From Pampas to Patagonia: Human-Modified Environments Drive the Spread of the Argentine Ant Beyond Its Climatic Limits
Abstract
1. Introduction
2. Materials and Methods
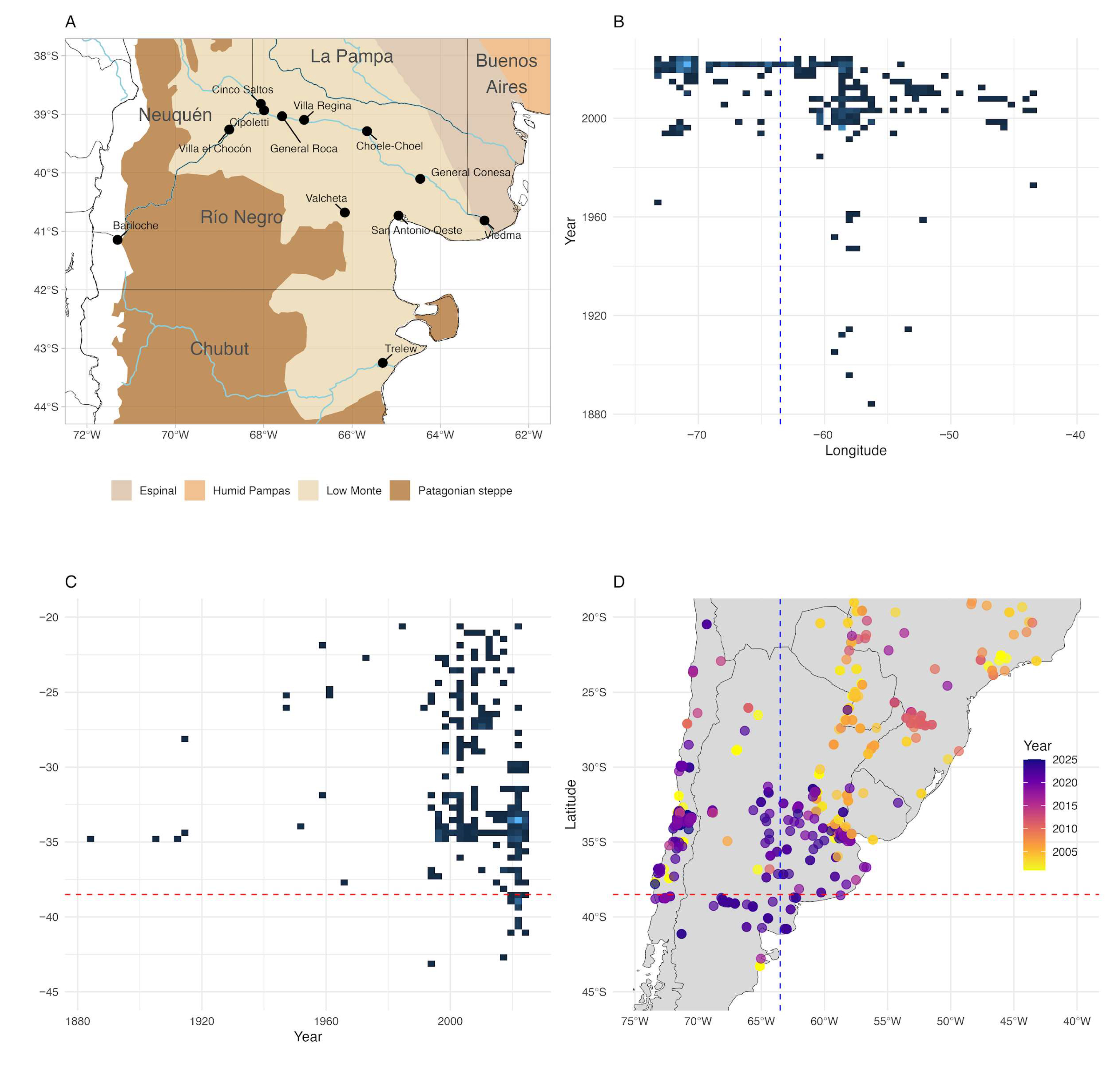
2.1. Study Area
2.2. Sampling Protocol
- Baiting: Groups of five sugar-based baits (0.5–1 g peanut butter each) were placed on 7 cm diameter plastic cards on the ground at 10 m intervals to attract common or dominant ants, particularly highly invasive species such as L. humile [23]. The baits were left in place for 30 min. During this time, all ants attracted to the baits were collected with soft forceps or hand-held aspirators. The ants were then preserved together in a 2 mL vial containing 96% ethanol.
- Manual Collection: Manual searches for ants were conducted at each site, extending up to 100 m at distances greater than 10 m from the baited area (to avoid disturbing ant activity in bait locations). All ants found on the ground, in shrubby vegetation, and on buildings (e.g., house walls, perimeter walls, flowerbeds in urban habitats) were collected with a hand-held aspirator and soft forceps. The ants were also preserved in separate 2 mL vials with 96% ethanol.
2.3. Argentine Ant Distribution Models
2.4. Genetic Analysis
3. Results
3.1. Argentine Ant Spread

3.2. Argentine Ant Distribution Models
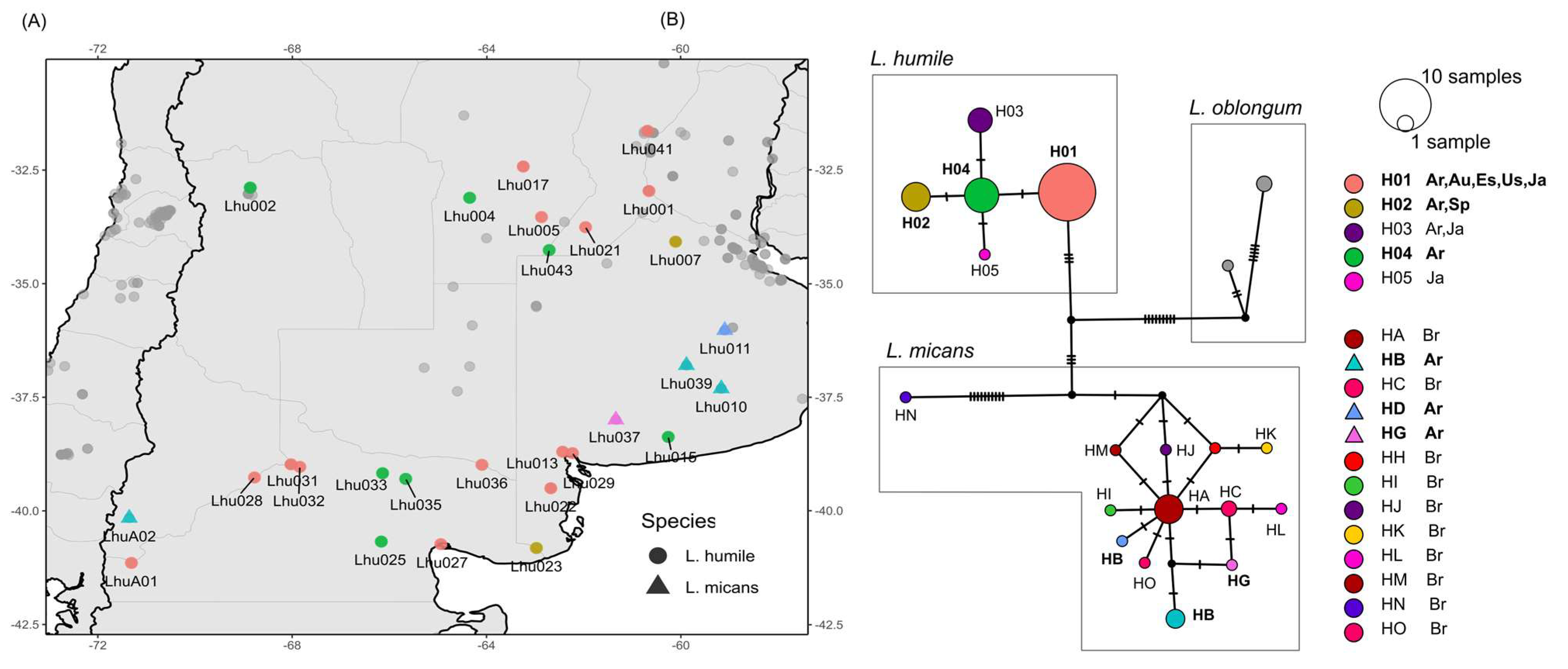
3.3. Invasive Haplotypes
4. Discussion
4.1. Argentine Ant Range Expansion
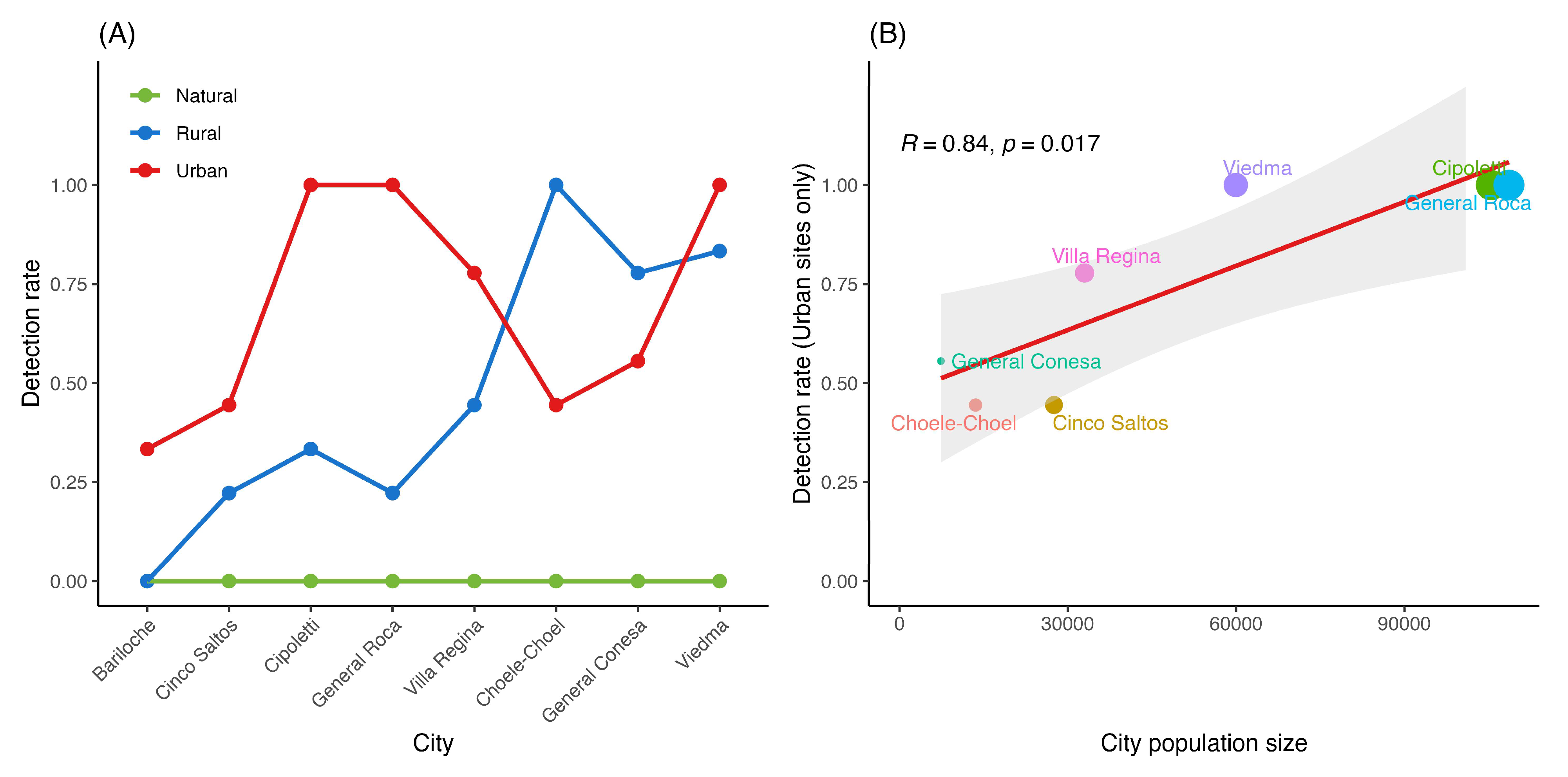
4.2. Abiotic Constraints and Urban Facilitation
4.3. Mitochondrial Signatures of Expansion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ Warning on Invasive Alien Species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and Rising Economic Costs of Biological Invasions Worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Roy, H.E.; Pauchard, A.; Stoett, P.J.; Renard Truong, T.; Meyerson, L.A.; Bacher, S.; Galil, B.S.; Hulme, P.E.; Ikeda, T.; Kavileveettil, S. Curbing the Major and Growing Threats from Invasive Alien Species Is Urgent and Achievable. Nat. Ecol. Evol. 2024, 8, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- IPBES. Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar]
- Hulme, M. Why We Disagree About Climate Change: Understanding Controversy, Inaction and Opportunity; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošík, V.; Wilson, J.R.; Richardson, D.M. A Proposed Unified Framework for Biological Invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef]
- Simberloff, D. Invasive Species: What Everyone Needs to Know; Oxford University Press: New York, NY, USA, 2013; ISBN 0-19-992203-9. [Google Scholar]
- Essl, F.; Bacher, S.; Blackburn, T.M.; Booy, O.; Brundu, G.; Brunel, S.; Cardoso, A.-C.; Eschen, R.; Gallardo, B.; Galil, B. Crossing Frontiers in Tackling Pathways of Biological Invasions. BioScience 2015, 65, 769–782. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Gaertner, M.; Wilson, J.R.U.; Cadotte, M.W.; MacIvor, J.S.; Zenni, R.D.; Richardson, D.M. Non-Native Species in Urban Environments: Patterns, Processes, Impacts and Challenges. Biol. Invasions 2017, 19, 3461–3469. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Y.; Zhang, H.; Qiu, D.; Zhu, Y. Characteristics of Invasive Alien Plants in Different Urban Areas: The Case of Kunshan City, Jiangsu Province, China. Front. Plant Sci. 2025, 16, 1539457. [Google Scholar] [CrossRef]
- Meineke, E.K.; Dunn, R.R.; Sexton, J.O.; Frank, S.D. Urban Warming Drives Insect Pest Abundance on Street Trees. PLoS ONE 2013, 8, e59687. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Von Der Lippe, M.; Kowarik, I. Do Cities Export Biodiversity? Traffic as Dispersal Vector across Urban–Rural Gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- McLean, P.; Gallien, L.; Wilson, J.R.U.; Gaertner, M.; Richardson, D.M. Small Urban Centres as Launching Sites for Plant Invasions in Natural Areas: Insights from South Africa. Biol. Invasions 2017, 19, 3541–3555. [Google Scholar] [CrossRef]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The Causes and Consequences of Ant Invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Rabitsch, W. The Hitchhiker’s Guide to Alien Ant Invasions. BioControl 2011, 56, 551–572. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Economo, E.P.; Guénard, B. The Global Spread and Invasion Capacities of Alien Ants. Curr. Biol. 2023, 33, 566–571.e3. [Google Scholar] [CrossRef]
- Chan, K.H.; Guénard, B. Ecological and Socio-Economic Impacts of the Red Import Fire Ant, Solenopsis invicta (Hymenoptera: Formicidae), on Urban Agricultural Ecosystems. Urban Ecosyst. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Ollier, S.; Liebhold, A.; Keller, L. Recent Human History Governs Global Ant Invasion Dynamics. Nat. Ecol. Evol. 2017, 1, 0184. [Google Scholar] [CrossRef]
- Angulo, E.; Hoffmann, B.D.; Taheri, A.; Renault, D. Economic Costs of Invasive Alien Ants Worldwide. Res. Sq. 2022, 24, 2041–2060. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Pooter, M. 100 of the World’s Worst Invasive Alien Species, a Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zeland, 2000. [Google Scholar]
- Calcaterra, L.A.; Cabrera, S.; Briano, J. Local Co-Occurrence of Several Highly Invasive Ants in Their Native Range: Are They All Ecologically Dominant Species? Insectes Sociaux 2016, 63, 407–419. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Wild, A.L.; Suarez, A.V.; Roura-Pascual, N.; Espadaler, X. Worldwide Spread of the Argentine Ant, Linepithema humile (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 187–194. [Google Scholar]
- Vogel, V.; Pedersen, J.S.; Giraud, T.; Krieger, M.J.B.; Keller, L. The Worldwide Expansion of the Argentine Ant. Divers. Distrib. 2010, 16, 170–186. [Google Scholar] [CrossRef]
- Schulze-Sylvester, M.; Corronca, J.A.; Paris, C.I. Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina. Insects 2018, 9, 11. [Google Scholar] [CrossRef]
- Bertelsmeier, C.; Keller, L. Bridgehead Effects and Role of Adaptive Evolution in Invasive Populations. Trends Ecol. Evol. 2018, 33, 527–534. [Google Scholar] [CrossRef]
- Diamond, S.E.; Chick, L.; Perez, A.B.E.; Strickler, S.A.; Martin, R.A. Rapid Evolution of Ant Thermal Tolerance across an Urban-Rural Temperature Cline. Biol. J. Linn. Soc. 2017, 121, 248–257. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Brotons, L.; Peterson, A.T.; Thuiller, W. Consensual Predictions of Potential Distributional Areas for Invasive Species: A Case Study of Argentine Ants in the Iberian Peninsula. Biol. Invasions 2009, 11, 1017–1031. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Suarez, A.V.; Gómez, C.; Pons, P.; Touyama, Y.; Wild, A.L.; Peterson, A.T. Geographical Potential of Argentine Ants (Linepithema humile Mayr) in the Face of Global Climate Change. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Roura-Pascual, N.; Hui, C.; Ikeda, T.; Leday, G.; Richardson, D.M.; Carpintero, S.; Espadaler, X.; Gómez, C.; Guénard, B.; Hartley, S.; et al. Relative Roles of Climatic Suitability and Anthropogenic Influence in Determining the Pattern of Spread in a Global Invader. Proc. Natl. Acad. Sci. USA 2011, 108, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xian, X.; Zhao, H.; Xue, L.; Chen, B.; Huang, H.; Wan, F.; Liu, W. Predicting the Potential Suitable Area of the Invasive Ant Linepithema humile in China under Future Climatic Scenarios Based on Optimized MaxEnt. Diversity 2022, 14, 921. [Google Scholar] [CrossRef]
- Pirk, G.I.; Werenkraut, V.; Lescano, M.N.; Elizalde, L.; Josens, R.B. “Hormiga Argentina” Linepithema humile; Ediciones INTA: Ciudad de Buenos Aires, Argentina, 2020. [Google Scholar]
- Werenkraut, V.; Lescano, M.; Elizalde, L.; Pirk, G.I. Citizen Science Quickly Reveals the Argentine Ant (Linepithema Humile) Distribution in an Invaded Urban Area and Provides Unexpected Findings. Sci. Rep. 2025; Submitted. [Google Scholar]
- Bejarán, R.A.; Camilloni, I.A. Objective Method for Classifying Air Masses: An Application to the Analysis of Buenos Aires’ (Argentina) Urban Heat Island Intensity. Theor. Appl. Climatol. 2003, 74, 93–103. [Google Scholar] [CrossRef]
- Barros, V.R.; Boninsegna, J.A.; Camilloni, I.A.; Chidiak, M.; Magrín, G.O.; Rusticucci, M. Climate Change in Argentina: Trends, Projections, Impacts and Adaptation. WIREs Clim. Change 2015, 6, 151–169. [Google Scholar] [CrossRef]
- Casadei, P.; Semmartin, M.; Garbulsky, M.F. Análisis Regional de Las Islas de Calor Urbano En La Argentina. Ecol. Austral 2021, 31, 190–203. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos (INDEC). Censo Nacional de Población, Hogares y Viviendas. Available online: https://www.indec.gob.ar/ (accessed on 2 January 2025).
- Matteucci, S.; Rodriguez, A.; Silva, M.; de Haro, C. Ecorregiones y Complejos Ecosistémicos Argentinos; Orientación Gráfica Editora S.R.L.: Buenos Aires, Argentina, 2012; pp. 309–348. [Google Scholar]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Schilman, P.E.; Lighton, J.R.B.; Holway, D.A. Water Balance in the Argentine Ant (Linepithema humile) Compared with Five Common Native Ant Species from Southern California. Physiol. Entomol. 2007, 32, 1–7. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A Solution to the Problem of Separation in Logistic Regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef]
- Firth, D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Guénard, B.; Weiser, M.D.; Gómez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) Database: Synthesizing Data on the Geographic Distribution of Ant Species (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 83–89. [Google Scholar]
- GBIF Occurrence Download for Linepithema humile. 2025. Available online: https://www.gbif.org/occurrence/download/0014376-250426092105405 (accessed on 1 September 2025).
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An Expanded Set of Bioclimatic and Topographic Variables Increases Flexibility and Improves Performance of Ecological Niche Modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Sánchez-Restrepo, A.F.; Chifflet, L.; Confalonieri, V.A.; Tsutsui, N.D.; Pesquero, M.A.; Calcaterra, L.A. A Species Delimitation Approach to Uncover Cryptic Species in the South American Fire Ant Decapitating Flies (Diptera: Phoridae: Pseudacteon). PLoS ONE 2020, 15, e0236086. [Google Scholar] [CrossRef]
- Tsutsui, N.D.; Suarez, A.V.; Holway, D.A.; Case, T.J. Relationships among Native and Introduced Populations of the Argentine Ant (Linepithema humile) and the Source of Introduced Populations. Mol. Ecol. 2001, 10, 2151–2161. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Clement, M.; Snell, Q.; Walke, P.; Posada, D.; Crandall, K. TCS: Estimating Gene Genealogies. In Parallel and Distributed Processing Symposium, International; IEEE Computer Society: Washington, DC, USA, 2002; Volume 2, 7p. [Google Scholar]
- Leigh, J.W.; Bryant, D. PopArt: Full-feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Perez, G.G. Capítulo 4. Una periodización para el estudio de las ciudades del Alto Valle. De la ciudad lineal a la conurbación neuquina. In Rompecabezas urbano: Producción de Desigualdades en Ciudades de la Norpatagonia; Perren, J., Casullo, F., Padín, N., Eds.; Aperturas; Editorial UNRN: Viedma, Argentina, 2020; pp. 137–162. ISBN 978-987-4960-28-3. [Google Scholar]
- Albert, A.; Anderson, J.A. On the Existence of Maximum Likelihood Estimates in Logistic Regression Models. Biometrika 1984, 71, 1–10. [Google Scholar] [CrossRef]
- Angulo, E.; Guenard, B.; Balzani, P.; Bang, A.; Frizzi, F.; Masoni, A.; Abril, S.; Suarez, A.V.; Hoffmann, B.; Benelli, G.; et al. The Argentine Ant, Linepithema humile: Natural History, Ecology and Impact of a Successful Invader. Entomol. Gen. 2024, 44, 41–61. [Google Scholar] [CrossRef]
- Gonzalez-Polo, M.; Austin, A.T. Spatial Heterogeneity Provides Organic Matter Refuges for Soil Microbial Activity in the Patagonian Steppe, Argentina. Soil Biol. Biochem. 2009, 41, 1348–1351. [Google Scholar] [CrossRef]
- Ipinza-Regla, J.; Castro, L.; Eissemann, R.; Morales, M.A. Factores Que Influyen En La Distribución de Nidos de La Hormiga Argentina Linepithema humile Mayr (Hymenoptera: Formicidae), En Un Ecosistema Precordillerano de La Zona Central de Chile. Neotrop. Entomol. 2010, 39, 686–690. [Google Scholar] [CrossRef]
- Hartley, S.; Harris, R.; Lester, P.J. Quantifying Uncertainty in the Potential Distribution of an Invasive Species: Climate and the Argentine Ant. Ecol. Lett. 2006, 9, 1068–1079. [Google Scholar] [CrossRef]
- Mothapo, N.P.; Wossler, T.C. Behavioural and Chemical Evidence for Multiple Colonisation of the Argentine Ant, Linepithema humile, in the Western Cape, South Africa. BMC Ecol. 2011, 11, 6. [Google Scholar] [CrossRef]
- Cooling, M.; Hartley, S.; Sim, D.A.; Lester, P.J. The Widespread Collapse of an Invasive Species: Argentine Ants (Linepithema humile) in New Zealand. Biol. Lett. 2012, 8, 430–433. [Google Scholar] [CrossRef]
- Ward, D.F.; Harris, R.J.; Stanley, M.C. Human-Mediated Range Expansion of Argentine Ants Linepithema humile (Hymenoptera: Formicidae) in New Zealand. Sociobiology 2005, 45, 401–407. [Google Scholar]
- Suhr, E.L.; O’Dowd, D.J.; Suarez, A.V.; Cassey, P.; Wittmann, T.A.; Ross, J.V.; Cope, R.C. Ant Interceptions Reveal Roles of Transport and Commodity in Identifying Biosecurity Risk Pathways into Australia. NeoBiota 2019, 53, 1–24. [Google Scholar] [CrossRef]
- Menke, S.B.; Booth, W.; Dunn, R.R.; Schal, C.; Vargo, E.L.; Silverman, J. Is It Easy to Be Urban? Convergent Success in Urban Habitats among Lineages of a Widespread Native Ant. PLoS ONE 2010, 5, e9194. [Google Scholar] [CrossRef]
- Tsutsui, N.D.; Suarez, A.V. The Colony Structure and Population Biology of Invasive Ants. Conserv. Biol. 2003, 17, 48–58. [Google Scholar] [CrossRef]
- Nair, R.R.; Gurvich, D.E.; Pereyra, M.; Sérsic, A.N. Clandestine Travelers, a Boon for South and a Bane for North? Warming-Induced Shifts in Global Invasion Potential of Argentine Ants. Biol. Invasions 2024, 26, 3369–3392. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, X.; Hu, X.; Feng, J. Niche Shifts Induce Major Changes in the Ranges of the World’s Worst Invasive Ant Species. Ecol. Evol. 2025, 15, e71754. [Google Scholar] [CrossRef]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting Future Invaders and Future Invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef]
- Whyte, B.A.; Sandidge, R.; Buellesbach, J.; Cash, E.I.; Scheckel, K.J.; Gibson, J.D.; Tsutsui, N.D. The Role of Body Size and Cuticular Hydrocarbons in the Desiccation Resistance of Invasive Argentine Ants (Linepithema humile). J. Exp. Biol. 2023, 226, jeb245578. [Google Scholar] [CrossRef]
- Guida-Johnson, B.; Zuleta, G.A. Land-Use Land-Cover Change and Ecosystem Loss in the Espinal Ecoregion, Argentina. Agric. Ecosyst. Environ. 2013, 181, 31–40. [Google Scholar] [CrossRef]
- López-Collar, D.; Cabrero-Sañudo, F.J.; Gil-Tapetado, D. The Urban Island: Climatic Suitability of Linepithema Humile (Hymenoptera: Formicidae) and the Role of Cities in the Invasion of the Western Palearctic. Integr. Zool. 2024; Early View. [Google Scholar] [CrossRef]
- Bianchi, A.R.; Cravero, S.A.C. Atlas Climático Digital de La República Argentina. 2010. Available online: https://www.argentina.gob.ar/sites/default/files/inta-atlas_climatico_digital_argentina-2010.pdf (accessed on 1 September 2025).
- Muñoz, L.I.J.; Schilman, P.E. Factores Bióticos y Abióticos Que Limitan La Distribución Austral de La Pequeña Hormiga de Fuego Wasmannia auropunctata. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2023. [Google Scholar]
- Coulin, C.; de la Vega, G.J.; Chifflet, L.; Calcaterra, L.A.; Schilman, P.E. Linking Thermo-Tolerances of the Highly Invasive Ant, Wasmannia Auropunctata, to Its Current and Potential Distribution. Biol. Invasions 2019, 21, 3491–3504. [Google Scholar] [CrossRef]
- Tsang, T.P.N.; Wong, M.K.L.; Cadotte, M.W.; Economo, E.P.; Guénard, B. Climate Change Can Exacerbate Ant Invasion Impacts by Unleashing Indoor Populations Into Outdoor Environments. Divers. Distrib. 2025, 31, e70041. [Google Scholar] [CrossRef]
- Wild, A.L. Taxonomy and Distribution of the Argentine Ant, Linepithema humile (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2004, 97, 1204–1215. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, L.A.; Chifflet, L.; Fernández, M.B.; Pirk, G.I.; Werenkraut, V.; Sánchez-Restrepo, A.F. From Pampas to Patagonia: Human-Modified Environments Drive the Spread of the Argentine Ant Beyond Its Climatic Limits. Diversity 2025, 17, 667. https://doi.org/10.3390/d17100667
Calcaterra LA, Chifflet L, Fernández MB, Pirk GI, Werenkraut V, Sánchez-Restrepo AF. From Pampas to Patagonia: Human-Modified Environments Drive the Spread of the Argentine Ant Beyond Its Climatic Limits. Diversity. 2025; 17(10):667. https://doi.org/10.3390/d17100667
Chicago/Turabian StyleCalcaterra, Luis A., Lucila Chifflet, María B. Fernández, Gabriela I. Pirk, Victoria Werenkraut, and Andrés F. Sánchez-Restrepo. 2025. "From Pampas to Patagonia: Human-Modified Environments Drive the Spread of the Argentine Ant Beyond Its Climatic Limits" Diversity 17, no. 10: 667. https://doi.org/10.3390/d17100667
APA StyleCalcaterra, L. A., Chifflet, L., Fernández, M. B., Pirk, G. I., Werenkraut, V., & Sánchez-Restrepo, A. F. (2025). From Pampas to Patagonia: Human-Modified Environments Drive the Spread of the Argentine Ant Beyond Its Climatic Limits. Diversity, 17(10), 667. https://doi.org/10.3390/d17100667






