Abstract
The present work provides an updated and corrected list of hosts for the five European species of pinnotherid crabs, taking into consideration all recent published works as well as new data obtained in the present study. In relation to the number of hosts, there are two groups of species, one composed by Pinnotheres pisum and Afropinnotheres monodi with the highest number of hosts, and a second group, with a reduced number of hosts and more specific taxa, consisting of three other species: Nepinnotheres pinnotheres, Pinnotheres bicristatus, and P. pectunculi. When studying the relationships between host preferences, host distributions, and pea crab distributions, we could not find a clear direct relationship between these parameters. Temperature is also probably an important influence and relevant factor responsible for the distribution of pinnotherid crabs, and this may be independent from the potential distribution due to the distribution of hosts.
1. Introduction
Five species of pinnotherids crabs (Brachyura, Pinnotheridae) have been recorded in European waters [1,2]. Three of them belong to the genus Pinnotheres Bosc, 1801: P. bicristatus García Raso and Cuesta, 2019 in Cuesta et al. [2], P. pectunculi Hesse, 1872, and P. pisum (Linnaeus, 1767)), and the other two to Afropinnotheres Manning, 1993, A. monodi Manning, 1993, and Neopinnotheres Manning, 1993, N. pinnotheres (Linnaeus, 1758). They all are members of the subfamily Pinnotherinae De Haan, 1833, and, like many of the pinnotherines, live symbiotically inside bivalves, except for N. pinnotheres, which also lives in the interior of sea squirts [3]. However, not all of them show the same host preferences, and two main types can be observed with respect to this behaviour. Three species show a restricted preference for a group of bivalves: this is the case for P. bicristatus, P. pectuncili, and N. pinnotheres. Pinnotheres bicristatus has only been recorded in the dog oyster, Anomia ephippium Linnaeus, 1758 [2], and a recent observation of one specimen inside a European flat oyster, Ostrea edulis Linnaeus, 1758 [4], requires further observations in order to consider it as a preferential host, and not an accidental one. Pinnotheres pectunculi has only been found in Glycymeris glycymeris (Linnaeus, 1758) and a few species of the family Veneridae Rafinesque, 1815 [1]. Nepinnotheres pinnotheres, although it is also found inside several species of ascidians, is only associated to three bivalve species of pen shells (Pinnidae Leach, 1819): Atrina pectinata (Linnaeus, 1767), Pinna nobilis Linnaeus, 1758, and Pinna rudis Linnaeus, 1758 [1,5]. However, A. monodi and P. pisum have been recorded in a long list of different bivalves [1,6,7]. Although these two species present some preferences for a few hosts where they are found in higher frequencies, they can live and reproduce inside a greater variety of different bivalves belonging to different families.
In the present study, the results of recent sampling surveys in the Gulf of Cádiz, currently the richest European area regarding the number of pinnotherid species, allow us to study the host preferences of these pea crabs, update and correct the latest known list of hosts [7], as well as analyse the implications that specificity or non-specificity for host selection and number of hosts could represent as a disadvantage or advantage regarding species’ distribution.
2. Materials and Methods
2.1. Study Area and Sample Collection
The study area is located in the southern part of the Iberian Peninsula, encompassing the area of the Gulf of Cádiz at 36° to 37.25° N and 6° to 7.5° W. The study area covers waters around the Gulf of Cádiz from the mouth of the Guadiana River that borders Portugal in Ayamonte (Huelva) to the Cape of Tarifa (Cádiz), with depths ranging from 3 to 800 m (Figure 1).
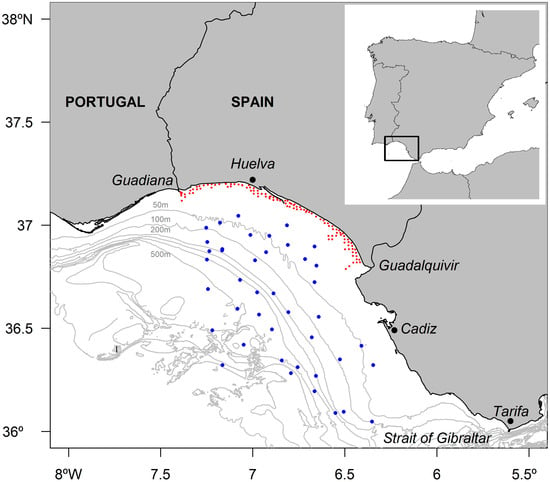
Figure 1.
Iberian Peninsula and the study area at the southwestern Spanish coast of the Gulf of Cádiz (black box). The locations of sampling stations during the surveys are shown as red (ACUVEN-2 and -3) and blue points (ARSA0318 and 0319).
Pinnotherids were collected using two types of sampling surveys conducted in the subtidal area (ACUVEN-3 and ACUVEN-2 surveys) and from the continental shelf and slope (ARSA0318 and ARSA0319 surveys) of the Gulf of Cádiz (Figure 1).
The ACUVEN-3 survey was carried out in May 2019, and it covered the whole subtidal area of the littoral of Huelva (3–15 m depth). The ACUVEN-2 survey was conducted in November 2020 in the protected subtidal area at the mouth of the Guadalquivir river. In both surveys, the sampling was conducted along transects perpendicular to the coast and separated by 1 nautical mile using a commercial hydraulic dredge with a rectangular cage (247–272 cm wide, 30 cm depth, and approximately 114 cm height), mounted upon two sledge runners. The spacing between the grid bars was 2 cm, and a hose connected to the front part of the cage was used to eject water under pressure. The sampling followed a systematic scheme where stations were located covering 3 depths of strata (3–6 m) (7–9 m) (9–12 m); for more details see Delgado et al. [8]. The ARSA0318 and ARSA0319 surveys were carried out in March 2018 and 2019, respectively, using bottom Baka 40/60 trawl gear with a 43.6 m footrope and a 60.1 m headline (an inner 20 mm mesh codend liner was used to prevent the escape of small individuals), and carried out from the bottom-trawler “Miguel Oliver”. Sampling was conducted in spring following a common protocol [9] and a random stratified scheme with 5 depths of strata (15–30, 31–100, 101–200, 201–500, and 501–800 m).
For each haul, sampling information (date, time, duration, initial and final longitude, latitude and depth, and the distance trawled) was recorded. All the species were identified to the lowest taxon possible, and were weighed, counted, and preserved in ethanol 70%.
A selection of the specimens collected during the ACUVEN-3, ACUVEN-2, AR-SA0318, and ARSA0319 surveys were deposited in the Marine Crustacean Collection (CRUST-IEOCD) at the Cádiz Oceanographic Centre of the Spanish Institute of Oceanography (IEO-CSIC) [10].
2.2. Environmental Variables
Water temperature (°C) and salinity (psu) data were measured at 1.5 m from the sea bottom and at each station using the YSI ProDSS handheld multiparameter instrument. Sediment samples were collected to study the grain size distribution of the sediment in each sampling station. These samples were collected using a Van Veen grab (ABT-SG-1000; 15 L, 1000 cm2). For the granulometric analysis, sediment samples of a similar weight (around 100 g) were dried in an oven for 48 h at 60 °C, sieved over a column of sieves (4, 2, 1, 0.5, 0.25, and 0.125 mm) and the fractions retained on each sieve were weighed (dry weight). The grain size data were analysed using the GRADISTAT package v 8.0 [11], which calculates, based on Folk and Ward [12] and on the method of moments, the grain size statistics (phi units; logarithmic mean).
2.3. Species Identification
Bivalve hosts were identified according to Muñoz et al. [13] and Gofas et al. [14]. Pea crabs were identified by their morphology using the key to European pinnotherids by Cuesta et al. [2], and confirmed by DNA barcoding, partially sequencing the gene of the mitochondrial cytochrome c oxidase subunit I (COI), following the same protocols in Perez-Miguel et al. [1]. All COI sequences obtained for the three species of pea crabs were deposited at Genbank under the accession codes PP907876-PP907878.
3. Results
Results from the ACUVEN-3 and -2 surveys in the subtidal depth range (3–16 m) indicated that the bivalves were represented by 42 species, which constituted the dominant group in the number of species (25.9%) in these samples. Three species of pinnotherids crabs were collected and identified during the surveys: P. pisum, P. bicristatus, and N. pinnotheres (see Table 1). The main host species for P. pisum was the bivalve from the family Cardiidae Acanthocardia paucicostata (G. B. Sowerby II, 1834), and for P. bicristatus it was the bivalve from the family Anomiidae Anomia ephippium (Linnaeus, 1758). Acanthocardia paucicostata showed a frequency of occurrence of 27.68% and a dominance of 0.15%. Anomia ephippium showed a frequency of occurrence of 17.86% and a dominance of 0.26%. Nepinnotheres pinnotheres was collected during the ARSA0318 and ARSA0319 surveys (depth range: 15–800 m) within the bivalve host Atrina fragilis (Pennant, 1777).

Table 1.
List of the pinnotherid species collected in the ACUVEN-3, ACUVEN-2, ARSA0318, and ARSA0319 surveys, indicating code: collection code, host: name of the bivalve host, N: number of pea crab specimens, Survey: survey, St: station, Depth: depth (m), T: temperature (°C), S: salinity (PSU), MGS: mean of the grain size in phi units, Sed: sediment type at each station, CS: coarse sand, FS: fine sand, MS: medium sand, VFS: very fine sand, and -: no data.
Although identification of the pinnotherids was firstly obtained based on their morphology, it was later confirmed by comparing the COI sequences (467–640 bp) obtained from the samples of these three species and those of the specimens deposited in GenBank through a BLAST search. All the sequences fitted between 99 and 100% with these sequences: MK468903-MK468907 of P. bicristatus, MT04612-MT046614, MT046616, and MG935291 of P. pisum, and MF134398.2 of N. pinnotheres.
The locations of the samples of pinnotherid crabs and their hosts during the ACUVEN-3 and -2, and ARSA0318 and 0319 surveys are shown in Figure 2. In the subtidal area, P. pisum (host: Acanthocardia paucicostata) mostly occurred in the eastern part of the Gulf of Cádiz, while P. bicristatus (host: Anomia ephippium) had a more western distribution. Nepinnotheres pinnotheres (host: Atrina fragilis) was found at depths between 83 and 94 m. Temperature and salinity varied between the stations during the ACUVEN-3 and -2 surveys and ranged between 14.47 and 20.70 °C, and 35.91 and 37.2, respectively (see Table 1). As can be observed, the stations linked to the host A. ephippium showed lower temperature records than the A. paucicostata stations. Additionally, a coarse grain sediment was observed at the stations in the eastern part of the subtidal area of the Gulf of Cádiz (A. ephippium samples) in comparison with the western part (A. paucicostata samples) (Table 1).
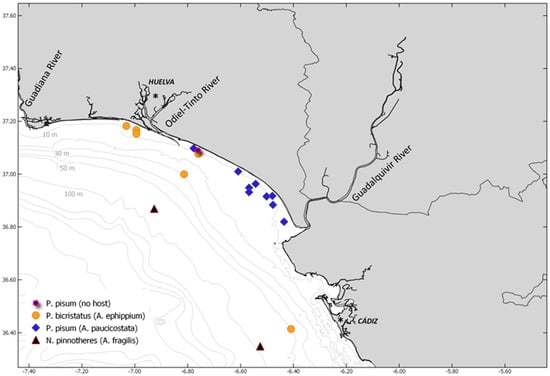
Figure 2.
Locations of pinnotherids crabs and hosts in the subtidal and shelf areas of the Gulf of Cádiz (southwestern Spain).
An updated and corrected list of hosts for European pinnotherids was compiled after checking the main literature, which contained data about hosts from the older to the most recent works [1,3,6,7,15,16,17,18,19,20,21], including new data obtained in the present study (Table 2).

Table 2.
Updated list of hosts of the European pinnotherid crabs. Underlined and in bold are the new host species for Nepinnotheres pinnotheres and Pinnotheres pisum obtained in the present study.
4. Discussion
After a detailed review of old and recent studies that provide data about the hosts of European pea crabs, we have made a consensus list (see Table 2) where we have deleted species erroneously assigned in some works, and we have added the recent new hosts [6,20] as well as the two new hosts as result of the present study.
In this updated list, P. pisum is the pea crab with the most known hosts, 29 species, even after eliminating Clausinella fasciata (da Costa, 1778), Dosinia lupinus (Linnaeus, 1758), Ensis ensis (Linnaeus, 1758), E. magnus Schumacher, 1817, and Nucula nitidosa Winckworth, 1930, which were mistakenly included on the list by de Gier and Becker [7]. They considered all species mentioned in Becker and Turkay [21] related with P. pisum, i.e., both those that were hosts and those that were not. They also included Acanthocardia echinata (Linnaeus, 1758), Cerastoderma edule (Linnaeus, 1758), and Chamelea gallina (Linnaeus, 1758) even though these species were considered “non-infested species” in Becker and Turkey ([21] Figure 1), but they are included in the consensus list (Table 2) because they have been recorded as P. pisum hosts in posterior works [1]. The only species mentioned by Becker and Turkey [21] as “non-infested species” and not included in the list by de Gier and Becker [7] was Venerupis corrugata (Gmelin, 1791) (as Venerupis senegalensis), also mentioned in Figure 1. The list of hosts for P. pisum has been completed with recently recorded hosts such as Fulvia fragilis (Forsskål, 1775) by Triay-Portella et al. [6], Glossus humanus (Linnaeus, 1758) by Marco-Herrero et al. [20], and now Acanthocardia paucicostata (present study).
The second species, in terms of the number of hosts, is A. monodi, with 13 known bivalve hosts. The list of hosts published by Perez-Miguel et al. [1] provided a total of 12 species, but a record from Magallana gigas (Thunberg, 1793) in Perez-Miguel’s unpublished Ph.D. thesis [22] has been included.
Nepinnotheres pinnotheres is the third species in terms of the number of known hosts. Another Pinnidae bivalve, Atrina fragilis, has been added in the present study and is the only bivalve family where specimens of this species have been recorded. The other hosts of this species are ascidians, and in this case, there are some doubts concerning Ascidia virginea Müller, 1776 and Phallusia mammillata (Cuvier, 1815), possibly bringing the number of ascidian host species up from three to five. Nepinnotheres pinnotheres is also associated with representatives from the genus Microcosmos, of which the number of included species is currently under dispute. The number of ascidian host species of N. pinnotheres might therefore be even higher. This species shows a preference for pinnid bivalves, bivalve species characterised by their large sizes.
Pinnotheres pectunculi is also an example of a species with an obvious preference in the selection of its hosts. It has been recorded mainly in Glycymeris glycimeris and in four species of venerids. Until recently, this species was only cited in the northeastern Atlantic, but Perez-Miguel et al. [1] have recorded it for the first time in the Mediterranean.
The European pea crab with the highest specialization in host selection is the recently described Pinnotheres bicristatus. It had only been recorded in Anomia ephippium [2], but recently it was also found inside A. ephippium, from the Western Mediterranean, where, additionally, a specimen was located in Ostrea edulis Linnaeus, 1758 [4]. This bivalve species is of commercial interest and until now no specimens of P. bicristatus have been recorded as a symbiont. More data will be needed to clarify whether this was an accidental host or not.
Considering the number and type of hosts, we could think a priori that species with a higher number of host species and less specific selection could present a wider distribution. This is right for some cases, such as P. pisum, which has a known distribution covering all Atlantic European waters, including the Canary Islands, the Mediterranean, and 29 known host species. However, N. pinnotheres, with a smaller number of hosts, presents a similar distribution, in the Atlantic, from Mauritania to Ireland, and the Mediterranean. In addition, A. monodi, with 13 different hosts, has a more limited known distribution, from Mauritania to Cascais in Portugal in the Atlantic waters and only a few records in the Alboran Sea in the Mediterranean [22]. Therefore, although the potential distribution, considering the distribution of their hosts, could be higher, the current known distribution of pea crabs does not mirror that of their hosts. For example, Mytilus spp. are the preferential hosts for P. pisum and A. monodi; however, although the distribution of these mussels covers the Atlantic (from Norway to Namibia) and the Mediterranean [23], P. pisum is not found in continental African Atlantic waters, and A. monodi is not found in mussel populations above Cascais, nor in the Mediterranean except for a few records in the Alboran Sea. Therefore, there are other parameters conditioning their distribution, and the main one is probably temperature. Pinnotheres pisum is adapted to cooler waters, and for this reason can be found in intertidal populations of mussels in the French Brittany, but not in the intertidal mussel populations in the Gulf of Cádiz. In fact, P. pisum is found in this southernmost area in subtidal species such as Chamelea gallina [1] and Acanthocardia paucicostata (see Table 1 and Figure 2 of the present study). Acanthocardia paucicostata has a wide distribution through the subtidal area of the coast of Huelva and lives continuously underwater, which ensures a more stable thermal regime. The presence of this infaunal bivalve with small siphons and a developed shell sculpture is also higher in areas of fine or very fine sediment close to river mouths (Figure 3, Table 1), especially to the Guadalquivir river, which has developed subtidal sedimentary formations. In the case of the pea crab P. bicristatus, its more westerly spatial distribution is linked to the presence of the bivalve A. ephippium that needs hard substates to settle on and is found attached to rocks or the shells of other bivalves. This habitat selectivity is consistent with the presence of coarser sediment, but it could also indicate a preference for the colder waters within the Gulf of Cádiz (Figure 3, Table 1).
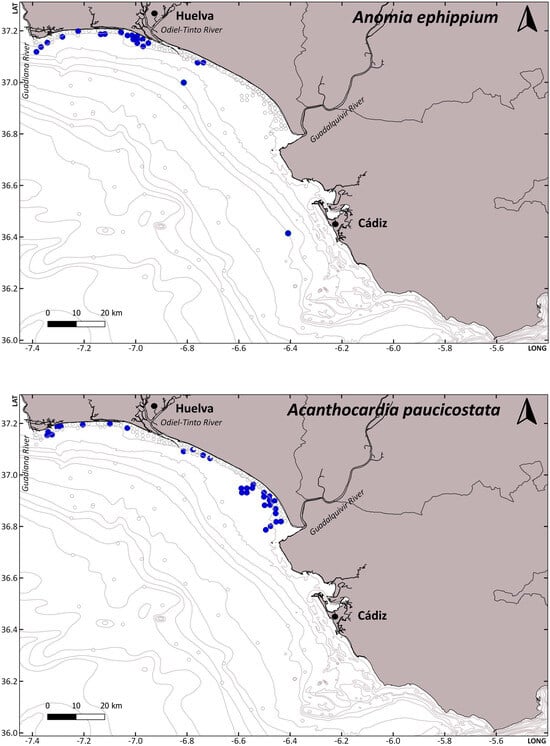
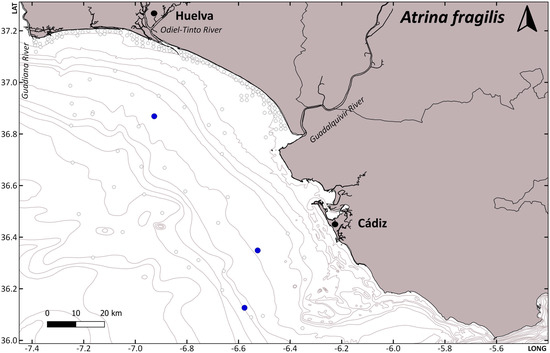
Figure 3.
Presence-absence maps of the hosts Anomia ephippium, Acanthocardia paucicostata, and Atrina fragilis along the Gulf of Cadiz. Presence (blue dots)-absence (white dots) maps. LAT: latitude and LONG: longitude. Data from ACUVEN-2, ACUVEN-3, and ARSA0318 surveys.
Differences in host depth distribution between P. pisum and P. bicristatus in the subtidal zone were very subtle (A. ephippium, 9.51 ± 1.15 m; A. paucicostata, 11.64 ± 2.26 m). However, the depth distribution range of A. ephippium is broader than that of A. paucicostata, conditioning the presence of P. bicristatus in the circalittoral area (up to 52 m). Additionally, the geographical differences observed in the distribution between P. pisum and P. bicristatus may not only be related to the higher presence of specific hosts, but also to environmental factors such as temperature (defining a more restricted thermal distribution range), the size of the bottom sediment, or the specific presence of other intermediate hosts. On the contrary, A. monodi has showed preferences for warmer waters, as has been already studied [24], and this could explain why it is not present in the northernmost European populations of mussels, although there is no justification based on low temperatures for its absence in Mediterranean waters. In this case, there are probably other reasons. For example, this species reaches the best reproductive output in mussel populations and they are the preferred host for soft females, but the males and hard females also need a primary host for their initial stages, such as Cerastodema spp., Scrobicularia plana (da Costa, 1778), etc. [25]. Therefore, the availability, or lack of, of these species in the Mediterranean, close to mussel populations, could be a decisive factor.
Population studies concerning European pea crabs are limited and, for this reason, it is not possible for us to be more conclusive about the reasons that justify the pea crab distributions. Although parameters such as temperature and the presence/absence of determined host species could play a decisive role.
The south of the Iberian Peninsula is the richest area in terms of the number of pea crab species; this might be due to the geographical position of this zone, which includes: the Gulf of Cádiz, the Strait of Gibraltar, the Alboran Sea, and the frontiers between the Atlantic and Mediterranean and between European and African waters. The increase in pea crab studies in the last few years in this zone may also provide an explanation. For example, the project that studied non-commercial bivalve species made it possible to discover and describe P. bicristatus (Cuesta et al., 2019) [2]. More studies in other zones of the Mediterranean and involving a higher number of bivalve species need to be performed to get a better understanding of the number of European pea crabs and their distributions. The consensus list of host species (Table 2) is a base line for future studies to start from. Besides this, the availability of DNA markers for all known European pea crabs in public databases such as Genbank and BOLD is also an important tool to confirm the species identification of any given species.
Author Contributions
Conceptualization, J.A.C.; methodology, J.A.C., A.R.d.l.R. and M.D.; field sampling, A.R.d.l.R., L.S. and S.R.; software, J.A.C. and S.R.; validation, J.A.C., S.R. and I.M.; formal analysis, J.A.C., S.R. and I.M.; investigation, J.A.C.; resources, J.A.C., L.S. and C.F.; data curation, J.A.C. and I.M.; writing—original draft preparation, J.A.C.; writing—review and editing, J.A.C. and M.D.; funding acquisition, J.A.C. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was developed within the framework of the project “VENUS” (“Estudio integral de los bancos naturales de moluscos bivalvos en el Golfo de Cádiz para su gestión sostenible y la conservación de sus hábitats asociados”) (0139_VENUS_5_E; INTERREG-POCTEP), cofinanced by the European Regional Development Fund (FEDER, Interreg V-A España-Portugal (POCTEP) 2014–2020 program). This study was also partially funded by the Spanish “Ministerio de Economía y Competitividad (MINECO)”, Plan Nacional I+D’, and the European FEDER funds through project AFROBIV (CGL2014-53557-P).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Information on the specimens collected and examined can be found in the CRUST_IEOCD Collection (https://www.gbif.org/es/dataset/317fd0a9-1bcf-4e94-bc3c-a685c4693c10) (accessed on 13 June 2024).
Acknowledgments
We would like to thank our colleagues at IEO (Centre of Cádiz), especially Miguel Cojan and Raimundo Blanco, for collaborating in the biological sampling and technical support. We would also like to thank the owners and the crew of the two ships “PITI-II” and “Miguel Oliver”, which were used in the sea surveys, for their support in sampling and data collection. We are grateful to three anonymous reviewers for their constructive comments, suggestions, and corrections, and to Carlos Sánchez (ICMAN-CSIC) for laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Perez-Miguel, M.; Drake, P.; García Raso, J.E.; Maman Menéndez, L.; Navas, J.I.; Cuesta, J.A. European Pinnotheridae (Crustacea, Decapoda, Brachyura): Species, distribution, host use and DNA barcodes. Mar. Biodiver. 2019, 49, 57–68. [Google Scholar] [CrossRef]
- Cuesta, J.A.; García Raso, J.E.; Abelló, P.; Marco-Herrero, E.; Silva, L.; Drake, P. A new species of pea crab from south-western Europe (Crustacea, Decapoda, Brachyura): Species description, geographical distribution and population structure with an identification key to European Pinnotheridae. J. Mar. Biol. Assoc. U. K. 2019, 99, 1141–1152. [Google Scholar] [CrossRef]
- Becker, C.; Türkay, M. Taxonomy and morphology of European pea crabs (Crustacea: Brachyura: Pinnotheridae). J. Nat. Hist. 2010, 44, 1555–1575. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Galimany, E.; Abelló, P.; Cuesta, J.A.; Drake, P.; Ramon, M. Updating hosts and distribution range of the pea crab Pinnotheres bicristatus (Brachyura: Pinnotheridae). Med. Mar. Sci. 2020, 21, 499–505. [Google Scholar] [CrossRef]
- Moreno, E.; Bacallado, J.J.; Pérez, J.M. Nueva contribución al conocimiento de los crustáceos decápodos de las islas Canarias. In Actas del II Simposio Ibérico de Estudio del Bentos Marino; Ros, J., Niell, F.X., Eds.; Cuadernos de Biología Marina del C.R.I.S.: Barcelona, Spain, 1982; Volume 3, pp. 213–219. [Google Scholar]
- Triay-Portella, R.; Perez-Miguel, M.; González, J.A.; Cuesta, J.A. On the presence of Pinnotheres pisum (Brachyura, Pinnotheridae) in the Canary Islands (NE Atlantic), its southernmost distribution limit. Crustaceana 2018, 91, 1397–1402. [Google Scholar] [CrossRef]
- de Gier, W.; Becker, C. A review of the ecomorphology of Pinnotherine pea crabs (Brachyura: Pinnotheridae), with an updated list of symbiont-host associations. Diversity 2020, 12, 431. [Google Scholar] [CrossRef]
- Delgado, M.; Silva, L.; Román, S.; Llorens, S.; Rodríguez-Rua, A.; Cojan, M. Spatial distribution patterns of striped venus clam (Chamelea gallina, L. 1758) natural beds in the Gulf of Cádiz (SW Spain): Influence of environmental variables and management considerations. Reg. Stud. Mar. Sci. 2023, 63, 103024. [Google Scholar] [CrossRef]
- Sobrino, I.; Jiménez, M.P.; Ramos, F.; Baro, J. Descripción de las Pesquerías Demersales de la Región Suratlántica Española; Technical report; IEO: Madrid, Spain, 1994; 151p. [Google Scholar]
- Muñoz, I. Colección de Crustáceos Marinos (CRUST-IEOCD). Version 1.31; Occurrence Dataset; Instituto Español de Oceanografía. Centro Oceanográfico de Cádiz (CSIC): Cádiz, Spain, 2024. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Gradistat: A grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landf. 2001, 26, 1237–1248. [Google Scholar] [CrossRef]
- Folk, R.L.; Ward, W.C. Brazos River bar: A study in the significance of grain size parameter. J. Sediment. Petrol. 1957, 27, 3–27. [Google Scholar] [CrossRef]
- Muñoz, J.L.; Sánchez, J.; Jiménez, F.; Martínez, M.; Torres, J.; Meneses, V. Guía de los Moluscos Marinos y Continentales del Campo de Gibraltar; de Andalucía, J., Ed.; Consejería de Medio ambiente y Ordenación del Territorio: Ornitour, Spain, 2018; 405p. [Google Scholar]
- Gofas, S.; Moreno, D.; Salas, C. Moluscos Marinos de Andalucía; Universidad de Málaga: Málaga, Spain, 2011; 800p. [Google Scholar]
- Pesta, O. Die Decapodenfauna der Adria; Deuticke, F., Ed.; Smithsonian Libraries and Archives: Liepzig, Germany, 1918; 500p. [Google Scholar]
- Bourdon, R. Inventaire de la faune marine de Roscoff. Decapodes-Stomatopodes; Editions de la Station Biologique de Roscoff: Roscoff, France, 1965; 45p. [Google Scholar]
- Adema, J.P.H.M. De Krabben van Nederland en België (Crustacea, Decapoda, Brachyura); Nationaal Natuurhistorisch Museum: Leiden, The Netherlands, 1991; 244p. [Google Scholar]
- Moro, L.; Herrera, R.; Ortea, J.; Riera, R.; Bacallado, J.J.; Martín, J. Aportaciones al conocimiento y distribución de los decápodos y estomatópodos (Crustacea: Malacostraca) de las islas Canarias. Rev. Acad. Canaria Cienc. 2014, 26, 33–82. [Google Scholar]
- Palacios Theil, E.; Cuesta, J.A.; Felder, D.L. Molecular evidence for non-monophyly of the pinnotheroid crabs (Crustacea: Brachyura: Pinnotheroidea), warranting taxonomic reappraisal. Inv. Syst. 2016, 30, 1–27. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Ramírez-Amaro, S.; Díaz, A.J.; Ordines, F.; Massutí, E. A new host record for the pea crab Pinnotheres pisum (Linnaeus, 1767) (Decapoda: Pinnotheridae) in the western Mediterranean, with an update on host species. Med. Mar. Sci. 2023, 24, 419–425. [Google Scholar] [CrossRef]
- Becker, C.; Türkay, M. Host specificity and feeding in European pea crabs (Brachyura, Pinnotheridae). Crustaceana 2017, 90, 819–844. [Google Scholar] [CrossRef]
- Perez Miguel, M. Efectos del Cangrejo Afropinnotheres monodi Manning, 1993 sobre las Especies de Bivalvos de Interés Comercial de la Península Ibérica. Ph.D Thesis, University of Cádiz, Cádiz, Spain, 2018. [Google Scholar]
- DecaNet. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 8 May 2024).
- Perez-Miguel, M.; Drake, P.; Cuesta, J.A. Temperature effect on the African pea crab Afropinnotheres monodi: Embryonic and larval developments, fecundity and adult survival. J. Exp. Mar. Biol. Ecol. 2020, 527, 151380. [Google Scholar] [CrossRef]
- Drake, P.; Marco-Herrero, E.; Subida, M.D.; Arias, A.M.; Cuesta, J.A. Host use pattern of the pea crab Afropinnotheres monodi: Potential effects on its reproductive success and geographical expansion. Mar. Ecol. Prog. Ser. 2014, 498, 203–215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).