Biodiversity and Seasonal Dynamics of Waterbirds in the Danube Wetland North of Kopački Rit
Abstract
1. Introduction
2. Materials and Methods
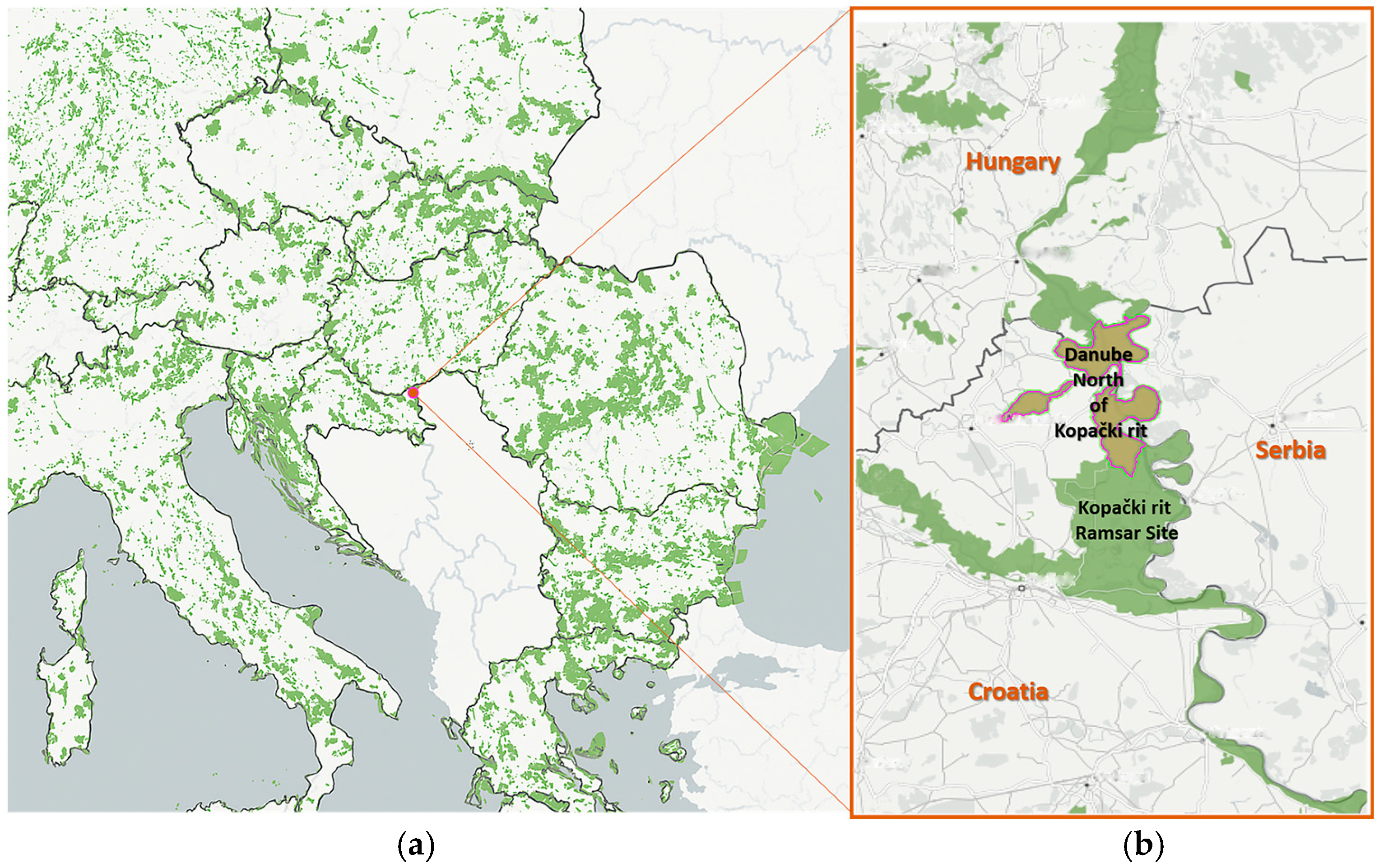
2.1. Study Area
- Topoljski dunavac (45°50′55″ N/18°47′45″ E) is a large hydro-accumulation, and it represents the biodiversity core of the entire study area. It was naturally created from an abandoned meander of the old Danube River. Through a system of canals, most notably the Šarkanj canal, it is irrigationally connected to the Danube. The dominant vegetation is reed (Phragmites australis), and the surrounding landscape primarily consists of arable land. The length of the transect is about 1400 m, and this is Monitoring Area 1 (Figure 2).
- Šarkanj (45°50′52″ N/18°48′17″ E) is predominantly characterized by willow (Salix spp.) vegetation and connected via an artificial canal to Topoljski dunavac in the west and the Danube River in the east. The Šarkanj canal is about 4 km long. The transect measures around 700 m and falls within Monitoring Area 2 (Figure 2).
- Šarkanj-Lorencov dunavac (45°51′11″ N 18°48′16″ E) includes mixed lowland forests, with the Lorencov dunavac canal playing an important ecological role as a biodiversity hotspot. Lorencov dunavac connects further with Mačkalučki dunavac, near the Danube River. This transect is approximately 700 m long and falls within Monitoring Area 2 (Figure 2).
- Lorencov-Mačkalučki dunavac (45°52′57″ N/18°49′05″ E) is located on the Mačkalučki dunavac side arm, which is close to the Danube River but not connected to it. The surrounding area is dominated by mixed forests, particularly stands of poplar (Populus spp.) and willow (Salix spp.). This transect measures approximately 1400 m, and this is Monitoring Area 3 (Figure 2).
2.2. Survey Implementation
2.3. Data Analysis
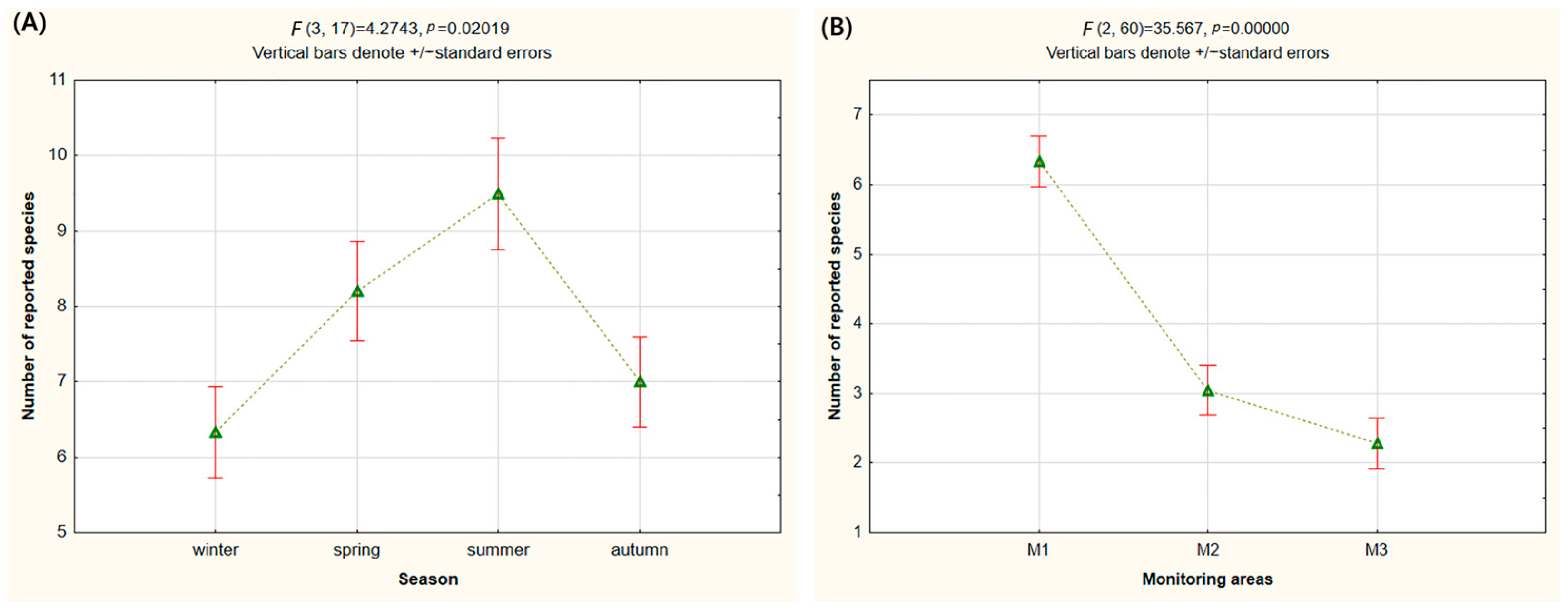
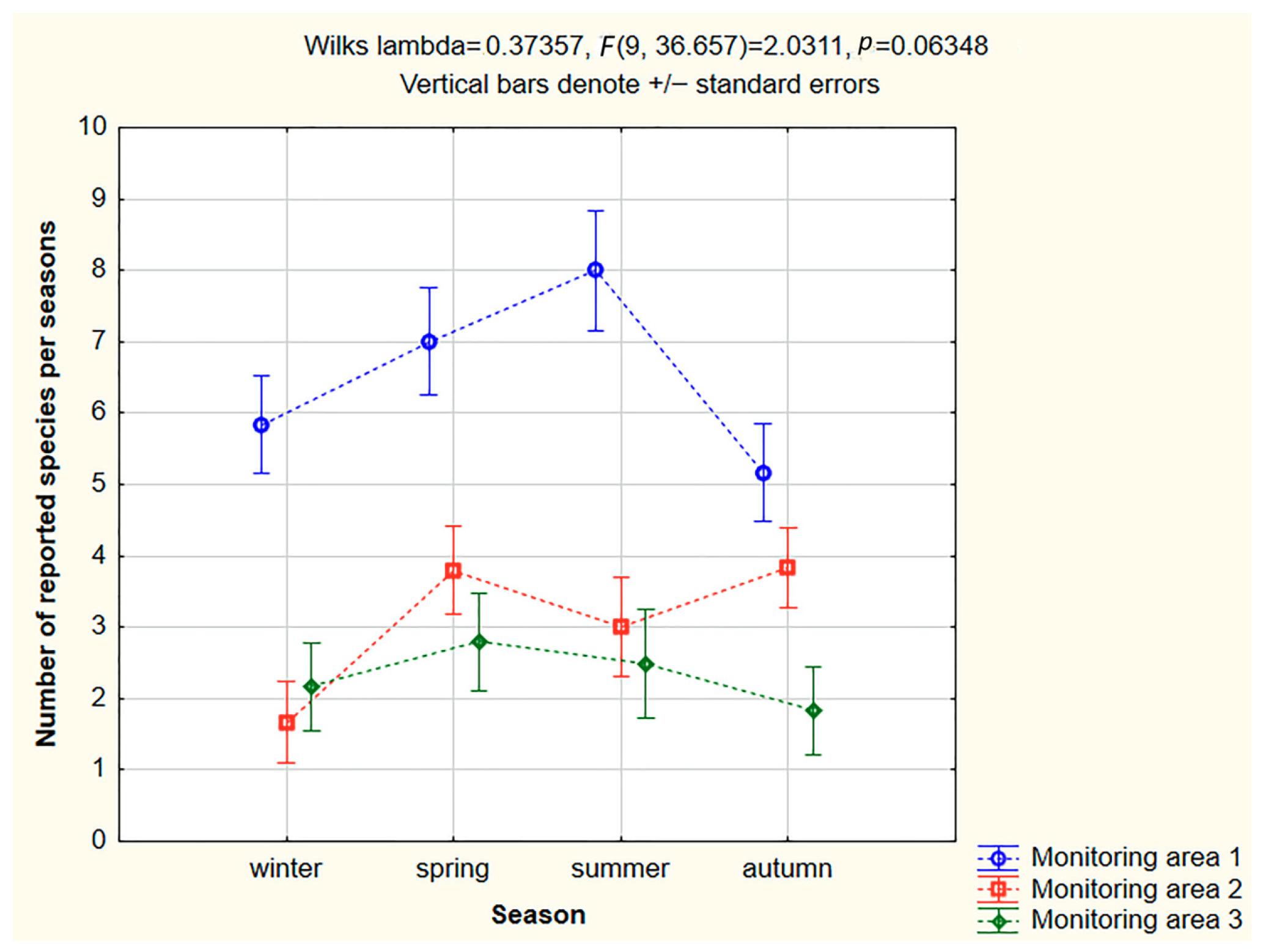
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetlands International Europe. Wetland Biodiversity. Available online: https://europe.wetlands.org/home-2/our-work/wetland-biodiversity/ (accessed on 30 August 2025).
- Luo, J.; Chen, Y.; Lin, L.; Liu, C.; Lei, G. Bird diversity and waterbird habitat preferences in relation to wetland restoration. Avian Res. 2019, 10, 21. [Google Scholar] [CrossRef]
- Morganti, M.; Manica, M.; Bogliani, G.; Gustin, M.; Luoni, F.; Trotti, P.; Perin, V.; Brambilla, M. Multi-species habitat models highlight the key importance of flooded reedbeds for inland wetland birds: Implications for management and conservation. Avian Res. 2019, 10, 15. [Google Scholar] [CrossRef]
- Bhowmik, S. Ecological and Economic Importance of Wetlands and Their Vulnerability: A Review. In Research Anthology on Ecosystem Conservation and Preserving Biodiversity; IGI Global: Hershey, PA, USA, 2022; pp. 11–27. [Google Scholar] [CrossRef]
- Lipton, D.; Rubenstein, M.A.; Weiskopf, S.R.; Carter, S.; Peterson, J.; Crozier, L.; Fogarty, M.; Gaichas, S.; Hyde, K.J.W.; Morelli, T.L.; et al. Ecosystems, Ecosystem Services, and Biodiversity; U.S. Global Change Research Program: Reston, VA, USA, 2018; pp. 268–321. [Google Scholar] [CrossRef]
- Kotarski, K.; Šelo-Šabić, S. A small state finding its way in the EU: Croatia and its approach to Brexit. J. Contemp. Eur. Stud. 2023, 31, 670–686. [Google Scholar] [CrossRef]
- Mužinić, J. The state of bird and nature protection in Croatia. Environmentalist 1995, 15, 188–195. [Google Scholar] [CrossRef]
- Pithart, D.; Petrov Rančić, I.; Kutleša, P.; Duplić, A. Study of freshwater ecosystem services in Croatia. Res. Rep. 2014, 71. Available online: https://www.researchgate.net/publication/316648208_Study_of_Freshwater_Ecosystem_Services_in_Croatia (accessed on 5 August 2025).
- Rožac, V.; Baković, A. RIS for Site No. 583: Nature Park Kopački Rit, Croatia; The Ramsar Sites Information Service: Gland, Switzerland, 2019; Available online: https://rsis.ramsar.org/ris/583 (accessed on 5 August 2025).
- Brundic, D.; Barbalić, D.; Omerbegović, V.; Schneider-Jacoby, M.; Tusić, Z. Alluvial wetlands preservation in Croatia: The experience of the Central Sava Basin flood control system. Period. Biol. 2000, 96, 348–356. Available online: https://www.researchgate.net/publication/237559423_ALLUVIAL_WETLANDS_PRESERVATION_IN_CROATIA_THE_EXPERIENCE_OF_THE_CENTRAL_SAVA_BASIN_FLOOD_CONTROL_SYSTEM (accessed on 5 August 2025).
- Liu, X.; Ho, L.; De Cock, A.; De Saeyer, N.; Pham, K.; Panique-Casso, D.; Forio, M.A.E.; Goethals, P.L.M. Water Quality and Its Influence on Waterbird Habitat Distribution: A Study Along the Lieve River, Belgium. Water 2025, 17, 595. [Google Scholar] [CrossRef]
- Amat, J.A.; Green, A.J. Waterbirds as bioindicators of environmental conditions. In Conservation Monitoring in Freshwater Habitats: A Practical Guide and Case Studies; Hurford, C., Schneider, M., Cowx, I., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 45–52. [Google Scholar] [CrossRef]
- Kralj, J. Ornitofauna Hrvatske tijekom posljednjih dvjesto godina. Larus 1997, 46, 1–112. [Google Scholar]
- Lukač, G. Popis ptica Hrvatske. Nat. Croat. 2007, 16 (Suppl. 1), 1–148. Available online: https://hrcak.srce.hr/file/49182 (accessed on 5 August 2025).
- Wang, Y.; Zhao, M.; Pei, W.; Guan, Q.; Liu, J.; Chen, Y.; Liu, J.; Zhang, Q. Research Overview on Isolated Wetlands. Water 2025, 17, 2013. [Google Scholar] [CrossRef]
- ICPDR. Kopački Rit—Wetland Paradise in Limbo Between Ongoing Threats and Future Protection. Danube Watch. Available online: https://www.icpdr.org/publications/kopacki-rit-wetland-paradise-limbo-between-ongoing-threats-and-future-protection (accessed on 30 August 2025).
- Mikuška, A.; Mikuška, T.; Kordić, M. Promjene u ornitofauni Kopačkog rita tijekom posljednjih 150 godina. Symp. Kopacki Rit 2022, 11, 124–125. Available online: https://www.croris.hr/crosbi/publikacija/prilog-skup/811126 (accessed on 30 June 2025).
- Mikuška, J.; Mikuška, T.; Romulić, M. Vodič kroz biološku raznolikost Kopačkog rita—Ptice. Matica Hrvat. Osijek 2002. [Google Scholar]
- Mikuška, A.; Mikuška, T. Ornitofauna Biljskog i Kopačkog rita. Symp. Kopacki Rit. 2021, 10, 138–139. [Google Scholar]
- Mikuska, T.; Mikuska, A.; Bjedov, D.; Ledinščak, J.; Tomik, A.; Šetina, N.; Podravec, D. NATURA 2000 status does not guarantee the protection of Croatian spoonbill breeding population. In Proceedings of the X International Workshop Eurasian Spoonbill International Expert Group, Zadar, Croatia, 3–7 October 2022; pp. 55–63. Available online: https://storkibisspoonbill.org/wp-content/uploads/2023/10/Special_Publication_2023.pdf#page=56 (accessed on 30 June 2025).
- Vereš, M.; Škoro, M.; Rožac, V.; Damjanović, I.; Romanjek, K.; Kučera, S.; Bolšec, B.; Marušić, M.; Agić, I.; Popijač, A. Preliminary data from the monitoring of ornithofauna in the ecological network site Danube North of Kopački rit in the period from 2020 to 2022. In Proceedings of the 12th Symposium with International Participation Kopački Rit Past, Present, Future 2023, Osijek, Croatia, 28–29 September 2023; Available online: https://www.researchgate.net/publication/374914385 (accessed on 30 June 2025).
- Damjanović, I.; Teni, M.; Uranjek, N.; Galić, A.; Prskalo, M. Bird fauna of the Middle Danube oxbow lakes: Study site Topoljski Dunavac. In Proceedings of the 12th Symposium with International Participation Kopački Rit Past, Present, Future 2023, Osijek, Croatia, 28–29 September 2023; Available online: https://pp-kopacki-rit.hr/wp-content/uploads/2023/09/Prijelom-jucer-danas-sutra-ZA-WEB.pdf (accessed on 30 June 2025).
- Natura 2000. 2025. Available online: https://natura2000.eea.europa.eu/?views=Sites_View (accessed on 20 June 2025).
- DaWetRest—Danube Wetlands Restoration Project. DaWetRest—Danube Wetlands and Flood Plains Restoration Through Systemic, Community Engaged and Sustainable Innovative Actions. 2025. Available online: https://dawetrest.eu/ (accessed on 20 June 2025).
- Wetlands International. Waterbird Population Estimates, Fifth Edition: Summary Report. 2012. Available online: https://www.wetlands.org/publication/waterbird-populations-estimates-fifth-edition/ (accessed on 5 August 2025).
- Svensson, L.; Mullarney, K.; Zetterström, D. Collins Bird Guide: The Most Complete Guide to the Birds of Britain and Europe; HarperCollins: London, UK, 2010; 448p. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; 117p. [Google Scholar]
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species: Search Results for “Larus”. Available online: https://www.iucnredlist.org/search?query=larus&searchType=species (accessed on 20 June 2025).
- European Parliament. Council of the European Union Directive 2009/147/ECon the Conservation of Wild Birds. Off. J. Eur. Union 2009, L20, 7–25. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009L0147 (accessed on 5 August 2025).
- Council of Europe. Convention on the Conservation of European wildlife and Natural Habitats (Bern Convention). Eur. Treaty Ser. 1979, 104. Available online: https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=104 (accessed on 5 August 2025).
- Radović, A.; Gavrilović, M.; Palcu, T.; Ćalić, J. Utilizing remote sensing data for species distribution modeling of birds in Croatia. Biodiversity 2025, 17, 399. [Google Scholar] [CrossRef]
- Observation.org. Available online: https://hr.observation.org/pages/about/ (accessed on 4 September 2025).
- eBird—Hrvatska. Available online: https://ebird.org/region/HR (accessed on 22 May 2025).
- Mikuška, J.; Bogdanović, T.; Mikuška, T.; Mikuška, A.; Šalić, V. Size and distribution of breeding colonies of Grey Heron (Ardea cinerea) in lowland Croatia. Acrocephalus 2005, 26, 37–40. Available online: https://www.researchgate.net/publication/375888083 (accessed on 30 June 2025).
- Dumbović Mazal, V.; Pintar, V.; Zadravec, M. Prvo Izvješće o Brojnosti i Rasprostranjenosti Ptica u Hrvatskoj Sukladno Odredbama Direktive o Pticama. 2019. 49p. Available online: https://www.haop.hr/sites/default/files/uploads/dokumenti/03_prirodne/izvjesca/DumbovicMazal2019_Prvo_izvjesce_art12_ptice_F%20(002).pdf (accessed on 30 June 2025).
- Jakubas, D.; Manikowska, B. The response of Grey Herons Ardea cinerea to changes in prey abundance. Bird Study 2011, 58, 487–494. [Google Scholar] [CrossRef]
- Piria, M. Utjecaj velikog vranca (Phalacrocorax carbo sinensis) na riblji stok—Pregled. Croat. J. Fish. 2014, 72, 164–173. [Google Scholar] [CrossRef][Green Version]
- Opačak, A.; Florijančić, T.; Horvat, D.; Ozimec, S.; Bodakoš, D. Diet spectrum of great cormorants (Phalacrocorax carbo sinensis L.) at the Donji Miholjac carp fishponds in eastern Croatia. Eur. J. Wildl. Res. 2004, 50, 173–178. [Google Scholar] [CrossRef]
- Tutiš, V.; Kralj, J.; Ćiković, D.; Barišić, S. Crvena Knjiga Ptica Hrvatske. 2013, pp. 10–243. Available online: https://www.haop.hr/sites/default/files/uploads/dokumenti/03_prirodne/crvene_knjige_popisi/Crvena_knjiga_ptica_web.pdf (accessed on 5 August 2025).
- Occurrence Data from GBIF. 2025. Available online: https://doi.org/10.15468/2gbkf0 (accessed on 5 August 2025).
- Hill, D.; Wright, R.; Street, M. Survival of Mallard ducklings Anas platyrhynchos and competition with fish for invertebrates on a flooded gravel quarry in England. Ibis 1987, 129, 159–167. [Google Scholar] [CrossRef]
- Cox, R.R.; Hanson, M.A.; Roy, C.C.; Euliss, N.H., Jr.; Johnson, D.H.; Butler, M.G. Mallard Duckling Growth and Survival in Relation to Aquatic Invertebrates. J. Wildl. Manag. 1998, 62, 124–133. Available online: https://digitalcommons.unl.edu/usgsnpwrc/224 (accessed on 6 August 2025). [CrossRef]
- Nummi, P.; Sjöberg, K.; Pöysä, H.; Elmberg, J. Individual foraging behaviour indicates resource limitation: An experiment with Mallard ducklings. Can. J. Zool. 2000, 78, 1891–1895. [Google Scholar] [CrossRef]
- Marteau, B.; Sundell, J.; Pesonen, R.; Nummi, P. Duckling body mass increases with abundant aquatic invertebrates: Experimental approach. Glob. Ecol. Conserv. 2025, 58, e03490. [Google Scholar] [CrossRef]
- Jurčević, I.; Mikuška, J. Wintering Anatidae community structure on the Drava River in Croatia. Limnol. Rep. 2004, 35, 633–638. Available online: https://www.croris.hr/crosbi/publikacija/prilog-skup/496496 (accessed on 30 June 2025).
- Barbraud, C.; Mathevet, R. Is commercial reed harvesting compatible with breeding purple herons Ardea purpurea in the Camargue, southern France? Environ. Conserv. 2000, 27, 334–340. [Google Scholar] [CrossRef]
- Morganti, M.; Viganò, E.; Berlusconi, A.; Cioccarelli, S.; Fasola, M. Post-fledging habitat selection of a Purple Heron Ardea purpurea revealed by GPS/GSM telemetry. Avocetta 2021, 45, 135–145. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kwon, I.K.; Yoo, J.C. Foraging Habitat Preferences of Herons and Egrets. J. Ecol. Environ. 2007, 30, 237–244. [Google Scholar] [CrossRef]
- Cieślińska, K.; Manikowska-Ślepowrońska, B.; Zbyryt, A.; Jakubas, D. Foraging Habitat Availability and the Non-Fish Diet Composition of the Grey Heron (Ardea cinerea) at Two Spatial Scales. Animals 2024, 14, 2461. [Google Scholar] [CrossRef]
- Petkov, N. Habitat characteristics assessment of the wetlands with breeding Ferruginous Duck Aythya nyroca and Pochard A. ferina in Bulgaria. Acrocephalus 2012, 32, 127–134. [Google Scholar] [CrossRef][Green Version]
- Vodopija, M.; Pavlinec, Ž.; Kralj, J. Factors affecting nest attendance in Common Terns. Larus 2024, 59, 63–82. [Google Scholar] [CrossRef]
- Kralj, J.; Martinović, M.; Jurinović, L.; Szinai, P.; Sütő, S.; Preiszner, B. Geolocator study reveals east African migration route of Central European Common Terns. Avian Res. 2020, 11, 6. [Google Scholar] [CrossRef]
- Alerstam, T.; Bäckman, J.; Grönroos, J.; Olofsson, P.; Strandberg, R.; Sjöberg, S. Migration of black terns Chlidonias niger and common terns Sterna hirundo between south Sweden and the Atlantic coast of Africa. J. Avian Biol. 2025, 2025, e03348. [Google Scholar] [CrossRef]
- Heneberg, P. Soil particle composition of Eurasian Kingfishers’ (Alcedo atthis) nest sites. Acta Zool. Acad. Sci. Hung. 2004, 50, 185–193. Available online: http://actazool.nhmus.hu/50/3/heneberg.pdf (accessed on 30 August 2025).
- Delić, A.; Grlica, I.D. Birds of the Končanica fish-ponds, Croatia. Nat. Croat. 2003, 12, 63–91. Available online: https://hrcak.srce.hr/file/17568 (accessed on 30 August 2025).
- Forman, D.W.; Brain, P.F. Reproductive strategies used by moorhens (Gallinula chloropus) colonizing an artificial wetland habitat in south Wales. J. Nat. Hist. 2004, 38, 389–401. [Google Scholar] [CrossRef]
- Kosicki, J.Z.; Profus, P.; Dolata, P.T.; Tobółka, M. Food composition and energy demand of the White Stork Ciconia ciconia breeding population. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Tryjanowski, P., Sparks, T.H., Jerzak, L., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006. [Google Scholar]
- Bocheński, M.; Jerzak, L. Behaviour of the White Stork Ciconia ciconia: A review. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Tryjanowski, P., Sparks, T.H., Jerzak, L., Eds.; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 295–324. Available online: https://www.researchgate.net/publication/303234014 (accessed on 5 August 2025).
- Battisti, C.; Cento, M.; Circosta, A.; Coppola, M.; Muratore, S. Resurrecting seasonal dynamics in waterbirds after wetland restoration: Before-after monitoring highlights the role of a single dominant species. Wetl. Ecol. Manag. 2023, 31, 203–211. [Google Scholar] [CrossRef]
- Wu, J.; Goodale, E.; Li, W.; Zou, F.; Zhang, Q. Patterns in the global diversity of mixed-species bird flocks in relation to environmental factors. Biol. Divers. 2024, 1, 44–53. [Google Scholar] [CrossRef]
- Festetics, A.; Leisler, B. Ecology of waterfowl in the region of Lake Neusiedl, Austria, particularly in the World Wildlife Fund Seewinkel Reserve. Wildfowl 1968, 19, 83–95. Available online: https://www.nationalparkneusiedlersee.at/sites/default/files/1968-ecology_of_waterfowl_in_the_region_of_lake_neusiedl_festetics-a.pdf (accessed on 5 August 2025).
- Ramsar Secretariat. Information Sheet on Ramsar Wetlands (RIS): Neusiedler See–Seewinkel (Site No. 271), Austria. Updated December 2005 (Originally Completed April 2003). Ramsar Sites Information Service. Available online: https://rsis.ramsar.org/ris/271 (accessed on 9 September 2025).
- Heiler, G.; Hein, T.; Schiemer, F.; Bornette, G. Hydrological connectivity and flood pulses as the central aspects for the integrity of a river-floodplain system. Regul. Rivers Res. Manag. 1995, 11, 351–361. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Wenskus, F.; Hecht, C.; Horchler, P.; Januschke, K.; Rieland, G.; Scholz, M.; Weber, A.; Hering, D. Unravelling direct and indirect effects of river-floodplain connectivity on biodiversity: Insights from the Elbe River floodplains. Biodivers. Conserv. 2025, 34, 2829–2850. [Google Scholar] [CrossRef]
- Hein, T.; Schwarz, U.; Habersack, H.; Nichersu, I.; Preiner, S.; Willby, N.; Weigelhofer, G. Current status and restoration options for floodplains along the Danube River. Sci. Total Environ. 2016, 543, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef]
- Gopal, B. Biodiversity in wetlands. In The Wetlands Handbook; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 2, pp. 65–95. [Google Scholar] [CrossRef]
- De Groot, D.; Brander, L.; Finlayson, C.M. Wetland ecosystem services. In The Wetland Book; Springer: Dordrecht, The Netherlands, 2018; pp. 323–333. [Google Scholar] [CrossRef]
- Siuta, M.; Nedelciu, C.E. Report on Socio-Economic Benefits of Wetland Restoration in Central and Eastern Europe; CEEweb for Biodiversity: Budapest, Hungary, 2016; Available online: https://www.ceeweb.org/ducuments/publications/report_on_socio_economic_benefits_of_wetland_restoration_in_CEE.pdf (accessed on 30 August 2025).
- ICPDR. Danube River Basin Management Plan—Update 2021. Available online: https://www.icpdr.org/tasks-topics/tasks/river-basin-management/danube-river-basin-management-plan-2021 (accessed on 30 August 2025).


| Taxa | IUCN [28] /EU Bird Directive [29] /(Bern Convention) [30] | Frequency of Occurence Throughout Season (n = Number of Field Observation Conducted in Certain Season) | |||||
|---|---|---|---|---|---|---|---|
| W (n = 6) | SP (n = 5) | S (n = 4) | S (n = 6) | Mean | SD | ||
| ANSERIFORMES | |||||||
| Anatidae | |||||||
| Anas crecca | LC/II/(III) | 0.17 | 0.00 | 0.00 | 0.00 | 0.04 | 0.09 |
| Anas platyrhynchos | LC/II/(III) | 22.67 | 22.40 | 21.50 | 8.25 | 18.71 | 6.99 |
| Aythya nyroca | LC/I/(III) | 0.00 | 0.00 | 3.25 | 0.00 | 0.81 | 1.63 |
| Bucephala clangula | LC/II/(III) | 3.83 | 0.00 | 0.00 | 0.00 | 0.96 | 1.92 |
| Cygnus olor | LC/II/(III) | 5.00 | 3.80 | 8.75 | 18.75 | 9.08 | 6.79 |
| Mergus merganser | LC/II/(III) | 18.00 | 3.80 | 0.00 | 5.25 | 6.76 | 7.81 |
| Anser anser | LC/II/(III) | 0.00 | 0.00 | 2.25 | 54.75 | 14.25 | 27.02 |
| PODICIPEDIFORMES | |||||||
| Podicipedidae | |||||||
| Podiceps cristatus | LC(III) | 0.00 | 0.00 | 0.00 | 0.50 | 0.13 | 0.25 |
| CHARADRIIFORMES | |||||||
| Scolopacidae | |||||||
| Actitis hypoleucos | LC(II) | 0.00 | 4.20 | 0.00 | 0.00 | 1.05 | 2.10 |
| Tringa ochropus | LC(II) | 2.17 | 0.20 | 0.25 | 0.00 | 0.66 | 1.02 |
| Sternidae | |||||||
| Chlidonias niger | LC/I/(II) | 0.00 | 0.00 | 4.00 | 0.00 | 1.00 | 2.00 |
| Sterna hirundo | LC/I/(II) | 0.00 | 6.60 | 0.00 | 0.00 | 1.65 | 3.30 |
| Laridae | |||||||
| Chroicocephalus ridibundus | LC/I/(III) | 0.00 | 0.00 | 0.00 | 19.00 | 4.75 | 9.50 |
| Larus sp. | 0.17 | 0.00 | 0.50 | 5.00 | 1.42 | 2.4 | |
| CICONIIFORMES | |||||||
| Ardeidae | |||||||
| Ardea alba | LC/I/(III) | 1.67 | 5.00 | 12.25 | 1.75 | 5.17 | 4.97 |
| Ardea cinerea | LC(III) | 7.17 | 16.20 | 39.75 | 21.00 | 21.03 | 13.73 |
| Ardea purpurea | LC/I/(II) | 0.00 | 0.60 | 1.75 | 1.50 | 0.96 | 0.81 |
| Ardeola ralloides | LC/I/(II) | 0.00 | 0.40 | 0.00 | 0.25 | 0.16 | 0.20 |
| Egretta garzetta | LC/I/(II) | 0.00 | 2.60 | 0.00 | 0.00 | 0.65 | 1.30 |
| Nycticorax nycticorax | LC/I/(II) | 0.00 | 6.00 | 11.75 | 9.00 | 6.69 | 5.04 |
| Ciconiidae | |||||||
| Ciconia ciconia | LC/I/(II) | 0.00 | 0.20 | 0.50 | 0.00 | 0.18 | 0.24 |
| Ciconia nigra | LC/I/(II) | 0.00 | 0.20 | 0.00 | 0.00 | 0.05 | 0.10 |
| Rallidae | |||||||
| Fulica atra | NT/II/(III) | 2.33 | 0.00 | 0.00 | 0.00 | 0.58 | 1.17 |
| Gallinula chloropus | LC/II/(III) | 0.00 | 0.00 | 3.75 | 1.50 | 1.31 | 1.77 |
| PELECANIFORMES | |||||||
| Phalacrocoracidae | |||||||
| Phalacrocorax carbo | LC(III) | 18.33 | 5.00 | 10.50 | 44.25 | 19.52 | 17.37 |
| CORACIIFORMES | |||||||
| Alcedinidae | |||||||
| Alcedo atthis | VU/I/(II) | 0.17 | 0.20 | 3.00 | 3.25 | 1.66 | 1.70 |
| Frequency of Occurrence per Monitoring Areas (n = Number of Field Observation Conducted in Certain Monitoring Area) | |||||
|---|---|---|---|---|---|
| Taxa | M1 (n = 21) | M2 (n = 21) | M3 (n = 21) | Mean | SD |
| ANSERIFORMES | |||||
| Anatidae | |||||
| Anas crecca | 0.00 | 0.05 | 0.00 | 0.02 | 0.03 |
| Anas platyrhynchos | 10.76 | 3.48 | 3.24 | 5.83 | 4.27 |
| Aythya nyroca | 0.43 | 0.00 | 0.19 | 0.21 | 0.22 |
| Bucephala clangula | 1.10 | 0.00 | 0.00 | 0.37 | 0.64 |
| Cygnus olor | 5.95 | 0.71 | 0.90 | 2.52 | 2.97 |
| Mergus merganser | 2.95 | 1.81 | 2.29 | 2.35 | 0.57 |
| Anser anser | 10.81 | 0.05 | 0.00 | 3.62 | 6.23 |
| PODICIPEDIFORMES | |||||
| Podicipedidae | |||||
| Podiceps cristatus | 0.00 | 0.00 | 0.10 | 0.03 | 0.06 |
| CHARADRIIFORMES | |||||
| Scolopacidae | |||||
| Actitis hypoleucos | 1.00 | 0.00 | 0.00 | 0.33 | 0.58 |
| Tringa ochropus | 0.62 | 0.00 | 0.10 | 0.24 | 0.33 |
| Sternidae | |||||
| Chlidonias niger | 0.76 | 0.00 | 0.00 | 0.25 | 0.44 |
| Sterna hirundo | 1.57 | 0.00 | 0.00 | 0.52 | 0.91 |
| Laridae | |||||
| Chroicocephalus ridibundus | 3.57 | 0.05 | 0.00 | 1.21 | 2.05 |
| Larus sp. | 1.10 | 0.00 | 0.00 | 0.37 | 0.64 |
| CICONIIFORMES | |||||
| Ardeidae | |||||
| Ardea alba | 2.05 | 1.57 | 0.71 | 1.44 | 0.68 |
| Ardea cinerea | 9.76 | 5.14 | 2.57 | 5.82 | 3.64 |
| Ardea purpurea | 0.76 | 0.00 | 0.00 | 0.25 | 0.44 |
| Ardeola ralloides | 0.14 | 0.00 | 0.00 | 0.05 | 0.08 |
| Egretta garzetta | 0.57 | 0.05 | 0.00 | 0.21 | 0.32 |
| Nycticorax nycticorax | 5.33 | 0.05 | 0.00 | 1.79 | 3.06 |
| Ciconiidae | |||||
| Ciconia ciconia | 0.10 | 0.05 | 0.00 | 0.05 | 0.05 |
| Ciconia nigra | 0.00 | 0.00 | 0.05 | 0.02 | 0.03 |
| Rallidae | |||||
| Fulica atra | 0.67 | 0.00 | 0.00 | 0.22 | 0.39 |
| Gallinula chloropus | 1.00 | 0.00 | 0.00 | 0.33 | 0.58 |
| PELECANIFORMES | |||||
| Phalacrocoracidae | |||||
| Phalacrocorax carbo | 12.19 | 1.71 | 3.43 | 5.78 | 5.62 |
| CORACIIFORMES | |||||
| Alcedinidae | |||||
| Alcedo atthis | 0.67 | 0.57 | 0.05 | 0.43 | 0.33 |
| Season | n | Monitoring Area 1 | Monitoring Area 2 | Monitoring Area 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| Winter | 6 | 3.589 | 6.376 | 4.521 | 1.101 | 1.738 | 2.456 | 1.976 | 0.326 | 1.583 | 4.696 | 2.735 | 1.355 |
| Spring | 5 | 3.335 | 6.823 | 4.557 | 1.525 | 2.090 | 4.209 | 3.239 | 0.947 | 1.961 | 3.597 | 2.845 | 0.599 |
| Summer | 4 | 4.098 | 7.365 | 6.270 | 1.472 | 1.419 | 5.044 | 2.816 | 1.950 | 2.324 | 2.814 | 2.547 | 0.248 |
| Autumn | 6 | 1.606 | 4.724 | 3.070 | 1.041 | 1.755 | 3.652 | 2.766 | 0.784 | 1.755 | 2.219 | 1.902 | 0.213 |
| Total | 21 | 1.606 | 7.365 | 4.448 | 1.613 | 1.419 | 5.044 | 2.730 | 1.034 | 1.583 | 4.696 | 2.526 | 0.793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedić, Z.; Nicolae, R.; Popescu, S.; Rožac, V.; Nikolić, V. Biodiversity and Seasonal Dynamics of Waterbirds in the Danube Wetland North of Kopački Rit. Diversity 2025, 17, 669. https://doi.org/10.3390/d17100669
Nedić Z, Nicolae R, Popescu S, Rožac V, Nikolić V. Biodiversity and Seasonal Dynamics of Waterbirds in the Danube Wetland North of Kopački Rit. Diversity. 2025; 17(10):669. https://doi.org/10.3390/d17100669
Chicago/Turabian StyleNedić, Zlatko, Raluca Nicolae, Stefan Popescu, Vlatko Rožac, and Vera Nikolić. 2025. "Biodiversity and Seasonal Dynamics of Waterbirds in the Danube Wetland North of Kopački Rit" Diversity 17, no. 10: 669. https://doi.org/10.3390/d17100669
APA StyleNedić, Z., Nicolae, R., Popescu, S., Rožac, V., & Nikolić, V. (2025). Biodiversity and Seasonal Dynamics of Waterbirds in the Danube Wetland North of Kopački Rit. Diversity, 17(10), 669. https://doi.org/10.3390/d17100669








