The Aging of Urban Gardens Can Enhance Their Role as Refuges for Local Ant Species
Abstract
1. Introduction
2. Materials and Methods
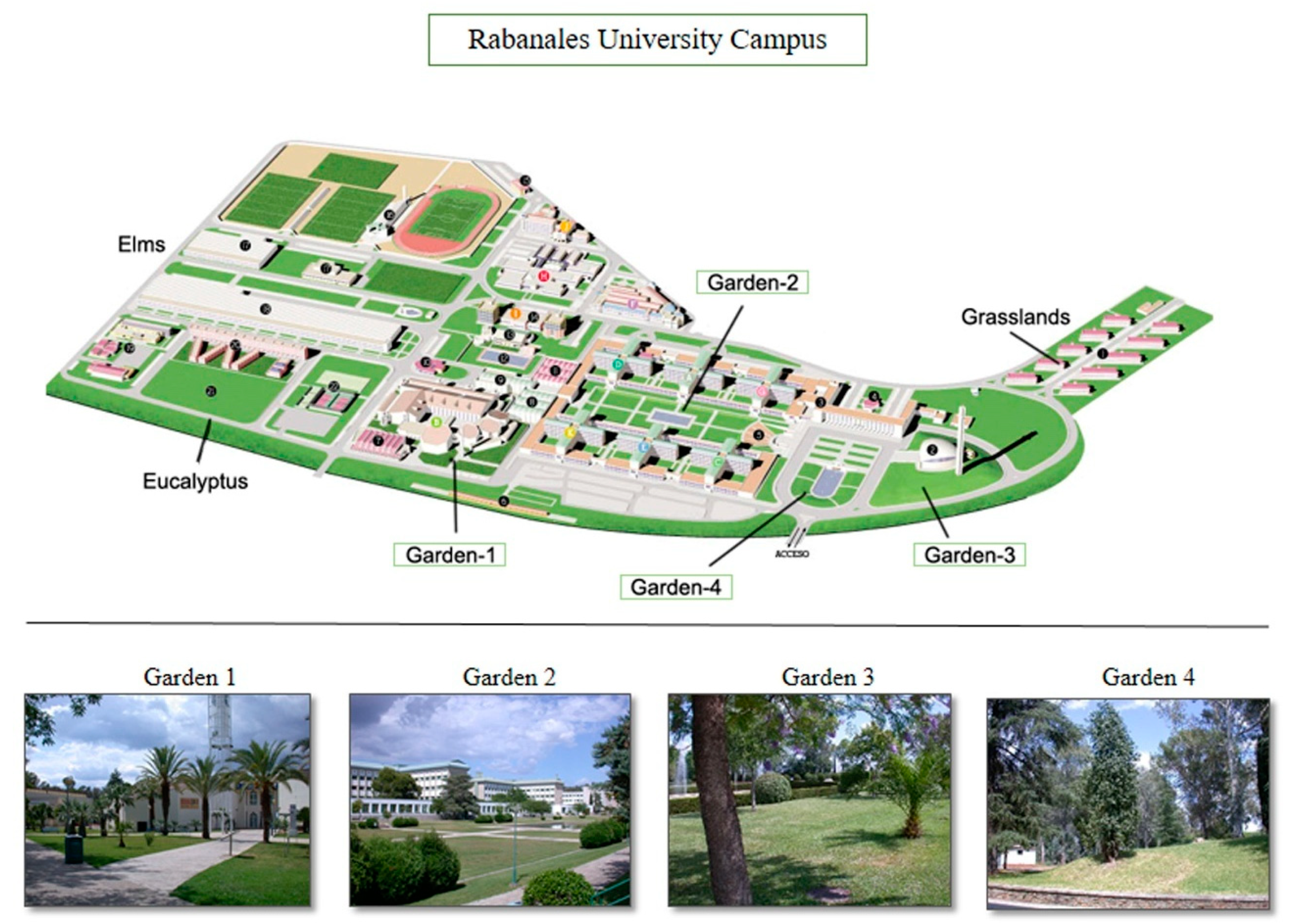
2.1. Study Areas
2.2. Sampling Method
2.3. Statistical Analyses
2.3.1. Comparing Ant Communities Among Campus Habitats
2.3.2. Species–Habitat Association
2.3.3. Species Population Dynamic over the Years
3. Results
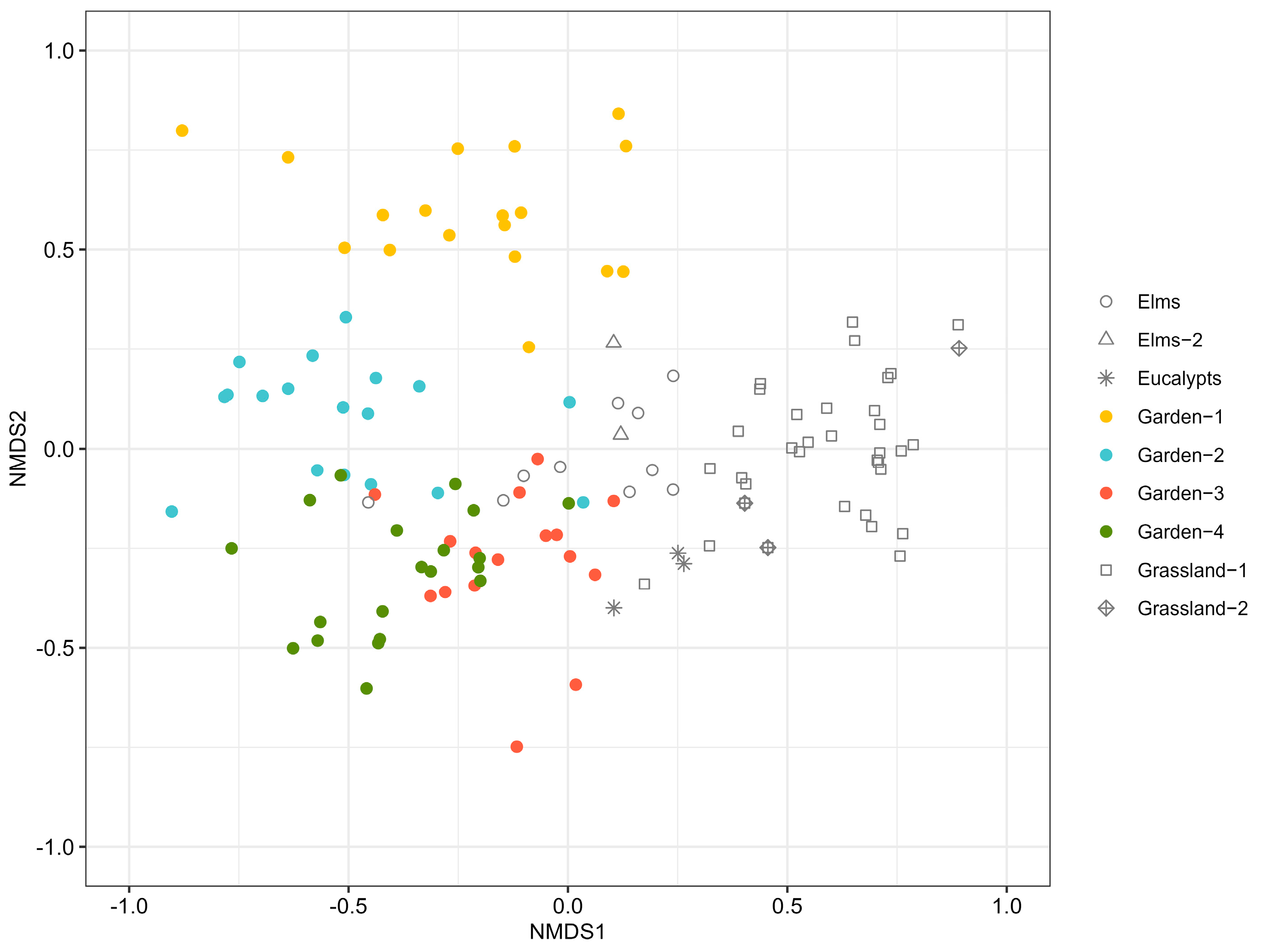
3.1. Comparing Ant Communities Among Campus Habitats
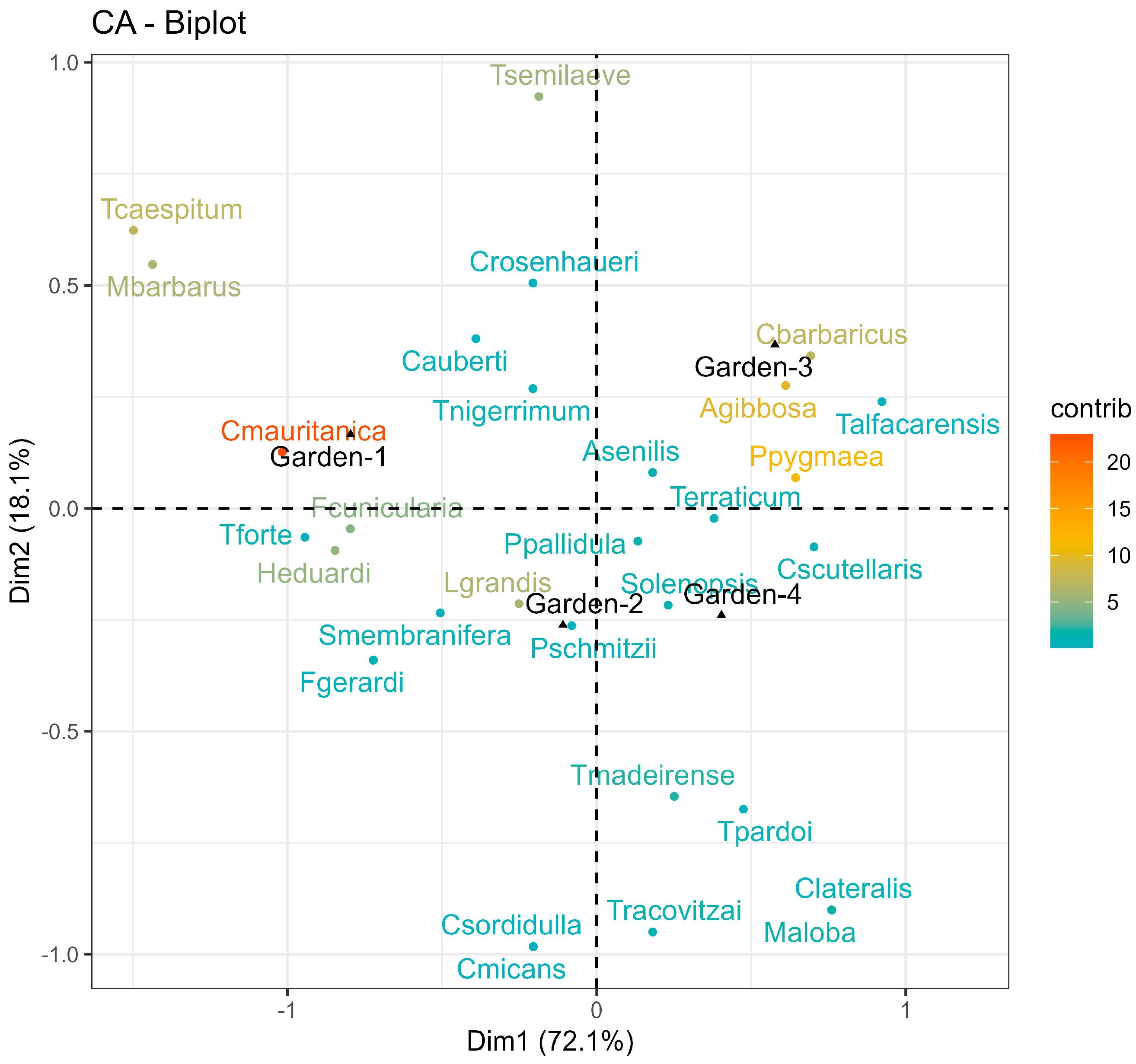
3.2. Species–Habitat Association
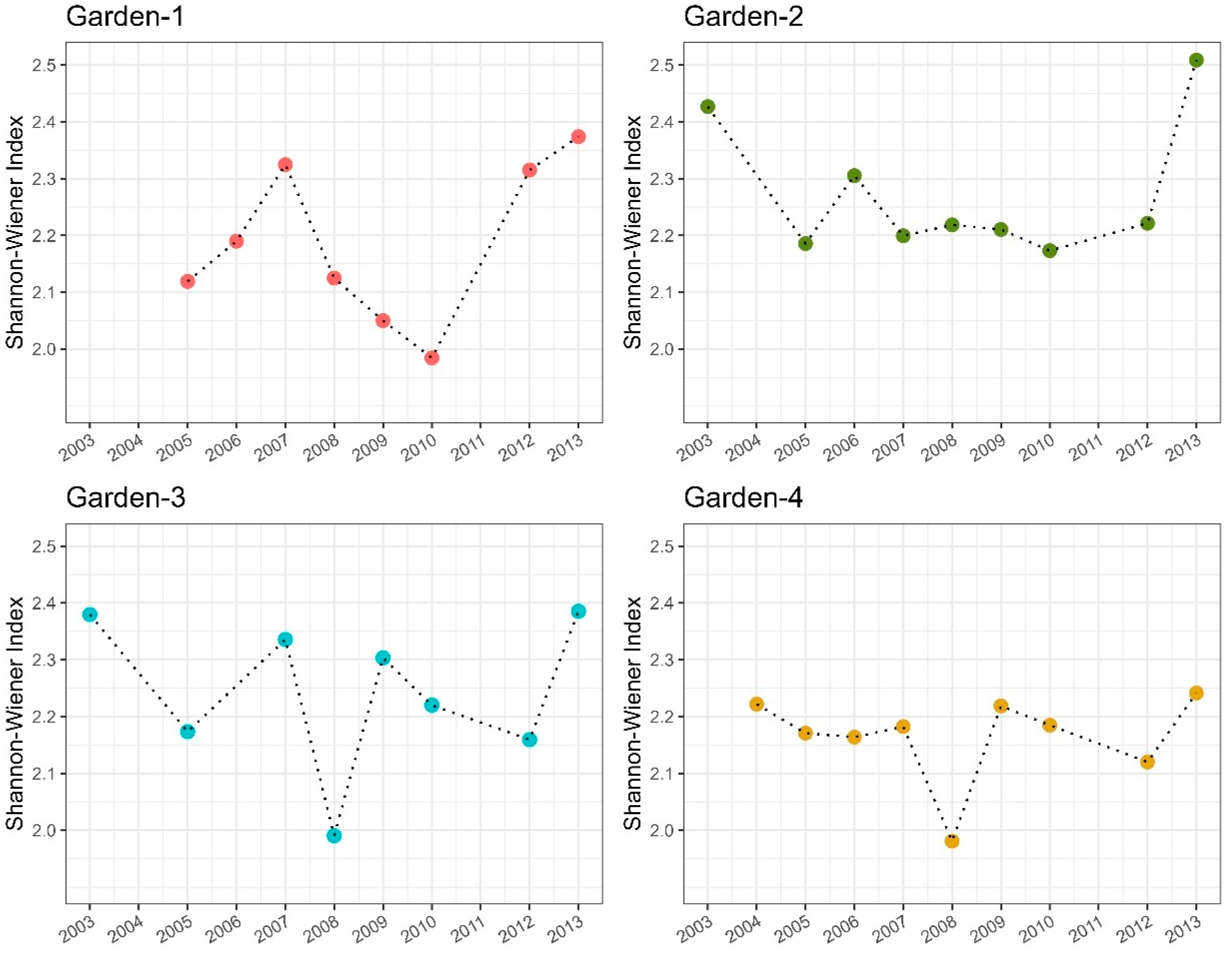
3.3. Relationship Between Species Abundance and Habitat Maturity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szulkin, M.; Munshi-South, J.; Chamantier, A. Urban Evolutionary Biology; Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Rega-Brodsky, C.C.; Aronson, M.F.J.; Piana, M.R.; Carpenter, E.S.; Hahs, A.K.; Herrera-Montes, A.; Nilon, C.H. Urban biodiversity: State of the science and future directions. Urban Ecosyst. 2022, 25, 1083–1109. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Maák, I.; Schmid, S.; Chodzik, P.; Czaczkes, T.J.; Witek, M.; Casacci, L.P.; Sánchez-Garcia, D.; Lörincz, A.; Kochanowski, M.; et al. Urban abiotic stressors drive changes in the foraging activity and colony growth of the black garden ant Lasius niger. Sci. Total Environ. 2024, 915, 170157. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Munshi-South, J. Evolution of life in urban environments. Science 2017, 358, eaam8327. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Tichý, L.; Danihelka, J.; Fajmon, K.; Hájek, O.; Kintrová, K.; Láníková, D.; Otýpková, Z.; Řehořek, V. Biotic homogenization of Central European urban floras depends on residence time of alien species and habitat types. Biol. Conserv. 2012, 145, 179–184. [Google Scholar] [CrossRef]
- Brassard, F.; Leong, C.M.; Chan, H.H.; Guénard, B. High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World. Diversity 2021, 13, 358. [Google Scholar] [CrossRef]
- Langemeyer, J.; Gómez-Baggethun, E. Urban biodiversity and ecosystem services. In Urban Biodiversity—From Research to Practice; Ossola, A., Niemelä, J., Eds.; Routledge: Oxon, UK; New York, NY, USA, 2018; pp. 36–53. [Google Scholar]
- Guénard, B.; Cardinal-De Casas, A.; Dunn, R.R. High diversity in an urban habitat: Are some animal assemblages resilient to long-term anthropogenic change? Urban Ecosyst. 2015, 18, 449–463. [Google Scholar] [CrossRef]
- Menke, S.B.; Guénard, B.; Sexton, J.O.; Weiser, M.D.; Dunn, R.R.; Silverman, J. Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; an example from ants. Urban Ecosyst. 2011, 14, 135–163. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Rutkowski, T.; Witek, M.; Ślipiński, P.; Babik, H.; Czechowski, W. Three categories of urban green areas and the effect of their different management on the communities of ants, spiders and harvestmen. Urban Ecosyst. 2020, 23, 803–818. [Google Scholar] [CrossRef]
- Reyes-López, J.L.; Carpintero, S. Comparison of the exotic and native ant communities (Hymenoptera: Formicidae) in urban green areas at inland, coastal and insular sites in Spain. Eur. J. Entomol. 2014, 111, 421–428. [Google Scholar] [CrossRef]
- Ślipiński, P.; Zmihorski, M.; Czechowski, W. Species Diversity and nestedness of Ant Assemblages in an Urban Environment. Eur. J. Entomol. 2012, 109, 197–206. [Google Scholar] [CrossRef]
- Llewellyn, T.; Gaya, E.; Murrell, D.J. Are Urban Communities in successional Stasis? A Case Study on Epiphytic Lichen Communities. Diversity 2020, 12, 330. [Google Scholar] [CrossRef]
- Vepsäläinen, K.; Ikonen, H.; Koivula, M.J. The structure of ant assemblages in an urban area of Helsinki, southern Finland. Ann. Zool. Fenn. 2008, 45, 109–127. [Google Scholar] [CrossRef]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems, 3rd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Davis, A.M.; Glick, T.F. Urban Ecosystems and Island Biogeography. Environ. Conserv. 1978, 5, 299. [Google Scholar] [CrossRef]
- Marzluff, J.M. Island biogeography for an urbanizing world: How extinction and colonization may determine biological diversity in human-dominated landscapes. Urban Ecosyst. 2005, 8, 157–177. [Google Scholar] [CrossRef]
- Dunn, R.R.; Burger, J.R.; Carlen, E.J.; Koltz, A.M.; Light, J.E.; Martin, R.A.; Munshi-South, J.; Nichols, L.M.; Vargo, E.L.; Yitbarek, S.; et al. A Theory of City Biogeography and the Origin of Urban Species. Front. Conserv. Sci. 2022, 3, 761449. [Google Scholar] [CrossRef]
- King, J.R.; Andersen, A.N.; Cutter, A.D. Ants as bioindicators of habitat disturbance: Validation of the functional group model for Australia's humid tropics. Biodivers. Conserv. 1998, 7, 1627–1638. [Google Scholar] [CrossRef]
- Andersen, A.N.; Hoffmann, B.D.; Müller, W.J.; Griffiths, A.D. Using ants as bioindicators in land management simplifying assessment of ant community responses. J. Appl. Ecol. 2002, 39, 8–17. [Google Scholar] [CrossRef]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Ivanov, K.; Keiper, J. Ant (Hymenoptera: Formicidae) diversity and community composition along sharp urban forest edges. Biodivers. Conserv. 2010, 19, 3917–3933. [Google Scholar] [CrossRef]
- Buczkowski, G.; Richmond, D.S. The effect of urbanization on ant abundance and diversity: A temporal examination of factors affecting biodiversity. PLoS ONE 2012, 7, e41729. [Google Scholar] [CrossRef]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants of Poland with Reference to the Myrmecofauna of Europe; Fauna Polonia 4; Natura Optima Dux Foundation: Warsaw, Poland, 2012. [Google Scholar]
- Roig, X.; Espadaler, X. Propuesta de grupos funcionales de hormigas para la Península Ibérica y Baleares, y su uso como bioindicadores Proposal of functional groups of ants for the Iberian Peninsula and Balearic Islands, and their use as bioindicators. Iberomyrmex 2010, 2, 28–29. [Google Scholar]
- Andersen, A.N. A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. J. Biogeogr. 1995, 22, 15–29. [Google Scholar] [CrossRef]
- Arnán, X.; Cerdá, X.; Retana, J. Partitioning the impact of environment and spatial structure on alpha and beta components of taxonomic, functional, and phylogenetic diversity in European ants. PeerJ 2015, 3, e1241. [Google Scholar] [CrossRef] [PubMed]
- Cabrero-Sañudo, F.J.; Cañizares García, R.; Caro-Miralles, E.; Gil-Tapetado, D.; Grzechnik, S.; López-Collar, D. Seguimiento de artrópodos bioindicadores en áreas urbanas: Objetivos, experiencias y perspectivas. Ecosistemas 2022, 31, 2340. [Google Scholar] [CrossRef]
- Carpintero, S.; Reyes-López, J.L. Effect of park age, size, shape and insolation on ant assemblages in two cities of. Southern Spain. Entomol. Sci. 2014, 17, 41–51. [Google Scholar] [CrossRef]
- Miguelena, G.J.; Baker, P.B. Effects of urbanization on the diversity, abundance, and composition of ant assemblages in an arid city. Environ. Entomol. 2019, 48, 836–846. [Google Scholar] [CrossRef]
- Tinaut Ranera, J.A. Estudio de los Formícidos de Sierra Nevada. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1981. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2010. Available online: https://www.R-project.org/ (accessed on 23 August 2024).
- Oksanen, J.; Simpson, G.L.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, B.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology package. R Package Version 2.0-10. 2024. Available online: http://CRAN.R-project.org/package=vegan (accessed on 23 August 2024).
- AntWiki. AntWiki: The Ants of the World. Available online: https://www.antwiki.org (accessed on 13 January 2025).
- Hormigas.org. Hormigas.org: The Ants of Spain. Available online: https://www.hormigas.org (accessed on 13 January 2025).
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package Version 1.0.5. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 August 2024).
- Trigos-Peral, G.; Reyes-López, J.L. Las hormigas como bioindicadores para la gestión de zonas verdes urbanas en el sur de la Península Ibérica. Iberomyrmex 2016, 8, 37–38. [Google Scholar]
- Wetterer, J.K. Worldwide Spread of the Moorish Sneaking Ant, Cardiocondyla mauritanica (Hymenoptera: Formicidae). Sociobiology 2012, 59, 985–997. [Google Scholar]
- Taheri, A.; Wetterer, J.K.; Reyes-López, J. Tramp ants of Tangier, Morocco. Trans. Am. Entomol. Soc. 2017, 143, 299–304. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, UK, 1990. [Google Scholar]
- Jiménez-Carmona, F.; Heredia-Arévalo, A.M.; Reyes-López, J.L. Ants (Hymenoptera: Formicidae) as an indicator group of human environmental impact in the riparian forests of the Guadalquivir river (Andalusia, Spain). Ecol. Indic. 2020, 118, 106762. [Google Scholar] [CrossRef]
- Lőrincz, Á.; Habenczyus, A.A.; Kelemen, A.; Ratkai, B.; Tölgyesi, C.; Lőrinczi, G.; Frei, K.; Bátori, Z.; Maák, I.E. Wood-pastures promote environmental and ecological heterogeneity on a small spatial scale. Sci. Total Environ. 2024, 906, 167510. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Villareal, D.; Lekberg, Y.; Hierro, J. Exotic success following disturbance explained by weak native resilience and ruderal exotic bias. J. Ecol. 2023, 111, 2412–2423. [Google Scholar] [CrossRef]
- Cotton, J. Diversity, abundance, and species composition of ants in urban green spaces. Urban Ecosyst. 2010, 13, 25–441. [Google Scholar]
- Stukalyuk, S.; Maák, I.E. The influence of illumination regimes on the structure of ant (Hymenoptera, Formicidae) community composition in urban habitats. Insectes Sociaux 2023, 70, 423–437. [Google Scholar] [CrossRef]
- Penick, C.A.; Savage, A.M.; Dunn, R.R. Stable isotopes reveal links between human food inputs and urban ant diets. Proc. R. Soc. B-Biol. Sci. 2015, 282, art 20142608. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Witek, M.; Csata, E.; Chudzik, P.; Heinze, J. Urban diet as potential cause of low body fat content in female ant sexuals. Myrmecol. News 2024, 34, 181–190. [Google Scholar]
- Gaber, H.; Ruland, F.; Jeschke, J.; Bernard-Verdier, M. Behavioural changes in the city: The common black garden ant defends aphids more aggressively in urban environments. Ecol. Evol. 2024, 4, e11639. [Google Scholar] [CrossRef]
- Vidal-Cordero, J.M.; Angulo, E.; Molina, F.P.; Boulay, R.; Cerdá, X. Long-term recovery of Mediterranean ant and bee communities after fire in southern Spain. Sci. Total Environ. 2023, 887, 164132. [Google Scholar] [CrossRef] [PubMed]
- Zina, V.; Ordeix, M.; Franco, J.C.; Ferreira, M.T.; Fernandes, M.R. Ants as Bioindicators of Riparian Ecological Health in Catalonian Rivers. Forests 2021, 12, 625. [Google Scholar] [CrossRef]
- Gollan, J.R.; Lobry de Bruyn, L.; Reid, N.; Smith, D.; Wilkie, L. Can ants be used as ecological indicators of restoration progress in dynamic environments? A case study in a revegetated riparian zone. Ecol. Indic. 2011, 11, 1517–1525. [Google Scholar] [CrossRef]
- Hill, J.G.; Summerville, K.S.; Brown, R.L. Habitat associations of ant species (Hymenoptera: Formicidae) in a heterogeneous Mississippi landscape. Environ. Entomol. 2008, 37, 453–463. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Espadaler, X.; Wetterer, A.L.; Cabral, S.G.M. Native and exotic ants of the Azores (Hymenoptera: Formicidae). Sociobiology 2004, 44, 265–297. [Google Scholar]
- Yamaguchi, T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan I. Analysis of ant species richness. Ecol. Res. 2004, 19, 209–216. [Google Scholar] [CrossRef]
- Clarke, K.M.; Fisher, B.L.; LeBuhn, G. The influence of urban park characteristics on ant (Hymenoptera, Formicidae) communities. Urban Ecosyst. 2008, 11, 317–334. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; De Simoni, L.; Galassi, D.M.P. Island biogeography of insect conservation in urban green spaces. Environ. Conserv. 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Radchenko, A.G.; Elmes, G.W. Myrmica Ants (Hymenoptera: Formicidae) of the Old World; Fauna Mundi 3; Natura Optima dux Foundation: Warszawa, Poland, 2010. [Google Scholar]
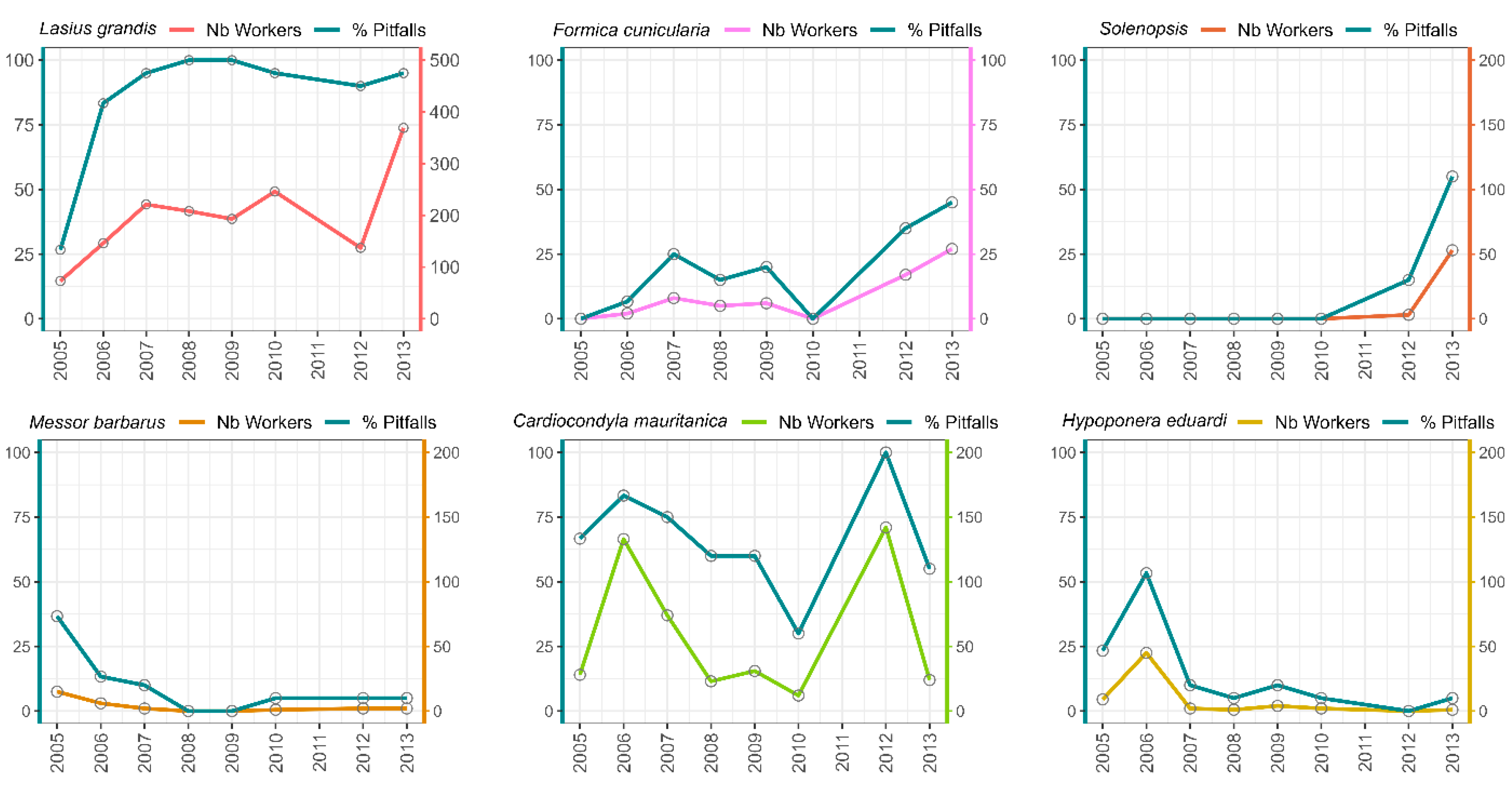
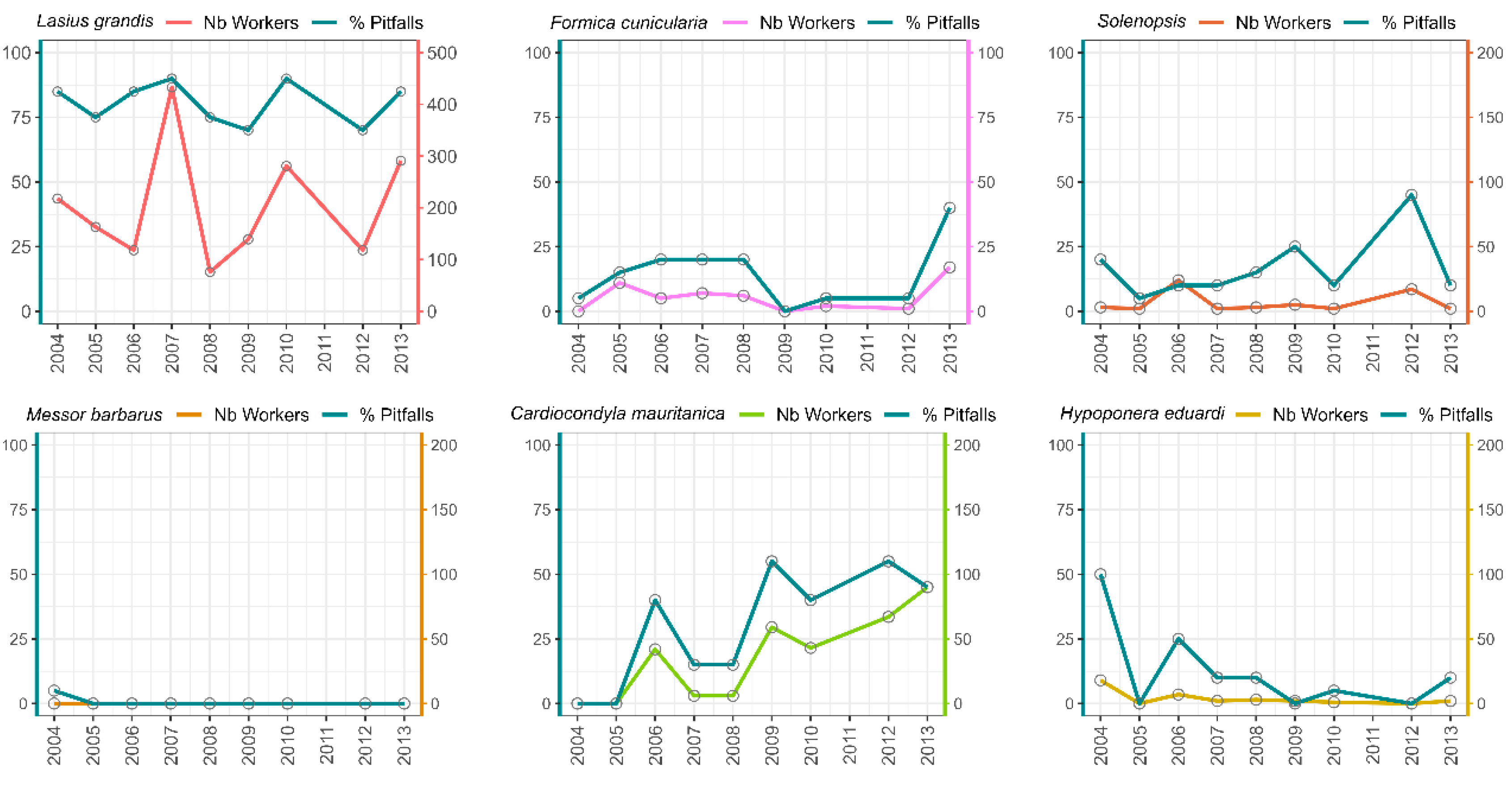
| Species | Garden 1 | Garden 2 | Garden 3 | Garden 4 | Total |
|---|---|---|---|---|---|
| Aphaenogaster gibbosa (Latreille, 1798) | 14 | 11 | 80 | 56 | 161 |
| Aphaenogaster senilis Mayr, 1853 | 31 | 48 | 54 | 44 | 177 |
| Camponotus barbaricus Emery, 1905 | 0 | 21 | 55 | 16 | 92 |
| Camponotus lateralis (Olivier, 1792) | 0 | 0 | 0 | 1 | 1 |
| Camponotus micans (Nylander, 1856) | 0 | 1 | 0 | 0 | 1 |
| Cardiocondyla mauritanica Forel, 1890 | 107 | 53 | 3 | 5 | 168 |
| Cataglyphis rosenhaueri Santschi, 1925 | 2 | 2 | 2 | 0 | 6 |
| Crematogaster auberti Emery, 1869 | 2 | 2 | 2 | 0 | 6 |
| Crematogaster scutellaris (Olivier, 1792) | 0 | 2 | 4 | 5 | 11 |
| Crematogaster sordidula (Nylander, 1849) | 0 | 1 | 0 | 0 | 1 |
| Formica cunicularia Latreille, 1798 | 30 | 25 | 3 | 0 | 58 |
| Formica gerardi Bondroit 1917 | 2 | 3 | 0 | 0 | 5 |
| Hypoponera eduardi (Forel, 1894) | 26 | 20 | 1 | 1 | 48 |
| Lasius grandis Forel, 1909 | 141 | 137 | 38 | 107 | 423 |
| Messor barbarus Linnaeus, 1767 | 18 | 0 | 0 | 0 | 18 |
| Myrmica aloba Forel, 1909 | 0 | 0 | 0 | 10 | 10 |
| Pheidole pallidula (Nylander, 1849) | 79 | 100 | 100 | 127 | 406 |
| Plagiolepis pygmaea (Latreille, 1798) | 1 | 56 | 98 | 64 | 219 |
| Plagiolepis schmitzii Forel, 1895 | 8 | 15 | 6 | 6 | 35 |
| Solenopsis sp. cfr | 14 | 29 | 21 | 33 | 97 |
| Strumigenys membranifera (Emery, 1869) | 5 | 7 | 1 | 0 | 13 |
| Tapinoma erraticum (Latreille, 1798) | 4 | 13 | 14 | 14 | 45 |
| Tapinoma madeirense Forel, 1895 | 1 | 18 | 4 | 12 | 35 |
| Tapinoma nigerrimum group (Nylander, 1856) | 13 | 8 | 8 | 4 | 33 |
| Temnothorax alfacarensis Tinaut & Reyes-López, 2020 | 0 | 0 | 1 | 1 | 2 |
| Temnothorax pardoi Tinaut, 1987 | 0 | 3 | 1 | 4 | 8 |
| Temnothorax racovitzai Bondroit, 1918 | 0 | 6 | 0 | 4 | 10 |
| Tetramorium caespitum (Linnaeus, 1758) | 23 | 0 | 0 | 0 | 23 |
| Tetramorium forte Forel, 1904 | 4 | 1 | 0 | 0 | 5 |
| Tetramorium semilaeve André, 1883 | 23 | 1 | 22 | 1 | 47 |
| Species | Greenery Category | Indicator Value (%) | p |
|---|---|---|---|
| (a) Gardens vs. natural/seminatural areas. | |||
| Lasius grandis | Garden | 85.1 | 0.001 |
| Pheidole pallidula | Garden | 79.2 | 0.001 |
| Cardiocondyla mauritanica | Garden | 70.7 | 0.001 |
| Plagiolepis pygmaea | Garden | 69.3 | 0.002 |
| Solenopsis spp. | Garden | 63.9 | 0.021 |
| Formica cunicularia | Garden | 56.5 | 0.001 |
| Hypoponera eduardi | Garden | 54.9 | 0.001 |
| Tapinoma erraticum | Garden | 52.8 | 0.013 |
| Strumigenys membranifera | Garden | 40.8 | 0.005 |
| Temnothorax pardoi | Garden | 35.4 | 0.015 |
| Messor barbarus | Natural/Seminatural | 87.9 | 0.001 |
| Plagiolepis shcmitzii | Natural/Seminatural | 79.1 | 0.001 |
| Tetramorium semilaeve | Natural/Seminatural | 77.2 | 0.001 |
| Aphaenogaster gibbosa | Natural/Seminatural | 76.4 | 0.001 |
| Aphaenogaster senilis | Natural/Seminatural | 76.2 | 0.001 |
| Tapinoma nigerrimum | Natural/Seminatural | 70.4 | 0.001 |
| Crematogaster auberti | Natural/Seminatural | 64.2 | 0.001 |
| Temnothorax tyndalei | Natural/Seminatural | 57.2 | 0.001 |
| Temnothorax alfacarensis | Natural/Seminatural | 54.9 | 0.001 |
| Camponotus micans | Natural/Seminatural | 54.3 | 0.001 |
| Cataglyphis velox | Natural/Seminatural | 51.9 | 0.001 |
| Cataglyphis rosenhaueri | Natural/Seminatural | 45.3 | 0.009 |
| Camponotus pilicornis | Natural/Seminatural | 43.9 | 0.001 |
| Tetramorium forte | Natural/Seminatural | 41.2 | 0.025 |
| Goniomma hispanicum | Natural/Seminatural | 36.7 | 0.003 |
| Messor celiae | Natural/Seminatural | 31 | 0.011 |
| (b) Young garden (maturity status = 0) vs. mature gardens (unwatered, maturity status = 1; watered, maturity status = 2). | |||
| Cardiocondyla mauritanica | 0 | 73.9 | 0.001 |
| Messor barbarus | 0 | 71.1 | 0.001 |
| Hypoponera eduardi | 0 | 60.3 | 0.001 |
| Tetramorium semilaeve | 0 | 57.2 | 0.007 |
| Tetramorium caespitum | 0 | 52.7 | 0.002 |
| Plagiolepis pygmaea | 1 | 68.8 | 0.001 |
| Solenopsis spp. | 1 | 61.5 | 0.027 |
| Formica cunicularia | 1 | 59.6 | 0.002 |
| Strumigenys membranifera | 1 | 50.9 | 0.024 |
| Temnothorax racovitzai | 1 | 44.5 | 0.025 |
| Aphaennogaster gibbosa | 2 | 74.7 | 0.001 |
| Camponotus barbarus | 2 | 62.5 | 0.006 |
| Myrmica aloba | 2 | 37.3 | 0.045 |
| Garden 1 | Garden 2 | Garden 3 | Garden 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | z | p | z | p | z | p | z | p | |
| Number of workers | L. grandis | 2.57 | 0.01 | 0.20 | 0.838 | 1.07 | 0.284 | −1.03 | 0.305 |
| F. cunicularia | 2.82 | 0.005 | 0.48 | 0.634 | 0 | 1 | - | - | |
| Solenopsis spp. | 2.57 | 0.024 | 0.13 | 0.896 | −0.53 | 0.598 | 0.43 | 0.664 | |
| C. mauritanica | −0.8 | 0.936 | 2.59 | 0.009 | 1.18 | 0.237 | −3.23 | 0.001 | |
| H. eduardi | −3.14 | 0.002 | −2.39 | 0.017 | 0 | 1 | 0 | 1 | |
| M. barbarus | −1.84 | 0.06 | - | - | - | - | - | - | |
| Number of pitfalls | L. grandis | 2.27 | 0.024 | −0.05 | 0.957 | −0.74 | 0.459 | −1.42 | 0.157 |
| F. cunicularia | 2.72 | 0.05 | 0.83 | 0.404 | 0.002 | 0.998 | - | - | |
| Solenopsis spp. | 2.86 | 0.004 | 1.19 | 0.232 | −1.83 | 0.06 | 1.58 | 0.113 | |
| C. mauritanica | −0.44 | 0.659 | 2.90 | 0.004 | 0.88 | 0.381 | 0.82 | 0.413 | |
| H. eduardi | −3.11 | 0.002 | −2.05 | 0.041 | 0.001 | 0.999 | −0.001 | 0.999 | |
| M. barbarus | −2.69 | 0.007 | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trigos-Peral, G.; Reyes-López, J.L. The Aging of Urban Gardens Can Enhance Their Role as Refuges for Local Ant Species. Diversity 2025, 17, 64. https://doi.org/10.3390/d17010064
Trigos-Peral G, Reyes-López JL. The Aging of Urban Gardens Can Enhance Their Role as Refuges for Local Ant Species. Diversity. 2025; 17(1):64. https://doi.org/10.3390/d17010064
Chicago/Turabian StyleTrigos-Peral, Gema, and Joaquín L. Reyes-López. 2025. "The Aging of Urban Gardens Can Enhance Their Role as Refuges for Local Ant Species" Diversity 17, no. 1: 64. https://doi.org/10.3390/d17010064
APA StyleTrigos-Peral, G., & Reyes-López, J. L. (2025). The Aging of Urban Gardens Can Enhance Their Role as Refuges for Local Ant Species. Diversity, 17(1), 64. https://doi.org/10.3390/d17010064







