef]
- Sun, J.; Ma, B.; Lu, X. Grazing Enhances Soil Nutrient Effects: Trade-Offs between Aboveground and Belowground Biomass in Alpine Grasslands of the Tibetan Plateau. Land Degrad. Dev. 2018, 29, 337–348. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, D.; Chen, H.Y.H.; Seabloom, E.W.; Han, G.; Wang, S.; Yu, G.; Li, Z.; Niu, S. Biodiversity Alleviates the Decrease of Grassland Multifunctionality under Grazing Disturbance: A Global Meta-Analysis. Glob. Ecol. Biogeogr. 2022, 31, 155–167. [Google Scholar] [CrossRef]
- Abdalla, K.; Mutema, M.; Chivenge, P.; Everson, C.; Chaplot, V. Grassland Degradation Significantly Enhances Soil CO2 Emission. CATENA 2018, 167, 284–292. [Google Scholar] [CrossRef]
- Ma, L.; Yao, Z.; Zheng, X.; Zhang, H.; Wang, K.; Zhu, B.; Wang, R.; Zhang, W.; Liu, C. Increasing Grassland Degradation Stimulates the Non-Growing Season CO2 Emissions from an Alpine Grassland on the Qinghai–Tibetan Plateau. Environ. Sci. Pollut. Res. 2018, 25, 26576–26591. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-Quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Uddin, M.E.; Kebreab, E. Review: Impact of Food and Climate Change on Pastoral Industries. Front. Sustain. Food Syst. 2020, 4, 543403. [Google Scholar] [CrossRef]
- Walker, J.W.; Heitschmidt, R.K.; Dowhower, S.L. Some Effects of a Rotational Grazing Treatment on Cattle Preference for Plant Communities. J. Range Manag. 1989, 42, 143–148. [Google Scholar] [CrossRef]
- Bailey, D.W.; VanWagoner, H.C.; Weinmeister, R. Individual Animal Selection Has the Potential to Improve Uniformity of Grazing on Foothill Rangeland. Rangel. Ecol. Manag. 2006, 59, 351–358. [Google Scholar] [CrossRef]
- Stephenson, M.B.; Bailey, D.W.; Howery, L.D.; Henderson, L. Efficacy of Low-Stress Herding and Low-Moisture Block to Target Cattle Grazing Locations on New Mexico Rangelands. J. Arid Environ. 2016, 130, 84–93. [Google Scholar] [CrossRef]
- Stevens, D.R.; Thompson, B.R.; Johnson, P.; Welten, B.; Meenken, E.; Bryant, J. Integrating Digital Technologies to Aid Grassland Productivity and Sustainability. Front. Sustain. Food Syst. 2021, 5, 602350. [Google Scholar] [CrossRef]
- Kelemen, A.; Török, P.; Valkó, O.; Deák, B.; Tóth, K.; Tóthmérész, B. Both Facilitation and Limiting Similarity Shape the Species Coexistence in Dry Alkali Grasslands. Ecol. Complex. 2015, 21, 34–38. [Google Scholar] [CrossRef]
- Tóth, E.; Deák, B.; Valkó, O.; Kelemen, A.; Miglécz, T.; Tóthmérész, B.; Török, P. Livestock Type Is More Crucial Than Grazing Intensity: Traditional Cattle and Sheep Grazing in Short-Grass Steppes. Land Degrad. Dev. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Dövényi, Z. Magyarország kistájainak katasztere [Inventory of Microregions in Hungary]; MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; p. 876. (In Hungarian) [Google Scholar]
- Király, G. Új Magyar Füvészkönyv: Magyarország Hajtásos Növényei; Határozókulcsok. [New Hungarian Herbal. The Vascular Plants of Hungary. Identification Key]; Aggteleki Nemzeti Park Igazgatóság: Aggtelek, Hungary, 2009; ISBN 978-963-87082-9-8. (In Hungarian) [Google Scholar]
- Balázs, F. A gyepek termésbecslése növényszociológia alapján. [Estimating grass yields based on plant sociology]. Agrártudomány 1949, 1, 26–35. [Google Scholar]
- Balázs, F. A gyepek botanikai és gazdasági értékelése. [Botanical and economic evaluation of grasslands]. A Keszthelyi Mezőgazdasági Akadémia Kiadványai. 1960, 8, 3–23. [Google Scholar]
- Szentes, S.; Turcsányi-Járdi, I.; Sipos, L.; Penksza, K.; Kende, Z.; Saláta-Falusi, E.; Szabó-Szöllösi, T.; Kevi, A.; Balogh, D.; Bajnok, M.; et al. Feed Values for Grassland Species and Method for Assessing the Quantitative and Qualitative Characteristics of Grasslands. Earth 2025, 6, 119. [Google Scholar] [CrossRef]
- Penksza, K.; Saláta, D.; Fűrész, A.; Penksza, P.; Fuchs, M.; Pajor, F.; Sipos, L.; Saláta-Falusi, E.; Wagenhoffer, Z.; Szentes, S. Are Hungarian Grey Cattle or Hungarian Racka Sheep the Best Choice for the Conservation of Wood-Pasture Habitats in the Pannonian Region? Agronomy 2024, 14, 846. [Google Scholar] [CrossRef]
- Simon, T. A Magyarországi Edényes Flóra Határozója. [Key to the Vascular Flora of Hungary], 4th ed.; Tankönyvkiadó: Budapest, Hungary, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 30 April 2025).
- Wickham, H. ggplot2: Elegant graphics for data analysis. J. Stat. Softw. 2010, 35, 1–3. [Google Scholar]
- Li, W.; Su, T.; Shen, Y.; Ma, H.; Zhou, Y.; Lu, Q.; Wang, G.; Liu, Z.; Li, J. Effects of warming seasonal rotational grazing on plant communities’ structure and diversity in desert grassland. Ecol. Evol. 2023, 13, e9748. [Google Scholar] [CrossRef] [PubMed]
- Portugal, T.B.; Szymczak, L.S.; de Moraes, A.; Fonseca, L.; Mezzalira, J.C.; Savian, J.V.; Zubieta, A.S.; Bremm, C.; de Faccio Carvalho, P.C.; Monteiro, A.L.G. Low-Intensity, High-Frequency Grazing Strategy Increases Herbage Production and Beef Cattle Performance on Sorghum Pastures. Animals 2022, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Calcagno, V.; Hector, A.; Connolly, J.; Harpole, W.S.; Reich, P.B.; Scherer-Lorenzen, M.; Schmid, B.; Tilman, D.; Van Ruijven, J.; et al. High plant diversity is needed to maintain ecosystem services. Nature 2011, 477, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Han, X.; Zhang, Z.; Sun, O. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 997–1008. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Z.; Li, Q.; He, L.; Tian, J.; Ding, W.; Liu, T.; Gao, Y.; Zhang, J.; Han, D.; et al. Grazing impacts on soil enzyme activities vary with vegetation types in the forest-grassland ecotone of northeastern china. Forests 2023, 14, 2292. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Wang, Y.; Xu, W.; Liu, A.; Ma, Z.; Mi, Z.; Zhang, Z.; Wang, S.; He, J.S. Warm- and cold-season grazing affect soil respiration differently in alpine grasslands. Agric. Ecosyst. Environ. 2017, 248, 136–143. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Xu, J.; Wei, W.; Xue, P.; Yan, H. Response of soil nutrients and stoichiometry to grazing management in alpine grassland on the Qinghai-Tibet Plateau. Soil Tillage Res. 2021, 206, 104822. [Google Scholar] [CrossRef]
- He, M.; Pan, Y.; Zhou, G.; Barry, K.E.; Fu, Y.; Zhou, X. Grazing and global change factors differentially affect biodiversity-ecosystem functioning relationships in grassland ecosystems. Glob. Change Biol. 2022, 28, 5492–5504. [Google Scholar] [CrossRef]
- Mipam, T.; Chen, S.; Liu, J.; Miehe, G.; Tian, L. Short-term yak-grazing alters plant-soil stoichiometric relations in an alpine grassland on the eastern tibetan plateau. Plant Soil 2019, 458, 125–137. [Google Scholar] [CrossRef]
- Liu, X.; Bo, Y.; Zhang, J.; He, Y. Classification of C3 and C4 Vegetation Types Using MODIS and ETM+ Blended High Spatio-Temporal Resolution Data. Remote Sens. 2015, 7, 15244–15268. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; McINTYRE, S.; Falczuk, V.; Casanoves, F.; Milchunas, D.G.; Skarpe, C.; Rusch, G.; Sternberg, M.; Noy-Meir, I.; et al. Plant Trait Responses to Grazing—A Global Synthesis. Glob. Change Biol. 2007, 13, 313–341. [Google Scholar] [CrossRef]
- Bullock, J.M.; Pakeman, R.J. Grazing of Lowland Heath in England: Management Methods and Their Effects on Healthland Vegetation. Biol. Conserv. 1997, 79, 1–13. [Google Scholar] [CrossRef]
- Loucougaray, G.; Bonis, A.; Bouzillé, J.-B. Effects of Grazing by Horses and/or Cattle on the Diversity of Coastal Grasslands in Western France. Biol. Conserv. 2004, 116, 59–71. [Google Scholar] [CrossRef]
- Niu, W.; Ding, J.; Fu, B.; Zhao, W.; Eldridge, D. Global Effects of Livestock Grazing on Ecosystem Functions Vary with Grazing Management and Environment. Agric. Ecosyst. Environ. 2025, 378, 109296. [Google Scholar] [CrossRef]
- Vova, O.; Kappas, M.; Renchin, T.; Fassnacht, S. Extreme climate event and its impact on landscape resilience in gobi region of mongolia. Remote Sens. 2020, 12, 2881. [Google Scholar] [CrossRef]
- Hayes, G.; Holl, K. Cattle grazing impacts on annual forbs and vegetation composition of mesic grasslands in california. Conserv. Biol. 2003, 17, 1694–1702. [Google Scholar] [CrossRef]
- Magyar, V.; Penksza, K.; Szentes, S. Comparative investigations of biomass composition in differently managed grasslands of the Balaton Uplands National Park, Hungary. Grassl. Stud. 2017, 15, 49–56. [Google Scholar] [CrossRef]
- Penksza, K.; Pajor, F.; Kevi, A.; Wagenhoffer, Z.; Sipos, L.; Salata-Falusi, E.; Penksza, P.; Poti, P.; Berke, J.; Salata, D.; et al. The Effect of Goat Grazing on the Biodiversity of Pannonian Dry Grassland. Diversity 2025, 17, 13. [Google Scholar] [CrossRef]
- Guo, Z.; Qin, F. An empirical analysis of the role of forage product trade on grassland quality and livestock production in china. Land 2022, 11, 1938. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, J.; Clark, C.M.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing alters ecosystem functioning and c:n:p stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Kotsonas, E.; Bakaloudis, D.; Vlachos, C.; Abraham, Ε.; Goutner, V. Effect of transhumant livestock grazing on pseudo-alpine grassland bird communities. Birds 2021, 2, 23–41. [Google Scholar] [CrossRef]
- Eldridge, D.; Poore, A.; Ruiz-Colmenero, M.; Letnic, M.; Soliveres, S. Ecosystem structure, function, and composition in rangelands are negatively affected by livestock grazing. Ecol. Appl. 2016, 26, 1273–1283. [Google Scholar] [CrossRef]
- Kohyani, P.T.; Bossuyt, B.; Bonte, D.; Hoffmann, M. Grazing impact on plant spatial distribution and community composition. Plant Ecol. Evol. 2011, 144, 19–28. [Google Scholar] [CrossRef]
- Wu, L.; He, N.; Wang, Y.; Han, X. Storage and dynamics of carbon and nitrogen in soil after grazing exclusion in Leymus chinensis grasslands of northern china. J. Environ. Qual. 2008, 37, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Külkamp, J.; Heiden, G.; Iganci, J.R.V. Endemic Plants from the Southern Brazilian Highland Grasslands. Rodriguésia 2018, 69, 429–440. [Google Scholar] [CrossRef]
- Pärtel, M.; Bruun, H.H.; Sammul, M. Biodiversity in Temperate European Grasslands: Origin and Conservation. In Grassland Science in Europe; Lund University: Lund, Sweden, 2005; pp. 1–14. [Google Scholar]
- Prangel, E.; Kasari-Toussaint, L.; Neuenkamp, L.; Noreika, N.; Karise, R.; Marja, R.; Ingerpuu, N.; Kupper, T.; Keerberg, L.; Oja, E.; et al. Afforestation and Abandonment of Semi-Natural Grasslands Lead to Biodiversity Loss and a Decline in Ecosystem Services and Functions. J. Appl. Ecol. 2023, 60, 825–836. [Google Scholar] [CrossRef]
- Prangel, E.; Reitalu, T.; Neuenkamp, L.; Kasari-Toussaint, L.; Karise, R.; Tiitsaar, A.; Soon, V.; Kupper, T.; Meriste, M.; Ingerpuu, N.; et al. Restoration of Semi-Natural Grasslands Boosts Biodiversity and Re-Creates Hotspots for Ecosystem Services. Agric. Ecosyst. Environ. 2024, 374, 109139. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szentes, S.; Pajor, F.; Penksza, K.; Saláta-Falusi, E.; Balogh, D.; Balogh, J.; Sári, L.; Balogh, P.; Bori, D.; Kárpáti, E.; et al. What Are the Effects of Cattle Grazing on Conservation and Forage Value Across Grazing Pressure Gradients in Alkali Grasslands? Diversity 2025, 17, 741. https://doi.org/10.3390/d17110741
Szentes S, Pajor F, Penksza K, Saláta-Falusi E, Balogh D, Balogh J, Sári L, Balogh P, Bori D, Kárpáti E, et al. What Are the Effects of Cattle Grazing on Conservation and Forage Value Across Grazing Pressure Gradients in Alkali Grasslands? Diversity. 2025; 17(11):741. https://doi.org/10.3390/d17110741
Chicago/Turabian StyleSzentes, Szilárd, Ferenc Pajor, Károly Penksza, Eszter Saláta-Falusi, Dániel Balogh, János Balogh, Leonárd Sári, Petra Balogh, Dániel Bori, Edina Kárpáti, and et al. 2025. "What Are the Effects of Cattle Grazing on Conservation and Forage Value Across Grazing Pressure Gradients in Alkali Grasslands?" Diversity 17, no. 11: 741. https://doi.org/10.3390/d17110741
APA StyleSzentes, S., Pajor, F., Penksza, K., Saláta-Falusi, E., Balogh, D., Balogh, J., Sári, L., Balogh, P., Bori, D., Kárpáti, E., Freiler-Nagy, Á., Orosz, S., Penksza, P., Szőke, P., Pintér, O., Szatmári, I., & Wagenhoffer, Z. (2025). What Are the Effects of Cattle Grazing on Conservation and Forage Value Across Grazing Pressure Gradients in Alkali Grasslands? Diversity, 17(11), 741. https://doi.org/10.3390/d17110741






