Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Work
2.4. Terminology
2.4.1. Nomenclature
2.4.2. Occurrence, Species Richness, and Abundance
2.5. Statistical Analysis
3. Results
3.1. Species Diversity and Community Assemblage
3.2. Hydromedusa Spatial Distribution (October 2005–October 2006)
3.2.1. Spatial Distribution of the Species Richness and Nominal Species
3.2.2. Spatial Distribution of Abundance
3.3. Hydromedusa Temporal Distribution (November 2005–October 2006)
3.3.1. Temporal Distribution of the Species Richness and Nominal Species
3.3.2. Temporal Distribution of Abundance
4. Discussion
4.1. Species Diversity, Abundance, and Medusae Assemblage
4.2. Hydromedusae Spatial Distribution
4.2.1. Coastal Versus Offshore Stations
4.2.2. Between Sites, and Reef Versus Non-Reef Zones
4.3. Temporal Distribution
4.4. Hydroidolina vs. the Known Local Hydroid Fauna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Hydromedusa Species Taxonomic List
- Remarks:
- (1)
- Classification according to WoRMS [34] except for Turritopsis chevalense kept instead of Oceania armata (see remark below).
- (2)
- (3)
- For detailed data on distribution and ecology of species in the whole Indian Ocean, see [3,4,5]. For the oldest Indo-Pacific literature and synonymy, see Kramp [1,78], and for more exhaustive literature, Arai & Brinckman-Voss [79]. Important knowledge about medusae is found in numerous articles by Bouillon from the fauna of Papua New Guinea [47], not cited here.
- (4)
- Global distribution of the species given only for the first Indian Ocean reports.
- (5)
- Materiel examined from the eight stations of this study, number of specimens for each species in brackets, dates of sampling provided.
- (6)
- (*) before species names corresponds to additional samples collected by hand or photographed in situ (qualitative samples). See Section 2.2. for more details.
- Class HYDROZOA
- Subclass HYDROIDOLINA (ex-HYDROIDOMEDUSAE)
- Order ANTHOATHECATA Haeckel, 1879 [34]
- Suborder APLANULATA Collins et al., 2005 [34]
| Family Corymorphidae Allman, 1872 Genus Corymorpha M. Sars, 1835 |
| Corymorpha bigelowi (Maas, 1905) Appendix B: Plate 1C |
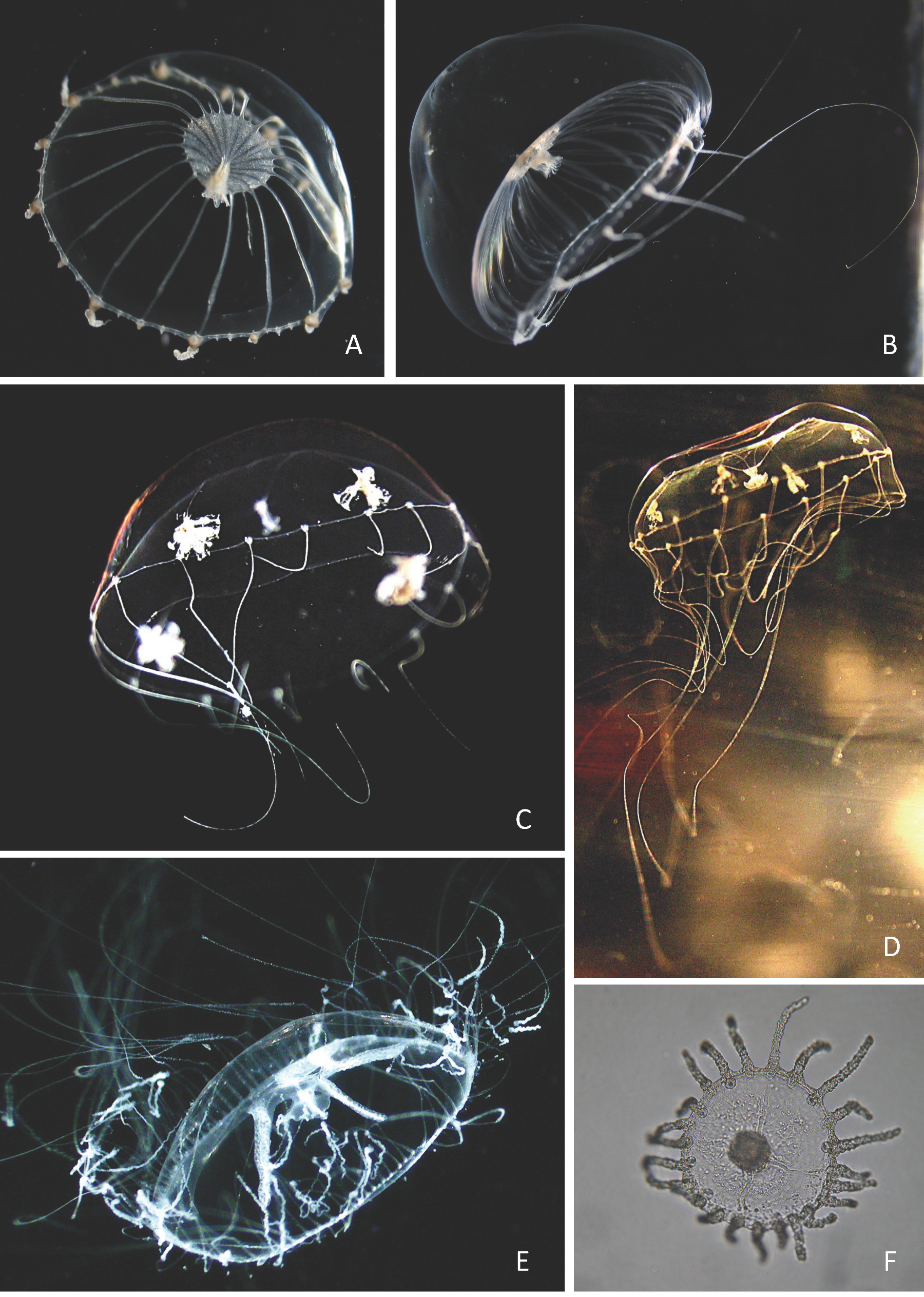
| References: [80]: 174, 198; [81]: 340, 370; [76]: 5; [1]: 14, Figure 26; [77]: 16, Figure 6; [3]: 2, 7, Figure 2, map 1; [4]: 273, Table 1; [17]: 32; [18]: 123, 128, 136; [5]: 33, 49, Table 2; [12]: 38; [19]: 46; [82]: 86; [13]: 82; [83]: 132; [84]: 171. Material examined: two immature medusae, ERMI-10 (1) 4 May 2006 and CAPL-10 (1) 1 May 2006. Seasonality: May. Remark: This species, attributed previously to the genus Euphysora [23], was recently included in the genus Corymorpha [85], as well as several other species checked previously in the Indian Ocean, E. abaxialis M. Sars, 1835, E. annulata Kramp, 1928, E. furcata Kramp, 1948, E. russelli Hamond, 1974 [1,15,17,19]. |
| Corymorpha forbesii (Mayer, 1894) |
| References: [6]: 50, plate 1 Figure 1 (Hybocodon); [81]: 368, 371; [1]: 13, Figure 22; [77]: 17, Figure 8; [3]: 3, 8, Figure 1, map 1; [4]: 274, Table 1; [16]: 119; [15]: 551; [8]: 84; [18]: 123, 128, 136; [5]: 33, 46, Figure 1, Table 2; [12]: 38; [10]: 151; [11]: 160; [19]: 47; [83]: 132; [84]: 171. Material examined: 26 specimens, juveniles and adults from all stations except ERMI-10, 1–2 specimens per sample. Seasonality: Obtained more or less regularly except in July in CAPL-10 (12). Remarks: (1) In the references above, species cited as Hybocodon or Vannuccia; (2) collected in the temperate and warm waters in all three oceans close to the water surface but rarely, except in the Caribbean [85] and in the western and northern Arabian Sea [16]. |
| Genus Euphysa Forbes, 1848 |
| Euphysa sp. |
| References: Three species reported in the Indian Ocean: E. aurata Forbes, 1848: [7]: 1; [77]: 16; [17]: 32; [8]: 84; [18]: 122, Table 3; [14]; 250; [10]: 151; [82]: 86; [83]: 132; [84]: 171; E. japonica (Maas, 1909): [10]: 151; E. tetrabrachia Bigelow, 1904: [78]: 38; [1]: 10, Figures 14–17; [10]: 151; [19]: 48; [83]: 132. Material examined: One juvenile specimen (SLEU-10, 4 November 2005). Seasonality: November. |
| Family Tubulariidae Goldfuss, 1818 Genus Hybocodon L. Agassiz, 1860 |
| Hybocodon sp. |
| References: after Hybocodon sp. [86]: 5, pl. 1, Figure 1, two species were checked in the Indian Ocean: H. atentaculatus Uchida, 1948: [10]: 151; [83]: 132; H. unicus (Browne, 1902): [6]: 50; [87]: 92, 94; [78]: 44; [8]: 84; [9]: 54; [10]: 151, [11]: 160; [82]: 86; [83]: 132; [84]: 171. Material examined: Two specimens from BOUC-50 (1), 16 December 2005, and CAPL-50 (1), 24 April 2006. Seasonality: April, December. Remarks: (1) Recently, hydroid specimens of this genus were found in Reunion Island for the first time in mesophotic depths [24]. |
| APLANULATA Incertae sedis [34] Genus Cnidocodon Bouillon, 1978 |
| Cnidocodon leopoldi Bouillon, 1978 Appendix B: Plate 1D |
| References: [88]: 33–37, Figures 1–3; [12]: 38; [14]: 250; [9]: 55; [10]: 151; [19]: 48; [82]: 86; [83]: 132; [84]: 170. Material examined: Two juvenile specimens from two stations, BOUC-10 (1) 13 February 2006 and ERMI-50 (1) 16 May 2006. Seasonality: February, May. Remarks: (1) hydroid polyp unknown; (2) Santhakumari [88] described juvenile and mature specimens from India and checked a few morphological differences from the original description of this species in the Pacific Ocean, of which more capitations on the tentacles, a character shared by the present specimens; (3) Bouillon [89] noticed an orange colour for the manubrium like in the medusae of this collection which differ from the brownish of the ones from India (maybe noticed on preserved material?). Distribution: Species described from Papua New Guinea ([89]: 255, Figure 4), firstly recorded in the Indian Ocean from Kerala and Karnataka coasts [88], then at Andaman and Nicobar [12], and it is also reported in the southeast of Africa [19] (data not reported in WoRMS [34]). The present collect (Reunion Island) confirms the presence of the species in the southwest of the Indian Ocean. |
| Suborder FILIFERA Kühn, 1913 Family Bougainvilliidae Lütken, 1850 Genus Bougainvillia Lesson, 1830 |
| Bougainvillia aurantiaca Bouillon, 1980 |
| References: First record. Material examined: One adult medusa with gonads, umbrella 1.1 mm in diameter, 0.7 mm in height, from one offshore station (ERMI-50, 16 March 2006). Seasonality: March. Remark: In Papua New Guinea, where it has been discovered, this species is seasonal, being present preferentially during the rainy season. The single specimen recorded here was also collected during the local rainy season. Distribution: Papua New Guinea, Mediterranean and New Zealand (after Schuchert [90] who reported two other doubtful references), and Reunion Island (this study). |
| Bougainvillia bitentaculata Uchida, 1925 |
| References: [12]: 38; [9]: 55; [10]: 151; [83]: 131; [84]: 170. Material examined: one immature medusa, umbrella diameter 1.4 mm and height 0.8 mm, from an offshore station (CAPL-50, 10 July 2006). Seasonality: July. Remark: Though not reported in the documented distribution of WoRMS [34], this species was already collected in the Indian Ocean (India), but this is the first report in the southwest. |
| Bougainvillia platygaster (Haeckel, 1879) |
| References: [91]: 9, Figure 1, pl. 3 Figures 1–6 (and pl. 7 Figures 3 and 4 for parasitic narcomedusa larvae); [76]: 18; [1]: 34, Figure 87; [77]: 19; [3]: 3, 11, Figure 5, map 2; [4]: 273, Table 1; [16]: 115, Figures 1, 2 and 5; [17]: 32, 33; [15]: 555; [8]: 84; [18]: 124; [5]: 34, 50, Figure 12, Table 2; [12]: 38; [9]: 55; [10]: 151. Material examined: One specimen (ERMI-50, 7 April 2006). Seasonality: April. Remark: It is the most frequent and abundant meroplanktonic species in the Indian Ocean together with Cytaeis tetrastyla, both being surface species, eurythermal and euryhaline with high oxygen requirements [16]. Biology: Asexual reproduction often reported [3,90,91]. |
| Bougainvillia principis (Steenstrup, 1850) |
| References: First record. Material examined: One immature medusa 2 mm high and wide from a coastal station (SLEU-10, 15 June 2006). Seasonality: June. Distribution: Artic (Indo-Pacific) and Papua New Guinea [92], Northern boreal [90], and Reunion Island (this study). |
| Family Cytaeididae L. Agassiz, 1862 Genus Cytaeis Eschsholtz, 1829 |
| Cytaeis nassa (Millard, 1959) |
| References: [93]: 307, Figure 3; [94]: 390, Figures 8 and 9, pl. 11; [95]: 31, pl. 5; [38]: 119, Figure 40; [18]: 123, 149; [69]: 127; [70]: app. 1. Material examined: Two juveniles from CAPL-50 (1) 27 March 2006 and CAPL-10 (1) 18 April 2006. Seasonality: March–April. Remark: This is the first sampling of the medusa from the plankton. Indeed, this species is known only from its polyp stage and juvenile medusae. Unfortunately, the present samplings concern juveniles as well, so the adult remains unknown. Distribution: Hydroid colonies were reported only from the Red Sea [38,96]) and the SWIO (Inhaca Island and Seychelles); Millard [38] cited also Madagascar and Mauritius, but without references; additional data are from Juan de Nova [69] and Glorieuses [70], two remote islands of the Mozambique Channel. In Reunion Island, colonies settled on shells of gastropods of the genus Nassa—like for other reports—were found on a sand beach back to a coral reef (Gravier-Bonnet unpublished data). |
| Cytaeis spp. (?Cytaeis tetrastyla, Appendix B: Plate 1A–B) |
| References: Two species reported; Cytaeis tetrastyla Eschscholtz, 1829: [97]: 135, pl. 1 Figure 1, pl. 4 Figure 12 (as C. herdmani, a synonym after Kramp [78]); [98]: 204, Figure 6; [80]: 178; [86]: 10, pl. 1, Figure 3; [6]: 53; [91]: 7; [81]: 340, 370; [78]: 63; [76]: 9–11; [1]: 26, Figure 64; [99]: 51, Figure 3, Table 1; [77]: 18; [3]: 3, 9, Figure 4; [4]: Table 1; [16]: 115, Figures 1, 3 and 4; [17]: 33; [8]: 85, Table 1; [18]: 123, 129; [5]: 33, 47, Figures 1 and 14, Table 2; [12]: 38, Figure 6; [14]: 250; [9]: 55, Figures 5 and 6; [10]: 151; [82]: 86; [13]: 82; [83]: 130; [84]: 170; Cytaeis vulgaris Agassiz & Mayer, 1899: [1]: 26, Figure 65; [8]: 84; [12]: 38; [14]: 250; [18]: 123; [10] 151. Moreover, Cytaeis spp. are reported by Buecher et al. ([19]: 39–40). Material examined: 26 medusae from all stations, except ERMI-10, 19 juveniles and immature medusae 0.5–1.1 mm wide, 7 adults with gonads 1.2–2.1 mm wide. Seasonality: Sporadic juveniles present from June to October, regularly but few, together adults in August–September, with one female in November. Remarks: (1) Cytaeis medusae are common in tropical plankton. In the Indian Ocean, Cytaeis tetrastyla is noted as the most frequent and abundant hydromedusa after the automedusae (refs. [5,12]; [16]: 115). (2) specimens of the present study can probably be identified as C. tetrastyla (Appendix B: Plate 1A–B). (3) C. tetrastyla is said to be circumglobally distributed in tropical and subtropical seas by Schuchert & Collins [100], but the hydroid polyp is still unknown. Biology: Presence of medusa buds on the manubrium of some adults in August–September. |
| Family Hydractiniidae L. Agassiz, 1862 Genus Podocorynoides Schuchert, 2007 |
| Podocorynoides minima (Trinci, 1903) |
| References: Under the name Hydractinia minima (Trinci, 1903): [2]: 7; [77]: 19; [3]: 3, 11; [5]: 34, 52. Material examined: Two specimens from two stations, ERMI-50 (1) 22 November 2005 and BOUC-50 (1) 17 October 2006. Seasonality: October–November. Remark: Cited also as Podocoryne/Podocoryna minima in the literature. |
| Genus Hydractinia Van Beneden, 1844 |
| Hydractinia sp. |
| Material examined: One specimen SLEU-10, 4 September 2006. Seasonality: September. |
| Hydractinia spp. |
| References: Hydractinia apicata (Kramp, 1959), also found under Podocoryne, and H. carnea (M. Sars, 1846) now accepted as Podocoryne and H. meteoris (Thiel, 1938) as Paracytaeis [34]: [78]: 67; [1]: 28; [2]: 5; [77]: 15; [3]: 3; [18]: 123, 129; [5]: 33–34; [19]: 40; H. ocellata (Agassiz & Mayer, 1902): [82]: 86; [83]: 131. Material examined: 21 specimens from all stations, except CAPL-10. Seasonality: January–March (austral summer). |
| Family Oceanidae Eschsholtz, 1829 Genus Turritopsis McCrady, 1857 |
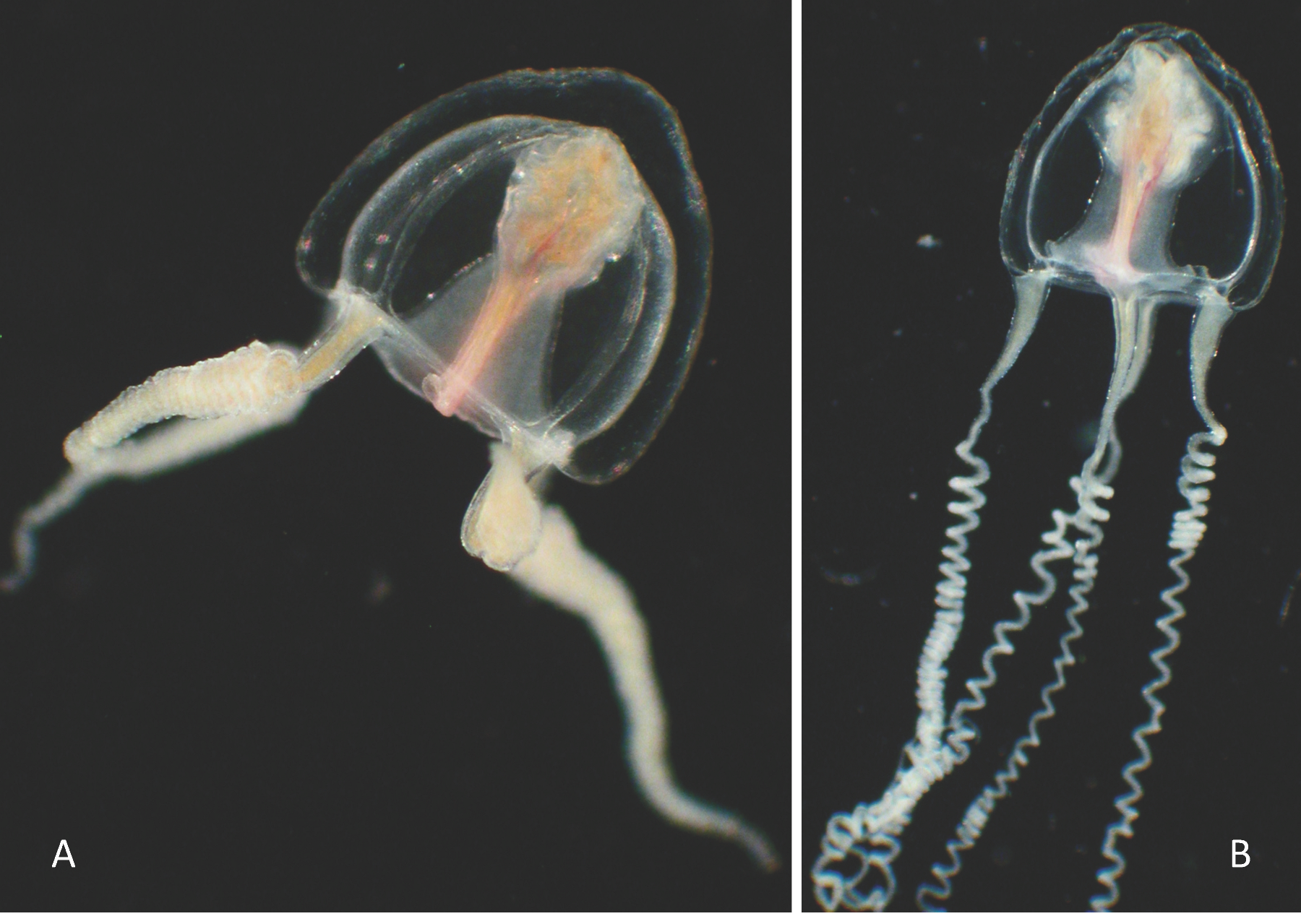
| Turritopsis chevalense (Thornely, 1904) Appendix B: Plate 1E–F |
| References: Under the name Turritopsis sp. or Turritopsis nutricula McCrady, 1857: [101]: 252 (as.); [98]: 209; [80]: 180; [87]: 92, 94; [78]: 66; [76]: 12; [1]: 27, Figure 66; [77]: 18; [3]: 5, 21; [17]: 33; [15]: 551; [18] 8a: 123; [5]: 33, 51. Material examined: Six mature medusae of diameter 0.5–1 mm, BOUC-10 (1) & BOUC-50 (3) 13 March 2006, CAPL-50 (2) 27 March 2006. Seasonality: March. Remarks: (1) Indian Ocean records of Turritopsis nutricula cited in references have been at a time attributed to T. chevalense Schuchert ([102]: 144), who said later it was a potentially valid species ([103]: 330). The species was not treated in Miglietta et al. [104]. Nowadays, however, T. chevalense is considered a synonym of Oceania armata Kölliker 1853 [34] after a more recent study [105] demonstrating the phylogenetic proximity of this medusa with large colonies attributed to T. chevalense from the Andaman Islands (Indian Ocean). In this study, however, when giving the list of synonyms for O. armata, Schuchert left an interrogative point before the original reference of T. chevalense [106]. The synonymy thus seems not yet confirmed. In addition, first, the colonies described by Thornely were from shallow waters in Ceylon (10–15 m), whereas those used for the phylogeny are from deep waters of the Andaman Sea (573 m) and, second, Bouillon ([18]: 123) checked together O. armata and T. nutricula in his list of the Seychelles hydromedusae. Moreover, we found colonies of two Turritopsis species in Reunion Island and in the Maldives ([72] and unpublished data). In Reunion Island, these species differ in the size of their nematocysts. Colonies of one of the species were until now sampled sterile, whereas the second released juvenile medusae, identical to those of this collection, provided highly vacuolated endodermal cells, the main character that separates Turritopsis from Oceania. In front of these considerations and presenting additional data, we have used here the name T. chevalense instead of O. armata, as we did previously for one sampled in shallow waters of Baa, a Maldives atoll [72]. It could match with specimens of the Turritopsis sp. from the Maldives, which is included in the “Turritopsis lata complex” of the phylogenetic tree of Miglietta et al. [61], where, once more, the T. chevalense of Andaman from deep waters matches with Oceania armata; (2) a second species, Turritopsis dohrni (Weisman, 1883) was reported in India [83], a species considered invasive [61]. Biology: Reverse development (medusa to polyp) occurred in the laboratory after the collect (S. Slobodov, unpublished observation), as already described for T. nutricula [107] and for T. dohrnii [108] but not obtained for Turritopsis sp.1 [61]. This process is thus not shared by all Turritopsis species. |
| Family Pandeidae Haeckel, 1879 Genus Amphinema Van Beneden, 1844 |
| Amphinema australis (Mayer, 1900) |
| References: [80]: 181 (Amphinema sp.); [19]: 41. Material examined: One medusa from one station (BOUC-10, 7 August 2006). Seasonality: August. Remark: This species is said to be “taxon inquirendum” [34]. Amphinema rugosum was reported in India [11,82]. |
| Amphinema dinema (Péron & Lesueur, 1810) |
| References: [80]: 181 (Amphinema sp.); [86]: 8, pl. 1, Figure 7; [6]: 52; [1]: 42, Figure 108; [3]: 3, 12, map 3; [18]: 124, 129; [5]: 34, 46; [83]: 132. Material examined: 27 juvenile medusae, from all stations, except CAPL-50. Seasonality: Present in plankton regularly in November–February with a max in December (10), sporadic in May–July. |
| Genus Leuckartiara Hartlaub, 1914 |
| Leuckartiara sp. |
| References: Five species recorded: L. annexa Kramp, 1957, L. gardineri Browne, 1916, L. hoepplii Hsu, 1928, L. octona (Fleming, 1823), L. zacae Bigelow, 1940. ([86]: 9, pl. 1, Figure 5; [109]; [6]: 52; [91]: 15, pl. 2 Figures 5 and 6; [81]: 342, 371; [78]: 103–106; [76]: 32–33; [1]: 45; [77]: 21; [3]: 13; [4]: Table 1; [17]: 34; [18]: 124; [5]: 34; [12]: 38; [14]: 250; [19]: 42–43; [13]: 82; [83]: 132; [84]: 171). Material examined: One juvenile specimen in January (CAPL-50, 23 January 2006). Seasonality: January. |
| Family Proboscidactylidae Hand & Hendrickson, 1950 Genus Proboscidactyla Brandt, 1835 |
| Proboscidactyla ornata (McCrady, 1859) |
| References: [110]: 727–728, pl. 54 Figures 1 and 2 (P. varians); [80]: 184 (P. tropica); [86]: 12, pl. 2, Figure 18; [6]: 57; [91]: pl. 3 Figure 7; [81]: 367, 371; [76]: 103; [1]: 108, Figure 290; [77]: 26, Figure 25; [3]: 5, 20, Figure 7; [4]: Table 1; [8]: 84; [18]: 127, 131; [5]: 36, 48, Figure 13, Table 2; [12]: 39; [14]: 251; [9]: 57; [10]: 151; [19]: 44; [13]: 82; [83]: 137; [84]: 172. Material examined: 22 juveniles and adults with medusa buds on manubrium from six stations (except coastal non-reef stations, CAPL-10 and BOUC-10). Seasonality: December–January (austral summer). Remarks: (1) P. varians Browne, 1905a described from the Maldives has been put in synonymy with P. ornata ([78]: 235; [34]); (2) P. tropica Browne, 1905a was reported by the author from the Amirantes islands [80]; (3) a third species described from Ceylon, P. minima Browne, 1905b ([97]: 136, pl. 2 Figure 3) is not mentioned in monographs [1,23,78] and is also absent from WoRMS [34]. Biology: Important asexual propagation by medusa-buds ([91]: 13). |
| Family Protiaridae Haeckel, 1879 Genus Halitiara Fewkes, 1882 |
| Halitiara formosa Fewkes, 1882 |
| References: [86]: 7, pl. 1, Figure 4; [78]: 102; [76]: 27; [1]: 40, Figure 102; [3]: 3, 13; [18]: 124; [5]: 34, 52. Material examined: Two anomalous juvenile medusae with five radial canals (BOUC-10, 17 July 2006). Seasonality: July. |
| Genus Protiara Haeckel, 1879 (cited as uncertain, nomen inquirendum, [34]) Protiara tetranema (Péron & Lesueur, 1810) Appendix B: Plate 2 A–B |
| References: First record. Remarks: Two species already checked in the Indian Ocean: P. haeckeli Hargitt, 1902: [2]: 9; [3]: 4, 14; [5]: 34; (2) P. tropica Bigelow, 1912: [76]: 24; [1]: 39, Figure 98; [2]: 9; [77]: 26; [3]: 4, 14; [4]: Table 1; [17]: 32, 34; [5]: 34; P. tropica Bigelow, 1912 is now transferred to the genus Pseudotiara Bouillon, 1980 [34]. Material examined: Three medusae in May, BOUC-10 (1) 8 May 2006, and CAPL-10 (2) 15 May 2006. Seasonality: May. Remarks: (1) hydroid unknown [23]; (2) as the genus, the species is considered uncertain [34] (taxon inquirendum); (3) one medusa was infested by a Phylliroe gastropode larva. |
| Suborder CAPITATA Kühn, 1913 Family Porpitidae Goldfuss, 1818 Genus Porpita Lamarck, 1801 |
| *Porpita porpita (Linnaeus, 1758) |
| References: [101]: 264 (Porpita lutkeana); [97]: 156 (Porpita sp.); [17]: 33; [15]: 551; [13]: 83; [83] 2020: 133; [84]: 171. Material examined: Three floating colonies noted in June–July 2006. Seasonality: Reported in Reunion Island on several occasions (Gravier-Bonnet, unpublished), either during the austral summer (December 2002, February 2003), the austral winter (August 2004, June–July 2006, this study), or the intermediate season (October 1987 and 2014, November 2000). Fertile colonies were checked in October 2014. Remark: According to WoRMS [34], the numerous Porpita species would nowadays be reduced to the two following, P. porpita and P. prunella Haeckel, 1888, the second belonging to the Pacific. |
| Genus Velella Lamarck, 1801 |
| Velella velella (Linnaeus, 1758) Appendix B: Plate 3A |
| References: [17]: 33; [18]: 122, 148; [13]: 83; 2 [83]: 133; [84]: 171. Material examined: One juvenile medusa (CAPL-10, 10 July 2006). Seasonality: July. Remark: In Reunion Island, floating colonies were checked being usually among those of Porpita in October 2014 (Gravier-Bonnet, unpublished) and on other occasions, not well documented. |
| Family Sphaerocorynidae Prévot, 1959 Genus Euphysilla Kramp, 1955 |
| Euphysilla pyramidata Kramp, 1955 |
| References: [1]: 17, Figure 33; [77]: 16, Figure 7; [3]: 2, 6, 21, Figure 1, map 1; [4]: Table 1; [17]: 32; [15]: 554, Figures 4–6; [18]: 122; [5]: 33, 47, Table 2; [19]: 48; [75]: 18, Figure 4. Material examined: One juvenile specimen (CAPL-50, 22 May 2006). Seasonality: May. Remarks: (1) Very recently, the hydroid polyp was described for the first time from the Maldives ([75]: 18, Figure 4), and it is similar to that of Sphaerocoryne; (2) E. pyramidata was suspected to be a complex of species [100]. |
| Family Teissieridae Bouillon, 1978 Genus Teissiera Bouillon, 1974 |
| Teissiera australe Bouillon, 1978 |
| References: [5]: 36, 47, Table 2; [12]: 39; [19]: 35. Material examined: One specimen (CAPL-10, 10 July 2006). Seasonality: July. |
| Teissiera sp. |
| References: In addition to T. australe, two other species were reported, T. milleporoides Bouillon, 1974, from the Seychelles ([18]: 123) and T. medusifera Bouillon, 1978, from SE of South Africa [19]. Material examined: Two juvenile specimens from two stations, BOUC-10 (28 February 2006) and ERMI-10 (2 March 2006). Seasonality: February–March. |
| Family Zancleidae Russell, 1953 Genus Halocoryne Hadzi, 1917 |
| Halocoryne frasca Boero, Bouillon & Gravili, 2000 |
| References: First record. Material examined: One adult specimen (SLEU-10, 12 October 2006). Seasonality: October. Distribution: Papua New Guinea and Malaysia [111], Reunion Island (Indian Ocean, present study). |
| Halocoryne spp. |
| References: A single species reported, Halocoryne orientalis (Browne, 1916): [80]: 171, 176, pl. 39 Figures 2 and 3; [1]: 21, Figure 48; [77]: 18; [3]: 9; [4]: Table 1; [18]: 123; [5]: 33, 45, Table 2; [12]: 39. Material examined: Three juvenile specimens from two stations, BOUC-50 (1) 28 October 2005, BOUC-50 (1) 13 January 2006, and CAPL-10 (1) 19 June 2006. Seasonality: January, June, October. |
| Genus Zanclea Gegenbaur, 1856 |
| *Zanclea medusopolypata Boero, Bouillon & Gravili, 2000 Appendix B: Plate 3B |
| References: First record. Material examined: One medusa collected in situ by hand (2007, precise date unknown). Seasonality: Unknown. Remark: This is only the fourth record of this rare species of which the polyp remains today unknown according to Schuchert & Collins [100] who described in the Gulf Stream another medusa budding polyps on its manubrium as well as Z. medusopolypata but with four tentacles instead of two. Distribution: Brazil ([112], Zanclea costata), Laing Island, Papua New Guinea [111], South China [113], recently found in Sagami Bay, Japan (Gaku Yamamoto pers. observation in 2023), and Reunion Island (Indian Ocean, present study). |
| Zanclea polymorpha Schuchert, 1996 |
| References: First record. Material examined: Six specimens from three stations, CAPL-50 (2) 22 May 2006, BOUC-50 (3) 22 May 2006, BOUC-10 (1) 12 June 2006. Seasonality: May–June. Remark: This is the first record of the species from the original description in the Pacific Ocean. Distribution: New Zealand [114] and Reunion Island (Indian Ocean, present study). |
| Zanclea ?sessilis (Gosse, 1853) |
| References: First record. Material examined: One specimen (CAPL-10, 8 December 2005). Seasonality: December. Distribution: NE Atlantic and Mediterranean [34] and Reunion Island (Indian Ocean, present study). |
| Zanclea spp. |
| References: In addition to Zanclea sp. ([87]: 92, 94), three species already reported Zanclea costata Gegenbaur, 1856: [98]: 199, Figure 3; [76]: 9; [1]: 21, Figure 47; [77]: 18; [3]: 5, 21; [16]: 120; [17]: 33; [18]: 123, 128; [5]: 33, 47, 51, Table 2; Zanclea dubia Kramp, 1959: [1]: 21, Figure 49; [2]: 5; [77]: 18; [3]: 5, 21; [4]: 279, Table 1; [5]: 33, 47, 51, Table 2; [12]: 36; [9]: 54; Zanclea implexa (Alder, 1856): [98]: 200, Figure 4. Material examined: 10 specimens from all stations, except BOUC-10, BOUC-50, and ERMI-50. Seasonality: July–December, plus one collected in May. Remarks: (1) Zanclea orientalis Browne, 1916 was reported ([78]: 55; [16]: 120) and was transferred to the genus Halocoryne [34]; (2) recently, the genus Zanclea was demonstrated to be polyphyletic [115]. |
| Genus Zanclella Boero & Hewitt, 1992 |
| Zanclella diabolica Boero, Bouillon & Gravili, 2000 Appendix B: Plate 3C–E |
| References: First record. Material examined: Nine juvenile specimens from two stations, CAPL-50 (8) 24 April 2006 and CAPL-10 (1) 1 May 2006. Seasonality: April–May. Remarks: (1) This is the second finding of the medusa of this rare species which is associated with a Bryozoan [44,111] like all other Zanclella species; (2) the polyp was reported only once from its discovery and with an question mark at Bunaken (Sulawesi) in July at 30–40 m depth [116]; (3) since, two Zanclella species related to Z. diabolica have been described [115] belonging, respectively, from the Maldives (sp. 1), in the Indian Ocean, and the Red Sea (sp. 2); (4) in Reunion Island, one more juvenile specimen was found two days after the collect on the bottom of a dish containing the remnants of a benthic sampling belonging from a mesophotic station (St Leu Bay, 97 m, 8/07/2022, Gravier-Bonnet, unpublished), implying probably the presence of benthic colonies nearby the station; (5) the juvenile medusae of the three species already known are very similar [115] for the two they described, the differences mainly based on the polyp phase. Juvenile medusae from Reunion Island look like the others, and the polyp phase not being found there until now, the name Z. diabolica was used, waiting for the capacity to give a proper comparison. Distribution: Papua New Guinea (Laing Island), Sulawesi (Bunaken), if confirmed, and Reunion Island (present study). |
| Family Zancleopsidae Bouillon, 1978 Genus Dicnida Bouillon, 1978 |
| *Dicnida rigida Bouillon, 1978 Appendix B, Plate 3F |
| References: First record of this monospecific genus. Material examined: One medusa, photographs by D. Caron (precise date unknown). Seasonality: Unknown. Remarks: (1) polyp unknown ([75]); (2) Wang et al. [117] revised the family Zancleopsidae that includes six Zancleopsis species and only one Dicnida (or two if the unidentified one reported from Japan would be a different species). Distribution: This rare medusa was described from Papua New Guinea ([89]: 257, Figures 5 and 6) and, later, one ?Dicnida sp. was reported from Japan [118,119,120,121]. |
| Subclass LEPTOTHECATA Haeckel, 1879 Family Aequoreidae Eschscholtz, 1829 Genus Aequorea Peron & Lesueur, 1810 |
| *Aequorea sp. Appendix B: Plate 4A–B |
| References: Ten species are already checked in the Indian Ocean; (1) Aequorea aequorea (Forskal, 1775): [76]: 95; [1]: 99, Figure 269a,b; [99]: 52, Table 1; [77]: 25; [3]: 5, 19–20, Figure 7, map 6; [4]: Table 1; [16]: 119; [17] 74: 32, 35; [18]: 126, 130; [5]: 35–36, Figure 13, Table 2; [12]: 39; [14]: 250; [9]: 57; [19]: 51–52; (2) A. australis Uchida, 1947: [76]: 96; [1]: 99, Figure 270; [77]: 25; [18]: 126, 130; [82]: 86; [83]: 132; (3) A. coerulescens (Brandt, 1838): [76]: 86; [1]: 98, Figure 266; [77]: 25; [3]: 5, 19–20, Figure 7, map 6; [4]: Table 1; [5]: 35–36, Figure 13, Table 2; (4) A. conica Browne, 1905: [97]: 145, pl. 1 Figure 2, pl. 2 Figures 16–18; [6]: 67; [87]: 92, 94; [81]: 360–362, 371; [76]: 99; [1]: 100, Figure 272; [99]: 52, Figure 3, Table 1; [3]: 5, 19–20, Figure 7, map 6; [4]: Table 1; [17]: 32, 35; [18]: 126; [5]: 35–36, Figure 13 Table 2; [12]: 39; [14]: 250; [9]: 57; [11]: 160; [19]: 51–52; [82]: 86; [83]: 132; [84]: 174; (5) A. globosa (Eschscholtz, 1829): [78]: 206; [76]: 98; [1]: 99, Figure 271; [3]: 5, 19–20, Figure 7, map 6; [18]: 126; [5]: 35–36, Figure 13 Table 2; [9]: 57; [19]: 51–52; (6) A. macrodactyla (Brandt, 1835): [80]: 171, 189; [91]: 38; [81]: 360–362, 371; [78]: 207; [76]: 87, Figure 8; [1]: 98, Figure 267; [99]: 52, Table 1; [77]: 25; [3]: 5, 19–20, Figure 7, map 6; [4]: Table 1; [17]: 32, 35; [15]: 551; [18]: 126; [5]: 35–36, Figure 13 Table 2; [12]: 39; [13]: 83; [84]: 174; (7) A. parva Browne, 1905: [97]: 146, pl. 2 Figures 5–7; [86]: 24, pl. 3, Figures 29–33; [87]: 92, 94; [78]: 207; [1]: 100, Figure 273; [77]: 26; [3]: 5, 19–20, Figure 7, map; [5]: 35–36, Figure 13 Table 2; [14]: 250; [9]: 57; (8) A. pensilis (Eschscholtz, 1829): [86] 2: 24; [6]: 67; [87]: 92, 93; [81]: 360–362, 371; [78]: 208; [76]: 92; [1]: 99, Figure 268; [3]: 5, 19–20, Figure 7, map 6; [4]: Table 1; [16]: 119; [15]: 551; [18]: 126; [5]: 35–36, Figure 13 Table 2; [9]: 57; [11]: 160; [19]: 51–52; [82]: 86; [83]: 132; (9) A. tenuis (Agassiz, 1862): [11]: 160; [82]: 86; [83]: 132; (10) A. vitrina Gosse, 1853: [83]: 132. Material examined: One medusa (28 September 2007). Seasonality: September. Remarks: (1) A. maldivensis Browne, 1905a ([110]: 732, pl. 56, Figures 4–12) is now classified as a synonym of A. macrodactyla [34]; (2) Aequorea genus is spelled “difficult” by Schuchert & Collins [100] who reported several species from the Gulf Stream. |
| Family Campanulariidae Johnston, 1836 Genus Pseudoclytia Mayer, 1900 |
| Pseudoclytia gardineri Browne, 1905 |
| References: Pseudoclytia gardineri: [110]: 731, pl. 55 Figures 1–3; [91]: 63; [76]: 64–65 (discussion on the validity of the genus as specimens are considered abnormal specimens of Phialidium); [17]: 34. Material examined: Four specimens: CAPL-10 (3) and CAPL-50 (1), 1 May 2006. Seasonality: May. Remarks: (1) The genus Pseudoclytia, characterized by the presence of five instead of four radial canals for Clytia (Phialidium), is now considered unaccepted [34] for the reason that the number of canals could be some abnormality, which is supported by recent studies on re-constitutive potential of such medusae (Sinigalia & Leclère, 10th Hydrozoan Workshop, 2023, oral communication). Mayer [122], however, created this genus because he found five radial canals in 70% of the Phialidium medusae he collected; in addition, the two well-preserved specimens [110], one male and one female, had five radial canals as well, being unlikely at the same time both abnormal forms. Pending more data, Pseudoclytia is thus provisionally considered here as valid; (2) however, a medusa belonging from the Indian Ocean with seven radial canals very regularly disposed (thus not regenerated) was described by Vanhöffen [98] as Phialidium heptactis: then is this number valid at a specific and/or generic level, the question remains open; (3) hydroid unknown [23]. |
| Genus Clytia Lamouroux, 1812 |
| Clytia hemisphaerica (Linnaeus, 1767) |
| References: [6]: 61; [87]: 92, 93; [78]: 67; [76]: 60; [1]: 76, Figure 201; [77]: 22, Figure 18; [3]: 4, 17; [17]: 34; [8]: 84; [18]: 125, 130; [5]: 35, 51, Table 2; [12]: 38, Figure 6; [14]: 250; [9]: 56, Figure 5; [10] 151, Figure 3; [11]: 160; [82]: 86; [83]: 136; [84]: 172. Material examined: One immature medusa (SLEU-10, 11 November 2005). Seasonality: November. Remark: Most of the references above and behind are under the genus name Phialidium. |
| Clytia mccradyi (Brooks, 1888) Appendix B: Plate 4C–D |
| References: [2]: 11, 27; [3]: 4, 17; [18]: 125; [5]: 35, 53; [12]: 38. Material examined: Three adult and three juvenile medusae from two stations, ERMI-10 (5) 3 February 2006, CAPL-10 (1) 3 April 2006. Seasonality: February, April. |
| Clytia spp. |
| References: Seven species of Clytia are reported in the Indian Ocean in addition to the two species cited before: (1) Clytia ambiga (Agassiz & Mayer, 1899): [77]: 22; (2) C. brunescens (Bigelow, 1904): [101]: 253, pl. 1 Figure 2; [78]: 165; [99]: 50, Table 1; [9]: 84; [10]: 151, [11]: 160; (3) C. globosa (Mayer, 1900): [78]: 166; [10]: 151, [11]: 160; [82]: 86; [83]: 136; (4) C. lomae (Torrey, 1909): [76]: 63; [17]: 34; (5) C. malayense (Kramp, 1961): [78]: 170; [76]: 62; [77]: 23; (6) C. rangiroae (Agassiz & Mayer, 1902): [76]: 61; [99]: 50, Table 1; [8]: 84; [18]: 125; (7) C. simplex (Browne, 1902): [78]: 171; [76]: 63; [3]: 4, 17; [15]: 551; [5]: 35, 53; [123]: 33, Figure 35; [12]: 38; [10]: 151, [11]: 160; [82]: 86; [83]: 136; Clytia sp.: [80]: 172, 187. In addition, Vanhöffen in 1911 reported from the Indian Ocean Phialidium phosphoricum Péron & Lesueur ([98]: 224) and described a new species Phialidium heptactis Vanhöffen, 1911 provided with seven radial canals ([98]: 225, Figure 15, pl. 22, Figure 11), two species not indexed in WoRMS [34]. Material examined: 170 medusae from all the stations. Seasonality: All year round. Remarks: (1) Clytia is the most speciose medusa-producing genus in Reunion Island, where nine species were separated recently in the deep mesophotic coastal area from benthic polyps [24]. This was also observed in Baa atoll (Maldives), where height species were reported [72]; (2) identification is problematic either for juveniles, very similar, and for adults for the lack of knowledge on life cycles. |
| Genus Obelia Péron & Lesueur, 1810 |
| Obelia sp. Appendix B: Plate 4F |
| References: [98]: 222 (O. geniculata); [86]: 15, pl. 2, Figure 22; [6]: 61; [91]: 33; [87]: 92, 94; [78]: 162; [76]: 59; [1]: 76, Figure 200; [99]: 50, Figures 1 and 3, Table 1; [77]: 22, Figure 17; [3]: 4, 16, Figure 1; [4]: 273, Table 1; [17]: 34; [8]: 84; [18]: 125, 130; [5]: 35, 53, Figure 1; [12]: 38; [9]: 55; [10]: 151; [11]: 160; [82]: 87 (Obelia sp. and O. dichotoma); [83]: 136. Material examined: Seven juvenile medusae from a single station, CAPL-10, (1) 27 March 2006, (1) 11 April 2006, (4) 7 August 2006, and (1) 17 October 2006. Seasonality: March, April, August, October. |
| Family Cirrholoveniidae Bouillon, 1984 Genus Cirrholovenia Kramp, 1959 |
| Cirrholovenia polynema Kramp, 1959 |
| References: [76]: 68; [1]: 79–80, Figure 213; [18]: 125, 130. Material examined: Four immature medusae (SLEU-10, 16 February 2006). Seasonality: February. |
| Cirrholovenia tetranema Kramp, 1959 |
| References: [1]: 79–80, Figure 214; [2]: 11, 28; [3]: 4, 17; [18]: 125, 130; [5]: 35, 53. Material examined: 11 immature medusae collected from three stations, ERMI-50 (1), SLEU-10 (8), SLEU-50 (2) on the same date (2 March 2006). Seasonality: March. Remark: The hydroid matching with this medusa is Egmundella amirantensis [95], a tiny species collected several times in Reunion Island, though sterile most of the time, and reported in the Maldives [72]. |
| Family Hebellidae Fraser, 1912 Genus Staurodiscus Haeckel, 1879 |
| Staurodiscus tetrastaurus Haeckel, 1879 |
| References: [86]: 14, pl. 2, Figures 14 and 19; [6]: 60; [87]: 92, 94; [78]: 148; [1]: 70, Figure 182; [3]: 4, 16; [18]: 129; [5]: 35, 53. Material examined: Two adult specimens from one station (ERMI-10, 2 March 2006). Seasonality: March. Remarks: (1) The study of Hebella species life cycles demonstrated that the medusae Staurodiscus belong to this genus [74,124] and that S. tetrastaurus is probably the medusa of Hebella scandens [125], but this is not evident on a recent phylogenetic tree [100]; (2) the widely distributed hydroid H. scandens is present in Reunion Island with a few other Hebella species; (3) the genus Toxorchis Haeckel, 1879 is considered now a synonym of Staurodiscus (refs. [23,34]; (4) six Staurodiscus and Toxorchis species were checked in the Indopacific ([1]: 69–71) including, for the SWIO, Staurodiscus polynema (Kramp, 1959) found around Madagascar ([76]: 56–57) and on the east coast of Africa ([19]: 61). |
| Family Laodiceidae Agassiz, 1862 Genus Laodicea Lesson, 1843 |
| Laodicea indica Browne, 1905 Appendix B: Plate 4E |
| References: [97]: 136, pl. 1 Figure 5, pl. 4 Figures 7–11 (Laodice indica); [6]: 59; [80]: 343, 370; [78]: 140; [76]: 52; [1]: 66, Figure 172; [77]: 22, Figure 17; [3]: 4, 15, Figur 6, Table 1, map 5; [4]: Table 1; [17]: 32, 34; [18]: 124, 129, 152, Figure 9; [5]: 34, 46, Figure 11, Table 2; [13]: 83. Material examined: Three adult specimens from three stations (CAPL-50, BOUC-10, BOUC-50) on the same date (3 April 2006). Seasonality: April. Remark: The life cycle of this species was described for the first time from Papua New Guinea [126]. |
| *Laodicea ?undulata (Forbes & Goodsir, 1853) |
| References: [78]: 147; [91]: 27; [19]: 60; [83]: 137. Material examined: Six juvenile specimens with one kept alive and having exhibited reverse development during rearing in the laboratory (S. Slobodov pers. obs.): 28 November 2005 (1 specimen), 29 November 2005 (2), 3 February 2006 (4). Seasonality: February, November. Remarks: (1) With some doubt, the specific name L. undulata is used here as it is the single species of this genus whose reverse development was described [127]; (2) in Papua New Guinea, this species is sometimes found with the parasite narcomedusa Cunina octonaria ([128]: 192). |
| Laodicea sp. |
| References: Four species reported in the Indian ocean; Laodicea fertilis (Lendenfeld, 1885): [1]: 66; [77]: 15, 22; L. fijiana Agassiz & Mayer, 1899: [1]: 66, Figure 171; [2]: 10; [3]: 15, map 5; [4]: Table 1; [16]: 119; [15]: 558, Figure 15; [5]: 34, Table 2; [19]: 59; L. marama Agassiz & Mayer, 1899: [1]: 67, Figure 173; L. pulchra Browne, 1902: [91]: 27, Table 2, pl. 4, Figure 7; [1]: 66, Figure 170; [19]: 60. Material examined: 12 juvenile specimens from all the stations, except BOUC-50 and SLEU-50. Seasonality: November, December, February, and June. Remark: If these juveniles are of the same species as the ones identified L. indica or L. ?undulata is unknown. |
| Family Lovenellidae Russell, 1953 Genus Eucheilota McCrady, 1859 |
| Eucheilota tropica Kramp, 1959 |
| References: [91]: 30; [78]: 175; [76]: 67; [1]: 80, Figure 222; [3]: 4, 17; [17]: 34; [8]: 84; [5]: 35, 53; [13]: 85; [84]: 173. Material examined: 16 juvenile specimens from one station (CAPL-10, 7 August 2006). Seasonality: August. Remark: More Eucheilota species have been already checked in the Indian Ocean, E. comata (Bigelow, 1909), E. maculata (Hartlaub, 1894), E. menoni (Kramp, 1959), E. paradoxica (Mayer, 1900) and E. ventricularis McCrady, 1859 (references: [86]: 17, pl. 1, Figure 9; [1]; [8]: 84–85, Table 1; [18]: 125, 130; [14]; [19]: 61; [82]: 86; [13]; [83]: 134; [84]: 173). |
| Family Phialellidae Russell, 1953 Genus Phialella Browne, 1902 |
| Phialella quadrata (Forbes, 1848) |
| References: [1]: 84, Figure 226; [76]: 65; [3]: 4, 17 (single doubtful specimen); [18]: 125, 130, Table 3; [5]: 35, 54 (single doubtful specimen); [83]: 135. Material examined: 11 medusae from three stations, ERMI-10 (1) and SLEU-50 (1), 22 November 2005; CAPL-10 (1), 22 May 2006; CAPL-10 (8), 10 July 2006. Seasonality: May, July, November. Remark: Phialella fragilis (Uchida, 1938) is also reported from India [11,82]. |
| Sub-Class TRACHYLINAE Haeckel, 1879 (ex- AUTOMEDUSAE) Order NARCOMEDUSAE Haeckel, 1879 Family Aeginidae Gegenbaur, 1857 Genus Aegina Eschscholtz, 1829 |
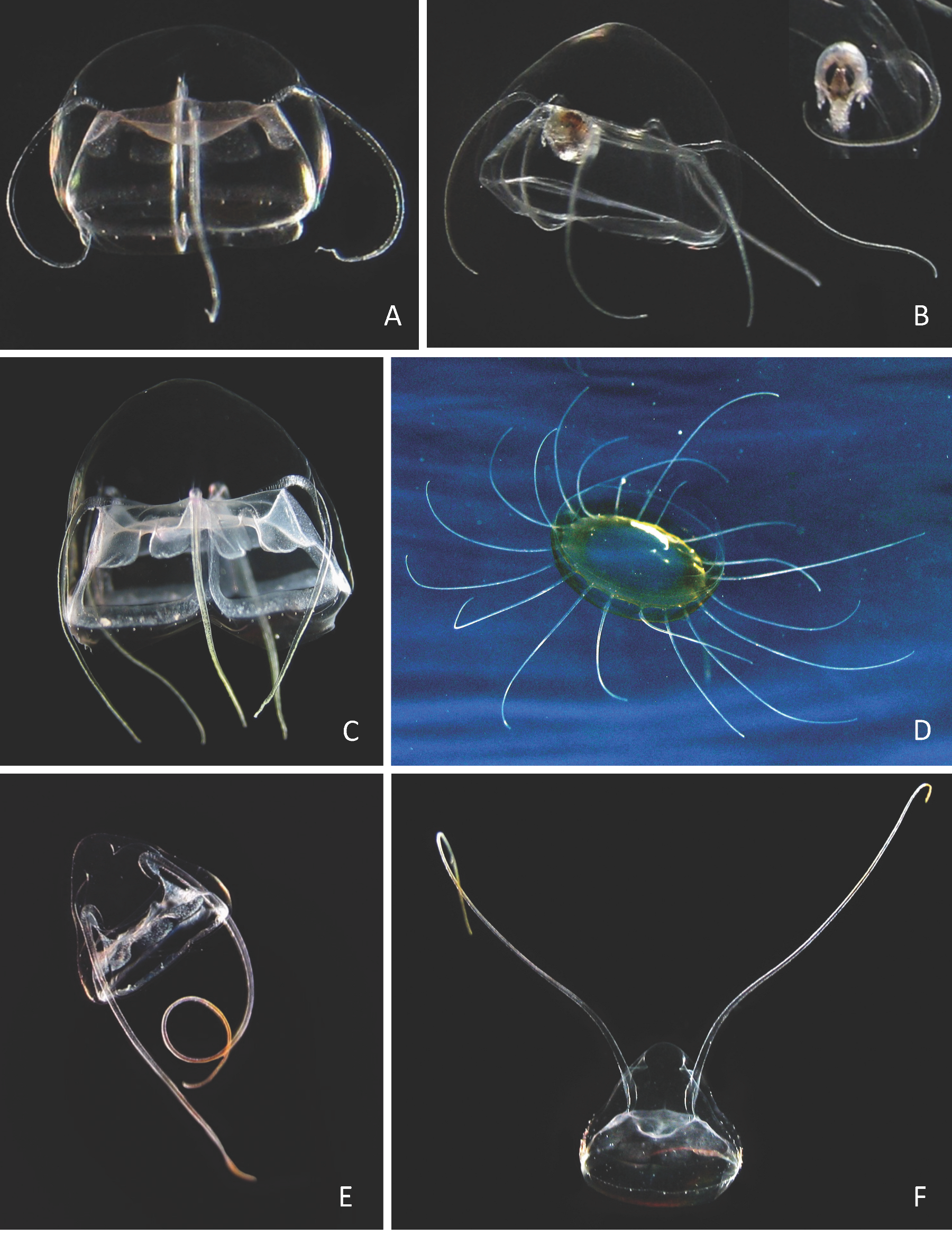
| Aegina citrea Eschscholtz, 1829 Appendix B: Plate 5A–C |
| References: [129]: 50; [80: 171, 200; [91]: 63; [81]: 370–371; [78]: 266; [76]: 139; [1]: 123, Figure 334; [3]: 6, 25, Figures 1 and 13, map 10; [4]: 275; [16]: 118–119; [8]: 84; [5]: 36, 44, Figure 8, Table 2; [12]: 39; [14]; 251; [9]: 60; [10]: 151; [19]: 30; [13]: 87; [84]: 180. Material examined: Three specimens, two juveniles about 1.5 mm in diameter and one immature 6 mm, from two offshore stations, BOUC-50 (2), 22 December 2005, and SLEU-50 (1), 19 December 2005. Seasonality: December. Remark: The study of specimens coming from Japanese seas and the Atlantic demonstrates that the genus Aegina and the family Aeginidae were polyphyletic [56] and to describe the new family Pseudaeginidae Lindsay, Bentlage & Collins, 2017, and the new genus Pseudaegina Lindsay, 2017, to include those with five to six tentacles, Aegina having only four. The photographs of the three specimens collected in Reunion Island revealed that they have different numbers of tentacles (4, 5, 6, see Plate 5 in Appendix B). It is thus possible that the larger one, with five tentacles, would have to be attributed to the genus Pseudaeginia, possibly P. pentanema (Kishinouye, 1910). However, the specimens of Pseudaeginia rhodina (Haeckel, 1879) belonging to the Atlantic Ocean [100] were separated into two forms, one with four and the other with five tentacles; thus, the number of tentacles could not be a secure character to separate the genera. |
| Genus Solmundella Haeckel, 1879 |
| Solmundella bitentaculata (Quoy & Gaimard, 1833) Appendix B: Plate 5E–F |
| References: [110]: 741, pl. 56 Figure 3; [97]: 153, pl. 4 Figures 1–6; [80]: 171–72, 201; [86]: 4, 28; [6]: 70; [91]: 64; [81]: 369, 371; [87]: 93; [78]: 270; [76]: 142; [1]: 124, Figure 338; [99]: 53, Figure 3, Table 1; [3]: 6, 26, Figures 1 and 4; [16]: 114; [77]: 28, Figures 31 and 42; [17]: 32, 36, Table 4; [15]: 552; [8]: 85, Table 1; [18]: 128, 131, 163; [5]: 36, 49, Figures 1 and 14, Table 2; [12]: 39, Figure 6; [14]: 251; [9]: 60, Figures 4–6; [10]: 151, Figures 3, 5 and 6; [11]: 160; [19]: 30; [82]: 87; [13]: 87; [83]: 142; [84]: 180. Material examined: 450 specimens of all stages, a common species from all stations. Seasonality: All year round. Remark: From phylogenetic data, clades found could represent three different species among the specimens studied from the Mediterranean and the Atlantic and Pacific Oceans [100]. Biology: In Papua New Guinea, the Narcomedusa Cunina peregrina was found as a parasite in this species ([128]: 193; [23]: 103, Figure 63). |
| Family Cuninidae Bigelow, 1913 Genus Cunina Eschscholtz, 1829 |
| Cunina sp. |
| References: Four Cunina species reported in the Indian Ocean, C. duplicata Maas, 1893, C. frugifera Kramp, 1948, C. octonaria McCrady, 1859, C. peregrina Bigelow, 1909 ([80]: 171–172, 179, 201; [91]: 81; [81]: 370–371; [76]: 151; [1]: 128, Figures 347–352; [99]: 53, Table 1; [2]: 5, 15, Figures 3 and 4, Table 1; [3]: 6, 27, Figures 15 and 16; [17]: 36; [15]: 552; [18]: 128, 131; [5]: 37, Figure 1, Table 2; [12]: 39; [14]: 51; [9]: 60; [10]: 151; [19]: 31; [82]: 86; [83]: 142; [84]: 180). One more, C. lativentris Gegenbaur, 1857, was considered a doubtful species [80] and is now a synonym of C. globosa Eschscholtz, 1829 [34]. Material examined: Two specimens of polypoid stage from manubrium and sub-umbrella of two Aglaura hemistoma medusae from two stations, BOUC-10 and SLEU-50 (18 September 2006). Seasonality: September. Remarks: (1) species present in the Red Sea ([77]: 28–29); (2) Browne ([80] collected an unidentified Cunina sp. near Mauritius Island. Biology: A specimen of Bougainvillia fulva Agassiz and Mayer, 1899, from the Chagos and Amirantes Islands was found “infested of Cunina buds at different stages of development…attached to the sub-umbrella close to the base of the stomach” [80]. Recently, such parasitism was also reported in Liriope tetraphylla in the South Atlantic [130]. For commensal and parasitic larvae of Narcomedusae, see Kramp ([91]: 89), and for the development stages of the parasitic Cunina species see [23,128]. |
| Family Solmarisidae Haeckel, 1879 Genus Pegantha Haeckel, 1879 |
| *Pegantha sp. Appendix B: Plate 5D |
| References: Five species were reported in the Indian Ocean, (1) Pegantha clara R.P. Bigelow, 1909: [91]: 73, text-Figures 12 and 13, pl. 6, Figure 3; [78]: 272; [76]: 147; [1]: 126, Figures 339 and 340; [3]: 6, 26; [16]: 119; [77]: 28, Figures 26 and 42; [8]: 60; [5]: 36, 45, Table 2; [12]: 39, 42; [14]: 251; [9]: 60; [10]: 151 (deep species); [19]: 33; (2) Pegantha laevis H.B. Bigelow, 1909: [91]: 69, text-Figure 11, pl. 6, Figure 2; [78]: 273; [76]: 147; [1]: 126, Figure 341; [3]: 6, 26; [77]: 28, Figure 33; [17]: 36; [5]: 37, 46, Table 2; [19]: 33; (3) Pegantha martagon Haeckel, 1879: [91]: 67, text-Figure 10, pl. 6, Figure 1; [78]: 273; [76]: 145; [1]: 127, Figure 342; [3]: 6, 26; [16]: 114, 119; [77]: 28, Figure 32; [17]: 36; [8]: 84; [5]: 37, 45, 49, Table 2; [19]: 33; (4) Pegantha rubiginosa (Kölliker, 1853): [91]: 76, pl. 6, Figure 4; [78]: 276; [1]: 127, Figures 343 and 344; [19]: 33; (5) Pegantha triloba Haeckel, 1879: [91]: 77; [78]: 276; [76]: 149; [1]: 127, Figure 345; [3]: 6, 27, Figure 14; [16]: 114, 119; [77]: 28, Figure 33; [17]: 36; [5]: 37, 49, Table 2; [131]: 616, Figure 1. Material examined: One medusa photographed (18 September 2006). Seasonality: September. Remark: Pegantha simplex Bigelow, 1904, described from the Maldives ([101]: 260, Pl. 5, Figures 19 and 20) is nowadays considered a synonym of P. martagon [34]. Biology: according to Vannucci & Navas [3], many of the Narcomedusae of the genera Cunina and Pegantha have larvae living as commensals or parasites in the gastral cavity of other Narco- or Trachymedusae (rarely in Anthomedusae). |
| Genus Solmaris Haeckel, 1879 |
| Solmaris sp. |
| References: Two species reported in the Indian Ocean, S. lenticulaHaeckel, 1879 and S. rhodoloma Brandt, 1838: [80]: 171–172, 199; [91]: 77; [87]: 93–94; [76]: 278; [1]: 128, Figure 346; [2]: 5, 15; [3]: 6, 27; [15]: 551; [18]: 128, Table 3; [5]: 37, 54; [131]: 618, Figure 3; [9]: 60; [10]: 151; [19]: 34. Material examined: Two specimens collected the same day (18 September 2006) from two stations, BOUC-10 and SLEU-50, the last being a medusa about 3 cm in diameter. Seasonality: September. Biology: Medusae sometimes found with parasitic juveniles of Cunina [131]. |
| Order LIMNOMEDUSAE Kramp, 1938 Family Geryoniidae Eschscholtz, 1829 Genus Geryonia Péron & Lesueur, 1810 |
| Geryonia proboscidalis (Forskål, 1775) Appendix B: Plate 6A–B |
| References: [132]: 84 (Geryonia from Zanzibar); [80]: 171–72, 199; [91]: 62; [78]: 237; [76]: 136; [1]: 122, Figure 332; [99]: 52, Table 1; [3]: 5, 20, map 7; [4]: Table 1; [77]: 26; [17]: 32, 35, Table 4; [15]: 551; [5]: 36, 48, Table 2; [12]: 39; [14]: 251; [9]: 58; [10]: 151; [11]: 160; [19]: 35; [82]: 86; [83]: 141; [84]: 179. Material examined: 12 medusae juveniles up to 5 mm in diameter and 2 immature adults, about 14 mm, captured in May and September, from all stations, by 1 per sample. Seasonality: All year round. Remarks: (1) Browne [80] collected near Mauritius Island this warm-water species present in all oceans; (2) in the articles cited, the genus Geryonia is included in the Trachymedusae Order, as it was usual; it has been recently transferred into the Limnomedusae after the building of phylogenetic trees [34,133]. |
| Genus Liriope Lesson, 1843 |
| Liriope tetraphylla (Chamisso & Eysenhardt, 1821) |
| References: [132]: 82; [101]: 258–260, pl. 4 Figures 15 and 16, pl. 5 Figures 17 and 18 (as L. indica & L. hemisphaericus); [110]: 738, pl. 54 Figure 3; [97]: 152; [80]: 171–72, 198; [86]: 4, 28; [6]: 70; [91]: 63; [81]: 368, 371; [87]: 93; [78]: 238; [76]: 129; [1]: 122, Figure 333; [99]: 52, Figure 3, Table 1; [3]: 5, 21, Figures 1 and 4; [4]: 273, Figure 1; [16]: 114; [77]: 26, Figures 26 and 42; [17]: 32, 35, Table 4; [15]: 551; [8]: 85, Table 1; [18]: 127, 131, 162; [5]: 36, 47, Figures 1 and 14, Table 2; [12]: 39, Figure 6; [14]; [9]: 58, Figures 2, 5 and 6; [10]: 151, Figures 3, 4 and 6; [11]: 160; [19]: 35; [82]: 87; [13]: 87; [83]: 141; [84]: 179. Material examined: 674 specimens of all stages, a common species from all stations. Seasonality: Present in all samples all year. Remarks: (1) This holoplanktonic species, present in all oceanic warm and temperate waters, was reported by Browne [80] near Mauritius Island which is the nearest island from Reunion; (2) in the articles cited, the genus Liriope is included in the Trachymedusae Order, as it was usual; it has been recently transferred into the Limnomedusae after the building of phylogenetic trees [34,133]; (3) worldwide cryptic lineages inside L. tetraphylla were put in evidence very recently by Miglietta & Pruski ([134]: Figures 1 and 3). Biology: (1) for direct development, see Bouillon et al. ([23]: 76, Figure 51); (2) many L. tetraphylla collected in Papua New Guinea were sampled with parasite Narcomedusa larvae, of which Cunina peregrina ([128]: 193; [135]: 251). |
| Order TRACHYMEDUSAE Haeckel, 1866 Family Rhopalonematidae Russell, 1953 Genus Aglaura Péron & Lesueur, 1810 |
| Aglaura hemistoma Péron & Lesueur, 1810 Appendix B: Plate 6E–F |
| References: [132]: 78; [110]: 739; [80]: 171–72, 196; [6]: 69; [81]: 368, 371; [87]: 93; [78]: 251; [76]: 127; [1]: 122, Figure 331; [99]: 52, Table 1; [3]: 5, Figures 1 and 22; [4]: 273, Figure 1; [16]: 114; [77]: 26, Figures 27 and 42; [17]: 32, 35, Table 4; [15]: 551; [8]: 85, Table 1; [18]: 127, 131, 162; [5]: 36, 47, Figure 1, Table 2; [12]: 39, Figures 3 and 6; [14]; [9]: 58, Figures 3 and 5; [10]: 151, Figures 2 and 3; [11]: 160; [19]: 35; [82]: 87; [83]: 141; [84]: 180. Material examined: 1546 specimens of all stages present in all samples, a common species. Seasonality: All year round. Remarks: (1) present in warm and temperate waters of all oceans, the most abundant species in some collections [3,4], predominantly epipelagic but found at all depths [76]; (2) species collected near Mauritius Island [80]. Biology: (1) life cycle in Bouillon et al. ([23]: 75, Figure 50); (2) 10% of the medusae found in Papua New Guinea had parasites Narcomedusa larvae, of which Cunina peregrina ([89]: 482; [128]: 193; [135]: 251). |
| Genus Amphogona Browne, 1905 |
| Amphogona apsteini (Vanhöffen, 1902) Appendix B: Plate 5C |
| References: [110]: 740, pl. 54 Figure 5, pl. 55 Figure 5, pl. 56 Figure 1, pl. 57 Figures 10–15; [80]: 171–72, 197; [78]: 252; [76]: 123, Figures 12 and 13; [1]: 118, Figure 319; [3]: 5, 22; [18]: 127, 131; [5]: 36, 129; [14]; [10]: 151; [82]: 86; [83]: 141. Material examined: Four specimens from three stations, CAPL-10 (2) 20 March 2006, CAPL-50 (1) 19 June 2006, and SLEU-10 (1) 12 October 2006. One adult female medusa had umbrella diameter about 5 mm, 40 tentacles, and 8 statocysts (CAPL-50, 19 June 2006). Seasonality: March, June, October. Biology: Cases of hermaphroditism occur occasionally in this species [80]. |
| Amphogona pusilla Hartlaub, 1909 |
| References: [78]: 253; [76]: 124; [1]: 119, Figure 320. Material examined: Seven specimens from three stations, CAPL-10 (1) 15 May 2006, ERMI-10 (1) 15 June 2006, BOUC-10 (1) 17 July 2006, and BOUC-10 (4) 31 July 2006. Two adult medusae with gonads captured the 31 June 2006 had umbrella diameter of 3.5 and 4 mm. Seasonality: May–June–July. |
| Amphogona sp. |
| References: In addition to A. apsteini and A. pusilla, a third species, A. apicata Kramp, 1957, is reported by: [78]: 252; [16]: 114, 119; [5]; [14]: 251; [9]: 58; [82]: 86; [83]: 141. Material examined: Five specimens from BOUC-50 (1) 22 May 2006, BOUC-10 (1) 29 May 2006, CAPL-10 (2) 05 June 2006, and BOUC-10 (1) 10 July 2006. Seasonality: May–June–July. |
| Genus Rhopalonema Gegenbaur, 1857 |
| Rhopalonema velatum Gegenbaur, 1857 Appendix B: Plate 6D |
| References: [132]: 59; [80]: 171–72, 193; [91]: 52, Table 1, pl. 7 Figures 5–7; [81]: 368, 371; [87]: 93–94; [78]: 262; [76]: 110; [1]: 114, Figure 307; [3]: 6, 24, Figures 1 and 4; [16]: 114; [77]: 27, Figures 29 and 42; [17]: 32, 35; [15]: 551; [8]: 84; [18]: 127, 131, 162; [5]: 36, 48, Figures 1 and 14, Table 2; [12]: 39, Figures 2, 4 and 6; [14]; [9]: 57, Figure 5; [10]: 151, Figure 3; [19]: 36; [82]: 87; [83]: 141; [84]: 180. Material examined: 114 specimens, juveniles and adults up to 2.7 mm in diameter, from all stations (from 1 to 13 specimens in a single sample). Seasonality: Present episodically in October–April and regularly from May until October. Remarks: (1) this epipelagic species is present in all inventories in warm and temperate waters around the world; (2) species collected near Mauritius Island [80]. Biology: Kramp ([91]: 89, pl. 7 Figures 5–7) reported parasitic larvae of Narcomedusae on R. velatum, and the parasite Cunina peregrina was found in Papua New Guinea ([128]: 193). |
| Genus Sminthea Gegenbaur, 1857 |
| Sminthea eurygaster Gegenbaur, 1857 |
| References: [80]: 194; [91]: 55; [81]: 368, 371; [78]: 264; [1]: 116, Figure 314; [3]: 6, 24, Figures 1 and 11, map 10; [17]: 32, 36; [5]: 36, 49, Figures 1 and 6, Table 2. Material examined: One specimen about 1 mm in diameter from offshore (CAPL-50, 22 May 2006). Seasonality: May. Remarks: (1) present in the Red Sea ([77]: 28, Figures 30 and 42); (2) for Kramp [1], this species is eurybathic and either epipelagic during the cold periods or mesopelagic during warming periods of the superficial waters. |
| Family Halicreatiidae Fewkes, 1886 Genus Haliscera Vanhöffen, 1902 |
| Haliscera conica Vanhöffen, 1902 |
| References: [132]: 72, pl. 9 Figure 6, pl. 11 Figure 33; [78]: 246; [131]: 622–623, Figure 11. Material examined: Two juvenile specimens, 0.5–0.8 mm in diameter, from two offshore stations, CAPL-50 (1) 4 September 2006 and SLEU-50 (1) 2 October 2006. Seasonality: September–October. Remark: (1) considered as a deep oceanic species, and collected even in the Antarctic, its presence in Reunion Island waters in September is probably a sign of rising cold waters from the south; (2) two other species, Haliscera bigelowi Kramp, 1947 and H. racovitzae (Maas, 1906), were reported, respectively, from the Arabian Sea [136] and the Indian Ocean Central System [5]. Distribution in the Indian Ocean: The original record is from New Amsterdam, a French island in the very south, the present one (Reunion Island, this study) being the first for a more centered area. |
Appendix B. Photo Plates of Hydromedusa Species
Plate 1—Anthomedusae. A–B: Cytaeis ?tetrastyla, A, juvenile with oral tentacles in extension, B, male and one medusa with medusa buds; C: Corymorpha (ex-Euphysora) bigelowi; D: Cnidocodon leopoldi; E–F: Turritopsis chevalense (profile and close-up views from below).
Plate 2—Anthomedusae. A–B: Protiara tetranema (juvenile with tentacles contracted and one male with tentacles in extension).
Plate 3—Anthomedusae. A: Velella velella; B: Zanclea medusopolypata (notice the cnidophores and the extension rate of the manubrium with multiple polyp buds); C–E: Zanclella diabolica (under compound microscope: medusa, cnidophores and one exumbrellar nematocyst); F: Dicnida rigida.
Plate 4—Leptomedusae. A–B: Aequorea sp. (from top and profile); C–D: Clytia mccrady (notice the bundles of gonothecae on the radial canals); E: Laodicea indica; F: Obelia sp.
Plate 5—Narcomedusae. A–C: Aegina citrea with (A) four, (B) five, and (C) six tentacles (detail of a crustacean parasite in B); D: Pegantha sp.; E–F: Solmundella bitentaculata.
Plate 6—Limno- and Trachymedusae. A–B: Geryonia proboscidalis (below and profile); C: Amphogona apsteni; D: Rhopalonema velatum; E–F: Aglaura hemistoma (profile and close-up views).
References
- Kramp, P.L. The Hydromedusae of the Pacific and Indian Oceans: Sections 2 and 3; Brill: Leiden, The Netherlands, 1968; p. 200. [Google Scholar]
- Navas-Pereira, D. New Records of Hydromedusae from the Indian Ocean. Contrib. Inst. Oceanogr. Univ. São Paulo 1971, 22, 1–33. [Google Scholar]
- Vannucci, M.; Navas, D. On the Ecology of Indian Ocean Hydromedusae. IOBC Handb. 1973, 5, 1–54. [Google Scholar]
- Vannucci, M.; Navas, D. Distribution of Hydromedusae in the Indian Ocean. In The Biology of the Indian Ocean; Zeitzschel, B., Gerlach, S.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1973; pp. 273–281. ISBN 978-3-642-65468-8. [Google Scholar]
- Navas-Pereira, D.; Vannucci, M. The Hydromedusae and Water Masses of the Indian Ocean. Bolletino Inst. Oceanogr. 1991, 39, 25–60. [Google Scholar] [CrossRef]
- Nair, K.K. Medusae of the Trivandrum Coast. Part I. Systematics. Bull. Cent. Res. Inst. Univ. Travancore Ser. C Nat. Sci. 1951, 20, 47–75. [Google Scholar]
- Vannucci, M.; Santhakumari, V. New Records of Hydromedusae from the Shelf Area off the Kerala Coast. J. Mar. Biol. Assoc. India 1969, 11, 40–43. [Google Scholar]
- Santhakumari, V. Distribution of Hydromedusae along the South West Coast of India. Mahasagar 1977, 10, 83–86. [Google Scholar]
- Santhakumari, V. Species Composition, Distribution and Abundance of Hydromedusae in the Exclusive Economic Zone of the East Coast of India. Publ. Seto Mar. Biol. Lab. 1997, 38, 53–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santhakumari, V.; Nair, V.R. Distribution of Hydromedusae from the Exclusive Economic Zone of the West and East Coasts of India. Indian J. Mar. Sci. 1999, 28, 150–157. [Google Scholar]
- Santhakumari, V.; Tiwari, L.R.; Nair, V.R. Species Composition, Abundance and Distribution of Hydromedusae from Dharamtar Estuarine System, Adjoining Bombay Harbour. Indian J. Mar. Sci. 1999, 28, 158–162. [Google Scholar]
- Santhakumari, V. A Study of Medusae from Andaman and Nicobar Waters. J. Zool. Soc. Kerala 1993, 3, 37–43. [Google Scholar]
- Chakraborthy, O.; Raghunathan, C. Cnidaria: Hydrozoans. In Faunal Diversity of Biogeographic Zones: Islands of India; Chandra, K., Raghunathan, C., Eds.; Zoological Survey of India: Kolkata, India, 2018; pp. 81–90. [Google Scholar]
- Santhakumari, V. Medusae from the Sea around Laccadive Group of Islands (7-18 N/69-76′′E). In Proceedings of the Second Workshop on Scientific Results of FORV Sagar Sampada, New Delhi, India, 11 June 1996; pp. 249–255, ISBN 81-900656-0-2. [Google Scholar]
- Hamond, R. Some Medusae and Other Hydrozoa from the Indian Ocean and the Bass Strait. J. Nat. Hist. 1974, 8, 549–561. [Google Scholar] [CrossRef]
- Vannucci, M.; Navas, D. Patterns of Distribution of Arabian Sea Hydromedusae. In Special Publication Dedicated to Dr. N. K. Panikkar; Marine Biological Association of India: Kochi, India, 1973; pp. 110–121. [Google Scholar]
- Schmidt, H.-E.; Klinker, J. Hydromedusae (Coelenterata) from the Indian Ocean. Meteor Forschungsergebnisse Reihe D Biol. 1974, 18, 29–38. [Google Scholar]
- Bouillon, J. Hydroméduses de l’archipel Des Séchelles et Du Mozambique. Rev. Zool. Afr. 1978, 92, 117–173. [Google Scholar]
- Buecher, E.; Goy, J.; Gibbons, M.J. Hydromedusae of the Agulhas Current. Afr. Invertebr. 2005, 46, 27–69. [Google Scholar]
- Conway, D.V.P.; White, R.G.; Hugues-Dit-Ciles, J.; Gallienne, C.P.; Robins, D.B. Guide to the Coastal and Surface Zooplankton of the South-Western Indian Ocean; Occasional Publication of the Marine Biological Association of the United Kingdom: Plymouth, UK, 2003; Available online: http://plymsea.ac.uk/279/ (accessed on 11 July 2025)ISSN 0260-2784.
- Bourmaud, C.A.F.; Leclère, L.; Mangion, P.; Michonneau, F.; Pennober, G. Biodiversity of Réunion Coral Reefs. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; Volume 1, pp. 202–207. [Google Scholar]
- Bourmaud, C.A.F.; Abouïdane, A.; Boissier, P.; Leclère, L.; Mirault, E.; Pennober, G. Coastal and Marine Biodiversity of La Réunion. Indian J. Mar. Sci. 2005, 34, 98–103. [Google Scholar]
- Bouillon, J.; Gravili, C.; Pagès, F.; Gili, J.M.; Boero, F. An Introduction to Hydrozoa; Mémoires du Museum National d’Histoire Naturelle de Paris: Paris, France, 2006; Volume 194, pp. 1–591. ISBN 978-2-85653-580-6. [Google Scholar]
- Gravier-Bonnet, N.; Boissin, É.; Hoarau, L.; Plantard, P.; Loisil, C.; Ory, D.; Mulochau, T.; Chabanet, P.; Adjeroud, M.; Bourmaud, C.; et al. Diving into the Lower Mesophotic Coral Ecosystems (65–93 m Depth) of Reunion Island (Southwestern Indian Ocean) Sheds Light on the Hidden Diversity of Hydroids (Cnidaria, Hydrozoa). Mar. Biodivers. 2022, 52, 38. [Google Scholar] [CrossRef]
- McDougall, I. The Geochronology and Evolution of the Young Volcanic Island of Réunion, Indian Ocean. Geochim. Cosmochim. Acta 1971, 35, 261–288. [Google Scholar] [CrossRef]
- Duncan, R.A.; Backman, J.; Peterson, L. The Shipboard Scientific Party Reunion Hotspot Activitity through Tertiary Time: Initial Results from the Ocean Drilling Program, Leg 115. J. Volcanol. Geotherm. Res. 1989, 36, 193–198. [Google Scholar] [CrossRef]
- Cordier, E. Dynamique Hydrosédimentaire du Récif Frangeant de l’Hermitage/La Saline (La Réunion): Processus Physiques et Flux Sédimentaires. Ph.D. Thesis, Université de la Réunion, St. Dennis, France, 2007. [Google Scholar]
- Farrow, G.E.; Brander, K.M. Tidal Studies on Aldabra. Philos. Trans. R. Soc. B Biol. Sci. 1997, 260, 93–121. [Google Scholar] [CrossRef]
- Cordier, E.; Lézé, J.; Join, J.-L. Natural Tidal Processes Modified by the Existence of Fringing Reef on La Reunion Island (Western Indian Ocean): Impact on the Relative Sea Level Variations. Cont. Shelf Res. 2013, 55, 119–128. [Google Scholar] [CrossRef]
- Conand, F.; Marsac, F.; Tessier, E.; Conand, C. A Ten-Year Period of Daily Sea Surface Temperature at a Coastal Station in Reunion Island, Indian Ocean (July 1993–April 2004): Patterns of Variability and Biological Responses. West. Indian Ocean. J. Mar. Sci. 2008, 6. [Google Scholar] [CrossRef]
- Naim, O.; Cuet, P.; Mangar, V. Coral Reefs of the Mascarene Archipelago. In Coral Reefs of the Indian Ocean: Their Ecology and Conservation; Oxford University Press: Oxford, UK, 2000; pp. 353–381. [Google Scholar]
- Battistini, R.; Bourrouilh, F.; Chevalier, J.-P.; Coudray, J.; Denizot, M.; Faure, G.; Fisher, J.-C.; Guilcher, A.; Harmelin-Vivien, M.; Jaubert, J.; et al. Eléments de Terminologie Récifale Indopacifique. Téthys Stn. Mar. d’Endoume 1975. [Google Scholar]
- Montaggioni, L.F.; Faure, G. Récifs Coralliens des Mascareignes (Ocean Indien); Centre Universitaire de La Réunion: St. Dennis, France, 1980; p. 151. ISSN 0337-100X. [Google Scholar]
- Schuchert, P.; Choong, H.; Galea, H.; Hoeksema, B.; Lindsay, D.; Mańko, M.; Pica, D. World Hydrozoa Database. 2025. Available online: https://www.marinespecies.org/hydrozoa (accessed on 22 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Morandini, A.C.; Straehler-Pohl, I.; Jarms, G. World Atlas of Jellyfish; Dölling und Galitz Verlag: Hamburg, Germany, 2019; p. 816. [Google Scholar]
- Miglietta, M.P.; Rossi, M.; Collin, R. Hydromedusa Blooms and Upwelling Events in the Bay of Panama, Tropical East Pacific. J. Plankton Res. 2008, 30, 783–793. [Google Scholar] [CrossRef]
- Millard, N.A.H. Monograph on the Hydroida of Southern Africa. Ann. S. Afr. Mus. 1975, 68, 1–513. [Google Scholar]
- Mammen, T.A. On a Collection of Hydroids from South India. I Suborder Athecata. J. Mar. Biol. Assoc. India 1963, 5, 27–61. [Google Scholar]
- Mammen, T.A. On a Collection of Hydroids from South India. II. Suborder Thecata (Excluding Family Plumulariidae). J. Mar. Biol. Assoc. India 1965, 7, 1–57. [Google Scholar]
- Mammen, T.A. On a Collection of Hydroids from South India. III Family Plumulariidae. J. Mar. Biol. Assoc. India 1967, 7, 291–324. [Google Scholar]
- Di Camillo, C.G.; Bavestrello, G.; Cerrano, C.; Gravili, C.; Piraino, S.; Puce, S.; Boero, F. Hydroids (Cnidaria, Hydrozoa): A Neglected Component of Animal Forests. In Marine Animal Forests; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 397–427. [Google Scholar] [CrossRef]
- Puce, S.; Calcinai, B.; Bavestrello, G.; Cerrano, C.; Gravili, C.; Boero, F. Hydrozoa (Cnidaria) Symbiotic with Porifera: A Review. Mar. Ecol. 2005, 26, 73–81. [Google Scholar] [CrossRef]
- Puce, S.; Bavestrello, G.; Di Camillo, C.; Boero, F. Symbiotic Relationships between Hydroids and Bryozoans. Symbiosis 2007, 44, 137–143. [Google Scholar]
- Puce, S.; Camillo, C.G.D.; Bavestrello, G. Hydroids Symbiotic with Octocorals from the Sulawesi Sea, Indonesia. J. Mar. Biol. Assoc. United Kingd. 2008, 88, 1643–1654. [Google Scholar] [CrossRef]
- Buecher, E.; Gibbons, M.J. Observations on the Diel Vertical Distribution of the Hydromedusae in the Southen Benguela. Afr. J. Mar. Sci. 2003, 25, 231–238. [Google Scholar] [CrossRef]
- Bouillon, J. Hydroméduses de La Mer de Bismarck (Papouasie, Nouvelle-Guinée). 2—Limnomedusa, Narcomedusa, Trachymedusa et Laingiomedusa (Sous-Classe Nov.). Cah. Biol. Mar. 1978, 19, 473–483. [Google Scholar]
- Buecher, E.; Gibbons, M.J. Temporal Persistence in the Vertical Structure of the Assemblage of Planktonic Medusae in the NW Mediterranean Sea. Mar. Ecol. Prog. Ser. 1999, 189, 105–115. [Google Scholar] [CrossRef]
- Goy, J. Hydromedusae of the Mediterranean Sea. In Developments in Hydrobiology, Proceedings of the Coelenterate Biology: Recent Research on Cnidaria and Ctenophora; Williams, R.B., Cornelius, P.F.S., Hughes, R.G., Robson, E.A., Eds.; Springer: Dordrecht, The Netherlands, 1991; Volume 66, pp. 351–354. [Google Scholar]
- Bernard, P.; Berline, L.; Gorsky, G. Long Term (1981–2008) Monitoring of the Jellyfish Pelagia noctiluca (Cnidaria, Scyphozoa) on Mediterranean Coasts (Principality of Monaco and French Riviera). J. Oceanogr. Res. Data 2011, 4, 1–10. [Google Scholar]
- Zampardi, S.; Licandro, P.; Milisenda, G.; Scannella, D.; Piraino, S.; Boero, F. Citizen Science Substantiates Jellyfish Occurrence in the Mediterranean Sea. Sci. Rep. 2025, 15, 21641. [Google Scholar] [CrossRef]
- Nair, S.R.S.; Nair, V.R.; Achuthankutty, C.T.; Madhupratap, M. Zooplankton Composition and Diversity in Western Bay of Bengal. J. Plankton Res. 1981, 3, 493–508. [Google Scholar] [CrossRef]
- González-Duarte, M.M.; Megina, C.; López-González, P.J.; Galil, B. Cnidarian Alien Species in Expansion. In The Cnidaria, Past, Present and Future: The World of Medusa and Her Sisters; Goffredo, S., Dubinsky, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 139–160. ISBN 978-3-319-31305-4. [Google Scholar]
- Bouillon, J.; Boero, F. Synopsis of the Families and Genera of the Hydromedusae of the World, with a List of the Worldwide Species. Thalass. Salentina 2000, 24, 296. [Google Scholar]
- Boosten, M.; Sant, C.; Da Silva, O.; Chaffron, S.; Guidi, L.; Leclère, L. Independent Transitions to Fully Planktonic Life Cycles Shaped the Global Distribution of Medusozoans in the Epipelagic Zone. Proc. Natl. Acad. Sci. USA 2025, 122, e2415979122. [Google Scholar] [CrossRef]
- Lindsay, D.J.; Grossmann, M.M.; Bentlage, B.; Collins, A.G.; Minemizu, R.; Hopcroft, R.R.; Miyake, H.; Hidaka-Umetsu, M.; Nishikawa, J. The Perils of Online Biogeographic Databases: A Case Study with the ‘Monospecific’ Genus Aegina (Cnidaria, Hydrozoa, Narcomedusae). Mar. Biol. Res. 2017, 13, 494–512. [Google Scholar] [CrossRef]
- Zheng, L.; He, J.; Lin, Y.; Cao, W.; Zhang, W. 16S rRNA Is a Better Choice than COI for DNA Barcoding Hydrozoans in the Coastal Waters of China. Acta Oceanol. Sin. 2014, 33, 55–76. [Google Scholar] [CrossRef]
- Moura, C.J.; Harris, D.J.; Cunha, M.R.; Rogers, A.D. DNA Barcoding Reveals Cryptic Diversity in Marine Hydroids (Cnidaria, Hydrozoa) from Coastal and Deep-Sea Environments. Zool. Scr. 2008, 37, 93–108. [Google Scholar] [CrossRef]
- Schuchert, P. High Genetic Diversity in the Hydroid Plumularia Setacea: A Multitude of Cryptic Species or Extensive Population Subdivision? Mol. Phylo. Evol. 2014, 76, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boissin, E.; Hoareau, T.B.; Postaire, B.; Gravier-Bonnet, N.; Bourmaud, C.A.-F. Cryptic Diversity, Low Connectivity and Suspected Human-Mediated Dispersal among 17 Widespread Indo-Pacific Hydroid Species of the South-Western Indian Ocean. J. Biogeogr. 2018, 45, 2104–2117. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Maggioni, D.; Matsumoto, Y. Phylogenetics and Species Delimitation of Two Hydrozoa (Phylum Cnidaria): Turritopsis (McCrady, 1857) and Pennaria (Goldfuss, 1820). Mar. Biodivers. 2019, 49, 1085–1100. [Google Scholar] [CrossRef]
- Bouillon, J.; Barnett, T.J. The Marine Fauna of New Zealand: Hydromedusae (Cnidaria: Hydrozoa); NIWA Biodiversity Memoir, 113; New Zealand Oceanographic Institute: Wellington, New Zealand, 1999; ISBN 0-478-08485-4. [Google Scholar]
- Gibbons, M.J.; Janson, L.A.; Ismail, A.; Samaai, T. Life Cycle Strategy, Species Richness and Distribution in Marine Hydrozoa (Cnidaria: Medusozoa). J. Biogeogr. 2010, 37, 441–448. [Google Scholar] [CrossRef]
- Puente-Tapia, F.A.; Espinosa-Fuentes, M. de la L.; Zavala-García, F.; Olguín-Jacobson, C.; Flores-Coto, C. Spatial Distribution of Medusae (Cnidaria) Assemblages in the Southern Gulf of Mexico (Dry Season). Community Ecol. 2022, 23, 137–162. [Google Scholar] [CrossRef]
- Nogueira Júnior, M.; da Costa, B.S.P.; Martinez, T.A.; Brandini, F.P.; Miyashita, L.K. Diversity of Gelatinous Zooplankton (Cnidaria, Ctenophora, Chaetognatha and Tunicata) from a Subtropical Estuarine System, Southeast Brazil. Mar. Biodivers. 2019, 49, 1283–1298. [Google Scholar] [CrossRef]
- Gili, J.-M.; Hughes, R.G. The Ecology of Marine Benthic Hydroids. Oceanogr. Mar. Biol. Annu. Rev. 1995, 33, 351–426. [Google Scholar]
- Genzano, G.N.; Mianzan, H.W.; Bouillon, J. Hydromedusae (Cnidaria: Hydrozoa) from the Temperate Southwestern Atlantic Ocean: A Review. Zootaxa 2008, 1750, 1–18. [Google Scholar] [CrossRef]
- Pruski, S.; Miglietta, M.P. Fluctuation and Diversity of Hydromedusae (Hydrozoa, Cnidaria) in a Highly Productive Region of the Gulf of Mexico Inferred from High Frequency Plankton Sampling. PeerJ 2019, 7, e7848. [Google Scholar] [CrossRef] [PubMed]
- Gravier-Bonnet, N.; Bourmaud, C.A.-F. Hydroids (Cnidaria, Hydrozoa) of Coral Reefs: Preliminary Results on Community Structure, Species Distribution and Reproductive Biology in Juan de Nova Island (Southwest Indian Ocean). West. Indian Ocean. J. Mar. Sci. 2006, 5, 123–132. [Google Scholar] [CrossRef]
- Gravier-Bonnet, N.; Bourmaud, C.A.-F. Hydroids (Cnidaria, Hydrozoa) of Coral Reefs: Preliminary Results on Community Structure, Species Distribution and Reproductive Biology in the Îles Glorieuses (Southwest Indian Ocean). In Proceedings of the 10th ICRS, Okinawa, Japan, 28 June–2 July 2004; pp. 188–196. [Google Scholar]
- Gravier-Bonnet, N.; Bourmaud, C.A.-F. Trois mille Hydraires sous les mers. Univers Maoré 2007, 7, 30–35. [Google Scholar]
- Gravier-Bonnet, N.; Bourmaud, C.A. Hydroids (Cnidaria, Hydrozoa) of Baa Atoll (Indian Ocean, Maldives Archipelago). Atoll Res. Bull. 2012, 590, 85–123. [Google Scholar]
- Migotto, A.E.; Cabral, A.S. Lafoeina amirantensis (Cnidaria: Hydrozoa, Campanulinoidea), the Hydroid Stage of the Medusa Cirrholovenia tetranema (Cnidaria: Hydrozoa, Lovenelloidea). Zootaxa 2005, 919, 1–16. [Google Scholar] [CrossRef]
- De Andrade, L.P.; Migotto, A.E. Is There a Link between Hebella Hydroids (Hydrozoa, Lafoeidae) and Staurodiscus Medusae (Hydrozoa, Laodiceidae)? In Proceedings of the VII Colacmar Congresso Latino-Americano sobre Ciencias do Mar; 1997; Volume 1, pp. 35–36. Available online: https://www.researchgate.net/profile/Alvaro-Migotto/publication/281712393_IS_THERE_A_LINK_BETWEEN_HEBELLA_HYDROIDS_HYDROZOA_LAFOEIDAE_AND_STAURODISCUS_MEDUSAE_HYDROZOA_LAODICEIDAE/links/55f5755708ae6a34f6624f8b/IS-THERE-A-LINK-BETWEEN-HEBELLA-HYDROIDS-HYDROZOA-LAFOEIDAE-AND-STAURODISCUS-MEDUSAE-HYDROZOA-LAODICEIDAE.pdf (accessed on 28 September 2025).
- Maggioni, D.; Schuchert, P.; Arrigoni, R.; Hoeksema, B.W.; Huang, D.; Strona, G.; Seveso, D.; Berumen, M.L.; Montalbetti, E.; Collins, R.; et al. Integrative Systematics Illuminates the Relationships in Two Sponge-Associated Hydrozoan Families (Capitata: Sphaerocorynidae and Zancleopsidae). Contrib. Zool. 2021, 90, 487–525. [Google Scholar] [CrossRef]
- Kramp, P.L. The Hydromedusae of the Pacific and Indian Oceans; Dana Report 63; Andr. Fred. Host & Son: Copenhagen, Denmark, 1965; pp. 1–161. [Google Scholar]
- Schmidt, H.-E. Die Hydromedusen (Hydrozoa: Coelenterata) des Roten Meeres und seiner angrenzenden Gebiete. Meteor Forschungsergebnisse Reihe D Biol. 1973, 15, 1–35. [Google Scholar]
- Kramp, P.L. Synopsis of the Medusae of the World. J. Mar. Biol. Assoc. United Kingd. 1961, 40, 7–382. [Google Scholar] [CrossRef]
- Arai, M.N.; Brinckmann-Voss, A. Hydromedusae of British Columbia and Puget Sound. Can. Bull. Fish. Aquac. Sci. 1980, 204, 1–192. [Google Scholar]
- Browne, E.T. Medusae from the Indian Ocean. Trans. Linn. Soc. Lond. 2nd Ser. Zool. 1916, 17, 169–210. [Google Scholar] [CrossRef]
- Kramp, P.L. Hydromedusae in the Indian Museum. Rec. Indian Mus. 1958, 53, 339–376. [Google Scholar] [CrossRef]
- Nagale, P.; Bhave, V.; Apte, D. A Review of Hydrozoa from Maharashtra. In Proceedings of the National Seminar on Biodiversity & Conservation of Coastal and Marine Ecosystems of India, Mumbai, India, 13–15 September 2012; pp. 84–88, ISBN 978-81-925489-0-6. [Google Scholar]
- Chakraborthy, O.; Raghunathan, C. Notes of seven Aglaopheniids (Cnidaria: Hydrozoa: Aglaopheniidae) from Andaman and Nicobar Islands with three new records to India. Zootaxa 2020, 4790, 291–317. [Google Scholar] [CrossRef]
- Chakraborthy, O.; Raghunathan, C. Cnidaria: Hydrozoa. In Deep Sea Faunal Diversity in India; Chandra, K., Raghunathan, C., Pillai, H., Purushothaman, J., Mondal, T., Eds.; Zoological Survey of India: Kolkata, India, 2021; pp. 167–185. [Google Scholar]
- Schuchert, P. The European Athecate Hydroids and Their Medusae (Hydrozoa, Cnidaria): Capitata Part 2. Rev. Suisse De Zool. 2010, 117, 337–555. [Google Scholar] [CrossRef]
- Menon, M.G.K. The Hydromedusae of Madras; Bulletin of the Madras Government Museum: Chennai, India, 1932; pp. 1–32. [Google Scholar]
- Ganapati, P.N.; Nagabhushanam, R. Seasonal Distribution of the Hydromedusae off the Visakhapatnam Coast. Andhra Univ. Mem. Oceanogr. 1958, 2, 91–99. [Google Scholar]
- Santhakumari, V. A New Record of Cnidocodon leopoldi Bouillon (Anthomedusae, Hydrozoa) from Indian Ocean with a Note on Its Variation. Bull. Dep. Mar. Sci. Univ. Cochin 1986, 14, 33–37. [Google Scholar]
- Bouillon, J. Hydroméduses de La Mer de Bismarck (Papouasie, Nouvelle-Guinée). Partie 1: Anthomedusae Capitata (Hydrozoa-Cnidaria). Cah. De Biol. Mar. 1978, 19, 249–297. [Google Scholar]
- Schuchert, P. The European Athecate Hydroids and Their Medusae (Hydrozoa, Cnidaria): Filifera Part 2. Rev. Suisse De Zool. 2007, 114, 195–396. [Google Scholar] [CrossRef]
- Kramp, P.L. The Hydromedusae from the Discovery Collections. Discovery Report 1957, 29, 1–128. [Google Scholar]
- Bouillon, J.; Claereboudt, M.; Seghers, G. Hydromeduses de La Baie de Hansa (Mer de Bismarck; Papouasie Nouvelle-Guinee) Repartition, Condition Climatiques et Hydrologiques. Indo-Malay. Zool. 1986, 3, 105–152. [Google Scholar]
- Millard, N.A.H. Hydrozoa from Ships’ Hulls and Experimental Plates in Cape Town Docks. Ann. S. Afr. Mus. 1959, 45, 239–256. [Google Scholar]
- Rees, W.J. Hydroids of the Family Cytaeidae, L. Agassiz, 1862. Bull. Br. Mus. Nat. Hist. 1962, 8, 379–400. [Google Scholar]
- Millard, N.A.H.; Bouillon, J. Hydroids from the Seychelles (Coelenterata). Ann. du Musée R. d’Afrique Cenrale Tervuren 1973, 206, 1–106. [Google Scholar]
- Vervoort, W. The Hydroida and Chondrophora of the Israel South Red Sea Expedition, 1962. Rep. Isr. South Red Sea Exped. 1967, 25, 18–54. [Google Scholar]
- Browne, E.T. Report on the Medusae (Hydromedusae, Scyphomedusae and Ctenophora) Collected by Professor Herdman, at Ceylon, in 1902. Ceylon Pearl Oyster Fish. Suppl. Rep. 1905, 27, 1–166. [Google Scholar]
- Vanhöffen, E. Die Anthomedusen Und Leptomedusen Der Deutschen Tiefsee-Expedition, 1898–1899. Wiss. Ergeb. Valdivia 1911, 19, 191–233. [Google Scholar]
- Vannucci, M.; Santhakumari, V.; dos Santos, E.P. The Ecology of Hydromedusae from the Cochin Area. Mar. Biol. 1970, 7, 49–58. [Google Scholar] [CrossRef]
- Schuchert, P.; Collins, R. Hydromedusae Observed during Night Dives in the Gulf Stream. Rev. Suisse Zool. 2021, 128, 237–356. [Google Scholar] [CrossRef]
- Bigelow, H.B. Medusae from the Maldive Islands. Bull. Mus. Comp. Zool 1904, 39, 245–269. [Google Scholar]
- Schuchert, P. Hydroids (Cnidaria, Hydrozoa) of the Danish Expedition to the Kei Islands. Steenstrupia 2003, 27, 137–256. [Google Scholar]
- Schuchert, P. Revision of the European Athecate Hydroids and Their Medusae (Hydrozoa, Cnidaria): Families Oceanidae and Pachycordylidae. Rev. Suisse Zool. 2004, 111, 315–369. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Piraino, S.; Kubota, S.; Schuchert, P. Species in the Genus Turritopsis (Cnidaria, Hydrozoa): A Molecular Evaluation. J. Zool. Syst. Evol. Res. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- Schuchert, P. The Polyps of Oceania armata Identified by DNA Barcoding (Cnidaria, Hydrozoa). Zootaxa 2016, 4175, 539–555. [Google Scholar] [CrossRef]
- Thornely, L.R. Report on the Hydroida Collected by Professor Herdman, at Ceylon, in 1902. Ceylon Pearl Oyster Fish. Suppl. Rep. 1904, 2, 107–126. [Google Scholar]
- Bavestrello, G.; Sommer, C.; Sarà, M. Bi-Directional Conversion in Turritopsis nutricula (Hydrozoa). Sci. Mar. 1992, 56, 137–140. [Google Scholar]
- Piraino, S.; Boero, F.; Aeschbach, B.; Schmid, V. Reversing the Life Cycle: Medusae Transforming into Polyps and Cell Transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa). Biol. Bull. 1996, 190, 302–312. [Google Scholar] [CrossRef]
- Bigelow, H.B. Medusae of the Templeton Crocker and Eastern Pacific, Zaca Expeditions, 1936–1938. Zoologica 1940, 25, 281–321. [Google Scholar] [CrossRef]
- Browne, E.T. Hydromedusae, with a Revision of the Williadae and Petasidae. In The Fauna and Geography of the Maldive and Laccadive Archipelagoes; Cambridge University Press: Cambridge, UK, 1904; Volume 2, pp. 722–749. [Google Scholar]
- Boero, F.; Bouillon, J.; Gravili, C. A Survey of Zanclea, Halocoryne and Zanclella (Cnidaria, Hydrozoa, Anthomedusae, Zancleidae) with Description of New Species. Ital. J. Zool. 2000, 67, 93–124. [Google Scholar] [CrossRef]
- Navas-Pereira, D. New Record of Budding in Zanclea costata (Anthomedusae, Zancleidae). Dusenia 1984, 14, 89–93. [Google Scholar]
- Du, F.Y.; Xu, Z.Z.; Huang, J.Q.; Guo, D.H.; Jiang, Y.; Lin, Z.J. Four New Species and Two New Records of Hydrozoa from the Northern of South China Sea and Its Adjacent Waters, China (Cnidaria, Automedusa, Hydroidomedusa). Acta Zootaxonomica Sin. 2009, 34, 854–861. [Google Scholar]
- Schuchert, P. The Marine Fauna of New Zealand: Athecate Hydroids and Their Medusae (Cnidaria: Hydrozoa). Mem. Oceanogr. Inst. 1996, 106, 1–159. [Google Scholar]
- Maggioni, D.; Arrigoni, R.; Galli, P.; Berumen, M.L.; Seveso, D.; Montano, S. Polyphyly of the Genus Zanclea and Family Zancleidae (Hydrozoa, Capitata) Revealed by the Integrative Analysis of Two Bryozoan-Associated Species. Contrib. Zool. 2018, 87, 87–104. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Bavestrello, G.; Valisano, L.; Puce, S. Spatial and Temporal Distribution in a Tropical Hydroid Assemblage. J. Mar. Biol. Assoc. United Kingd. 2008, 88, 1589–1599. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Huang, J.; Guo, D.; Lin, M.; Xia, Z. Taxonomic Notes on Hydroidomedusae (Cnidaria) from South China Sea III: Family Rathkeidae and Zancleopsidae. Zool. Syst. 2016, 41, 392–403. [Google Scholar] [CrossRef]
- Kubota, S. Hydromedusan Fauna of the Nansei Islands. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; pp. 197–201. [Google Scholar]
- Kubota, S. Hydromedusae (Cnidaria, Hydrozoa), Including Species New to Japan, Collected between April and September, 1997, in Tanabe Bay, Wakayama Prefecture, Japan. Publ. Seto Mar. Biol. Lab. 2008, 21, 40–48. [Google Scholar]
- Kubota, S.; Iwao, K. Cnidarian Medusa Collected from Coastal Region of Akajima Island, Kerama Islands, Okinawa, Japan. Midoriishi 2002, 13, 19–22. [Google Scholar]
- Kubota, S.; Gravili, C. A List of Hydromedusae (Excluding Siphonophora, Milleporidae and Actinulidae) in Japan. Nankiseibutsu 2007, 49, 189–204. [Google Scholar]
- Mayer, A.G. Some Medusae from the Tortugus, Florida. Bull. Mus. Comp. Zool. Harv. Coll. 1900, 37, 13–82. [Google Scholar]
- Pagès, F.; Gili, J.-M.; Bouillon, J. Medusae (Hydrozoa, Scyphozoa, Cubozoa) of the Benguela Current (Southeastern Atlantic). Sci. Mar. 1992, 56, 1–64. [Google Scholar]
- De Andrade, L.P.; Migotto, A.E. O Ciclo de Vida de Hebella Furax (Cnidaria, Hydrozoa, Lafoeidae); III Simposio sobre Oceanographia; Instituto Oceanográfico da USP: São Paulo, Brazil, 1996; p. 29. [Google Scholar]
- Migotto, A.E.; De Andrade, L.P. The Life Cycle of Hebella furax (Cnidaria: Hydrozoa): A Link between a Lafoeid Hydroid and a Laodiceid Medusa. J. Nat. Hist. 2000, 34, 1871–1888. [Google Scholar] [CrossRef]
- Bouillon, J.; Boero, F.; Fraschetti, S. The Life Cycle of Laodicea indica (Laodiceidae, Leptomedusae, Cnidaria). Hydrobiologia 1991, 530/531, 151–157. [Google Scholar] [CrossRef]
- De Vito, D.; Piraino, S.; Schmich, J.; Bouillon, J.; Boero, F. Evidence of Reverse Development in Leptomedusae (Cnidaria, Hydrozoa): The Case of Laodicea undulata (Forbes and Goodsir 1851). Mar. Biol. 2006, 149, 339–346. [Google Scholar] [CrossRef]
- Bouillon, J. Considerations Sur Le Developpement Des Narcomeduses et Sur Leur Position Phylogenetique. Indo-Malay. Zool. 1987, 4, 189–278. [Google Scholar]
- Vanhöffen, E. Die Narcomedusen. Wiss. Ergebn. Valdivia 1908, 19, 41–74. [Google Scholar]
- Puente-Tapia, F.A.; Castiglioni, F.; Failla Siquier, G.; Genzano, G. Spatial Distribution of Medusa Cunina octonaria and Frequency of Parasitic Association with Liriope tetraphylla (Cnidaria: Hydrozoa: Trachylina) in Temperate Southwestern Atlantic Waters. Zool. Stud. 2020, 59, e57. [Google Scholar] [CrossRef]
- van der Spoel, S. A Hypothesis on Mesozoic Vicariance in Hydromedusae. J. Plankton Res. 1996, 18, 615–634. [Google Scholar] [CrossRef]
- Vanhöffen, E. Die Craspedoten Medusen Der Deutschen Tiefsee-Expedition, 1898–1899. I Trachymedusen. Wiss. Ergebn. Valdivia 1902, 3, 54–86. [Google Scholar]
- Collins, A.G.; Bentlage, B.; Lindner, A.; Lindsay, D.; Haddock, S.H.D.; Jarms, G.; Norenburg, J.L.; Jankowski, T.; Cartwright, P. Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with New Insights on the Evolution of Some Problematical Taxa. J. Mar. Biol. Assoc. 2008, 88, 1673–1685. [Google Scholar] [CrossRef]
- Miglietta, M.P.; Pruski, S. Cryptic Species in Time and Space: An Assessment of Cryptic Diversity within Eight Nominal Species of Hydrozoa (Cnidaria). Proc. R. Soc. B Biol. Sci. 2023, 290, 20230851. [Google Scholar] [CrossRef]
- Bouillon, J.; Seghers, G.; Boero, F. Notes Additionnelles Sur Les Méduses de Papouasie Nouvelle-Guinée (Hydrozoa, Cnidaria). III. Indo-Malay. Zool. 1988, 5, 225–253. [Google Scholar]
- Ezhilarasan, P.; Sampathkumar, P.; Kannathasan, A.; Balamuruganhy, K. Occurrence of Haliscera bigelowi Kramp, 1947 (Hydromedusae: Trachymedusae: Halicreatidae) in Northern Arabian Sea, India. Check List 2012, 8, 924–926. [Google Scholar] [CrossRef][Green Version]
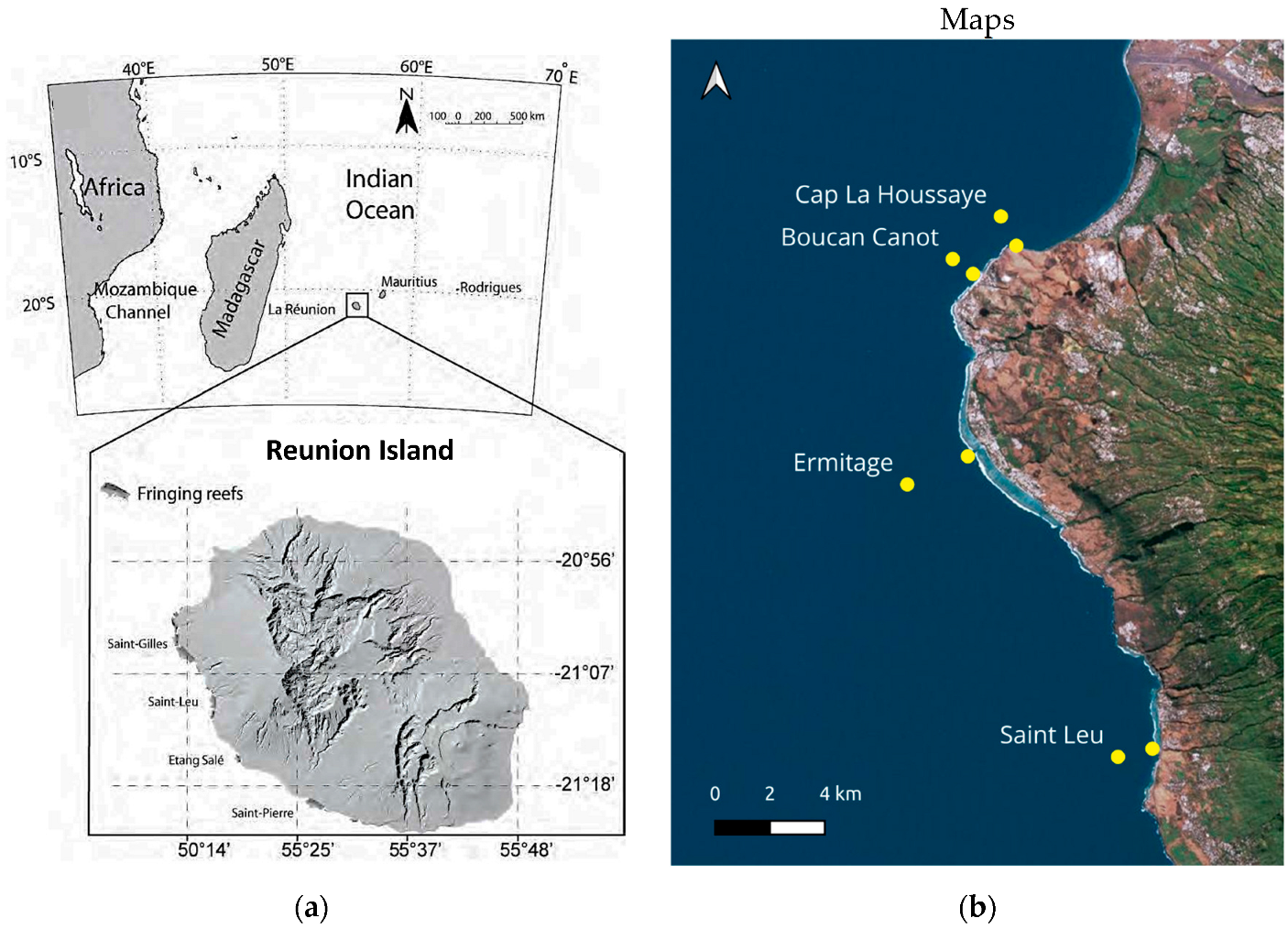


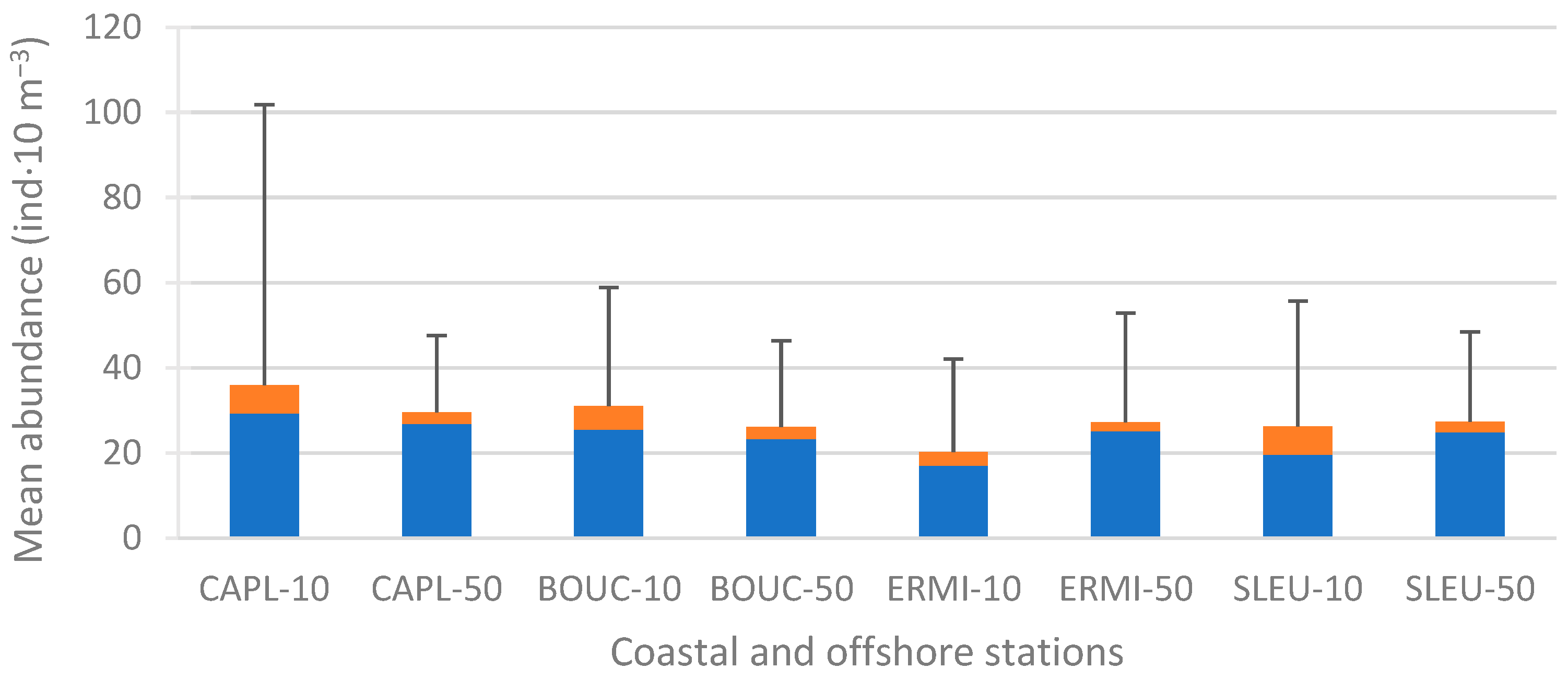
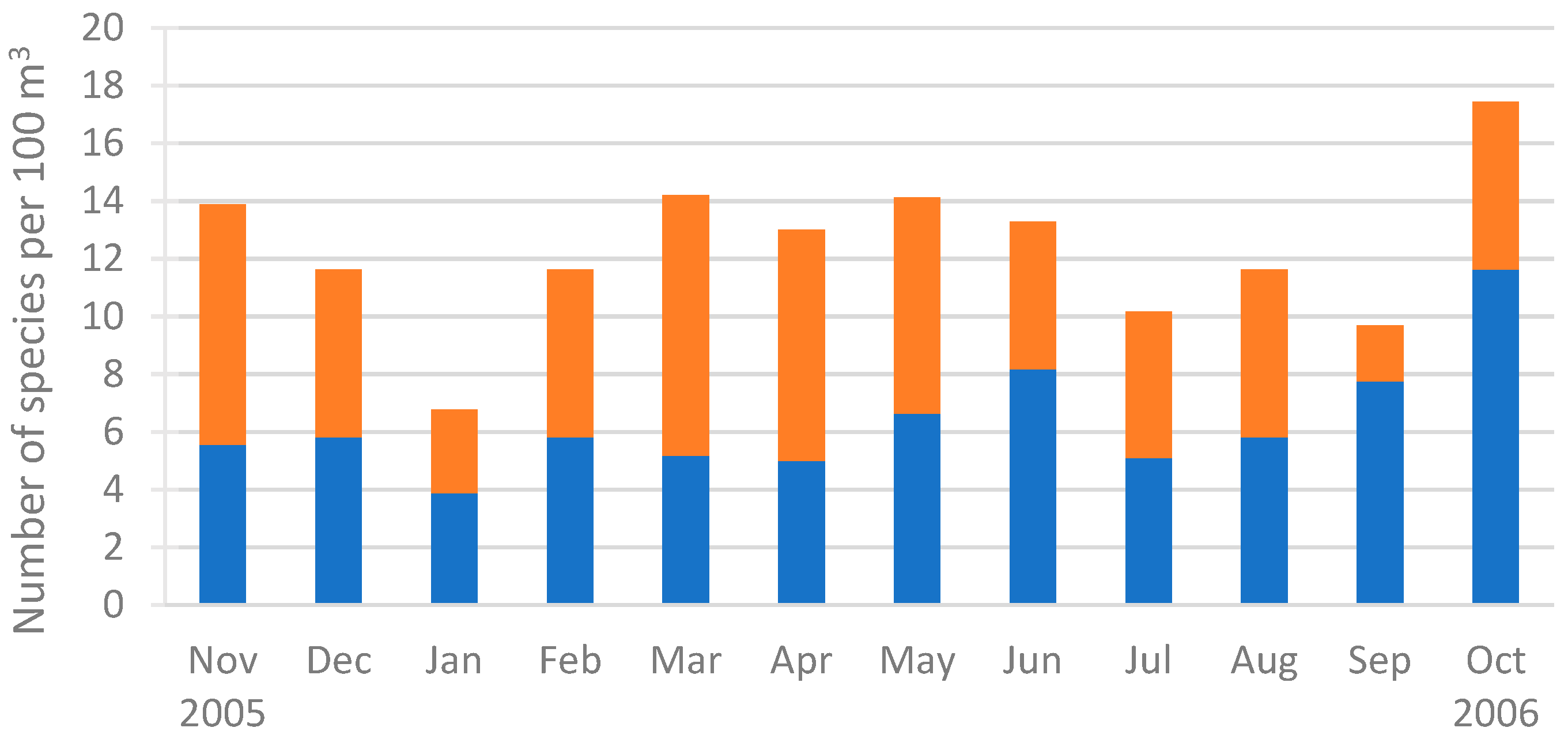
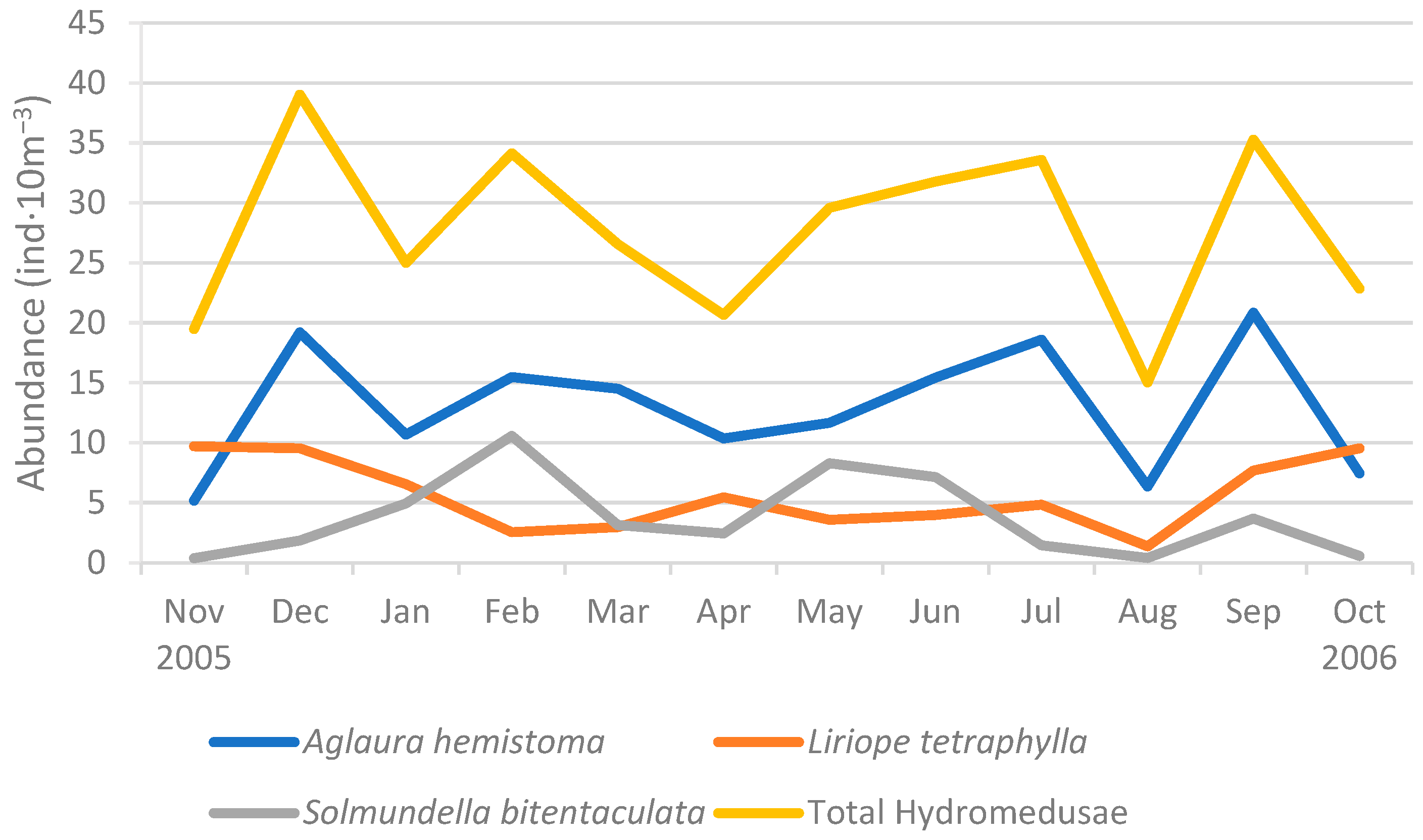
| Station Abbreviation | Site (Depth/ Localization) | Latitude (S) | Longitude (E) | Geomorphology (Bottom Nature) |
|---|---|---|---|---|
| CAPL-10 | Cap La Houssaye (10 m/coastal) | 21°01′03.0″ | 055°14′18.2″ | Coral bank (basalt slope) |
| CAPL-50 | Cap La Houssaye (50 m/offshore) | 21°00′28.1″ | 055°13′59.0″ | Coral bank (lower sloping platform 1) |
| BOUC-10 | Boucan Canot (10 m/coastal) | 21°01′35.9″ | 055°13′23.0″ | Reef platform (basalt slope) |
| BOUC-50 | Boucan Canot (50 m/offshore) | 21°01′18.0″ | 055°12′57.6″ | Reef platform (lower sloping platform) |
| ERMI-10 | Ermitage (10 m/coastal) | 21°05′12.2″ | 055°13′13.9″ | Coral reef (spurs and grooves) |
| ERMI-50 | Ermitage (50 m/offshore) | 21°05′44.8″ | 055°11′57.0″ | Coral reef (lower sloping platform) |
| SLEU-10 | Saint Leu (10 m/coastal) | 21°11′01.5″ | 055°17′03.2″ | Coral reef (spurs and grooves) |
| SLEU-50 | Saint Leu (50 m/offshore) | 21°11′10.7″ | 055°16′19.5″ | Coral reef (lower sloping platform) |
| Site-depth (m) | CAPL-10 | CAPL-50 | BOUC-10 | BOUC-50 | ERMI-10 | ERMI-50 | SLEU-10 | SLEU-50 | Total |
| Number of samples | 40 | 40 | 40 | 39 | 26 | 27 | 28 | 27 | 267 |
| Site-depth (m) | CAPL-10 | CAPL-50 | BOUC-10 | BOUC-50 | ERMI-10 | ERMI-50 | SLEU-10 | SLEU-50 | Total |
| Hydromedusae | 464 | 636 | 401 | 549 | 169 | 396 | 237 | 397 | 3249 |
| Taxa | Families | Genera | Species | Specimens |
|---|---|---|---|---|
| Anthomedusae | 13 | 20 | 27 | 181 |
| Leptomedusae | 6 | 8 | 11 | 247 |
| Hydroidolina indet | - | - | - | 194 |
| Total Hydroidolina | 19 | 28 | 38 | 622 |
| Narcomedusae | 3 | 4 | 4 | 457 |
| Limnomedusae | 1 | 2 | 2 | 685 |
| Trachymedusae | 2 | 5 | 7 | 1679 |
| Total Trachylinae | 6 | 11 | 13 | 2821 |
| Total Hydromedusae | 25 | 39 | 51 | 3443 |
| Species or Taxa | Number of Specimens | Occurrences (%) |
|---|---|---|
| Hydroidolina | ||
| Clytia spp. | 170 | 24.0 |
| Corymorpha forbesii | 26 | 6.0 |
| Cytaeis spp. | 26 | 6.7 |
| Amphinema dinema | 22 | 5.6 |
| Proboscidactyla ornata | 22 | 3.3 |
| Hydractinia spp. | 21 | 3.3 |
| Eucheilota tropica | 16 | 0.4 |
| Laodicea sp. | 12 | 3.4 |
| Cirrholovenia tetranema | 11 | 1.1 |
| Phialella quadrata | 11 | 1.5 |
| Trachylinae | ||
| Aglaura hemistoma | 1546 | 83.2 |
| Liriope tetraphylla | 673 | 74.2 |
| Solmundella bitentaculata | 450 | 50.6 |
| Rhopalonema velatum | 114 | 25.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourmaud, C.A.-F.; Slobodov, S.; Guilhaumon, F.; Goy, J.; Gravier-Bonnet, N. Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution. Diversity 2025, 17, 694. https://doi.org/10.3390/d17100694
Bourmaud CA-F, Slobodov S, Guilhaumon F, Goy J, Gravier-Bonnet N. Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution. Diversity. 2025; 17(10):694. https://doi.org/10.3390/d17100694
Chicago/Turabian StyleBourmaud, Chloé A.-F., Sergey Slobodov, François Guilhaumon, Jacqueline Goy, and Nicole Gravier-Bonnet. 2025. "Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution" Diversity 17, no. 10: 694. https://doi.org/10.3390/d17100694
APA StyleBourmaud, C. A.-F., Slobodov, S., Guilhaumon, F., Goy, J., & Gravier-Bonnet, N. (2025). Medusae (Cnidaria) of Reunion Island (South West Indian Ocean): Diversity, Abundance and Distribution. Diversity, 17(10), 694. https://doi.org/10.3390/d17100694