Effects of Land Use on the Community Structure of Aquatic Invertebrate in Subtropical Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurements of Environmental Variables
2.3. Macrobenthic Invertebrate Sampling
2.4. Statistical Analysis
3. Results
3.1. Local Environmental Variations
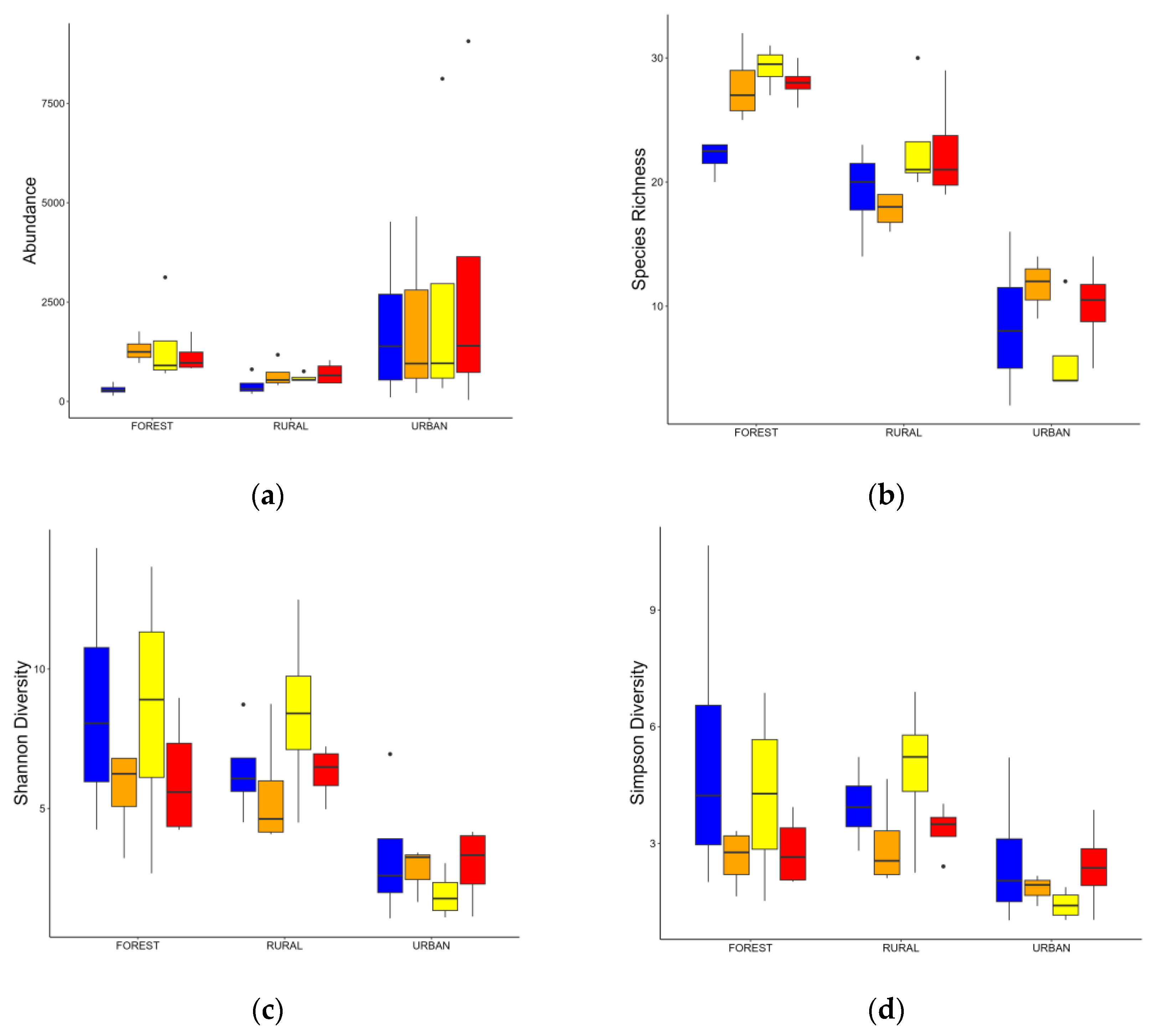
3.2. Changes in the Macroinvertebrates Community
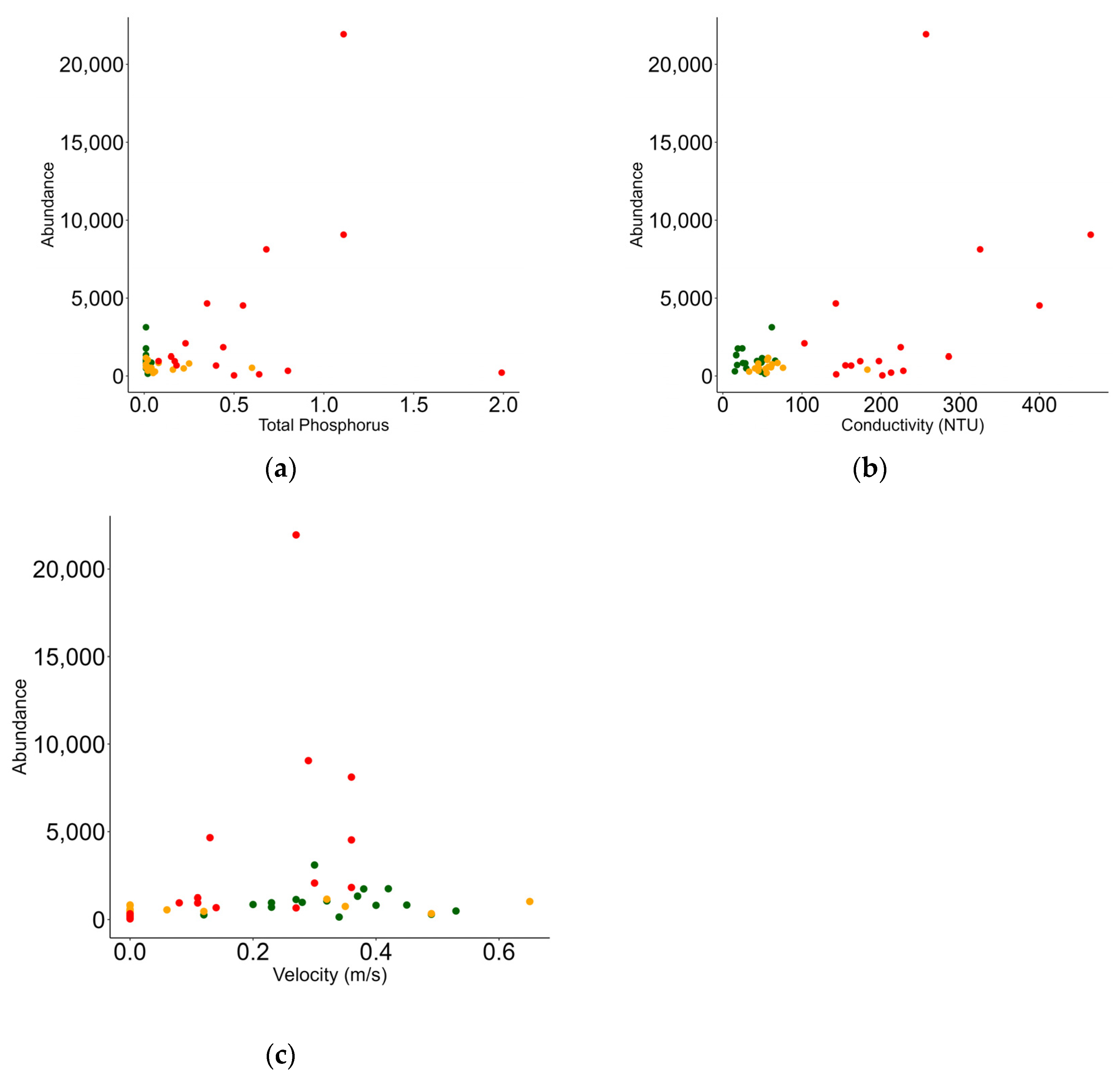
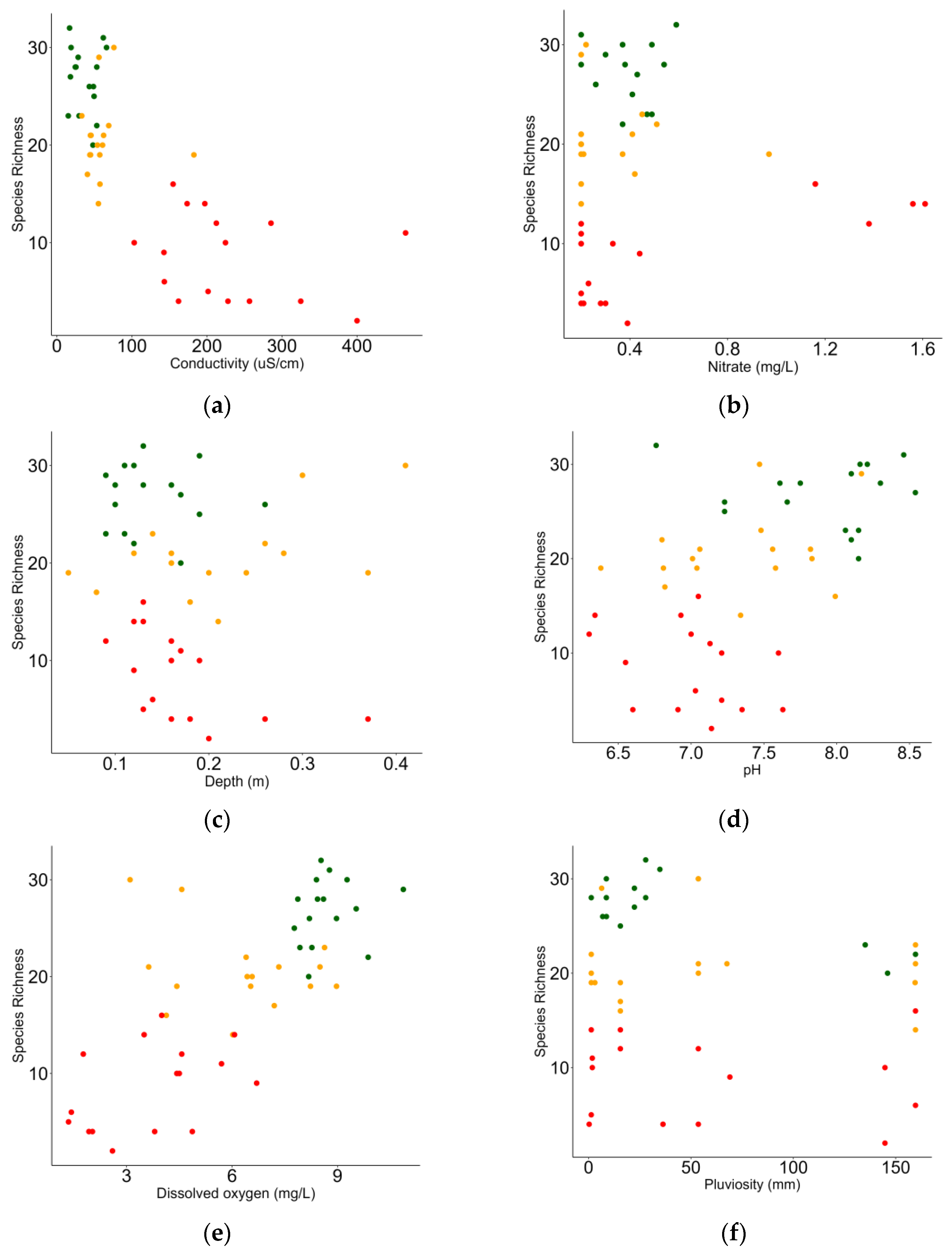
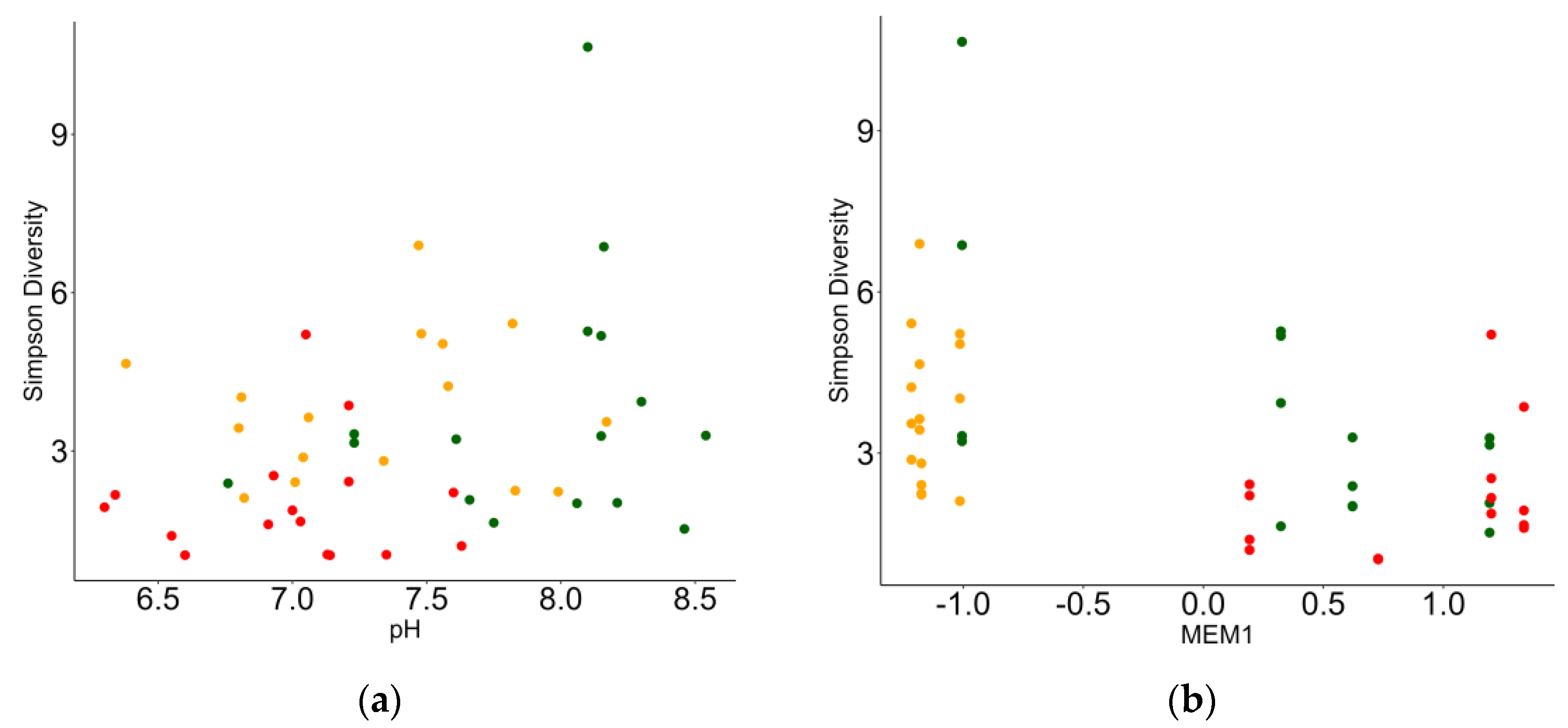
3.3. Relationship between Macrobenthic Invertebrates and Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Taxa | Forest | Rural | Urban | |
|---|---|---|---|---|
| Megaloptera | Corydalidae | 43 | 4 | 0 |
| Odonata | Aeshnidae | 21 | 8 | 1 |
| Dicteriadidae | 23 | 17 | 3 | |
| Calopterigydae | 73 | 58 | 17 | |
| Libellulidae | 153 | 47 | 5 | |
| Gomphidae | 16 | 22 | 0 | |
| Megapodagrionidae | 53 | 12 | 0 | |
| Coenagrionidae | 6 | 3 | 0 | |
| Caenidae | 97 | 14 | 0 | |
| Ephemeroptera | Baetidae | 402 | 572 | 0 |
| Leptohyphidae | 163 | 74 | 0 | |
| Leptophlebiidae | 439 | 94 | 0 | |
| Plecoptera | Perlidae | 236 | 13 | 0 |
| Gripopterygidae | 355 | 25 | 0 | |
| Trichoptera | Hydroptilidae | 124 | 185 | 0 |
| Hydropsychidae | 387 | 157 | 90 | |
| Philopotamidae | 35 | 2 | 0 | |
| Leptoceridae | 153 | 15 | 20 | |
| Calamoceratidae | 196 | 0 | 0 | |
| Lmnephilidae | 12 | 20 | 0 | |
| Helicopsychidae | 8 | 0 | 6 | |
| Polycentropodidae | 12 | 0 | 0 | |
| Diptera | Tipulidae | 124 | 41 | 21 |
| Tabanidae | 17 | 12 | 5 | |
| Psychodidae | 4 | 0 | 0 | |
| Empididae | 227 | 56 | 60 | |
| Chironomidae | 10.298 | 4.537 | 51.803 | |
| Ceratopogonidae | 403 | 201 | 356 | |
| Syrphidae | 3 | 1 | 0 | |
| Simuliidae | 366 | 819 | 466 | |
| Blephariceridae | 3 | 0 | 0 | |
| Chaoboridae | 0 | 13 | 0 | |
| Culicidae | 0 | 9 | 0 | |
| Coleoptera | Haliplidae | 9 | 0 | 24 |
| Elmidae | 782 | 147 | 47 | |
| Girinidae | 16 | 26 | 1 | |
| Staphylinidae | 13 | 2 | 0 | |
| Hydrophilidae | 5 | 2 | 0 | |
| Dytiscidae | 14 | 19 | 1 | |
| Psephenidae | 100 | 0 | 0 | |
| Hemiptera | Gerridae | 15 | 25 | 0 |
| Corixidae | 63 | 17 | 0 | |
| Pleidae | 7 | 2 | 0 | |
| Mesoveliidae | 63 | 73 | 4 | |
| Belostomatidae | 25 | 149 | 3 | |
| Veliidae | 76 | 12 | 7 | |
| Lepidoptera | Pyralidae | 35 | 22 | 0 |
| Crambidae | 6 | 0 | 0 | |
| Neuroptera | Osmylidae | 0 | 8 | 0 |
| Heteroptera | Naucoridae | 18 | 0 | 0 |
| Arthropoda | Collembola | 123 | 449 | 22 |
| Acari | Hydracarina | 267 | 305 | 38 |
| Crustacea | Ostracoda | 19 | 372 | 0 |
| Macrobrachium | 20 | 7 | 6 | |
| Annelida | Oligochaeta | 354 | 352 | 567 |
| Hirudinidae | 29 | 76 | 3.366 | |
| Nematoda | Nematoda | 23 | 27 | 33 |
| Basommatophora | Planorbidae | 21 | 102 | 68 |
| Ancylidae | 10 | 27 | 9 | |
| Physidae | 0 | 0 | 2 | |
| Caenogastropoda | Thiaridae | 24 | 32 | 249 |
| Architaenioglossa | Ampullariidae | 0 | 5 | 39 |
| Stylommatophora | Camaenidae | 0 | 0 | 1 |
| Veneroida | Pisidiidae | 0 | 223 | 0 |
| Sphaeriidae | 13 | 56 | 88 | |
| Amphipoda | Hyalellidae | 7 | 0 | 3 |
| Littorinimorpha | Hydrobiidae | 0 | 0 | 11 |
References
- Downing, J.A.; Cole, J.J.; Duarte, C.M.; Middelburg, J.J.; Melack, J.M.; Prairie, Y.T.; Kortelainen, P.; Strigegl, W.H.; Tranvik, L.J. Global abundance and size distribution of streams and rivers. Inland Waters 2012, 2, 229–236. [Google Scholar] [CrossRef]
- Wohl, E. The significance of small streams. Front. Earth Sci. 2017, 11, 447–456. [Google Scholar] [CrossRef]
- Baumgartner, S.D.; Robinson, C.T. Changes in macroinvertebrate trophic structure along a land-use gradient within a lowland stream network. Aquat. Sci. 2017, 79, 407–418. [Google Scholar] [CrossRef]
- Bohn Vieira, I.C.; Soares Correa da Costa, V.; de Mello Cionek, V.; Olinto Branco, J.; Werneck Ribeiro, E.A. Predileção de riachos para o monitoramento da qualidade da água: Um serviço ecossistêmico de provisão na bacia hidrográfica do rio Itajaí-Mirim (Brasil) [Predilection of streams for water quality monito ring: An ecosystem provision service in the Itajaí-mirim river basin (Brazil). Finisterra 2022, 57, 95–108. [Google Scholar] [CrossRef]
- Meyer, J.M.; Kaplan, L.A.; Newbold, D.; Strayer, D.L.; Woltemade, C.J.; Zedler, J.B.; Beilfuss, R.; Carpenter, Q.; Semlitsch, R.; Watzin, M.C.; et al. Where Rivers Are Born: The Scientific Imperative for Defending Small Streams and Wetlands; Sierra Club and American Rivers: Oakland, CA, USA, 2007; Available online: https://www.researchgate.net/publication/313728461_Where_Rivers_are_Born_the_Scientific_Imperative_for_Defending_Small_Streams_and_Wetlands (accessed on 15 March 2024).
- Bohus, A.; Gál, B.; Barta, B.; Szivák, I.; Karádi-Kovács, K.; Boda, P.; Padisák, J.; Schmera, D. Effects of urbanization-induced local alterations on the diversity and assemblage structure of macroinvertebrates in low-order streams. Hydrobiologia 2023, 850, 881–899. [Google Scholar] [CrossRef]
- Ferreira, V.; Albariño, R.; Larrañaga, A.; LeRoy, C.J.; Masese, F.O.; Moretti, M.S. Ecosystem services provided by small streams: An overview. Hydrobiologia 2023, 850, 2501–2535. [Google Scholar] [CrossRef]
- Finlay, J.C. Stream size and human influences on ecosystem production in river networks. Ecosphere 2011, 2, 1–21. [Google Scholar] [CrossRef]
- Palmer, M.A.; Reidy Liermann, C.A.; Nilsson, C.; Flörke, M.; Alcamo, J.; Lake, P.S.; Bond, N. Climate change and the world’s river basins: Anticipating management options. Front. Ecol. Environ. 2008, 6, 81–89. [Google Scholar] [CrossRef]
- Lafage, D.; Bergman, E.; Eckstein, R.L.; Österling, E.M.; Sadler, J.P.; Piccolo, J.J. Local and landscape drivers of aquatic-to terrestrial subsidies in riparian ecosystems: A worldwide meta-analysis. Ecosphere 2019, 10, e02697. [Google Scholar] [CrossRef]
- Lemes da Silva, A.L.; Lemes, W.P.; Andriotti, J.; Petrucio, M.M.; Feio, M.J. Recent land-use changes affect stream ecosystem processes in a subtropical island in Brazil. Austral Ecol. 2020, 45, 644–658. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Burdon, F.J.; Munz, N.A.; Reyes, M.; Focks, A.; Joss, A.; Räsänen, K.; Altermatt, F.; Eggen, R.I.L.; Stamm, C. Agriculture versus wastewater pollution as drivers of macroinvertebrate community structure in streams. Sci. Total Environ. 2019, 659, 1256–1265. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M.; Verdonschot, R.C.M. The role of stream restoration in enhancing ecosystem services. Hydrobiologia 2023, 850, 2537–2562. [Google Scholar] [CrossRef]
- Santos, J.I.; Silva, C.; Gonçalves, F.J.M.; Pereira, J.L.; Castro, B.B. Macroinvertebrate community structure and ecological status in Portuguese streams across climatic and water scarcity gradients. Hydrobiologia 2023, 850, 967–984. [Google Scholar] [CrossRef]
- Luiza-Andrade, A.; Silva, R.R.; Shimano, Y.; Faria, A.P.J.; Cardoso, M.N.; Brasil, L.S.; Ligeiro, R.; Martins, R.T.; Hamada, N.; Juen, L. Niche breadth and habitat preference of Ephemeroptera, Plecoptera, and Trichoptera (Insecta) in streams in the Brazilian Amazon. Hydrobiologia 2022, 849, 4287–4306. [Google Scholar] [CrossRef]
- Loomer, H.A.; Kidd, K.A.; Erdozain, M.; Benoy, G.A.; Chambers, P.A.; Culp, J.M. Stream macroinvertebrate community responses to an agricultural gradient alter consumer-driven nutrient dynamics. Hydrobiologia 2023, 850, 315–334. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, phosphorus, and eutrophication in streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Cummins, K.W.; Klug, M.J. Feeding ecology of stream invertebrates. Annu. Rev. Ecol. Syst. 1979, 10, 147–172. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Arias, M.; Bonetto, C.; Fanelli, S.L.; Scenna, L.; Miglioranza, K.S.B.; Mugni, H. Macroinvertebrate assemblages in lowland streams under horticultural impact (Buenos Aires, Argentina). Hydrobiologia 2023, 850, 399–416. [Google Scholar] [CrossRef]
- Iñiguez-Armijos, C.; Hampel, H.; Breuer, L. Land-use effects on structural and functional composition of benthic and leaf-associated macroinvertebrates in four Andean streams. Aquat. Ecol. 2018, 52, 77–92. [Google Scholar] [CrossRef]
- Scotti, A.; Füreder, L.; Marsoner, T.; Tappeiner, U.; Stawinoga, A.E.; Bottarin, R. Effects of land cover type on community structure and functional traits of alpine stream benthic macroinvertebrates. Freshw. Biol. 2020, 65, 524–539. [Google Scholar] [CrossRef]
- Oester, R.; dos Reis Oliveira, P.C.; Moretti, M.S.; Altermatt, F.; Bruder, A. Leaf-associated macroinvertebrate assemblage and leaf litter breakdown in headwater streams depend on local riparian vegetation. Hydrobiologia 2023, 850, 3359–3374. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Li, J.; Tan, L.; Rizo, E.Z.; Han, B.P. The seasonal patterns of aquatic insect composition and diversity in a disturbed (sub)tropical river revealed by linear and nonlinear approaches with occurrence and abundance data. Hydrobiologia 2023, 850, 3949–3963. [Google Scholar] [CrossRef]
- de Donnová, S.; Petruželová, J.; Kintrová, K.; Sorfová, V.; Polásková, V.; Straka, M.; Vrba, J.; Bojková, J. Rapid macroinvertebrate colonisation in restored channelised streams contiguous with natural stream reaches. Hydrobiologia 2022, 849, 4135–4152. [Google Scholar] [CrossRef]
- Martins, R.T.; Brito, J.; Dias-Silva, K.; Leal, C.G.; Leitão, R.P.; Oliveira, V.C.; Oliveira-Júnior, J.M.B.; de Paula, F.R.; Roque, F.O.; Hamada, N.; et al. Congruence and responsiveness in the taxonomic compositions of Amazonian aquatic macroinvertebrate and fish assemblages. Hydrobiologia 2022, 849, 2281–2298. [Google Scholar] [CrossRef]
- Bohn Vieira, I.C.; Correa da Costa, V.S.; Cionek, V.d.M.; Werneck Ribeiro, E.A.; Branco, J.O. Ecologia de riachos: Delineando planos amostrais para compreensão dos efeitos de uso da terra sobre comunidades aquáticas. Gaia Sci. 2022, 16, 33–52. [Google Scholar]
- Wiederkehr, F.; Wilkinson, C.L.; Zeng, Y.; Yeo, D.C.J.; Ewers, R.M.; O’Gorman, E.J. Urbanisation affects ecosystem functioning more than structure in tropical streams. Biol. Conserv. 2020, 249, 108634. [Google Scholar] [CrossRef]
- Gabetti, A.; Maganza, A.; Mossotto, C.; Rizzioli, B.; Esposito, G.; Bertoli, M.; Pizzul, E.; Bozzetta, E.; Prearo, M.; Pastorino, P. Macrobenthic Assemblages and the Influence of Microhabitat in a High-Mountain Lake (Northwest Italy). Diversity 2024, 16, 329. [Google Scholar] [CrossRef]
- Sistema de Informação de Recursos Hídricos do Estado de Santa Catarina (SIRHESC). Comitê Bacia Hidrográfica do Itajaí [Itajaí Hydrographic Basin Committee]. Available online: http://www.sirhesc.sds.sc.gov.br/sirhsc/index.jsp?idEmpresa=14 (accessed on 14 November 2023).
- Homechin, M., Jr.; Beaumord, A.C. Caracterização da Qualidade das Águas do Trecho Médio do Rio Itajaí-Mirim, Santa Catarina [Characterization of the Water Quality of the Middle Stretch of the Itajaí-Mirim River, Santa Catarina]. In Proceedings of the Anais do VIII Congresso de Ecologia do Brasil [VIII Brazil Ecology Congress Annals], Caxambú, Minas Gerais, Brazil, 23–28 September 2007; Available online: https://www.seb-ecologia.org.br/revistas/indexar/anais/viiiceb/pdf/1740.pdf (accessed on 20 January 2024).
- Comitê do Itajaí. Caderno Síntese: Para Que a Água Continue a Trazer Benefícios Para Todos Plano de Recursos Hídricos da Bacia do Itajaí [Synthesis Notebook: So that Water Continues to Bring Benefits to Everyone]. Brazil, Blumenau. 2010. Available online: https://www.aguas.sc.gov.br/index.php?option=com_k2&view=item&layout=item&id=1904&Ite%02mid=248&jsmallfib=1&dir=JSROOT/DHRI/Planos+de+Bacias/Plano+da+Bacia+Hidrografica+do+Rio+Ita%02ja%C3%AD&sort_by=name&sort_as=desc (accessed on 15 January 2024).
- Ab’Sáber, A.N. Os Domínios de Natureza no Brasil: Potencialidades Paisagísticas [The Domains of Nature in Brazil: Landscape Potential], 1st ed.; Ateliê Editorial: São Paulo, Brazil, 2003; p. 144. Available online: https://edisciplinas.usp.br/pluginfile.php/7993715/mod_resource/content/1/AB%20SABER-Os%20dom%C3%ADnios%20de%20natureza.pdf (accessed on 12 December 2023).
- Instituto Brasileiro de Geografia e Estatística. Cidades [Cities]. Available online: https://cidades.ibge.gov.br (accessed on 15 March 2024).
- Burliga, A.L.; Torgan, L.C.; de Nobrega, E.A.; Beaumord, A.C.; da Costa, C.O.; Yamauti, D.V. Diatomáceas Epilíticas do Rio Itajaí-Mirim, Santa Catarina, Brasil [Epilithic Diatoms from the Itajaí-Mirim River, Santa Catarina, Brazil]. Acta Sci. Biol. Sci. 2008, 27, 415–421. [Google Scholar] [CrossRef]
- Strahler, A.N. Quantitative Analysis of Water shed Geomorphology. Trans. Am. Geophys. Union 1957, 38, 913–920. [Google Scholar] [CrossRef]
- de Sousa Sobrinho, J.; Lima, M.L.; Ucker, F.E.; dos Santos, F.C.V.; Freire, C.L. Comparação entre o método do flutuador e o molinete hidrométrico para estimativa de vazões em condutos livres [Comparison between the float method and the hydrometric windlass for estimating flow rates in free conduits]. Rev. Uniaraguaia 2019, 14, 103–110. Available online: https://sipe.uniaraguaia.edu.br/index.php/REVISTAUNIARAGUAIA/article/view/591/Vol14-2-art-9 (accessed on 15 March 2024).
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária. Protocolo de Coleta e Preparação de Amostras de Macroinvertebrados Bentônicos em Riachos [Protocol for Collecting and Preparing Samples of Benthic Macroinvertebrates in Streams]. Comunicado Técnico. São Paulo: Jaguariúna, Brazil. 2004. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPMA/5841/1/comunicado_19.pdf (accessed on 10 February 2021).
- Dudgeon, D. The impacts of human disturbance on stream benthic invertebrates and their drift in North Sulawesi, Indonesia. Freshw. Biol. 2006, 51, 1710–1729. [Google Scholar] [CrossRef]
- Fernández, H.R.; Dominguéz, E. Guia Para Determinación de los Artropodos Bentônicos Sudamericanos [Guide for Determining South American Benthic Arthropods]; UNT: Tucumán, Argentina, 2001; 282p. [Google Scholar]
- Mugnai, R.; Nessimian, J.L.; Baptista, D.F. Manual de Identificação de Macroinvertebrados Aquáticos do Estado do Rio de Janeiro [Identification Manual for Aquatic Macroinvertebrates in the State of Rio de Janeiro]; Technical Books: Rio de Janeiro, Brazil, 2010; 176p. [Google Scholar]
- Jones, F.C. Taxonomic sufficiency: The influence of taxonomic resolution on freshwater bioassessments using benthic macroinvertebrates. Environ. Rev. 2008, 16, 45–69. [Google Scholar] [CrossRef]
- Kokesh, B.S.; Kidwell, S.M.; Walther, S.M. Detecting strong spatial and temporal variation in macrobenthic composition on an urban shelf using taxonomic surrogates. Mar. Ecol. Prog. Ser. 2022, 682, 13–30. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Mykrä, H.; Heino, J. Decreased habitat specialization in macroinvertebrate assemblages in anthropogenically disturbed streams. Ecol. Complex. 2017, 31, 181–188. [Google Scholar] [CrossRef]
- Metcalfe, J. Biological water quality assessment of running waters based on macroinvertebrate communities: History and present status in Europe. Environ. Pollut. 1989, 60, 101–139. [Google Scholar] [CrossRef]
- Rimcheska, B.; Vidinova, Y. Diversity and structure of macroinvertebrate communities in permanent small streams and rivers in Eastern Balkans. Hydrobiologia 2023, 850, 3341–3357. [Google Scholar] [CrossRef]
- Masese, F.O.; Achieng, A.O.; O’Brien, G.C.; McClain, M.E. Macroinvertebrate taxa display increased fidelity to preferred biotopes among disturbed sites in a hydrologically variable tropical river. Hydrobiologia 2020, 848, 321–343. [Google Scholar] [CrossRef]
- Cope, N.J.; Winterbourn, M.J. Competitive interactions between two successful molluscan invaders of freshwaters: An experimental study. Aquat. Ecol. 2004, 38, 83–91. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Durán-Rodríguez, O.Y.; Valencia-Espinosa, J.A.; Torres-Olvera, M.J.; Pineda-López, R.F.; Jones, R.W.; Ramírez-Herrejón, J.P. Spatial and temporal organization of aquatic insect assemblages in two subtropical river drainages. Hidrobiológica 2022, 32, 127–140. [Google Scholar] [CrossRef]
- Winterton, S.L.; Zhao, J.; Garzón-Orduña, I.J.; Wang, Y.; Liu, Z. The phylogeny of lance lacewings (N europtera: O smylidae). Syst. Entomol. 2017, 42, 555–574. [Google Scholar] [CrossRef]
- Skalskaya, I.A.; Bakanov, A.I.; Flerov, B.A. Study on zooperiphyton and zoobenthos of a small river. Inland Water Biol. 2008, 1, 84–92. [Google Scholar] [CrossRef]
- Wilkinson, D.M. The Disturbing History of Intermediate Disturbance. Oikos 1999, 84, 145–147. [Google Scholar] [CrossRef]
- Tamaris-Turizo, C.E.; Gómez Arrieta, K. Feeding preferences of Phylloicus sp. (Trichoptera: Calamoceratidae) in a Neotropical river of the Sierra Nevada de Santa Marta, Colombia. Rev. Biol. Trop. 2020, 68, 79–91. [Google Scholar] [CrossRef]
- Mwaijengo, G.N.; Vanschoenwinkel, B.; Dube, T.; Njau, K.N.; Brendonck, L. Seasonal variation in benthic macroinvertebrate assemblages and water quality in an Afrotropical river catchment, northeastern Tanzania. Limnologica 2020, 82, 125780. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; McKnight, U.S.; Loinaz, M.C.; Thomsen, N.I.; Olsson, M.E.; Bjerg, P.L.; Binning, P.J.; Kronvang, B. A catchment scale evaluation of multiple stressor effects in headwater streams. Sci. Total Environ. 2013, 442, 420–431. [Google Scholar] [CrossRef]
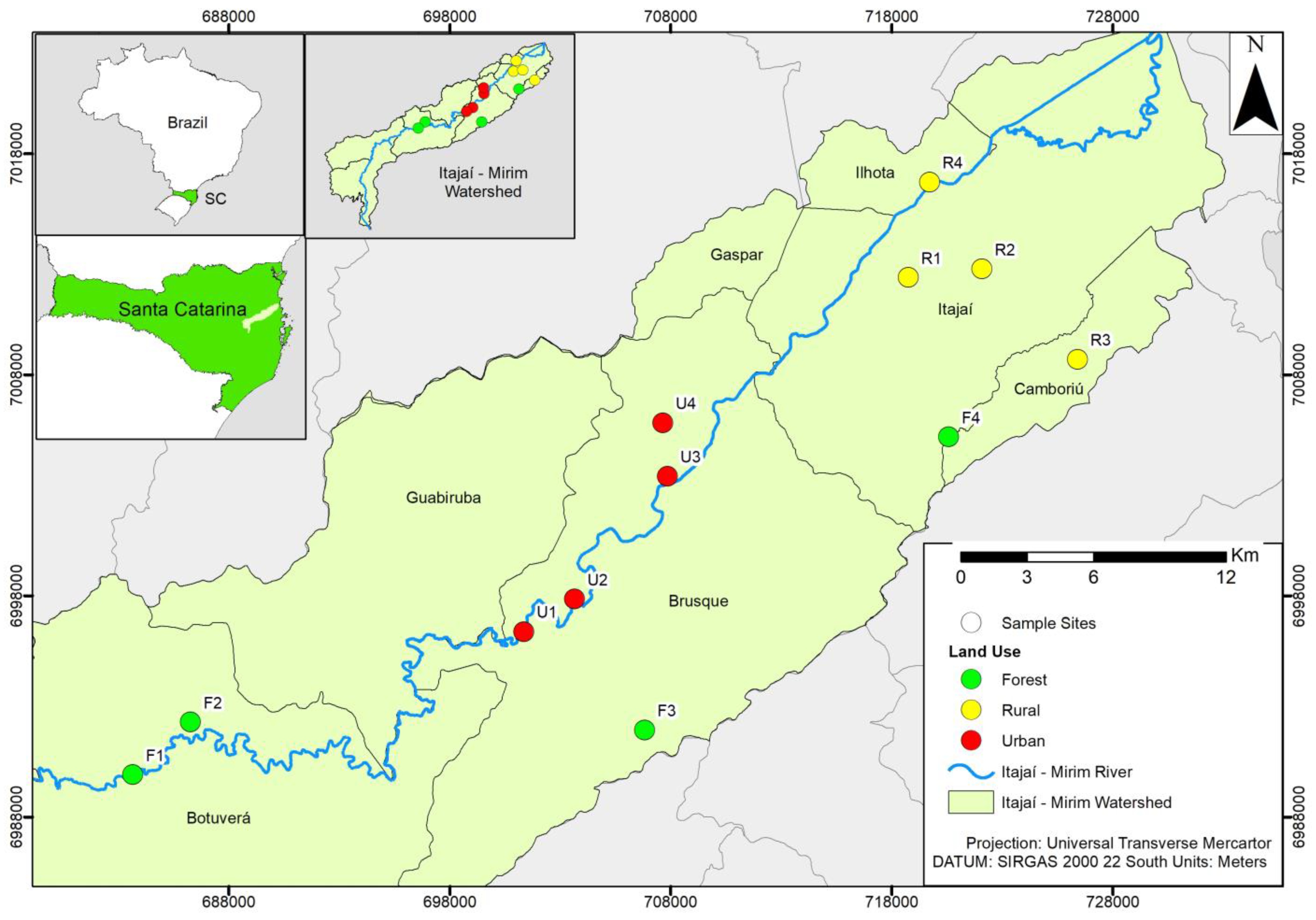

| Site | Longitude | Latitude | Site Description |
|---|---|---|---|
| F 1 | 49°8′48″ O | 27°11′59″ S | Stream upstream of the basin, a shaded, lotic environment with rapids and few backwaters, near the main tributary. |
| F 2 | 49°07′12″ O | 27°10′45″ S | Stream upstream of the watershed, partially shaded, lotic environment with rapids and backwaters, heterogeneous substrate. Located after the waterfall, on private property. |
| F 3 | 48°54′45″ O | 27°10′47″ S | Stream in the intermediate region, between upstream and downstream of the main course of the river in the watershed, shaded and lotic environment, heterogeneous substrate, presence of downstream dwellings. |
| F 4 | 48°46′34″ O | 27°03′29″ S | Stream downstream of the basin, partially shaded, lotic environment with rapids and backwaters, heterogeneous substrate, far from the main tributary, with animal breeding in the surrounding area. Located on private property. |
| R 1 | 48°47′44″ O | 26°59′36″ S | Stream downstream of the basin, without shading, lentic environment, homogeneous substrate, distant from the main tributary. |
| R 2 | 48°46′03″ O | 26°58′58″ S | Stream downstream of the basin, without shading, lentic environment, homogeneous substrate with the presence of periphyton, near the main tributary, surrounded by rice plantations. |
| R 3 | 48°43′05″ O | 27°01′32″ S | Stream downstream of the basin, partially shaded, intermediate flow environment, heterogeneous substrate, located on private property, with animal breeding in the surrounding area. |
| R 4 | 48°47′13″ O | 26°57′15″ S | Stream downstream of the basin, without shading, intermediate flow environment, homogeneous substrate, fine. Stream partially piped (rural road), with a steep slope. |
| U 1 | 48°58′06″ O | 27°08′25″ S | Stream in the intermediate region, between upstream and downstream of the main course of the river, without shading, a lentic environment, and a homogeneous substrate. Water with a foul odor, the formation of supernatant oil, and pigmentation. Stream in the allotment region, with a steep slope. |
| U 2 | 48°56′38″ O | 27°07′45″ S | Stream in the intermediate region, between upstream and downstream of the main course of the river, shaded, with intermediate flow and heterogeneous substrate. Presence of supernatant oil in the water, surrounded by banana plantations and partially piped. |
| U 3 | 48°90′40″ O | 27°04′34″ S | Stream in the intermediate region, between upstream and downstream of the main course of the river, partially shaded, with intermediate flow and homogeneous substrate. Water with a foul odor, Chinonomidae larvae with red pigmentation in the flow, and the presence of direct sewage in the site. |
| U 4 | 48°54′23″ O | 27°03′15″ S | Stream in the intermediate region, between upstream and downstream of the main course of the river, without shading, environment and intermediate flow, homogeneous substrate, with steep slope. Water with a foul odor, pigmentation, and the presence of larvae of the Hirudinea group in the flow, with direct sewage in the site |
| Land Use | Season | Temp (°C) | pH (Unit) | DO (mg/L−1) | Cond (µS cm−1) | TP (mg/L−1) | NO3 (mg/L−1) | NO2 (mg/L−1) | NH4 (mg/L−1) |
|---|---|---|---|---|---|---|---|---|---|
| Forest | Summer | 23.11 ± 0.56 | 8.32 ± 0.21 | 9.61 ± 0.89 | 43.62 ± 23.99 | 0.01 ± 0 | 0.33 ± 0.09 | 0.05 ± 0 | 0.11 ± 0.01 |
| Fall | 17.21 ± 0.64 | 8.12 ± 0.04 | 8.56 ± 0.88 | 36.53 ± 17.44 | 0.02 ± 0 | 0.38 ± 0.13 | 0.05 ± 0 | 0.10 ± 0 | |
| Winter | 16.95 ± 0.87 | 7.95 ± 0.36 | 8.60 ± 0.26 | 36.57 ± 16.93 | 0.02 ± 0 | 0.33 ± 0.12 | 0.10 ± 0 | 0.10 ± 0 | |
| Spring | 16.67 ± 0.56 | 7.24 ± 0.40 | 8.09 ± 0.34 | 33.58 ± 15.34 | 0.01 ± 0 | 0.45 ± 0.14 | 0.10 ± 0 | 0.10 ± 0 | |
| Rural | Summer | 23.45 ± 0.50 | 7.67 ± 0.18 | 6.38 ± 2.32 | 61.17 ± 12.47 | 0.17 ± 0.29 | 0.21 ± 0.01 | 0.05 ± 0 | 0.18 ± 0.13 |
| Fall | 18.03 ± 0.26 | 7.37 ± 0.22 | 6.63 ± 2.30 | 44.56 ± 9.04 | 0.10 ± 0.10 | 0.36 ± 0.10 | 0.05 ± 0 | 0.10 ± 0 | |
| Winter | 17.93 ± 0.59 | 7.20 ± 0.65 | 5.50 ± 1.31 | 55.82 ± 10.31 | 0.04 ± 0.02 | 0.28 ± 0.15 | 0.10 ± 0 | 1.01 ± 1.38 | |
| Spring | 17.41 ± 0.19 | 7.06 ± 0.67 | 6.18 ± 2.31 | 84.49 ± 65.87 | 0.10 ± 0.10 | 0.45 ± 0.36 | 0.10 ± 0 | 1.37 ± 1.82 | |
| Urban | Summer | 23.87 ± 0.19 | 7.22 ± 0.33 | 3.82 ± 1.27 | 250.11 ± 70.90 | 0.51 ± 0.29 | 0.52 ± 0.57 | 0.09 ± 0.04 | 7.79 ± 2.83 |
| Fall | 18.44 ± 0.32 | 7.11 ± 0.08 | 3.13 ± 1.39 | 200.29 ± 134.97 | 0.40 ± 0.22 | 0.53 ± 0.42 | 0.23 ± 0.26 | 3.90 ± 3.09 | |
| Winter | 18.32 ± 0.10 | 7.22 ± 0.28 | 4.39 ± 2.14 | 272.00 ± 129.01 | 0.53 ± 0.42 | 0.55 ± 0.70 | 0.11 ± 0.01 | 4.80 ± 4.12 | |
| Spring | 17.87 ± 0.42 | 6.45 ± 0.14 | 3.48 ± 2.28 | 196.36 ± 49.31 | 0.91 ± 0.83 | 0.63 ± 0.62 | 0.12 ± 0.02 | 5.31 ± 3.37 |
| Land Use | Season | Wid (m) | Vel (m/s) | Dep (m) | Pluv (mm) |
|---|---|---|---|---|---|
| Summer | 2.64 ± 1.23 | 0.30 ± 0.07 | 0.14 ± 0.04 | 33.27 ± 14.76 | |
| Forest | Fall | 2.88 ± 0.42 | 0.37 ± 0.18 | 0.12 ± 0.03 | 144.00 ± 11.66 |
| Winter | 1.88 ± 0.12 | 0.34 ± 0.10 | 0.12 ± 0.03 | 6.35 ± 3.50 | |
| Spring | 3.42 ± 1.36 | 0.32 ± 0.08 | 0.18 ± 0.06 | 19.99 ± 9.52 | |
| Summer | 2.86 ± 1.39 | 0.10 ± 0.16 | 0.21 ± 0.13 | 57.08 ± 6.98 | |
| Rural | Fall | 2.42 ± 1.10 | 0.12 ± 0.24 | 0.22 ± 0.05 | 159.65 ± 0.10 |
| Winter | 1.75 ± 1.35 | 0.19 ± 0.31 | 0.19 ± 0.11 | 2.54 ± 2.52 | |
| Spring | 1.81 ± 1.15 | 0.08 ± 0.16 | 0.21 ± 0.12 | 12.39 ± 6.22 | |
| Summer | 2.06 ± 0.92 | 0.19 ± 0.16 | 0.23 ± 0.11 | 44.96 ± 9.97 | |
| Urban | Fall | 1.41 ± 0.34 | 0.20 ± 0.16 | 0.17 ± 0.03 | 152.24 ± 8.61 |
| Winter | 1.44 ± 0.61 | 0.18 ± 0.17 | 0.15 ± 0.02 | 1.53 ± 0.29 | |
| Spring | 1.48 ± 0.47 | 0.13 ± 0.11 | 0.14 ± 0.02 | 25.06 ± 30.16 |
| Abundance | Richness | Shannon (H) | Simpson (D) | |
|---|---|---|---|---|
| Treatment | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Forest | 1038.06 ± 514.70 | 26.75 ± 1.96 | 4.68 ± 2.82 | 2.24 ± 1.91 |
| Rural | 591.81 ± 254.93 | 20.62 ± 3.64 | 5.45 ± 2.30 | 3.47 ± 1.80 |
| Urban | 2340.16 ± 3044.18 | 8.56 ± 4.51 | 2.21 ± 1.77 | 2.01 ± 113 |
| Estimate | Std. Error | Z value | Pr(>|z|) | |
|---|---|---|---|---|
| Intercept | 1.978 | 0.092 | 21.289 | <0.001 |
| Rural Use | −0.083 | 0.134 | −0.624 | 0.532 |
| Urban Use | −0.969 | 0.181 | −5.341 | <0.001 |
| Fixed Effects | Estimate | Std. Error | Z value | Pr(>|z|) |
|---|---|---|---|---|
| Intercept | 1.318 | 0.129 | 10.201 | <0.001 |
| Rural Use | 0.015 | 0.182 | 0.085 | 0.931 |
| Urban Use | −0.587 | 0.220 | −2.661 | <0.001 |
| Fixed Effects | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| Intercept | 5.859 | 0.228 | 25.672 | <0.001 |
| TP | 0.922 | 0.425 | 2.168 | 0.030 |
| Cond | 0.003 | 0.001 | 2.465 | 0.013 |
| Vel | 2.691 | 0.677 | 3.975 | <0.001 |
| Fixed Effects | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| Intercept | −11.338 | 10.450 | −1.085 | 0.284 |
| Cond | −0.040 | 0.008 | −5.030 | <0.001 |
| NO3 | 4.609 | 2.019 | 2.282 | 0.027 |
| Dep | 25.497 | 9.049 | 2.818 | 0.007 |
| pH | 2.878 | 1.410 | 2.041 | 0.047 |
| DO | 1.353 | 0.382 | 3.536 | 0.001 |
| Pluv | −0.030 | 0.010 | −2.766 | 0.008 |
| Fixed Effects | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| Intercept | 1.468 | 0.216 | 6.778 | <0.001 |
| Cond | −0.003 | <0.001 | −3.726 | <0.001 |
| NO3 | 0.536 | 0.228 | 2.355 | 0.018 |
| Wid | 0.139 | 0.058 | 2.367 | 0.017 |
| MEM1 | −0.196 | 0.069 | −2.842 | 0.004 |
| Fixed Effects | Estimate | Std. Error | t Value | Pr(>|t|) |
|---|---|---|---|---|
| Intercept | −1.020 | 1.080 | −0.944 | 0.345 |
| pH | 0.287 | 0.143 | 2.002 | 0.045 |
| MEM1 | −0.259 | 0.083 | −3.097 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohn, I.C.; Branco, J.O.; Cionek, V.d.M.; Costa, V.S.C.d.; Silva, A.L.L.d.; Ribeiro, E.A.W. Effects of Land Use on the Community Structure of Aquatic Invertebrate in Subtropical Streams. Diversity 2024, 16, 497. https://doi.org/10.3390/d16080497
Bohn IC, Branco JO, Cionek VdM, Costa VSCd, Silva ALLd, Ribeiro EAW. Effects of Land Use on the Community Structure of Aquatic Invertebrate in Subtropical Streams. Diversity. 2024; 16(8):497. https://doi.org/10.3390/d16080497
Chicago/Turabian StyleBohn, Isabel Cristina, Joaquim Olinto Branco, Vivian de Mello Cionek, Vinícius Soares Correa da Costa, Aurea Luiza Lemes da Silva, and Eduardo Augusto Werneck Ribeiro. 2024. "Effects of Land Use on the Community Structure of Aquatic Invertebrate in Subtropical Streams" Diversity 16, no. 8: 497. https://doi.org/10.3390/d16080497
APA StyleBohn, I. C., Branco, J. O., Cionek, V. d. M., Costa, V. S. C. d., Silva, A. L. L. d., & Ribeiro, E. A. W. (2024). Effects of Land Use on the Community Structure of Aquatic Invertebrate in Subtropical Streams. Diversity, 16(8), 497. https://doi.org/10.3390/d16080497








