Multidimensional Environmental Drivers of Bamboo Species Richness on Subtropical Islands
Abstract
1. Introduction
2. Materials and Methods
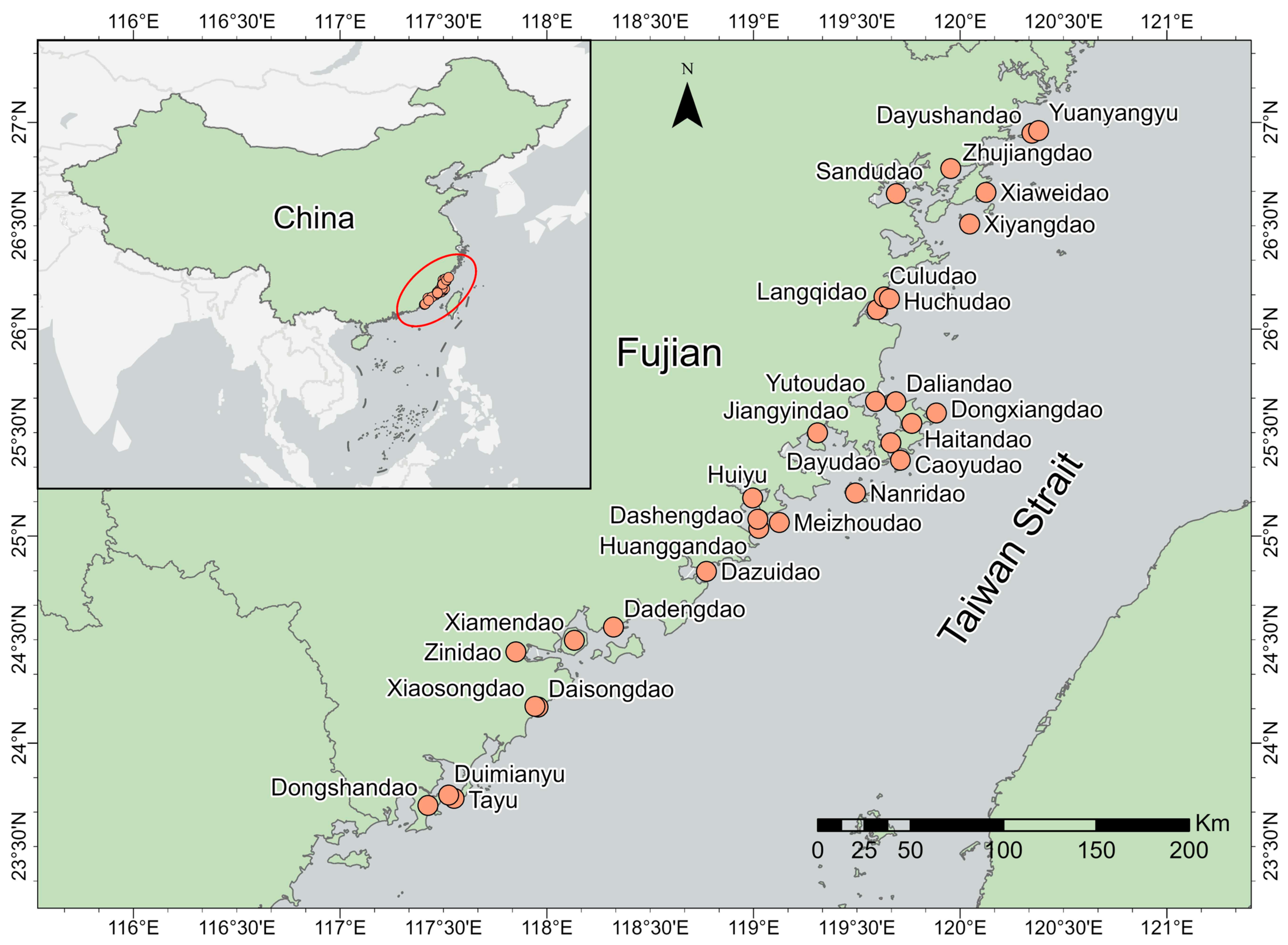
2.1. Study Area
2.2. Species Data Collection
2.3. Environmental Variables of Islands
2.3.1. Climate
2.3.2. Island Scale
2.3.3. Shape
2.3.4. Isolation
2.3.5. Development Intensity and Anthropogenic Disturbance
2.3.6. Habitat Heterogeneity
2.4. Data Analysis
3. Results and Analysis
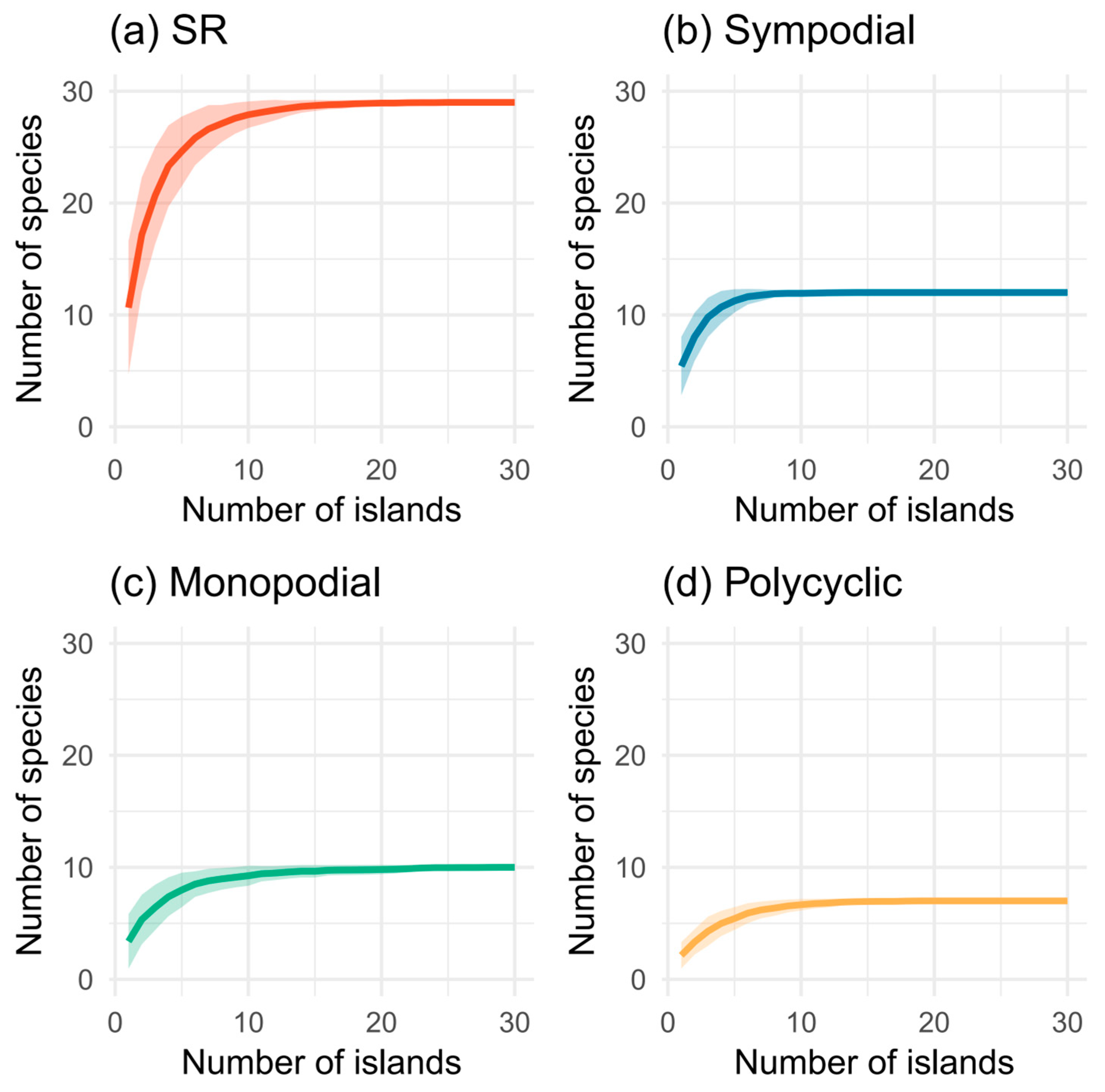
3.1. Composition of Bamboo Species on Islands
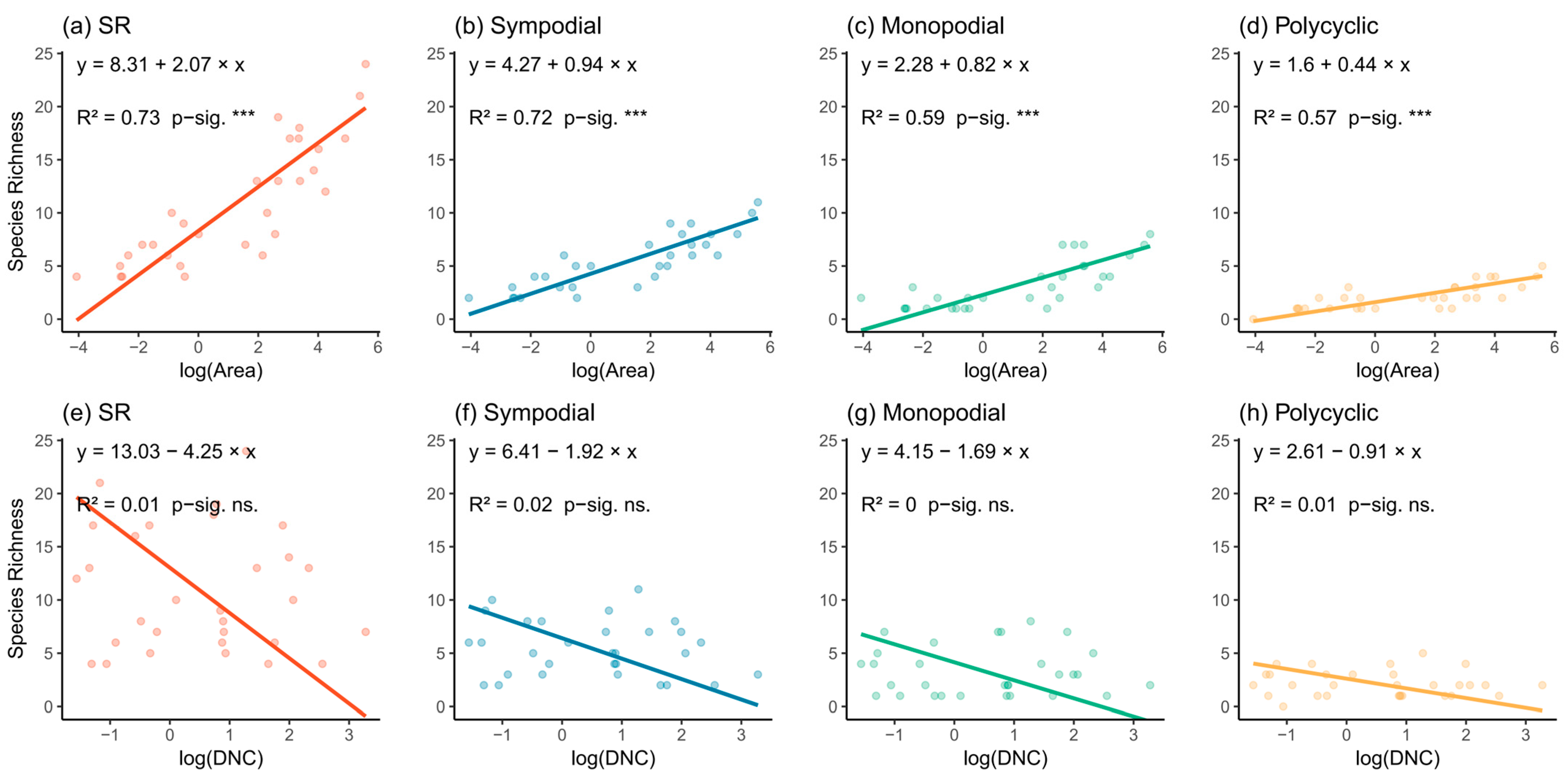
3.2. Relationship Between Species Richness, Island Area, and Isolation
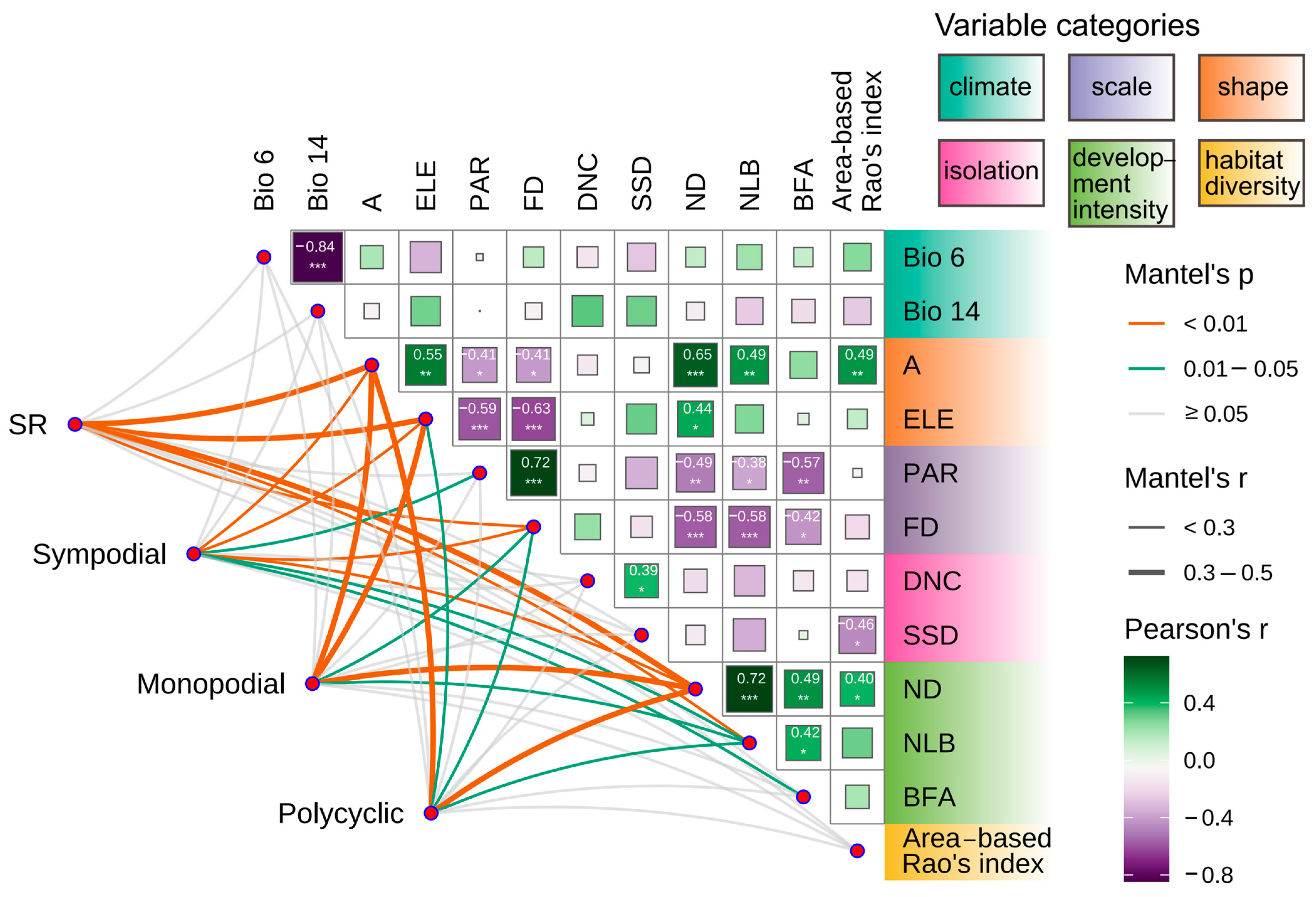
3.3. Bamboo Species and Island Environmental Factors
3.4. Multidimensional Driving Factors of Island Bamboo Species
4. Discussion
4.1. Area Effect
4.2. Isolation Effect
4.3. Development Intensity and Habitat Heterogeneity on Islands
4.4. Climatic Factors
4.5. Implications for the Conservation and Management of Island Bamboo Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Climate | Island Characteristic | Anthropogenic Disturbance | Q index | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Island | Bio 6 (°C) | Bio 14 (mm) | A (km2) | ELE (m) | PAR | FD | DNC (km) | SSD (km) | ND | NLB | BFA (%) | Alpha 1 | Alpha 2 | SR | Sympodial | Monopodial | Polycyclic |
| Caoyudao | 9.06 | 30 | 7.05 | 212.30 | 2.29 | 1.25 | 4.29 | 3.63 | 10 | 0 | 0.37 | 0.09 | 0.17 | 13 | 7 | 4 | 2 |
| Culudao | 7.42 | 37 | 14.40 | 232.60 | 1.42 | 1.19 | 0.26 | 0.26 | 9 | 1 | 0.49 | 0.17 | 0.23 | 13 | 6 | 4 | 3 |
| Dadengdao | 8.89 | 23 | 12.99 | 41.80 | 1.45 | 1.18 | 0.62 | 5.74 | 8 | 2 | 0.70 | 0.21 | 0.27 | 8 | 5 | 2 | 1 |
| Daisongdao | 9.09 | 25 | 0.36 | 27.30 | 7.71 | 1.28 | 0.40 | 0.40 | 6 | 1 | 0.79 | 0.16 | 0.22 | 6 | 3 | 1 | 2 |
| Daliandao | 8.77 | 32 | 9.96 | 238.50 | 2.03 | 1.24 | 7.88 | 1.18 | 6 | 1 | 0.17 | 0.09 | 0.17 | 10 | 5 | 3 | 2 |
| Dashengdao | 9.28 | 23 | 0.07 | 44.20 | 17.78 | 1.26 | 2.54 | 1.28 | 0 | 0 | 0.00 | 0.15 | 0.21 | 5 | 3 | 1 | 1 |
| Dayudao | 8.88 | 26 | 0.22 | 45.10 | 12.38 | 1.25 | 2.47 | 1.04 | 1 | 0 | 0.13 | 0.15 | 0.21 | 7 | 4 | 2 | 1 |
| Dayushandao | 5.13 | 40 | 21.22 | 541.40 | 1.41 | 1.18 | 6.62 | 5.27 | 6 | 0 | 0.22 | 0.13 | 0.18 | 7 | 8 | 7 | 2 |
| Dazuidao | 9.48 | 24 | 0.61 | 103.10 | 8.39 | 1.27 | 2.33 | 2.33 | 2 | 0 | 0.04 | 0.15 | 0.21 | 9 | 5 | 2 | 2 |
| Dongshandao | 10.35 | 26 | 220.18 | 274.30 | 0.51 | 1.20 | 0.31 | 0.31 | 35 | 5 | 0.24 | 0.19 | 0.26 | 1 | 10 | 7 | 4 |
| Dogxiangdao | 8.96 | 36 | 4.80 | 134.60 | 4.10 | 1.28 | 26.50 | 2.30 | 8 | 0 | 0.46 | 0.20 | 0.26 | 7 | 3 | 2 | 2 |
| Duimianyu | 10.74 | 27 | 0.10 | 25.80 | 20.71 | 1.30 | 5.78 | 0.49 | 2 | 0 | 0.01 | 0.10 | 0.16 | 6 | 2 | 3 | 1 |
| Haitandao | 8.77 | 32 | 267.13 | 438.20 | 0.48 | 1.24 | 3.59 | 1.20 | 51 | 2 | 0.62 | 0.24 | 0.31 | 4 | 11 | 8 | 5 |
| Huanggandao | 9.28 | 23 | 0.55 | 72.40 | 8.27 | 1.26 | 0.72 | 0.72 | 0 | 0 | 0.00 | 0.15 | 0.23 | 5 | 3 | 1 | 1 |
| Huchudao | 7.42 | 37 | 0.07 | 33.72 | 22.56 | 1.32 | 5.20 | 0.38 | 0 | 0 | 0.00 | 0.15 | 0.20 | 4 | 2 | 1 | 1 |
| Huiyu | 8.37 | 24 | 0.41 | 59.90 | 13.14 | 1.31 | 1.11 | 1.11 | 2 | 0 | 0.55 | 0.16 | 0.22 | 10 | 6 | 1 | 3 |
| Jiangyindao | 8.67 | 25 | 69.75 | 429.10 | 0.72 | 1.19 | 0.21 | 0.21 | 14 | 7 | 0.41 | 0.15 | 0.21 | 2 | 6 | 4 | 2 |
| Langqidao | 7.42 | 37 | 55.50 | 275.00 | 0.59 | 1.16 | 0.56 | 0.56 | 21 | 5 | 0.60 | 0.17 | 0.23 | 16 | 8 | 4 | 4 |
| Meizhoudao | 9.28 | 23 | 14.35 | 95.20 | 2.32 | 1.27 | 2.19 | 2.19 | 3 | 0 | 0.67 | 0.15 | 0.22 | 9 | 9 | 7 | 3 |
| Nanridao | 9.10 | 27 | 47.24 | 116.30 | 2.24 | 1.21 | 7.34 | 3.37 | 10 | 0 | 0.85 | 0.15 | 0.21 | 4 | 7 | 3 | 4 |
| Sandudao | 5.84 | 42 | 29.17 | 460.60 | 1.05 | 1.18 | 2.08 | 1.93 | 3 | 0 | 0.22 | 0.18 | 0.24 | 8 | 7 | 7 | 4 |
| Tayu | 10.74 | 27 | 1.01 | 91.30 | 6.82 | 1.24 | 2.44 | 1.16 | 3 | 0 | 0.26 | 0.19 | 0.26 | 8 | 5 | 2 | 1 |
| Xiamendao | 9.24 | 25 | 134.84 | 339.60 | 0.49 | 1.14 | 0.71 | 0.71 | 90 | 7 | 0.80 | 0.21 | 0.27 | 17 | 8 | 6 | 3 |
| Xiaosongdao | 9.09 | 25 | 0.02 | 26.00 | 30.37 | 1.29 | 0.35 | 0.35 | 0 | 1 | 0.00 | 0.20 | 0.27 | 4 | 2 | 2 | 0 |
| Xiaweidao | 6.23 | 46 | 0.08 | 35.51 | 22.42 | 1.29 | 0.27 | 0.27 | 0 | 0 | 0.00 | 0.16 | 0.22 | 4 | 2 | 1 | 1 |
| Xiyangdao | 6.23 | 46 | 29.80 | 221.00 | 0.72 | 1.21 | 10.23 | 6.99 | 5 | 0 | 0.25 | 0.07 | 0.11 | 13 | 6 | 5 | 2 |
| Yuanyangyu | 5.13 | 40 | 0.64 | 149.81 | 9.01 | 1.28 | 12.85 | 0.25 | 0 | 0 | 0.00 | 0.12 | 0.18 | 4 | 2 | 1 | 1 |
| Yutoudao | 8.76 | 28 | 8.56 | 77.90 | 2.57 | 1.26 | 2.40 | 0.25 | 17 | 0 | 0.86 | 0.09 | 0.15 | 6 | 4 | 1 | 1 |
| Zhujiangdao | 5.68 | 45 | 0.15 | 47.30 | 12.82 | 1.23 | 0.81 | 0.81 | 2 | 0 | 0.66 | 0.13 | 0.19 | 7 | 4 | 1 | 2 |
| Zinidao | 9.23 | 26 | 28.56 | 5.00 | 1.26 | 1.20 | 0.28 | 0.28 | 65 | 9 | 0.91 | 0.15 | 0.21 | 17 | 9 | 5 | 3 |
| Number | Number | Genus | Species |
|---|---|---|---|
| 1 | Poaceae | Dendrocalamus | Dendrocalamus latiflorus Munro |
| 2 | Poaceae | Bambusa | Bambusa vulgaris Schrad. ex J. C. Wendland |
| 3 | Poaceae | Bambusa | Bambusa oldhamii Munro |
| 4 | Poaceae | Bambusa | Bambusa ventricosa McClure |
| 5 | Poaceae | Bambusa | Bambusa blumeana Schult. and Schult. f. |
| 6 | Poaceae | Bambusa | Bambusa multiplex (Lour.) Raeusch. ex Schult. and Schult. f. |
| 7 | Poaceae | Bambusa | Bambusa albolineata (McClure) L. C. Chia |
| 8 | Poaceae | Bambusa | Bambusa emeiensis L. C. Chia and H. L. Fung |
| 9 | Poaceae | Schizostachyum | Schizostachyum funghomii McClure |
| 10 | Poaceae | Dendrocalamus | Dendrocalamus pulverulentus L. C. Chia and But |
| 11 | Poaceae | Bambusa | Bambusa textilis McClure |
| 12 | Poaceae | Bambusa | Bambusa multiplex (Lour.) Raeusch. ex Schult. and Schult. f. |
| 13 | Poaceae | Phyllostachys | Phyllostachys edulis (Carrière) J. Houz. |
| 14 | Poaceae | Phyllostachys | Phyllostachys sulphurea var. viridis R. A. Young |
| 15 | Poaceae | Phyllostachys | Phyllostachys rivalis H. R. Zhao and A. T. Liu |
| 16 | Poaceae | Phyllostachys | Phyllostachys iridescens C. Y. Yao and S. Y. Chen |
| 17 | Poaceae | Phyllostachys | Phyllostachys vivax McClure |
| 18 | Poaceae | Phyllostachys | Phyllostachys rubicunda T. W. Wen |
| 19 | Poaceae | Phyllostachys | Phyllostachys makinoi Hayata |
| 20 | Poaceae | Phyllostachys | Phyllostachys nigra (Lodd. ex Lindl.) Munro |
| 21 | Poaceae | Bambusa | Bambusa cerosissima McClure |
| 22 | Poaceae | Phyllostachys | Phyllostachys heteroclada Oliv. |
| 23 | Poaceae | Indocalamus | Indocalamus tessellatus (Munro) P. C. Keng |
| 24 | Poaceae | Indocalamus | Indocalamus latifolius (Keng) McClure |
| 25 | Poaceae | Indocalamus | Indocalamus longiauritus Hand.-Mazz. |
| 26 | Poaceae | Pseudosasa | Pseudosasa amabilis (McClure) P. C. Keng ex S. L. Chen and al. |
| 27 | Poaceae | Pleioblastus | Pleioblastus maculatus (McClure) C. D. Chu and C. S. Chao |
| 28 | Poaceae | Pleioblastus | Pleioblastus chino var. hisauchii Makino |
| 29 | Poaceae | Shibataea | Shibataea nanpingensis var. fujianica (Z. D. Zhu and H. Y. Zhou) C. H. Hu |
References
- Patiño, J.; Whittaker, R.J.; Borges, P.A.; Fernández-Palacios, J.M.; Ah-Peng, C.; Araújo, M.B.; Ávila, S.P.; Cardoso, P.; Cornuault, J.; de Boer, E.J.; et al. A Roadmap for Island Biology: 50 Fundamental Questions after 50 Years of the Theory of Island Biogeography. J. Biogeogr. 2017, 44, 963–983. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Paulay, G. Biodiversity on Oceanic Islands: Its Origin and Extinction1. Am. Zool. 1994, 34, 134–144. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Whittaker, R.J.; Triantis, K.A.; Ladle, R.J. A General Dynamic Theory of Oceanic Island Biogeography. J. Biogeogr. 2008, 35, 977–994. [Google Scholar] [CrossRef]
- Pinheiro, H.T.; Bernardi, G.; Simon, T.; Joyeux, J.-C.; Macieira, R.M.; Gasparini, J.L.; Rocha, C.; Rocha, L.A. Island Biogeography of Marine Organisms. Nature 2017, 549, 82–85. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. An Equilibrium Theory of Insular Zoogeography. Evolution 1963, 17, 373–387. [Google Scholar] [CrossRef]
- Heaney, L. Dynamic Disequilibrium: A Long-Term, Large-Scale Perspective on the Equilibrium Model of Island Biogeography. Glob. Ecol. Biogeogr. 2000, 9, 59–74. [Google Scholar] [CrossRef]
- Lomolino, M.V. A Call for a New Paradigm of Island Biogeography: Island Paradigms. Glob. Ecol. Biogeogr. 2000, 9, 1–6. [Google Scholar] [CrossRef]
- Kalmar, A.; Currie, D.J. A Global Model of Island Biogeography. Glob. Ecol. Biogeogr. 2006, 15, 72–81. [Google Scholar] [CrossRef]
- Whittaker, R.J. Scale, succession and complexity in island biogeography: Are we asking the right questions?: Scale, succession and complexity in island biogeography. Glob. Ecol. Biogeogr. 2000, 9, 75–85. [Google Scholar] [CrossRef]
- Triantis, K.A.; Mylonas, M.; Lika, K.; Vardinoyannis, K. A Model for the Species–Area–Habitat Relationship. J. Biogeogr. 2003, 30, 19–27. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J.; Ackerman, J.D. Tremblay Island Biogeography of Native and Alien Plant Species: Contrasting Drivers of Diversity across the Lesser Antilles. Divers. Distrib. 2020, 26, 1539–1550. [Google Scholar] [CrossRef]
- Kubota, Y.; Shiono, T.; Kusumoto, B. Role of Climate and Geohistorical Factors in Driving Plant Richness Patterns and Endemicity on the East Asian Continental Islands. Ecography 2015, 38, 639–648. [Google Scholar] [CrossRef]
- Yu, J.; Shen, L.; Li, D.D.; Guo, S.L. Determinants of Bryophyte Species Richness on the Zhoushan Archipelago, China. Basic Appl. Ecol. 2019, 37, 38–50. [Google Scholar] [CrossRef]
- Heatwole, H. Factors Affecting the Number of Species of Plants on Islands of the Great Barrier Reef, Australia. J. Biogeogr. 1991, 18, 213. [Google Scholar] [CrossRef]
- Valli, A.-T.; Kougioumoutzis, K.; Iliadou, E.; Panitsa, M.; Trigas, P. Determinants of Alpha and Beta Vascular Plant Diversity in Mediterranean Island Systems: The Ionian Islands, Greece. Nord. J. Bot. 2019, 37, e02156. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Ye, W.; Xing, F.-W. Bryophyte Diversity on a Tropical Continental Island (Hainan, China): Potential Vulnerable Species and Environmental Indicators. J. Bryol. 2019, 41, 350–360. [Google Scholar] [CrossRef]
- Irl, S.D.H.; Schweiger, A.H.; Steinbauer, M.J.; Ah-Peng, C.; Arevalo, J.R.; Beierkuhnlein, C.; Chiarucci, A.; Daehler, C.C.; Fernandez-Palacios, J.M.; Flores, O.; et al. Human Impact, Climate and Dispersal Strategies Determine Plant Invasion on Islands. J. Biogeogr. 2021, 48, 1889–1903. [Google Scholar] [CrossRef]
- Honnay, O.; Piessens, K.; Van Landuyt, W.; Hermy, M.; Gulinck, H. Satellite Based Land Use and Landscape Complexity Indices as Predictors for Regional Plant Species Diversity. Landsc. Urban Plan. 2003, 63, 241–250. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Triantis, K.A. Habitat Diversity, Ecological Requirements of Species and the Small Island Effect. Divers. Distrib. 2009, 15, 131–140. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Hortal, J. Time, Area and Isolation: Factors Driving the Diversification of Azorean Arthropods. J. Biogeogr. 2009, 36, 178–191. [Google Scholar] [CrossRef]
- Cabral, J.S.; Weigelt, P.; Kissling, W.D.; Kreft, H. Biogeographic, Climatic and Spatial Drivers Differentially Affect Alpha-, Beta- and Gamma-Diversities on Oceanic Archipelagos. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133246. [Google Scholar] [CrossRef]
- Liu, J.L.; Vellend, M.; Wang, Z.; Yu, M.J. High Beta Diversity among Small Islands Is Due to Environmental Heterogeneity Rather than Ecological Drift. J. Biogeogr. 2018, 45, 2252–2261. [Google Scholar] [CrossRef]
- Chi, Y.; Zhang, Z.; Gao, J.; Xie, Z.; Zhao, M.; Wang, E. Evaluating Landscape Ecological Sensitivity of an Estuarine Island Based on Landscape Pattern across Temporal and Spatial Scales. Ecol. Indic. 2019, 101, 221–237. [Google Scholar] [CrossRef]
- Jesse, W.A.M.; Behm, J.E.; Helmus, M.R.; Ellers, J. Human Land Use Promotes the Abundance and Diversity of Exotic Species on Caribbean Islands. Glob. Change Biol. 2018, 24, 4784–4796. [Google Scholar] [CrossRef]
- Ibanez, T.; Keppel, G.; Baider, C.; Birkinshaw, C.; Culmsee, H.; Cordell, S.; Florens, F.B.V.; Franklin, J.; Giardina, C.P.; Gillespie, T.W.; et al. Regional Forcing Explains Local Species Diversity and Turnover on Tropical Islands. Glob. Ecol. Biogeogr. 2018, 27, 474–486. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding Factors in the Detection of Species Responses to Habitat Fragmentation. Biol. Rev. 2005, 81, 117. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- McIntyre, S.; Lavorel, S.; Landsberg, J.; Forbes, T.D.A. Disturbance Response in Vegetation–towards a Global Perspective on Functional Traits. J. Veg. Sci. 1999, 10, 621–630. [Google Scholar] [CrossRef]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xing, L.; Liu, T.; Han, N.; Liu, Y.; Zhu, D.; Zheng, J.; et al. Spatiotemporal Evolution and Impacts of Climate Change on Bamboo Distribution in China. J. Environ. Manag. 2019, 248, 109265. [Google Scholar] [CrossRef] [PubMed]
- Cupertino-Eisenlohr, M.A.; Vinicius-Silva, R.; Meireles, L.D.; Eisenlohr, P.V.; Meira-Neto, J.A.A.; Santos-Goncalves, A.P. Stability or Breakdown under Climate Change? A Key Group of Woody Bamboos Will Find Suitable Areas in Its Richness Center. Biodivers. Conserv. 2017, 26, 1845–1861. [Google Scholar] [CrossRef]
- Bhandawat, A.; Sharma, V.; Sharma, H.; Sood, A.; Sharma, R.K. Development and Crosstransferability of Functionally Relevant Microsatellite Markers in Dendrocalamus Latiflorus and Related Bamboo Species. J. Genet. 2015, 94, 48–55. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Raven, P.; Hong, D.Y. Flora of China; Botanical Garden Press: St. Louis, MO, USA, 2013. [Google Scholar]
- Hanjiao, G.; Cancan, Z.; Jinsong, W.; Xuewen, S.; Ruixue, X.; Bin, L.; Fusheng, C.; Wensheng, B. Variation in basic morphological and functional traits of Chinese bamboo. Biodivers. Sci. 2019, 27, 585–594. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Zhang, Z.E. The Value and Conservation Progress of Bamboo Biodiversity in China. Bamboo Res. J. 2003, 17, 7–12. [Google Scholar]
- Yu, M.J.; Hu, G.; Feeley, K.J.; Wu, J.G.; Ding, P. Richness and Composition of Plants and Birds on Land-Bridge Islands: Effects of Island Attributes and Differential Responses of Species Groups. J. Biogeogr. 2012, 39, 1124–1133. [Google Scholar] [CrossRef]
- Hu, G.; Feeley, K.J.; Wu, J.; Xu, G.; Yu, M. Determinants of Plant Species Richness and Patterns of Nestedness in Fragmented Landscapes: Evidence from Land-Bridge Islands. Landsc. Ecol. 2011, 26, 1405–1417. [Google Scholar] [CrossRef]
- Fang, J.; Wang, X.; Tang, Z. Local and Regional Processes Jointly Control Community Species Diversity: The Species Pool Hypothesis. Biodiversity 2009, 17, 605–612. [Google Scholar]
- Editorial Committee of the Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 2004. [Google Scholar]
- Wu, J.G. Effects of Changing Scale on Landscape Pattern Analysis: Scaling Relations. Landsc. Ecol. 2004, 19, 125–138. [Google Scholar] [CrossRef]
- Weigelt, P.; Kreft, H. Quantifying Island Isolation–Insights from Global Patterns of Insular Plant Species Richness. Ecography 2013, 36, 417–429. [Google Scholar] [CrossRef]
- Rocchini, D.; Marcantonio, M.; Ricotta, C. Measuring Rao’s Q Diversity Index from Remote Sensing: An Open Source Solution. Ecol. Indic. 2017, 72, 234–238. [Google Scholar] [CrossRef]
- Rocchini, D.; Thouverai, E.; Marcantonio, M.; Iannacito, M.; Da Re, D.; Torresani, M.; Bacaro, G.; Bazzichetto, M.; Bernardi, A.; Foody, G.M.; et al. Rasterdiv—An Information Theory Tailored R Package for Measuring Ecosystem Heterogeneity from Space: To the Origin and Back. Methods Ecol. Evol. 2021, 12, 1093–1102. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models; Irwin Professional Publishing: Chicago, IL, USA, 1996; ISBN 978-0-256-11736-3. [Google Scholar]
- Bivand, R.S.; Wong, D.W.S. Comparing Implementations of Global and Local Indicators of Spatial Association. Test 2018, 27, 716–748. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. J. Veg. Sci. 2020, 14, 6. [Google Scholar]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3—An R Package for Estimation and Inference about Allometric Lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Henseler, J.; Ringle, C.M.; Sinkovics, R.R. The Use of Partial Least Squares Path Modeling in International Marketing. In New Challenges to International Marketing; Emerald Group Publishing Limited: Leeds, UK, 2009; Volume 20, pp. 277–319. ISBN 978-1-84855-468-9. [Google Scholar]
- Šegota, T.; Chen, N.; Golja, T. The Impact of Self-Congruity and Evaluation of the Place on WOM: Perspectives of Tourism Destination Residents. J. Travel Res. 2022, 61, 800–817. [Google Scholar] [CrossRef]
- Lomolino, M.V. The species-area relationship: New challenges for an old pattern. Prog. Phys. Geogr.: Earth Environ. 2001, 25, 1–21. [Google Scholar] [CrossRef]
- Panitsa, M.; Tzanoudakis, D.; Triantis, K.A.; Sfenthourakis, S. Patterns of Species Richness on Very Small Islands: The Plants of the Aegean Archipelago. J. Biogeogr. 2006, 33, 1223–1234. [Google Scholar] [CrossRef]
- Santos, A.M.C.; Whittaker, R.J.; Triantis, K.A.; Borges, P.A.V.; Jones, O.R.; Quicke, D.L.J.; Hortal, J. Are Species–Area Relationships from Entire Archipelagos Congruent with Those of Their Constituent Islands? Glob. Ecol. Biogeogr. 2010, 19, 527–540. [Google Scholar] [CrossRef]
- Wang, W.; Tao, J.P.; Song, L.X.; Ran, C.Y. Progress and Prospects in the Population Ecology of Bamboo Species. Guihaia 2006, 4, 412–417. [Google Scholar]
- Chen, Q.B.; Gao, S.P.; Liu, L. Selection of Superior Bamboo Species for Pulp Production and the Industrial Development of Bamboo-Based Paper in Sichuan Province. Bamboo Res. J. 2002, 47–51. [Google Scholar]
- Gu, D.X.; Chen, S.L.; Zheng, W.M.; Mao, X.Q. A Review of Research on the Ecological Adaptability of Bamboo. Bamboo Res. J. 2010, 29, 17–23. [Google Scholar]
- Henneron, L.; Sarthou, C.; de Massary, J.; Ponge, J. Habitat diversity associated to island size and environmental filtering control the species richness of rock-savanna plants in neotropical inselbergs. Ecography 2019, 42, 1536–1547. [Google Scholar] [CrossRef]
- Brown, J.H.; Kodric-Brown, A. Turnover Rates in Insular Biogeography: Effect of Immigration on Extinction. Ecology 1977, 58, 445–449. [Google Scholar] [CrossRef]
- Wang, Z.; Chase, J.M.; Xu, W.; Liu, J.; Wu, D.; Zhang, A.; Wang, J.; Luo, Y.; Yu, M. Higher Trophic Levels and Species with Poorer Dispersal Traits Are More Susceptible to Habitat Loss on Island Fragments. Ecology 2024, 105, e4300. [Google Scholar] [CrossRef]
- Gu, H.-J.; Zhang, C.-C.; Chen, F.-S.; Huang, J.-H.; Wang, J.-S.; Bruelheide, H.; Trogisch, S.; Fang, X.-M.; Li, J.-J.; Bu, W.-S. The Bamboo Rhizome Evolution in China Is Driven by Geographical Isolation and Trait Differentiation. Forests 2021, 12, 1280. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Tan, X.; Wang, W. The Role of Habitat Diversity in Generating the Small-island Effect. Ecography 2020, 43, 1241–1249. [Google Scholar] [CrossRef]
- Chiarucci, A.; Bacaro, G.; Triantis, K.A.; Fernandez-Palacios, J.M. Biogeographical Determinants of Pteridophytes and Spermatophytes on Oceanic Archipelagos. Syst. Biodivers. 2011, 9, 191–201. [Google Scholar] [CrossRef]
- Jonsson, M.; Yeates, G.W.; Wardle, D.A. Patterns of Invertebrate Density and Taxonomic Richness across Gradients of Area, Isolation, and Vegetation Diversity in a Lake-Island System. Ecography 2009, 32, 963–972. [Google Scholar] [CrossRef]
- Negoita, L.; Fridley, J.; Lomolino, M.; Mittelhauser, G.; Craine, J.; Weiher, E. Isolation-driven functional assembly of plant communities on islands. Ecography 2015, 39, 1066–1077. [Google Scholar] [CrossRef]
- Swapna, M.; Dev, B.M. Understanding the Indian mainland–island biogeography through plant dispersal mechanism. Biodivers. Conserv. 2019, 28, 2063–2084. [Google Scholar] [CrossRef]
- Hamid, N.H.; Jawaid, M.; Abdullah, U.H.; Alomar, T.S. Monopodial and Sympodial Bamboos Grown in Tropic and Sub-Tropic Countries—A Review. Bioresources 2023, 18, 6499–6560. [Google Scholar] [CrossRef]
- Editorial Committee of China. Island Chronicles, The Island Chronicles of China, 1st ed.; China Ocean Press: Beijing, China, 2014. [Google Scholar]
- Xie, Y.; Huang, H.; Xie, X.; Ou, J.; Chen, Z.; Lu, X.; Kong, D.; Nong, L.; Lin, M.; Qian, Z.; et al. Landscape, Human Disturbance, and Climate Factors Drive the Species Richness of Alien Invasive Plants on Subtropical Islands. Plants 2024, 13, 2437. [Google Scholar] [CrossRef]
- Petit, S.; Griffiths, L.; Smart, S.S.; Smith, G.M.; Stuart, R.C.; Wright, S.M. Effects of area and isolation of woodland patches on herbaceous plant species richness across great britain. Landsc. Ecol. 2004, 19, 463–472. [Google Scholar] [CrossRef]
- Murakami, K.; Maenaka, H.; Morimoto, Y. Factors Influencing Species Diversity of Ferns and Fern Allies in Fragmented Forest Patches in the Kyoto City Area. Landsc. Urban Plan. 2005, 70, 221–229. [Google Scholar] [CrossRef]
- Tsiftsis, S. The complex effect of heterogeneity and isolation in determining alpha and beta orchid diversity on islands in the aegean archipelago. Syst. Biodivers. 2020, 18, 281–294. [Google Scholar] [CrossRef]
- Jones, G.P.; Barone, G.; Sambrook, K.; Bonin, M.C. Isolation promotes abundance and species richness of fishes recruiting to coral reef patches. Mar. Biol. 2020, 167, 167. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Celesti-Grapow, L.; Basnou, C.; Bazos, I.; Kokkoris, Y.; Blasi, C.; Vilà, M. Comparing naturalized alien plants and recipient habitats across an east–west gradient in the mediterranean basin. J. Biogeogr. 2010, 37, 1811–1823. [Google Scholar] [CrossRef]
- Dale, S. Islands in the forest: Effects of patch size and isolation on farmland bird species richness and community composition of farmland patches in forest landscapes. Landsc. Ecol. 2019, 34, 2823–2836. [Google Scholar] [CrossRef]
- Watling, J.I.; Donnelly, M.A. Fragments as Islands: A Synthesis of Faunal Responses to Habitat Patchiness. Conserv. Biol. 2006, 20, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sanders, N.J.; Liu, J.; Jin, T.; Zhou, H.; Lu, R.; Ding, P.; Si, X. β Diversity among Ant Communities on Fragmented Habitat Islands: The Roles of Species Trait, Phylogeny and Abundance. Ecography 2021, 44, 1568–1578. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological Impacts of Invasive Alien Plants: A Meta-Analysis of Their Effects on Species, Communities and Ecosystems: Ecological Impacts of Invasive Alien Plants. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Otto, R.; Escudero, C.; Fernández-Lugo, S.; Arteaga, M.; Delgado, J.D.; Fernández-Palacios, J.M. Do anthropogenic corridors homogenize plant communities at a local scale? A case studied in tenerife (canary islands). Plant Ecol. 2010, 209, 23–35. [Google Scholar] [CrossRef]
- García-Palacios, P.; Gross, N.; Gaitán, J.; Maestre, F.T. Climate Mediates the Biodiversity–Ecosystem Stability Relationship Globally. Proc. Natl. Acad. Sci. USA 2018, 115, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Irl, S.D.H.; Obermeier, A.; Beierkuhnlein, C.; Steinbauer, M.J. Climate Controls Plant Life-Form Patterns on a High-Elevation Oceanic Island. J. Biogeogr. 2020, 47, 2261–2273. [Google Scholar] [CrossRef]
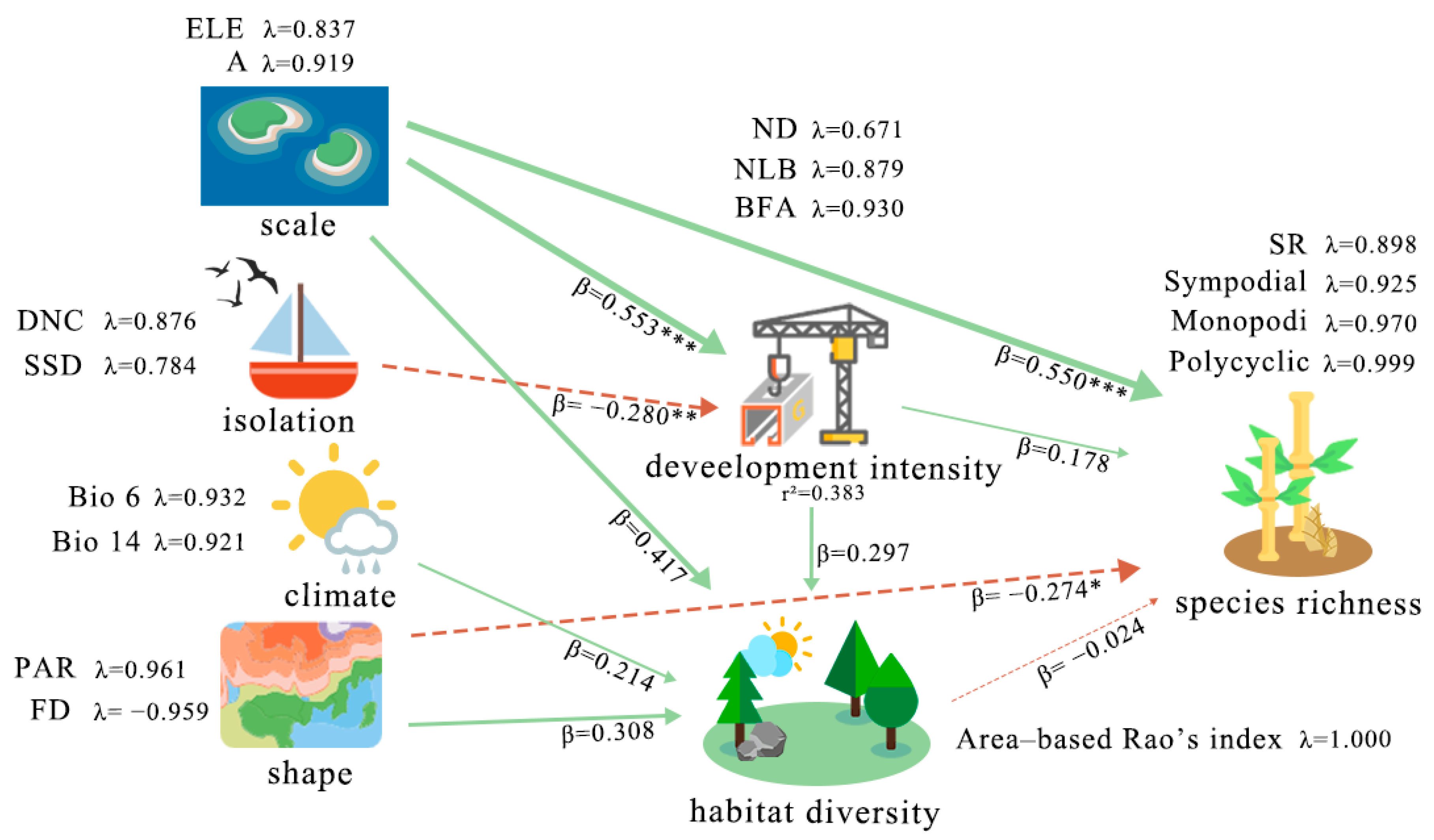
| Construct | Type | Items | Loadings | CR | AVE |
|---|---|---|---|---|---|
| Isolation | Reflective | DNC | 0.876 | 0.817 | 0.691 |
| SSD | 0.784 | ||||
| Scale | Reflective | A | 0.919 | 0.872 | 0.773 |
| ELE | 0.837 | ||||
| Shape | Reflective | PAR | 0.932 | 0.924 | 0.858 |
| FD | 0.921 | ||||
| Climate | Reflective | Bio6 | 0.961 | 0.916 | 0.922 |
| Bio14 | −0.959 | ||||
| Development intensity | Reflective | ND | 0.93 | 0.871 | 0.696 |
| NLB | 0.879 | ||||
| BFA | 0.671 | ||||
| Habitat diversity | Reflective | Area-Based Rao’s Index | 1.000 | 1.000 | 1.000 |
| Species richness | Reflective | SR | 0.999 | 0.973 | 0.9 |
| Sympodial | 0.97 | ||||
| Monopodial | 0.925 | ||||
| Polycyclic | 0.898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, W.; Xie, Y.; Xie, X.; Chen, Z.; Deng, C.; Huang, H. Multidimensional Environmental Drivers of Bamboo Species Richness on Subtropical Islands. Diversity 2025, 17, 46. https://doi.org/10.3390/d17010046
Zhan W, Xie Y, Xie X, Chen Z, Deng C, Huang H. Multidimensional Environmental Drivers of Bamboo Species Richness on Subtropical Islands. Diversity. 2025; 17(1):46. https://doi.org/10.3390/d17010046
Chicago/Turabian StyleZhan, Weifeng, Yanqiu Xie, Xinran Xie, Zujian Chen, Chuanyuan Deng, and Hui Huang. 2025. "Multidimensional Environmental Drivers of Bamboo Species Richness on Subtropical Islands" Diversity 17, no. 1: 46. https://doi.org/10.3390/d17010046
APA StyleZhan, W., Xie, Y., Xie, X., Chen, Z., Deng, C., & Huang, H. (2025). Multidimensional Environmental Drivers of Bamboo Species Richness on Subtropical Islands. Diversity, 17(1), 46. https://doi.org/10.3390/d17010046






