Detection and Assessment of White Flowering Nectar Source Trees and Location of Bee Colonies in Rural and Suburban Environments Using Deep Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. UAV Data Collection
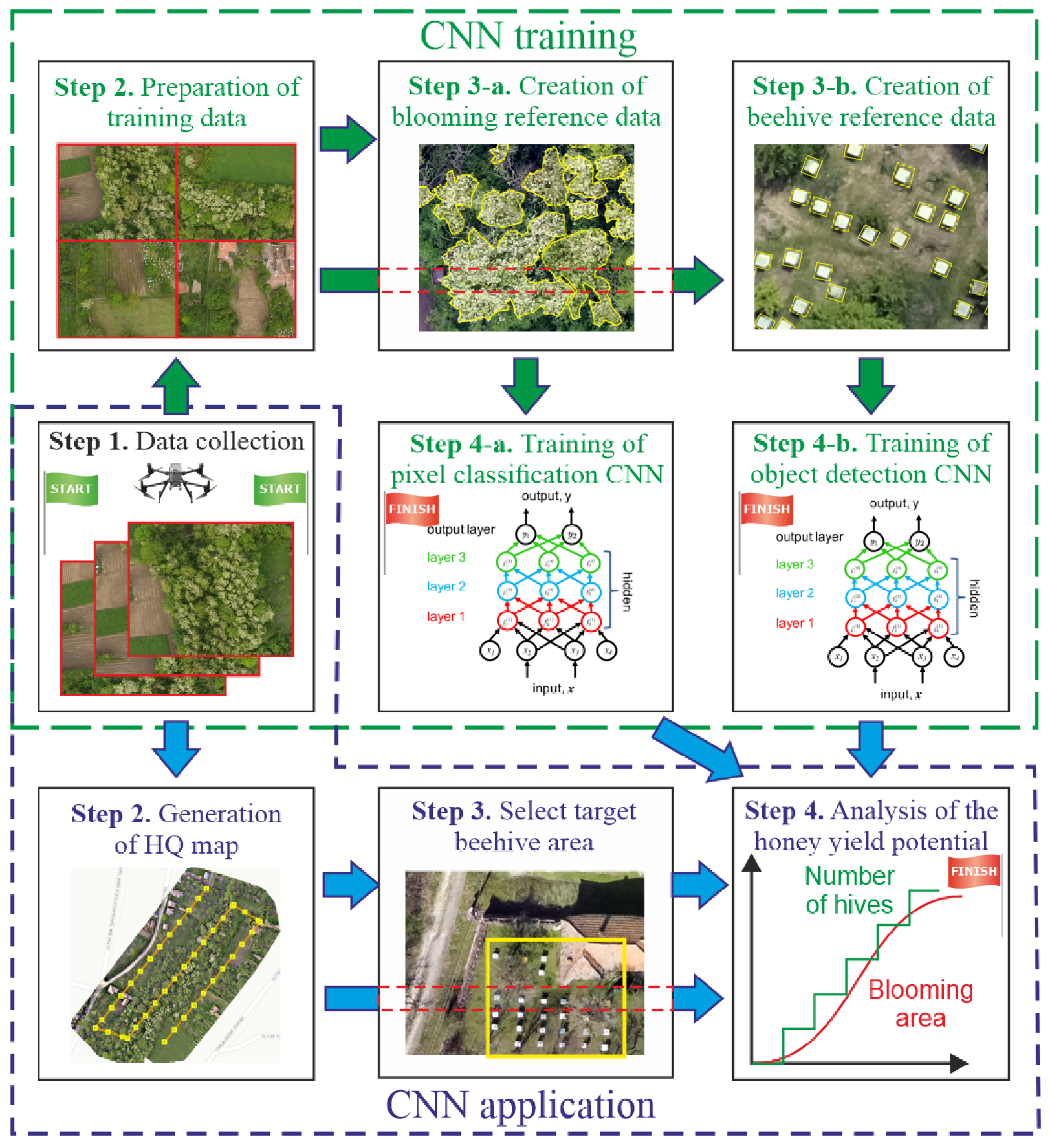
2.3. Data Processing
- Step 1. Collection of data with the use of a UAV—a drone should be used to make visual spectrum pictures of the area of interest, which should include both blooming trees and beehives;
- Step 2. Preparation of training data—at this stage, some of the UAV-obtained images, which contain many blooming trees and beehives, are selected for training and validation purposes. The selected photos could be combined into a single image to facilitate the training process.
- Step 3. Creation of reference data—this step has two aspects:
- ○
- Creating reference data for the identification of blooming trees. This includes marking the available blooming areas over the training image with polygons;
- ○
- Creating reference data for identification and counting of beehives. This includes marking all available beehives in the training image with rectangles.
- Step 4. Training convolutional neural networks for the following:
- ○
- Identification of blooming areas—considering these areas could be with random (polygonal) form, a pixel-based classification algorithm is chosen;
- ○
- Counting beehives—considering they have approximately the same form, an object detection algorithm should be chosen that works with regular rectangular areas.
- Step 1. Collection of data with the use of a UAV—this step could be common with the CNN training methodology, although it is also possible to use different image datasets.
- Step 2. Generation of a high-quality (HQ) map—the obtained drone images are combined into a giant orthomosaic for further analysis.
- Step 3. Selection of areas with beehives—parts of the HQ map containing beehives are selected for counting their number. This step is required to reduce the number of false positives, which might occur in rural and suburban environments. The necessity for this step is explained in the Results section of this study.
- Step 4. Application of the CNN and evaluation of the honey yield potential—this is conducted in three steps:
- ○
- The HQ map is analyzed using the trained pixel-based classification model to estimate the total area of blooming trees.
- ○
- The selected parts (from Step 3) of the HQ map are analyzed using the trained object-based detection model to estimate the total number of beehives.
- ○
- The obtained results are used to estimate the productive potential of the investigated area. The maximum honey yield expected from the experimental area was considered as the amount of honey yield to be harvested based on the nectar secretion potential of honey plants. The calculation of the expected honey yield is performed according to the method described in [4]:
3. Results and Discussion
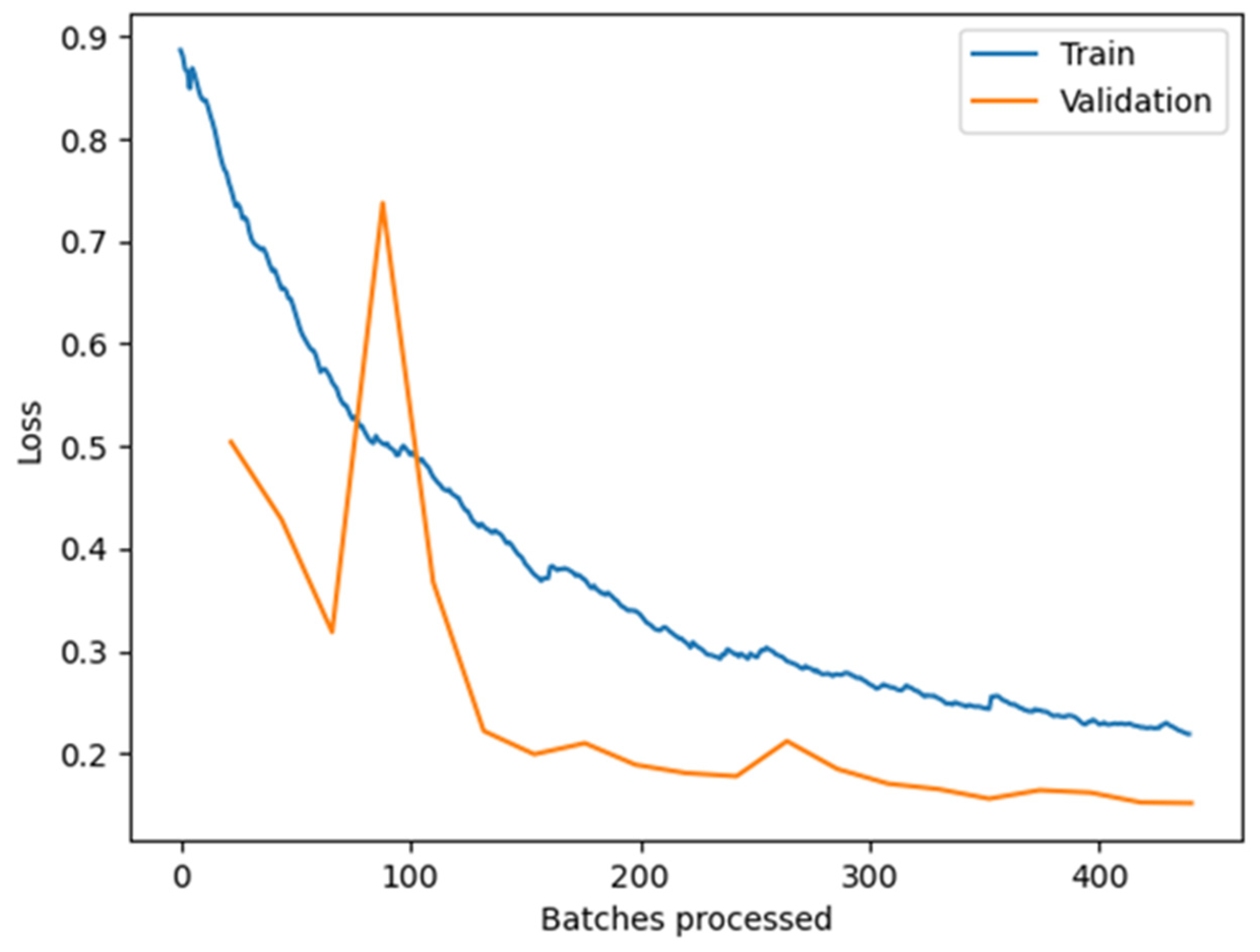
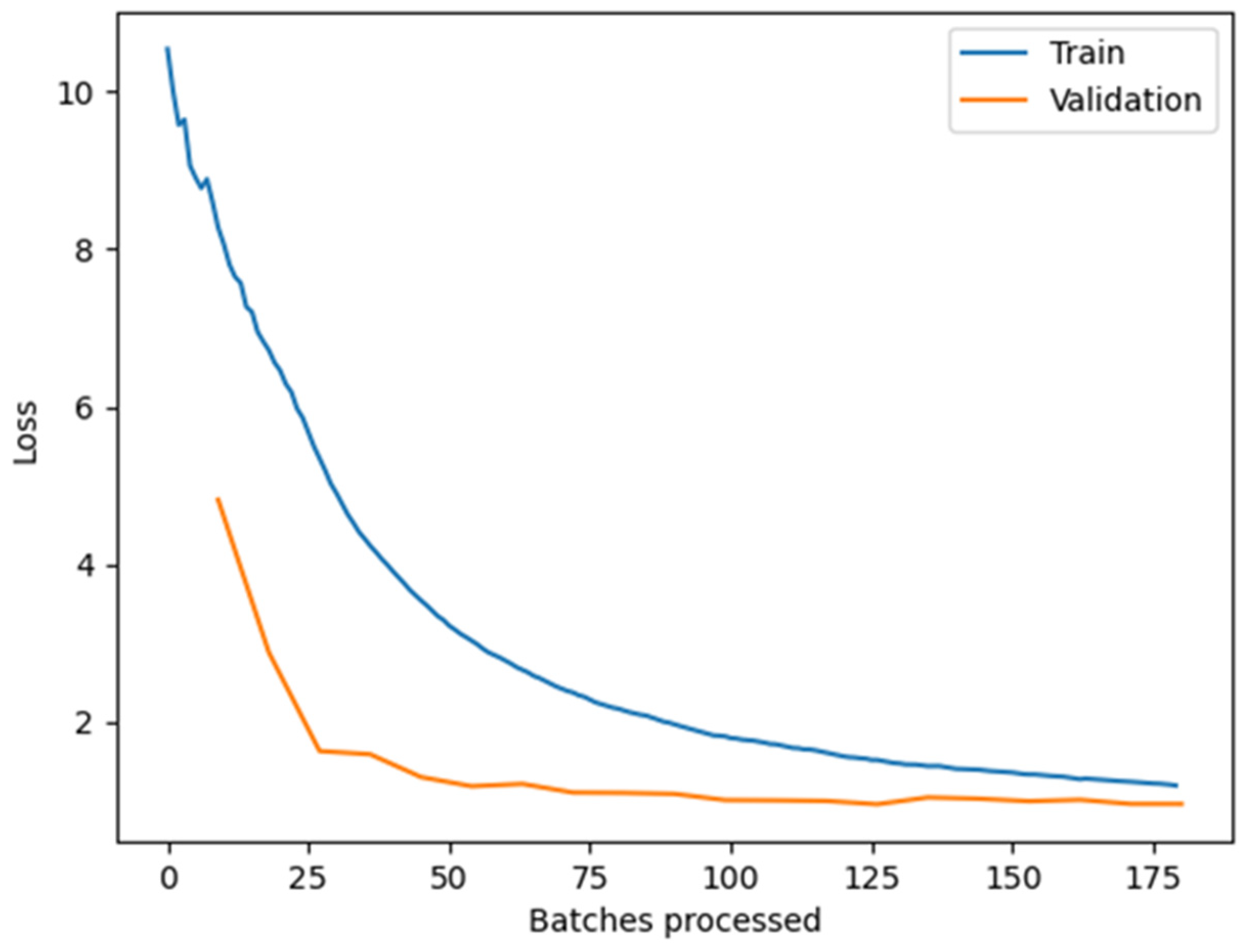
3.1. Training of a CNN for Blooming Trees Recognition
3.2. Training of a CNN for Identification of Beehives
3.3. Assessment of the Honey Production Potential
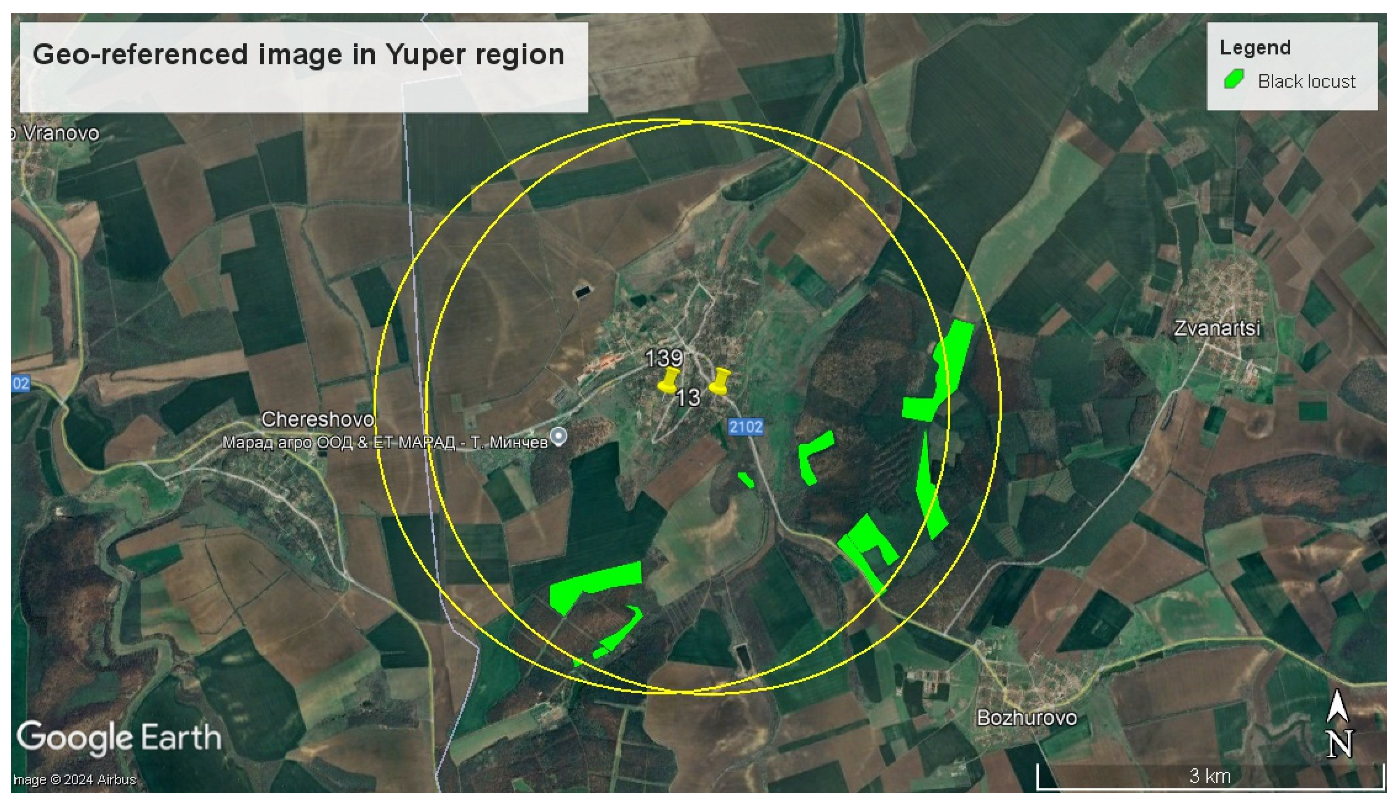
3.3.1. Estimation of the Number of Beehives
3.3.2. Estimation of the Area Blooming Trees
3.3.3. Analysis of the Honey Production Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Layek, U.; Das, U.; Karmakar, P. The pollination efficiency of a pollinator depends on its foraging strategy, flowering phenology, and the flower characteristics of a plant species. J. Asia-Pac. Entomol. 2022, 25, 101882. [Google Scholar] [CrossRef]
- Herbertsson, L.; Rundlöf, M.; Smith, G.H. The relation between oilseed rape and pollination of later flowering plants varies across plant species and landscape contexts. Basic Appl. Ecol. 2017, 24, 77–85. [Google Scholar] [CrossRef]
- Atanasov, A.Z.; Georgiev, S.G.; Vulkov, L.G. Parameter Estimation Analysis in a Model of Honey Production. Axioms 2023, 12, 214. [Google Scholar] [CrossRef]
- Zhelyazkov, P.; Atanasov, A.; Hristakov, I. Study on the honey productive potential of the bee forage species in Northeast part of Bulgaria in Silistra region. In Proceedings of the X International Scientific Symposium FMPMSA, Lublin, Poland, 20–22 November 2019; pp. 183–188. [Google Scholar]
- Lu, B.; He, Y. Species classification using Unmanned Aerial Vehicle (UAV)-acquired high spatial resolution imagery in a heterogeneous grassland. ISPRS J. Photogramm. Remote Sens. 2017, 128, 73–85. [Google Scholar] [CrossRef]
- Sheffield, K.J.; Clements, D.; Clune, D.J.; Constantine, A.; Dugdale, T.M. Detection of Aquatic Alligator Weed (Alternanthera philoxeroides) from Aerial Imagery Using Random Forest Classification. Remote Sens. 2022, 14, 2674. [Google Scholar] [CrossRef]
- Fariz, T.R.; Suhardono, S.; Sultan, H.; Rahmawati, D.; Arifah, E.Z. Land cover mapping in lake Rawa pening using Landsat 9 Imagery and Google Earth Engine. J. Environ. Sci. Educ. 2022, 2, 1–6. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Prodromou, M.; Hadjimitsis, D.; Christoforou, M. Detecting and distinguishing between apicultural plants using UAV multispectral imaging. PeerJ 2023, 11, e15065. [Google Scholar] [CrossRef]
- Ottoy, S.; Tziolas, N.; Van Meerbeek, K.; Aravidis, I.; Tilkin, S.; Sismanis, M.; Stavrakoudis, D.; Gitas, I.Z.; Zalidis, G.; De Vocht, A. Effects of Flight and Smoothing Parameters on the Detection of Taxus and Olive Trees with UAV-Borne Imagery. Drones 2022, 6, 197. [Google Scholar] [CrossRef]
- Torresani, M.; Kleijn, D.; de Vries, J.P.R.; Bartholomeus, H.; Chieffallo, L.; Gatti, R.C.; Moudrý, V.; Da Re, D.; Tomelleri, E.; Rocchini, D. A novel approach for surveying flowers as a proxy for bee pollinators using drone images. Ecol. Indic. 2023, 149, 110123. [Google Scholar] [CrossRef]
- Atanasov, A.Z.; Evstatiev, B.I.; Vladut, V.N.; Biris, S.-S. A Novel Algorithm to Detect White Flowering Honey Trees in Mixed Forest Ecosystems Using UAV-Based RGB Imaging. AgriEngineering 2024, 6, 95–112. [Google Scholar] [CrossRef]
- Ramon, J.P.E.; Michael, C.V.; Jomar, F.R. Determining the optimal distribution of bee colony locations to avoid overpopulation using mixed integer programming. J. Nat. Stud. 2010, 9, 79–82. [Google Scholar]
- Rebysarah, S.T.; Jomar, F.R.; Ramon, J.P.E.; Michael, C. Villadelrey Prediction of migration path of a colony of bounded-rational species foraging on patchily distributed resources. Adv. Stud. Biol. 2011, 3, 333–345. [Google Scholar]
- Yuce, B.; Packianather, M.S.; Mastrocinque, E.; Pham, T.D.; Lamiase, A. Honey bees inspired optimization method: The Bees Algorithm. Insects 2013, 4, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Gavina, M.K.A.; Rabajante, J.F.; Cervancia, C.R. Mathematical Programming Models for Determining the Optimal Location of Beehives. Bull. Math. Biol. 2014, 76, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Saritha, R.; Vinod Chandra, S.S. Multi Dimensional Honey Bee Foraging Algorithm Based on Optimal Energy Consumption. J. Inst. Eng. India 2017, 98, 527–531. [Google Scholar] [CrossRef]
- Aderinto, O.Y.; Azagbaekwue, A.; Bamigbola, M.O.; Oke, M.O.; Obayomi, A.A.; Salaudeen, O.L.; Aliu, O.T. Optimization of Honey Bee Production. Int. J. Math. Models Methods Appl. Sci. 2020, 14, 56–61. [Google Scholar] [CrossRef]
- Atanasov, A.; Georgiev, I. Evaluation of the places for creation of apiaries and optimal distribution of the bee colonies. INMATEH—Agric. Eng. 2021, 65, 373–380. [Google Scholar] [CrossRef]
- Atanasov, A.; Georgiev, I.; Hristakov, I.; Hristov, P. Application of mathematical model for apiaries location evaluation. In Proceedings of the 21st International Scientific Conference “Engineering for Rural Development”, Jelgava, Latvia, 25–27 May 2022; pp. 187–193. [Google Scholar] [CrossRef]
- Kumar, P.B.; Prasad, T.K. Application of Multi-Criteria Decision Analysis (MCDA) to Apiculture Potential Assessment: A Case Study of Thiruvananthapuram Corporation, Kerala, India. Trans. Inst. Indian Geogr. 2021, 43, 215–226. [Google Scholar]
- Roque, N.; Fernandez, P.; Silveira, C.; Vilas-Boas, M.; Anjos, O. Using Analytic Hierarchy Process to Assess Beekeeping Suitability in Portuguese Controlled Areas: A First Approach. Insects 2024, 15, 91. [Google Scholar] [CrossRef]
- Egerer, M.; Kowarik, I. Confronting the modern gordian knot of urban beekeeping. Trends Ecol. Evol. 2020, 35, 956–959. [Google Scholar] [CrossRef]
- Quiralte, D.; Zarzo, I.; Fernandez-Zamudio, M.-A.; Barco, H.; Soriano, J.M. Urban Honey: A Review of Its Physical, Chemical, and Biological Parameters That Connect It to the Environment. Sustainability 2023, 15, 2764. [Google Scholar] [CrossRef]
- Mahé, C.; Jumarie, C.; Boily, M. The countryside or the city: Which environment is better for the honeybee? Environ. Res. 2021, 195, 110784. [Google Scholar] [CrossRef] [PubMed]
- Jovetić, M.S.; Redžepović, A.S.; Nedić, N.M.; Vojt, D.; Ðurđić, S.Z.; Brčeski, I.D.; Milojković-Opsenica, D.M. Urban honey-the aspects of its safety. Arh. Hig. Rada Toksikol. 2018, 69, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jin, Y.; Brown, P. An enhanced bloom index for quantifying floral phenology using multi-scale remote sensing observations. ISPRS J. Photogramm. Remote Sens. 2019, 156, 108–120. [Google Scholar] [CrossRef]
- López-Granados, F.; Torres-Sánchez, J.; Jiménez-Brenes, F.M.; Arquero, O.; Lovera, M.; de Castro, A.I. An efficient RGB-UAV-based platform for field almond tree phenotyping: 3-D architecture and flowering traits. Plant Methods 2019, 15, 160. [Google Scholar] [CrossRef]
- Zhongyu, S.; Wenlong, J.; Xi, Q.; Long, Y. Identification and Monitoring of Blooming Mikania micrantha Outbreak Points Based on UAV Remote Sensing. Trop. Geogr. 2019, 39, 482–491. [Google Scholar] [CrossRef]
- Reckling, W.; Mitasova, H.; Wegmann, K.; Kauffman, G.; Reid, R. Efficient Drone-Based Rare Plant Monitoring Using a Species Distribution Model and AI-Based Object Detection. Drones 2021, 5, 110. [Google Scholar] [CrossRef]
- Haq, M.A.; Ahsan, A.; Gyani, J. Implementation of CNN for plant identification using UAV imagery. Int. J. Adv. Comput. Sci. Appl. 2023, 14. [Google Scholar] [CrossRef]
- Nasiri, V.; Darvishsefat, A.A.; Arefi, H.; Griess, V.C.; Sadeghi, S.M.M.; Borz, S.A. Modeling Forest Canopy Cover: A Synergistic Use of Sentinel-2, Aerial Photogrammetry Data, and Machine Learning. Remote Sens. 2022, 14, 1453. [Google Scholar] [CrossRef]
- Moysiadis, V.; Siniosoglou, I.; Kokkonis, G.; Argyriou, V.; Lagkas, T.; Goudos, S.K.; Sarigiannidis, P. Cherry Tree Crown Extraction Using Machine Learning Based on Images from UAVs. Agriculture 2024, 14, 322. [Google Scholar] [CrossRef]
- Williams, S.M.; Aldabashi, N.; Cross, P.; Palego, C. Challenges in Developing a Real-Time Bee-Counting Radar. Sensors 2023, 23, 5250. [Google Scholar] [CrossRef] [PubMed]
- Kulyukin, V.; Mukherjee, S. On Video Analysis of Omnidirectional Bee Traffic: Counting Bee Motions with Motion Detection and Image Classification. Appl. Sci. 2019, 9, 3743. [Google Scholar] [CrossRef]
- Florea, G.; Codreanu, N. Electronic System for the Management of a Beehive. In Proceedings of the 2022 IEEE 9th Electronics System-Integration Technology Conference (ESTC), Sibiu, Romania, 13–16 September 2022. [Google Scholar] [CrossRef]
- Voudiotis, G.; Moraiti, A.; Kontogiannis, S. Deep Learning Beehive Monitoring System for Early Detection of the Varroa Mite. Signals 2022, 3, 506–523. [Google Scholar] [CrossRef]
- Mrozek, D.; Gȯrny, R.; Wachowicz, A.; Małysiak-Mrozek, B. Edge-Based Detection of Varroosis in Beehives with IoT Devices with Embedded and TPU-Accelerated Machine Learning. Appl. Sci. 2021, 11, 11078. [Google Scholar] [CrossRef]
- Dirir, A.; Ignatious, H.; Elsayed, H.; Khan, M.; Adib, M.; Mahmoud, A.; Al-Gunaid, M. An Advanced Deep Learning Approach for Multi-Object Counting in Urban Vehicular Environments. Future Internet 2021, 13, 306. [Google Scholar] [CrossRef]
- Abeyrathna, R.M.R.D.; Nakaguchi, V.M.; Minn, A.; Ahamed, T. Recognition and Counting of Apples in a Dynamic State Using a 3D Camera and Deep Learning Algorithms for Robotic Harvesting Systems. Sensors 2023, 23, 3810. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, X.; Yang, H.; Lu, Y.; Liu, J.; Yu, X.; Feng, D.; Gao, K.; Xue, J.; Ming, B.; et al. Comparison and Optimal Method of Detecting the Number of Maize Seedlings Based on Deep Learning. Drones 2024, 8, 175. [Google Scholar] [CrossRef]
- P4 Multispectral. Available online: https://www.dji.com/bg/p4-multispectral (accessed on 20 July 2024).
- Visscher, P.K.; Seeley, T.D. Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef]
- Seeley, T.D.; Camazine, S.; Sneyd, J. Collective decision making in honey bees: How colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991, 28, 277–290. [Google Scholar] [CrossRef]



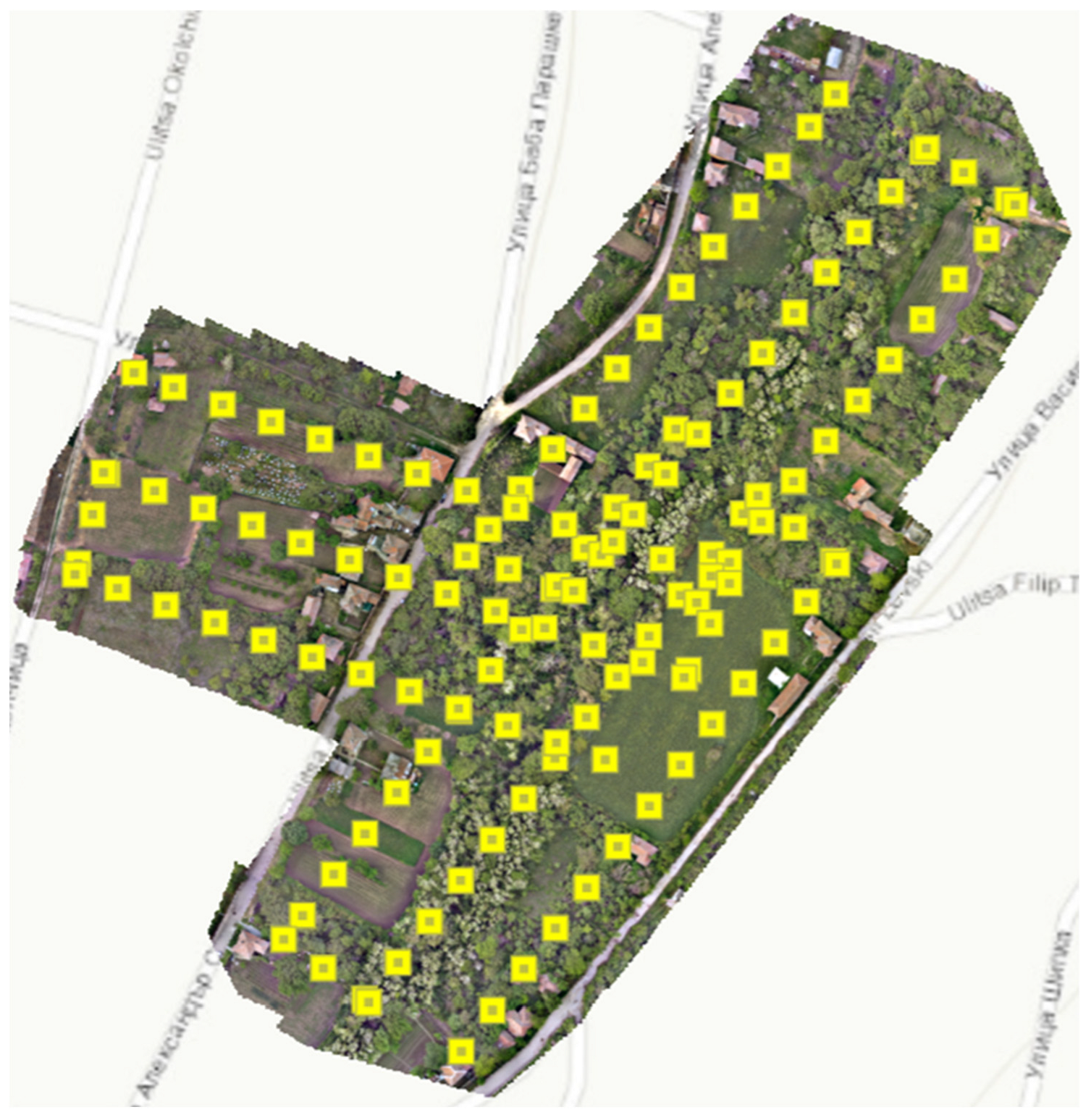


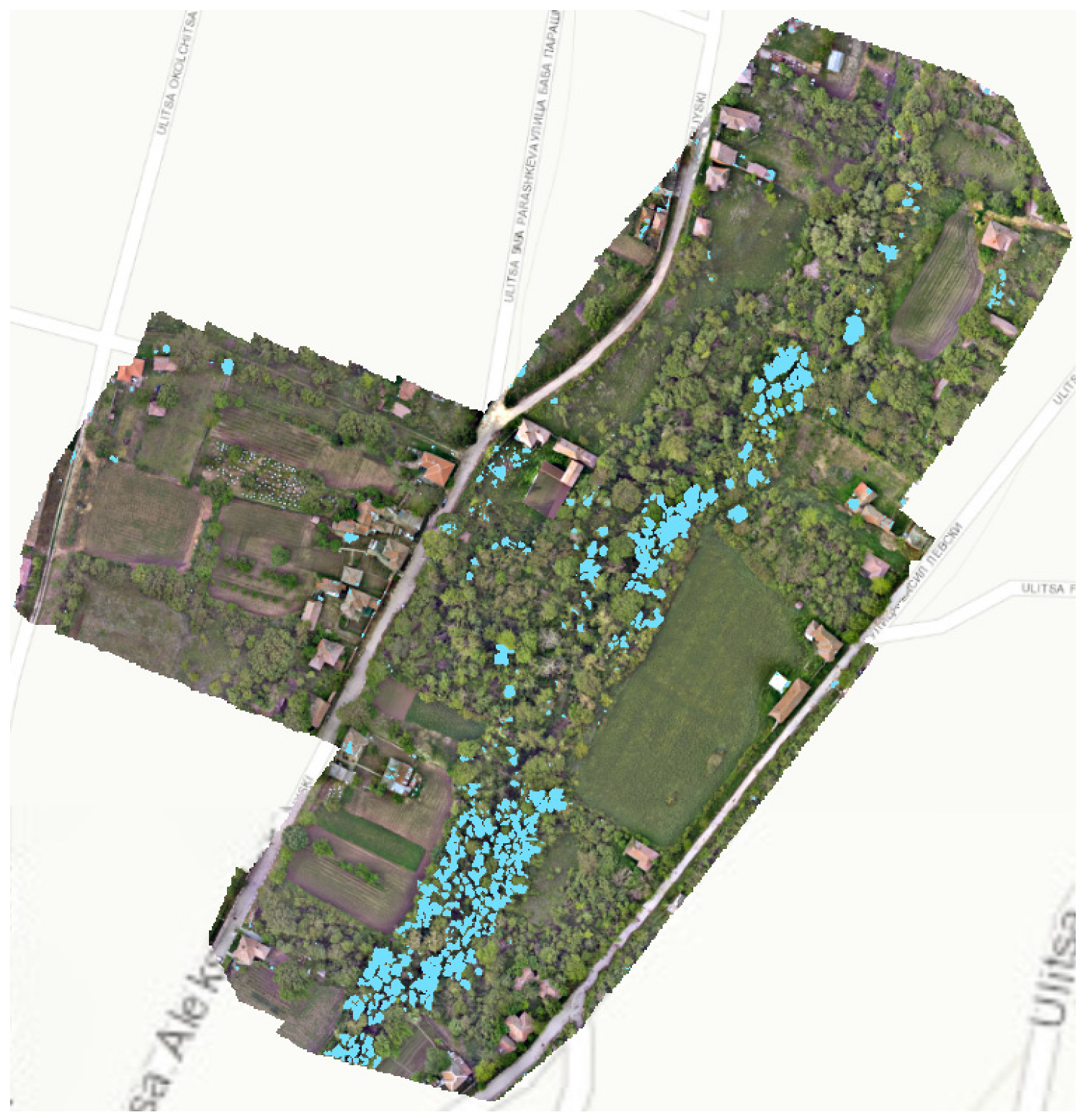

| Overlap Zone Inside the Village | |||||
|---|---|---|---|---|---|
| Forage Species | Area, ha | Number of Bee Colonies | Nectar Secretion Potential, kg ha−1 | Maximum Honey Yield Expected Potential, kg | Expected Honey Yield, kg Hive−1 per Season |
| Black locust | 0.365 | 149 | 300 | 54.75 | 0.367 |
| Overlap Zone outside the village | |||||
| Black locust | 38.18 | 149 | 300 | 5727 | 38.44 |
| Total | 38.807 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasov, A.Z.; Evstatiev, B.I.; Atanasov, A.I.; Hristakov, I.S. Detection and Assessment of White Flowering Nectar Source Trees and Location of Bee Colonies in Rural and Suburban Environments Using Deep Learning. Diversity 2024, 16, 578. https://doi.org/10.3390/d16090578
Atanasov AZ, Evstatiev BI, Atanasov AI, Hristakov IS. Detection and Assessment of White Flowering Nectar Source Trees and Location of Bee Colonies in Rural and Suburban Environments Using Deep Learning. Diversity. 2024; 16(9):578. https://doi.org/10.3390/d16090578
Chicago/Turabian StyleAtanasov, Atanas Z., Boris I. Evstatiev, Asparuh I. Atanasov, and Ivaylo S. Hristakov. 2024. "Detection and Assessment of White Flowering Nectar Source Trees and Location of Bee Colonies in Rural and Suburban Environments Using Deep Learning" Diversity 16, no. 9: 578. https://doi.org/10.3390/d16090578
APA StyleAtanasov, A. Z., Evstatiev, B. I., Atanasov, A. I., & Hristakov, I. S. (2024). Detection and Assessment of White Flowering Nectar Source Trees and Location of Bee Colonies in Rural and Suburban Environments Using Deep Learning. Diversity, 16(9), 578. https://doi.org/10.3390/d16090578







