Abstract
Comprehending the effects of climate change on the range of endangered species is essential for formulating successful conservation strategies. This research examines two nationally protected dung beetle species (Heliocopris dominus and Heliocopris bucephalus) in China to forecast their probable habitat range under present and future climate scenarios. Employing MaxEnt modeling with validated occurrence records and environmental variables, we discerned critical factors affecting their distribution and anticipated changes in habitat suitability. Results reveal that isothermality, temperature seasonality, maximum temperature of the warmest month, and annual precipitation are the principal environmental drivers. Presently, appropriate habitats are primarily located in southern Yunnan and Hainan, with future forecasts indicating a northward extension into additional areas. These findings offer critical insights for choosing conservation zones for these vulnerable species amid shifting climate conditions.
1. Introduction
Biodiversity denotes the comprehensive variety of life forms on Earth, comprising three levels: species diversity, genetic diversity, and ecosystem diversity [1]. Biodiversity, in terms of species richness, is predominantly shown by the quantity of species in a specific area and their distribution patterns, serving as a core measure of biodiversity [2]. Increased species richness can bolster ecosystem stability, augment resilience to disturbances, promote adaptability to environmental changes, and guarantee the ongoing performance of vital ecological services. The interdependence of many species creates intricate food webs and ecological networks, sustaining material cycle and energy transfer throughout ecosystems [3]. Nonetheless, global biodiversity is presently undergoing a substantial reduction. Research indicates that the present pace of species extinction is 100 to 1000 times greater than the natural background rate [4]. The principal factors contributing to biodiversity loss encompass habitat destruction, over-exploitation, pollution, and climate change, all of which are instigated by human activity. Global climate change is unequivocally transforming ecosystems at an unprecedented rate, posing a significant threat to biodiversity and consistently diminishing its spatial patterns. Numerous critically endangered species are currently on the verge of extinction [5]. In response to this situation, international conservation efforts have been mobilized. Organizations such as the “International Union for Conservation of Nature (IUCN)” have released the IUCN Red List of Threatened Species (IUCN 1994, 2001) and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), respectively. Numerous nations have implemented laws specific to their biological resources [6]. In China, for instance, legal frameworks such as the Lists of Wild Animals under Special State Protection in China and the List of Terrestrial Wild Animals of Important Ecological, Scientific, and Social Value have been established to protect species included in these lists. Moreover, evaluating species’ probable habitats via suitability predictions has emerged as an essential instrument for biodiversity conservation, augmented by the creation of protected areas to preserve wildlife populations [7]. Consequently, the objective assessment of the rare and endangered condition of biological resources, alongside the identification of critical factors affecting biodiversity and the execution of precise habitat suitability predictions, has emerged as an essential component of biodiversity conservation initiatives.
Approximately one million insect species have been identified worldwide, constituting 70–80% of all known life forms [8], hence rendering insects an essential element of ecosystems. Insects inhabit various trophic levels and critical ecological niches, fulfiling essential functions in sustaining plant diversity, serving as food sources, decomposing organic matter such as dead wood, feces, and carcasses, expediting the breakdown of organic material, improving soil fertility and nutrient cycling (particularly carbon and nitrogen), and facilitating energy flow and trophic stability within ecosystems. In specific areas, including Europe and North America, insect biomass has decreased by almost 75% in recent decades [9]. Research indicates that insects have heightened sensitivity to environmental influences, such as climate change, potentially resulting in species extinction [10]. Dung beetles (Scarabaeinae, Scarabaeidae, Coleoptera) function as essential decomposers throughout ecosystems. This category is essential for material cycling and energy flow [11], nonetheless, it is susceptible to environmental and anthropogenic perturbations, which may result in population decreases, ecological niche collapse, and the deterioration of ecosystem services [12]. The genus Heliocopris Hope, 1837, is the largest in body size among the dung beetle tribe Coprini (Scarabaeinae) [13,14]. Their members predominantly consume the excrement of elephants, rhinoceroses, and hippopotamuses, hence earning the designation of elephant dung beetles. More than 60 species have been documented globally, predominantly found in the tropical and subtropical areas of Africa and Asia. Only six species exist in Asia [15]. Four species have been documented in China: H. dominus (Bates, 1868), H. bucephalus (Fabricius, 1775), H. midas (Fabricius, 1775), and H. gigas (Linnaeus, 1758) [16]. Heliocopris beetles possess a substantial body size and pronounced head and thorax horns, resulting in a striking and unique look that enhances their cosmetic value and renders them highly desirable in the insect collecting industry [17]. In Myanmar, the larvae and pupae of Heliocopris species are regarded as delicacy and are collected [18]. In recent years, numerous reports have indicated the smuggling of Heliocopris pupal cocoons (containing pupae or pre-pupal larvae) into the Yunnan border region of China from Myanmar for consumption purposes [19,20,21,22,23]. The ongoing trade operations, along with the limited biological niche of these beetles, have resulted in persistent population decreases. On 5 February 2021, the State Council of China formally announced the newly amended Lists of Wild Animals under Special State Protection [24]. Three species of Heliocopris—H. dominus, H. bucephalus, and H. midas—are designated as Class II nationally protected species. Nevertheless, contemporary research on these endangered beetles is predominantly confined to taxonomy and genetics [25]. There is a deficiency of studies addressing population dynamics and spatial distribution patterns in relation to environmental factors such as climate. The potential distribution area of wild populations is currently unidentified, necessitating immediate conservation measures for this group.
This study aims to project the current and future potential distributions of two ecologically significant dung beetle species in China—H. dominus and H. Bucephalus—under climate change scenarios, and to identify priority conservation areas for these taxa. The specific objectives are as follows:
- (1)
- To evaluate the conservation status of each species at the regional level based on the IUCN Red List Categories and Criteria;
- (2)
- To identify the environmental variables most influential in shaping the distribution of Heliocopris species;
- (3)
- To model currently suitable habitats and project future shifts in distribution under two climate scenarios (RCP2.6 and RCP8.5) for the 2050s and 2070s;
- (4)
- To delineate potential conservation priority areas and propose relevant management strategies.
2. Materials and Methods
2.1. Endangered Status Evaluation of Testing Species
The criteria for assessing the conservation status of endangered species are developed and promoted by the “International Union for Conservation of Nature (IUCN)”. These criteria are designed to evaluate the extinction risk of species based on past, present, and projected future threats, and to classify species into corresponding threat categories within a standardized system. The assessment is based on various threat factors, including population size and trends, number of mature individuals, geographic range, habitat loss or degradation, habitat fragmentation, impacts of climate change, invasive species, overharvesting or poaching, disease or pathogens, environmental pollution, human disturbance, decreased reproductive success, and changes in food chains or ecosystem functions. Specifically, these factors are classified as follows: A1: percentage decline in population size: A2: continuous population decline; A3: predicted future population decline; B1: extent of population distribution; B2: shrinkage or degradation of distribution range; C1: changes in population size; C2: decline in population size and distribution area; D1: reduction in habitat area; D2: decline in habitat quality; E1: predicted impact of climate change and long-term trends; F1: threats posed by invasive species; G1: impact of overhunting or overfishing; H1: impact of diseases on the population; I1: negative impact of pollution on habitats and populations; J1: impact of land reclamation and infrastructure development on species; K1: decline in reproductive success; L1: impact of ecosystem function changes on species. Biodiversity assessments are internationally conducted according to nine categories, namely: extinct (EX), extinct in the wild (EW), regionally extinct (RE), critically endangered (CR), endangered (EN), vulnerable (VU), near threatened (NT), least concern (LC), and data deficient (DD). Among these, the categories critically endangered (CR), endangered (EN), and vulnerable (VU) are collectively referred to as the “Threatened Categories”.
In this study, a total of 32 precisely georeferenced occurrence records of H. dominus and H. bucephalus, after rigorous filtering, were imported into the sRedList platform (https://sredlist.eu/#/home, (accessed on 9 April 2025)). A buffer radius of 5.0 km was applied to each occurrence point, a parameter determined based on documented adult dispersal capacity and habitat connectivity requirements to ensure the inclusion of core areas essential for migration and reproduction. Potential species distributions were refined by integrating altitude data from specimen collection records and digital elevation values extracted from the coordinate points, thereby excluding altitudinally unsuitable habitats and generating preliminary potential distribution maps. The Extent of Occurrence (EOO) was calculated using the minimum convex polygon method, while the Area of Occupancy (AOO) was estimated based on a standardized grid cell approach. These metrics were evaluated against IUCN Red List Criterion B1 (EOO < 20,000 km2 in conjunction with ongoing habitat decline) and Criterion B2 (AOO < 2000 km2 accompanied by increasing habitat fragmentation). Subsequent species distribution modeling involved the selection of relevant environmental predictors provided by the platform to delineate suitable habitat areas and estimate the current distribution range. A generation length of one year was specified, and remote sensing-derived trends in forest cover loss-based on Landsat-8 imagery-were incorporated to project future changes in habitat suitability and potential range contraction. Finally, the platform generated a comprehensive sRedList assessment report, which includes detailed evaluation logs, species metadata, a country-level distribution list, habitat preference descriptions, spatial distribution shapefiles, and a table of original occurrence records, culminating in a map classifying the species’ conservation status according to IUCN categories.
2.2. Species Information
2.2.1. Sources and Processing of Dung Beetle Distribution Data
The distribution data were primarily obtained from field surveys, literature records [26], and the GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.uzkjq8 (accessed on 1 October 2025), database (https://www.gbif.org/, accessed on 19 August 2025). Erroneous and duplicate distribution points were excluded, resulting in a final dataset comprising 32 unique distribution points with associated latitude, longitude, and elevation information (Table 1–Supplement File S1). To ensure compatibility with MaxEnt (v3.4.4) software, the distribution data were saved in .csv format. ArcGIS (v10.8; Esri Inc., Redlands, CA, USA) software was then employed to generate an electronic map depicting the distribution of endangered dung beetle species across China, with latitude and longitude converted to decimal degrees during the process.

Table 1.
Information on the distribution points of two Heliocopris species.
2.2.2. Source and Processing of Environmental Variable Data
The standard base map issued by the Ministry of Natural Resources was utilized in this study (available at: https://cloudcenter.tianditu.gov.cn/administrativeDivision/, (accessed on 11 August 2025)).
The base map approval number is: GS (2024) No. 0650. The 19 bioclimatic variables for both current and future climate conditions were obtained from the WorldClim database (Supplement File S2) (http://www.worldclim.org/). The future bioclimatic variables for the 2050s (2040–2060) and 2070s (2060–2080) were derived from the Representative Concentration Pathways (RCP) 2.6 and RCP8.5 emission scenarios, using the Common Climate System Model (CCSM4). These scenarios represent low and high greenhouse gas concentration pathways, respectively [27]. RCP2.6 represents a stringent mitigation scenario requiring rapid and significant reductions in global greenhouse gas emissions, with peak emissions occurring around 2020–2030 followed by a sharp decline. This pathway aims to limit global warming to below 2 °C above pre-industrial levels, aspiring toward the 1.5 °C target set by the Paris Agreement. It assumes the widespread adoption of carbon capture technologies and negative emissions strategies in the latter half of the century. RCP8.5 reflects a high-emissions scenario often referred to as the “business-as-usual” pathway. It projects continued growth in fossil fuel use, increased energy demand, and minimal climate policy intervention, leading to a radiative forcing of 8.5 W/m2 by 2100. Under this scenario, global average temperatures are projected to rise by over 4 °C compared to pre-industrial levels, resulting in severe and widespread climatic impacts. These scenarios are widely used in ecological modeling, including species distribution projections, to evaluate a range of potential climate impacts from best-case to worst-case outcomes. The environmental datasets downloaded from the WorldClim database have a spatial resolution of 2.5 arc-minutes. Using ArcGIS software, the 19 global bioclimatic variables were extracted by masking them with the vector map of China, with the geographic coordinate system set to WGS 1984. The extracted bioclimatic variables were subsequently converted into .asc (ASCII Grid) format for compatibility with MaxEnt software.
2.3. MaxEnt Model Construction and Evaluation
The distribution points of the two Heliocopris species and bioclimatic variables were imported into MaxEnt software, with environmental layers designated as the environmental variables and set to “continuous” for the variable type. During the modeling process, the distribution points were split into 75% for the training set and 25% for the test set, with the procedure repeated 10 times. The accuracy of the MaxEnt model predictions was evaluated using the Receiver Operating Characteristic (ROC) curve. In addition, response curves were generated, and the contribution of bioclimatic variables to the suitable habitat distribution of the species was analyzed using the Jackknife method. Permutation importance values were also calculated to assess the relevance of each variable. Predicted maps were generated with the output format set to “Logistic”. Permutation importance was calculated by randomly shuffling the values of a specific environmental variable (while holding other variables constant) and observing the resulting decrease in model accuracy, such as the AUC value. A greater reduction in accuracy indicates a more significant contribution of that variable to the model.
Model prediction accuracy was assessed using the ROC AUC, with values ranging from 0 to 1. Higher AUC values closer to 1 correspond to greater prediction accuracy. The final prediction results were based on the highest AUC value, with an AUC greater than 0.9 indicating high prediction accuracy. The following AUC value ranges correspond to different levels of model performance: (0.5, 0.6] represents failure, (0.6, 0.7] represents poor, (0.7, 0.8] represents fair, (0.8, 0.9] represents good, and (0.9, 1] represents excellent.
2.4. Classification of Suitable Habitat Levels
The predicted results from the MaxEnt model represent the probability of occurrence for H. dominus and H. bucephalus in China. After saving the output files in .asc or .txt format, they were imported into ArcGIS for further analysis, generating prediction maps of suitable habitats for these species. The MaxEnt simulation output values range from 0 to 1, reflecting the species’ probability of occurrence. Based on the actual distribution patterns of the dung beetles, the “Natural Breaks” method was employed to classify the predicted suitability values into four categories: high suitability (0.6–0.9), moderate suitability (0.3–0.6), low suitability (0.1–0.3), and non-suitable habitat (0–0.1).
3. Results
3.1. IUCN Assessment of the Endangered Status of Four Heliocopris Species
The IUCN assessment of H. dominus and H. bucephalus (Figure 1) showed that the two species were classified as LC or NT based on the population decline rate (A1) and range reduction rate (A2). This classification indicates that their overall population decline has not reached the threshold for Endangered (i.e., a decline of ≥50% over the past 10 years, as per the A1 criterion). Under the geographic range criteria, the species were still categorized as LC/NT based on both range (B1) and area occupied (B2). However, in specific subregions (B2), all species were classified as EN, indicating a potential risk of local extinction in these areas.

Figure 1.
The IUCN assessment grade chart of the genus Heliocopris. (A) IUCN assessment level chart of the Heliocopris dominus; (B) IUCN assessment level chart of the Heliocopris bucephalus. Among them, LC represents the Least Concern level; NT represents the Near Threatened level; EN represents the Endangered level; VU represents the Vulnerable level; CR represents the Critically Endangered level.
3.2. Contribution of Environmental Factor Variability to Distribution
The variable selection was conducted through a two-stage process comprising preliminary and refined screening. The initial screening retained variables with clear physiological significance based on ecological relevance and Pearson correlation coefficients (|r| > 0.8). The refined screening employed MaxEnt modeling and jackknife tests to eliminate redundant variables by assessing percentage contribution and permutation importance. This process yielded 6 core variables with low correlation, minimal redundancy, high predictive performance, and clear ecological interpretability. The six bioclimatic variables are Isothermality, Temperature Seasonality, Maximum Temperature of the Warmest Month, Annual Precipitation, Coefficient of Temperature Seasonality Variation, and Mean Temperature of the Coldest Quarter. Bio3 (Isothermality) and Bio4 (Temperature Seasonality) define temperature stability at diurnal and seasonal scales, respectively, which is crucial for the activity patterns and metabolic rates of ectothermic insects like dung beetles. Bio5 (Max Temperature of Warmest Month) and Bio11 (Mean Temperature of Coldest Quarter) represent the extreme thermal limits during summer and winter, setting the ultimate boundaries for survival and distribution. Bio12 (Annual Precipitation) serves as a proxy for overall ecosystem productivity, indirectly determining the abundance of mammalian dung resources. Bio15 (Precipitation Seasonality) captures intra-annual variation in water availability, which directly influences soil moisture conditions critical for nesting and larval development. The six variables collectively characterize the key environmental conditions limiting the geographic distribution of the Heliocopris from multiple dimensions—including thermal stability, extreme temperature tolerance, and water resource availability—thereby serving as predictors with high information content and clear ecological significance in the model. Using 32 existing distribution points and six selected bioclimatic variables, the MaxEnt software was employed to model and predict the potential suitable habitats for the two endangered Heliocopris species in China. After running the model 10 times, the average AUC values were 0.966 and 0.929, indicating that the predictions are both reliable and accurate, making them suitable for assessing the impact of climate change on the distribution of dung beetles in China.
The analysis revealed that H. dominus is most strongly dependent on the mean temperature of the coldest quarter (BIO11) for its distribution (52.1%), with its distribution being least influenced by isothermality (BIO3) (6.8%). The geographic distribution of H. bucephalus is primarily driven by temperature seasonality (BIO4) (48.5%), showing the weakest response to the mean diurnal temperature range (BIO2) (5.2%).
3.3. Prediction of Suitable Habitats for Dung Beetles in China Under Current Climatic Conditions
The predicted suitable habitats (Figure 2) reveal that, under current climatic conditions, the high-suitability habitats for the two Heliocopris species are predominantly concentrated in the southern regions of Yunnan and Hainan. H. dominus and H. bucephalus exhibit small populations in the southeastern part of Tibet (Figure 2A,B). Surrounding these high-suitability areas, moderate-suitability habitats extend outward, covering central and northern Yunnan, Guangxi, and Guangdong, southeastern Tibet, central Taiwan, as well as parts of Guangxi, Guizhou, and Yunnan provinces.
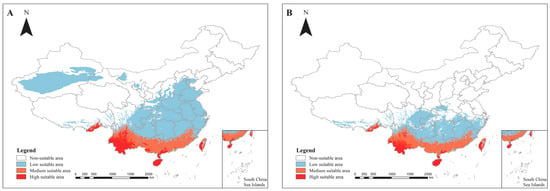
Figure 2.
Distribution of the suitability of genus Heliocopris in China under curent climatic conditions. (A) Prediction of the habitat suitability area for the Heliocopris dominus; (B) Prediction of the habitat suitability area for the Heliocopris bucephalus. The habitat suitability areas are represented by different colors: 0.6–0.9 is a high suitability area (red), 0.3–0.6 is a medium suitability area (orange), 0.1–0.3 is a low suitability area (blue), and 0–0.1 is an unsuitable area (white). Note: The map was produced using the standard base map with Review Number GS (2020) 4619, obtained from the Standard Map Service of the Ministry of Natural Resources (http://bzdt.ch.mnr.gov.cn). No alterations were made to the original base map.
3.4. Prediction of Suitable Habitats for Dung Beetles in China Under Future Climatic Conditions
Under the climate scenarios of RCP 2.6 and RCP 8.5 for 2050 and 2070, H. dominus is projected to experience significant northward expansion. In the 2050 RCP 8.5 scenario, the species will show sporadic increases and decreases in northern Tibet and western Chongqing (Figure 3B), with stable areas largely unchanged. For both the 2050 RCP 2.6 and RCP 8.5 scenarios, H. bucephalus is expected to expand predominantly into eastern Sichuan, Guizhou, central and northern Hunan, northern Jiangxi, and Zhejiang, with smaller population increases in Chongqing, Hubei, and Henan. These regions will display considerable variation in expansion and contraction (Figure 4A,B). In the 2070 RCP 2.6 scenario, the species is anticipated to expand into Xinjiang, northern Tibet, and northern Chongqing (Figure 4C).
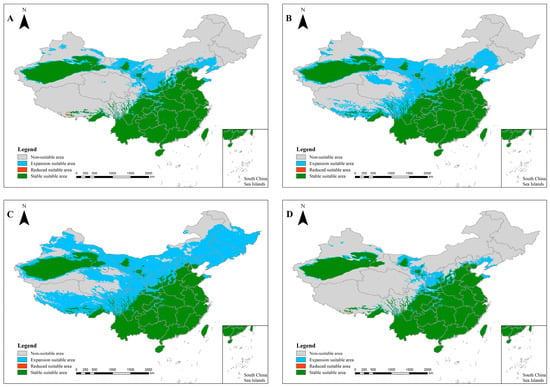
Figure 3.
Distribution of the suitability of Heliocopris dominus in China under future climatic conditions. (A): 2050 RCP 2.6 scenario; (B): 2050 RCP 8.5 scenario; (C): 2070 RCP 2.6 scenario; (D): 2070 RCP 8.5 scenario. Note: The map was produced using the standard base map with Review Number GS (2020) 4619, obtained from the Standard Map Service of the Ministry of Natural Resources (http://bzdt.ch.mnr.gov.cn). No alterations were made to the original base map.
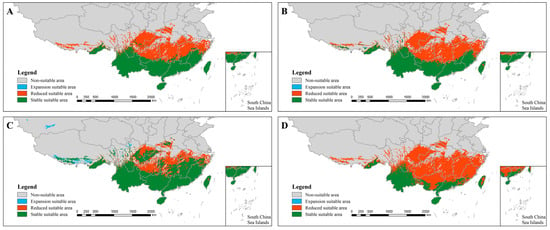
Figure 4.
Distribution of the suitability of Heliocopris bucephalus in China under future climatic conditions. (A): 2050 RCP 2.6 scenario; (B): 2050 RCP 8.5 scenario; (C): 2070 RCP 2.6 scenario; (D): 2070 RCP 8.5 scenario. Note: The map was produced using the standard base map with Review Number GS (2020) 4619, obtained from the Standard Map Service of the Ministry of Natural Resources (http://bzdt.ch.mnr.gov.cn). No alterations were made to the original base map.
4. Discussion and Conclusions
According to IUCN evaluations, H. dominus and H. bucephalus are classified as endangered in some specific subregions (criterion B2), indicating a significantly fragmented, diminishing, or variable global distribution. This classification highlights the significant conservation risks confronting their populations. This is probable because tropical rainforest remnants in these regions serve as the primary habitats for these species. Nonetheless, these ecosystems are progressively impacted by deforestation and agricultural expansion, resulting in habitat fragmentation, area reduction, and potential total loss [28]. Furthermore, associated dung beetle taxa depend significantly on the excrement of large herbivores, including elephants. Due to environmental degradation, the host animals have become endangered, resulting in food shortages and dwindling populations for the dependent dung beetles [29]. Conversely, Heliocopris species are famously challenging to breed in captivity, and wild populations face significant harvesting pressure due to their high demand in the beetle specimen market. Consequently, their population sizes vary and persist in diminishing.
This study revealed isothermality and temperature seasonality as the primary environmental variables affecting the potential distribution of Heliocopris species in China. The contribution rate was markedly superior to those of other factors. This research suggests that dung beetles have heightened sensitivity to temporal variations and stability in temperature, rather than to annual mean temperature or precipitation. The findings align with prior study on dung beetle ecology, demonstrating that these species, affected by host distribution and several ecological parameters, display a pronounced predilection for warm climatic conditions [30,31]. Elevated temperatures can enhance the beetles’ metabolism, augment foraging and reproductive effectiveness, whilst cold conditions frequently induce hibernation, diminishing survival rates [32]. Furthermore, dung beetle larvae mature within dung balls or soil, necessitating a specific humidity level to prevent desiccation. In humid circumstances, mammalian feces retains moisture, promoting eating and nesting, while in drier environments, feces desiccates quickly, diminishing its accessibility as a food resource [33]. Nonetheless, our model indicated that precipitation seasonality exerts minimal influence, although it imposes considerable limitations on the range of Heliocopris species. This may be ascribed to their inadequate adaptability to excessively damp or saturated circumstances [34]. Despite precipitation seasonality demonstrating the least relative influence weight, its extreme values can significantly restrict dispersion by impeding reproductive success due to soil moisture saturation. The distribution patterns of gigantic dung beetles, as a climate-sensitive indicator species, offer essential insights on the effects of climate change on decomposer ecosystems. In the context of impending global change, heightened variability in temperature may present a greater threat to their survival than the increase in average temperature.
Large mammals, including Asian elephants and wild buffaloes, which are significant suppliers of dung, are predominantly found in Yunnan (the habitat of Asian elephants) and previously in Hainan (the range of buffaloes) [35]. The anticipated favorable habitats for the two species roughly correspond with their documented distributions in China, particularly agreeing with verified occurrences in Yunnan and Hainan. Under prevailing climate circumstances, the anticipated habitat suitability for H. bucephalus and H. dominus is predominantly confined to tropical fringe zones, remaining comparatively low, with limited areas of high suitability primarily situated in southern Yunnan, Hainan, and Taiwan. This distribution pattern closely aligns with the tropical monsoon climate zone in China, suggesting that these species have specific hydrothermal requirements, inhabit a limited ecological niche, and are particularly susceptible to environmental alterations. This trend likely arises from their dependence on warm and humid tropical to subtropical climates. Low-latitude areas like Hainan and Taiwan provide consistent, warm, and humid conditions that fulfil the species’ biological needs [36]. Moreover, these areas preserve relatively unaltered primary or secondary forests, which offer crucial homes for dung beetles dependent on decomposing wood or the excrement of large herbivores. Conversely, human-induced deforestation in some locations has resulted in habitat deterioration and range contraction [37]. The model predicted minor areas of appropriate habitat in Tibet, perhaps due to climate similarities. Species distribution models predominantly rely on climatic variables, such as temperature and precipitation, potentially neglecting other critical limiting factors, including soil conditions and host availability. Certain river valley regions in southeastern Tibet may exhibit analogous annual temperature and precipitation patterns to those of southern China, resulting in erroneous suitability estimates. Alternatively, human-induced perturbations may be accountable. Although it is theoretically conceivable that H. dominus would endure in isolated, suitable habitats in Tibet if introduced, there is presently no empirical data to substantiate this claim [38,39]. Future climate projections for 2050 and 2070 (RCP 4.5 and RCP 8.5) indicate a rise and expansion of acceptable habitat areas for H. bucephalus and H. dominus, accompanied by a discernible trend of migration towards higher elevations or latitudes. H. dominus demonstrates a marked northward expansion trend, with its appropriate habitat anticipated to migrate considerably poleward under both warming scenarios between 2050 and 2070. Under the RCP8.5 scenario for 2050, fragmented new suitable regions arise in northern Tibet and western Chongqing, although the species’ core historical distribution, such as in Yunnan and Hainan, remains unchanged. This trend indicates that the species may react to climate change by a combination of range migration and in situ adaptation, with its potential range anticipated to grow considerably. Global warming may lead to low-elevation habitats surpassing the heat tolerance of beetles, necessitating population shifts to cooler, higher-altitude areas [40]. The expansion trajectory of H. bucephalus is more fragmented and extensive, with its suitable habitat predominantly expanding into heavily populated areas of central and eastern China, including eastern Sichuan, Guizhou, Hunan, Jiangxi, and Zhejiang. Significantly, even within the RCP2.6 scenario for 2070, there exists the possibility of a leapfrog spread into northwestern regions (e.g., Xinjiang and northern Tibet). The extensive and diverse changes in its distribution indicate that its future geographic range may face increased uncertainty. Likewise, the climate-induced population growth of H. bucephalus necessitates a thorough assessment grounded on the distinct movement patterns of its host species. Certain northern areas, including northern Sichuan and western Guizhou, may become newly viable as a result of winter warming [41]. Elevated precipitation in mountainous regions may enhance habitat humidity, but aridification in lowland areas could diminish habitat appropriateness [42]. Nonetheless, the migration rate of Heliocopris species may fail to match the tempo of climate change, leading to unoccupied yet climatically favorable regions [43].
Despite providing valuable preliminary insights, this study has several limitations. (1) The most significant constraint is the limited number of species occurrence records, which is inherent to the study of large-bodied and scarce dung beetles like Heliocopris in China. While we mitigated the risk of overfitting through rigorous variable selection, the small sample size may still affect the model’s accuracy and predictive power. Therefore, our models should be interpreted as identifying potential suitable habitats rather than providing definitive predictions. Future efforts should focus on expanding the occurrence database through continued field surveys and citizen science initiatives to refine these models. (2) Currently, research on the genetic diversity of species within the genus Heliocopris in China remains highly limited, with a lack of systematic population genomics or large-scale phylogeographic studies. Available information primarily stems from localized, taxonomically oriented investigations, which suggest that certain species may exhibit some degree of genetic differentiation at regional scales. However, due to constraints in sample size and geographical coverage, key characteristics-such as the distribution patterns of genetic diversity across China, historical population dynamics, and gene flow patterns-remain poorly elucidated. Indeed, dedicated studies on the genetic diversity of Heliocopris are notably scarce. Such research is essential to fill critical knowledge gaps that currently hinder a comprehensive assessment of the genus’ adaptive potential and response to environmental changes [44].
In summary, the preservation of the two Heliocopris species found in China encounters numerous obstacles, such as habitat degradation, human interference, and a lack of conservation awareness. To enhance the safeguarding of these endangered dung beetles and their closely related species, a thorough array of measures should be enacted. These encompass augmenting habitat protection to ensure secure and appropriate settings, intensifying regulatory supervision of critical regions and industries, rehabilitating degraded ecosystems, refining legislation, and constraining the administration of tourist destinations to mitigate human effect on wild Heliocopris populations. Moreover, the promotion of public education, community-based conservation initiatives, and active public engagement is essential to enhance awareness and cultivate a collaborative social environment that optimizes the effectiveness and accuracy of conservation activities [45]. Implementation plans may encompass: (1) the creation of new protected areas informed by the anticipated population dynamics of dung beetles under the climate scenarios for 2050 and 2070. Nature reserves must be established in critical habitats for endangered dung beetles, including forests, wetlands, and grasslands, with measures to restrict human activity and minimize wildlife disturbances; (2) the execution of ecological restoration projects in degraded habitats through methods such as afforestation, wetland restoration, and soil and water conservation, aimed at improving the ecological environment for endangered beetles and enhancing the survival prospects of their host groups, particularly large herbivores; (3) and the augmentation of environmental monitoring efforts through the application of advanced technologies such as artificial intelligence, satellite remote sensing, DNA analysis, and ecological monitoring, to continuously track the distribution, population, and ecological behaviors of wildlife [46], Artificial Intelligence (AI), satellite remote sensing, DNA technology, and ecological monitoring together constitute a comprehensive integrated monitoring system-a “sky-ground-space” network that operates across macro to micro scales. Satellite remote sensing provides large-scale surveillance of dynamic changes in suitable habitats such as forests and grasslands. Ecological monitoring techniques, including camera trapping and transect surveys, deliver ground-based verification and image datasets. These are analyzed through AI-driven deep learning and image recognition algorithms to automate species identification, population counts, and behavioral pattern analysis. In parallel, environmental DNA (eDNA) methods applied to soil and fecal samples enable detection of otherwise cryptic occurrences, offering particular utility in tracking rare and elusive species. By integrating these multi-source datasets through AI, the system facilitates real-time and precise assessment of species’ distributions, population trends, behavioral adaptations, and threatening factors, thereby delivering a robust scientific foundation for conservation decision-making.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100696/s1, Supplement File S1: Species occurrence point data and data sources; Supplement File S2: The 19 Bioclimatic Variable.
Author Contributions
Conceptualization, M.B. and Y.T.; methodology, M.B., Y.T. and X.W.; Data collection, N.Z. and M.L.; Data curation and spatial standardization, N.Z.; data analysis and processing, N.Z.; writing—original draft, N.Z.; writing—review and editing, Y.T., L.L., X.W. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key R&D Program of China (Grant No. 2022YFC2601200), National Natural Science Foundation of China (Grant No. 32200354), National Science & Technology Fundamental Resources Investigation Program of China (Grant No. 2022FY100500), the Survey of Wildlife Resources in Key Areas of Tibet (ZL202203601); and Rare and Endangered Species Survey Supervision and Industry Regulation Project.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We sincerely thank Yiping Luo from Ningxia University for her invaluable guidance on the procedures and analysis involved in IUCN assessments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lovejoy, T.E. Changes in biological diversity. In The Global 2000 Report to the President; U.S. Government Printing Office: Washington, DC, USA, 1980; Volume 2, pp. 327–332. [Google Scholar]
- Brondizio, E.S.; Díaz, S.; Settele, J.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Lu, Q.; Hao, X. Distribution records of Heliocopris bucephalus (Coleoptera: Scarabaeidae: Scarabaeinae), a national category II protected species, in Sichuan Province, China. J. Sichuan For. Sci. Technol. 2024, 45, 146–149. [Google Scholar]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Kapuka, A.; Dobor, L.; Hlásny, T. Climate change threatens the distribution of major woody species and ecosystem services provision in southern Africa. Sci. Total Environ. 2022, 850, 158006. [Google Scholar] [CrossRef]
- National People’s Congress. Wildlife Protection Law of the People’s Republic of China; China Legal Publishing House: Beijing, China, 2018. [Google Scholar]
- Xu, Z.; Hou, L.; Lei, Z.; Zhang, J.; Wu, Y.; Zhang, X.; Shi, T.; Zhang, S.; Du, C.; Pei, X. Study on suitability regionalization of Forsythia suspensa in Shanxi Province based on MaxEnt and ArcGIS. Chin. Wild Plant Resour. 2024, 43, 113–121. [Google Scholar]
- Zhang, R.; Ren, L.; Liu, X. Advances in insect diversity research. Biodivers. Sci. 2021, 29, 211–220. [Google Scholar]
- Goulson, D. Silent Earth: Averting the Insect Apocalypse; Jonathan Cape: London, UK, 2021. [Google Scholar]
- Ge, F.; Wu, K. Impacts of climate change on insect diversity and their adaptation mechanisms. Acta Entomol. Sin. 2021, 64, 589–602. [Google Scholar]
- Raine, E.H. Dung-Loving Dung Beetles. Explor. Nat. 2021, 4, 6. [Google Scholar]
- Qing, C.; Chen, S.; Deng, J.; Deng, X.; Li, Z.; Qiu, L. The effects of habitat amount, habitat quality, and meteorological factors on the species diversity of dung beetles in Chengdu. Ecol. Environ. Sci. 2024, 33, 708–719. [Google Scholar]
- Löbl, I.; Krell, F.T.; Král, D. Subfamily Scarabaeinae Latreille, 1802: Tribe Coprini Leach, 1815. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Volume 3: Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea, Byrrhoidea; Apollo Books: Stenstrup, Denmark, 2006; pp. 151–154. [Google Scholar]
- Král, D.; Bezděk, A. Subfamily Scarabaeinae Latreille, 1802: Tribe Coprini Leach, 1815. In Catalogue of Palaearctic Coleoptera, Rev. and Updated ed.; Löbl, I., Löbl, D., Eds.; Volume 3: Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea, Byrrhoidea; Brill: Leiden, The Netherlands, 2016; pp. 167–171. [Google Scholar]
- Sommer, D.; Sniegon, A.F.; Vais, M. First country records of the genus Heliocopris (Coleoptera: Scarabaeidae) from Chad and Mali. Acta Soc. Zool. Bohem. 2023, 86, 157–162. [Google Scholar]
- China Wildlife Conservation Association. Illustrated Handbook of National Key Protected Wildlife (Amphibians, Fishes, Insects and Others); Haixia Publishing House: Fuzhou, China, 2023. [Google Scholar]
- Larsen, T.H. Insect Collectors and Biodiversity: A Brief Review. Coleopt. Bull. 2012, 66, 309–312. [Google Scholar]
- Chen, K. The World of Dung Beetles; Owl Publishing House: Taipei, Taiwan, 2002. [Google Scholar]
- Arrow, G.J. The Fauna of British India. Coleoptera. Lamellicornia. Part III. Coprinae; Taylor & Francis: London, UK, 1931. [Google Scholar]
- Ma, Q. Smuggled Dung Beetles Seized in Yunnan. Xinhua News. 2016. Available online: https://hk.on.cc/cn/bkn/cnt/news/20160404/bkncn-20160404060927163-0404_05011_001.html (accessed on 4 April 2016).
- Yunnan Ruili Inspection and Quarantine Bureau Intercepts Batch of Live Insects Illegally Carried into Country. Sohu News. 2017. Available online: https://www.sohu.com/a/140212391_721669 (accessed on 12 May 2017).
- Kunming Customs. Border Biosafety Operation. Ningbo Customs District, P.R. China. 2023. Available online: http://ningbo.customs.gov.cn/kunming_customs/ztzl66/yqfkhlwlb74/gzcs/5023527/index.html (accessed on 13 April 2023).
- Feidian Video. Giant Dung Beetle Larvae Seized in Yunnan [Video]. Weibo, 12 April 2023. Available online: https://weibo.com/tv/show/1034:4889620538916900?from=old_pc_videoshow (accessed on 15 October 2024).
- National Forestry and Grassland Administration, Ministry of Agriculture and Rural Affairs. List of National Key Protected Wild Animals (Announcement No. 3, 2021) [Announcement]. 5 February 2021. Available online: http://www.forestry.gov.cn/main/5461/20210205/122418860831982.html (accessed on 15 October 2024).
- Wang, M.; Huang, D. Population genetic structure analysis of giant dung beetles in China based on mitochondrial genome data. Acta Zool. Sin. 2020, 41, 321–335. [Google Scholar]
- Bai, M.; Ahrens, D.; Yang, X.-K.; Ren, G.-D. Evolution of Scarabaeini (Coleoptera: Scarabaeidae: Scarabaeinae) and a discussion of its phylogenetic position based on morphological characters. Zootaxa 2008, 1752, 1–24. [Google Scholar]
- Zhang, H.; Shu, X.; Yu, X.; Zhu, R.; He, Q.; Zhao, M.; Yi, C. Prediction of suitable distribution area of Eupatorus gracilicornis Arrow in China based on MaxEnt model. J. Southwest Agric. 2024, 37, 1601–1610. [Google Scholar]
- Bishop, A.; Rangel, J.V.; Gaitan, J.J.; Rios, R.S.; Hortal, J.; Quesada, M.; Lughadha, E.N.; Barlow, J.; Louzada, J.; França, F.M.; et al. Megafauna declines reduce nutrient cycling by dung beetles. Global Ecol. Biogeogr. 2022, 31, 456–470. [Google Scholar]
- Garcia, R.; Arellano, L.; Basset, Y.; Davis, A.L.V.; Escobar, F.; Favila, M.E.; Halffter, G.; Krell, F.-T.; Lobo, J.M.; Scholtz, C.H.; et al. Climate change impacts on dung beetle distributions. Insect Conserv. Divers. 2020, 13, 321–335. [Google Scholar]
- Wang, Z.; Li, L.; Bai, M. Effects of temperature and humidity on the development of dung beetle larvae. J. Appl. Entomol. 2010, 47, 654–658. [Google Scholar]
- Yang, D.; Li, C.; Zhao, L. Dung beetle diversity and its relationship with environmental factors in the tropical region of Yunnan. Biodivers. Sci. 2007, 15, 285–291. [Google Scholar]
- Lobo, J.M.; Lumaret, J.P.; Jay-Robert, P. Habitat and climate preferences of Mediterranean dung beetles: Implications for conservation. J. Insect Conserv. 2011, 15, 75–85. [Google Scholar]
- Lumaret, J.P.; Galante, E.; Lumbreras, C.; Mena, J.; Bertrand, M.; Bernal, J.L.; Cooper, J.F.; Kadiri, N.; Crowe, D. Field effects of ivermectin residues on dung fauna. J. Appl. Ecol. 1993, 30, 428–436. [Google Scholar] [CrossRef]
- Halffter, G.; Edmonds, W.D. The Nesting Behavior of Dung Beetles; Instituto de Ecología: Mexico City, Mexico, 1982. [Google Scholar]
- Wang, L.H. Coevolution between dung beetles and large herbivores: Evidence from fecal resource use. Zool. Res. 2016, 37, 145–152. [Google Scholar]
- Zhang, W.; Li, Y.; Wang, X. Distribution patterns of endangered insects in China and their climatic drivers. Acta Ecol. Sin. 2018, 38, 4563–4572. [Google Scholar]
- Li, Z.Q.; Chen, H.; Liu, Y. Diversity of forest-dwelling insects in southern China: A case study of Hainan and Yunnan. Biodivers. Sci. 2020, 28, 234–245. [Google Scholar]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Wang, L. Impact of climate change on suitable habitats of dung beetles in Southwest China. Acta Ecol. Sin. 2023, 43, 1892–1903. [Google Scholar]
- Chen, Z.; Liu, Y.; Wu, X. Prediction of future distribution of an endangered insect using the MaxEnt model: A case study of Copris tripartitus. Biodivers. Sci. 2022, 30, 567–578. [Google Scholar]
- Huang, W.; Zhou, L.; Zhao, J. Potential impacts of climate change on the distribution of soil invertebrates in Yunnan, Southwest China. Chin. J. Appl. Ecol. 2021, 32, 2987–2996. [Google Scholar]
- Lin, F.; Zhen, X. Research progress on species migration lag under climate change. Acta Ecol. Sin. 2019, 38, 3124–3132. [Google Scholar]
- Tang, L.; Guo, X.; Bian, Z.; Gao, J.; Wang, L.; Li, Y.; Qiu, L. Comparative mitogenomic analysis of two dung beetles (Coleoptera, Scarabaeidae) and its implications for phylogenetic relationships. PeerJ 2023, 11, e14646. [Google Scholar]
- Yang, Y.; Zhang, D.; Xiao, J. Influencing factors and coping strategies of wildlife protection. Anhui Agric. Sci. Bull. 2025, 31, 53–56. [Google Scholar]
- Zhang, Y.; Mu, H.; Sun, Z. Intelligent technology promotes wildlife conservation. Science 2024, 76, 42–46. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).