Floristic Diversity and Natural Regeneration of Miombo Woodlands in the Rural Area of Lubumbashi, D.R. Congo
Abstract
1. Introduction
2. Materials and Methods
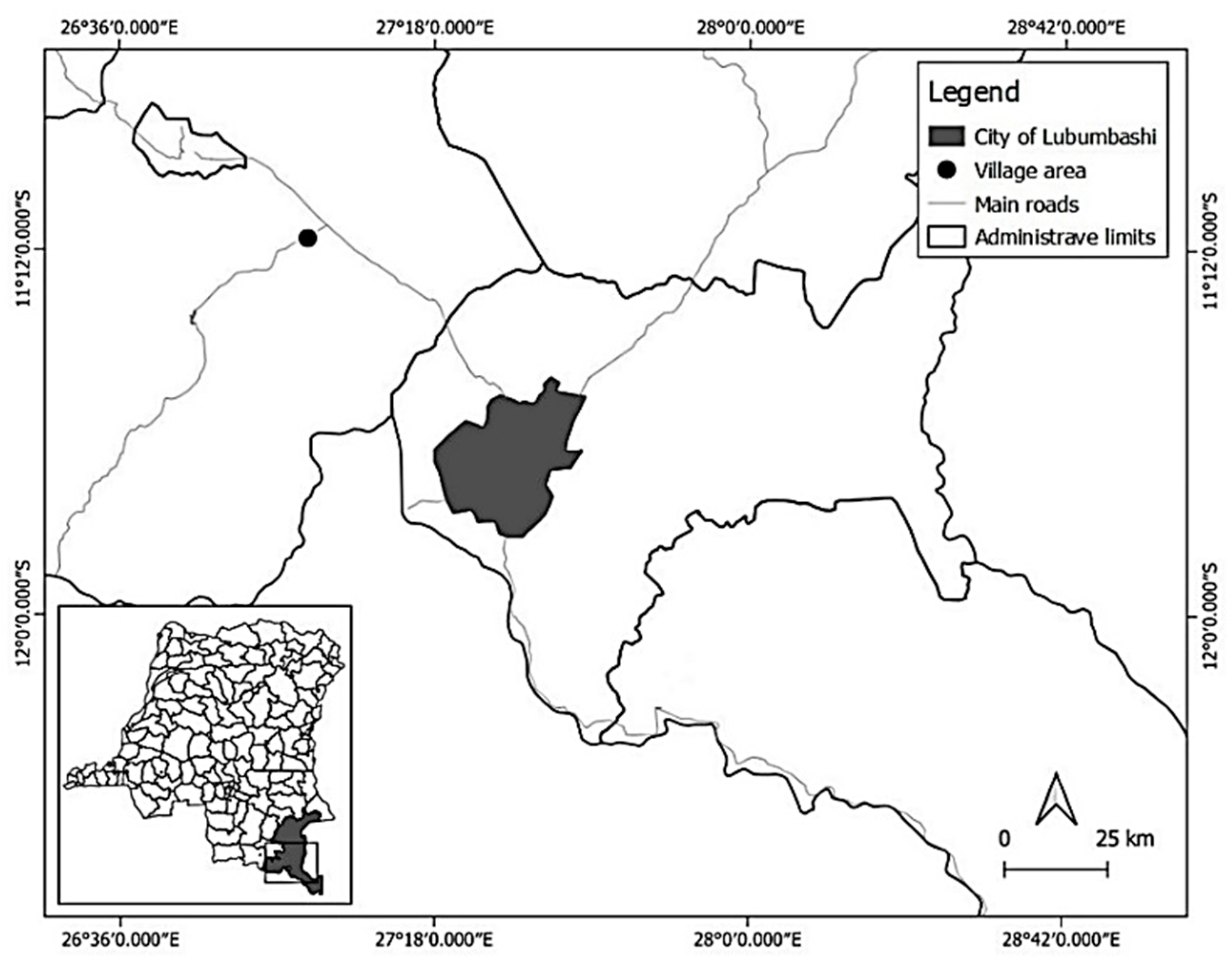
2.1. Study Area
2.2. Methods
2.2.1. Sampling and Data Collection
2.2.2. Data Analysis
3. Results
3.1. Floristic Characterization of the Three Habitats along an Anthropization Gradient
3.2. Natural Regeneration of Miombo Woody Species in the Three Habitats
3.3. Comparison of the Specific Richness of Woody Species Inventoried in Regeneration and Adult Stands
4. Discussion
4.1. Structure and Floristic Composition of Forest Strata and Stands along the Anthropization Gradient
4.2. Regeneration of Miombo Woody Species along the Anthropization Gradient
4.3. Similarity between Floristic Lists along the Anthropization Gradient
4.4. Implications for Sustainable Miombo Woodlands Restoration in Anthropized Landscapes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kalaba, F.K.; Quinn, C.H.; Dougill, A.J.; Vinya, R. Floristic composition, species diversity and carbon storage in charcoal and agriculture fallows and management implications in Miombo woodlands of Zambia. For. Ecol. Manag. 2013, 304, 99–109. [Google Scholar] [CrossRef]
- FAO. La Situation des forêts du monde 2022. In Des Solutions Forestières Pour une Relance verte et des Économies Inclusives, Résilientes et Durables; FAO: Rome, Italy, 2022; 180p. [Google Scholar] [CrossRef]
- Gonçalves, F.M.P.; Chisingui, A.V.; Luís, J.C.; Rafael, M.F.F.; Tchamba, J.J.; Cachissapa, M.J.; Caluvino, I.M.C.; Bambi, B.R.; Alexandre, J.L.M.; Chissingui, M.D.G.; et al. First vegetation-plot database of woody species from Huíla province, SW Angola. VCS 2021, 2, 109–116. [Google Scholar] [CrossRef]
- Berrahmouni, N.; Regato, P.; Parfondry, M. Global Guidelines for the Restoration of Degraded Forests and Landscapes in Drylands: Building Resilience and Benefiting Livelihoods; FAO: Rome, Italy, 2015; 173p. [Google Scholar]
- Ameja, L.G.; Ribeiro, N.S.; Sitoe, A.; Guillot, B. Regeneration and Restoration Status of Miombo Woodland Following Land Use Land Cover Changes at the Buffer Zone of Gile National Park’s Central Mozambique. Trees For. People 2022, 9, 100290. [Google Scholar] [CrossRef]
- Malaisse, F. How to Live and Survive in Zambezian Open Forest (Miombo ecoregion); Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2010; 424p. [Google Scholar]
- Chidumayo, E.N. Management implications of tree growth patterns in miombo woodlands of Zambia. For. Ecol. Manag. 2019, 436, 105–116. [Google Scholar] [CrossRef]
- Chirwa, P.W.; Larwanou, M.; Syampungani, S.; Babalola, F.D. Management and restoration practices in degraded landscapes of Eastern Africa and requirements for up-scaling. Int. For. Rev. 2015, 17, 20–30. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Mittermeier, C.G.; Brooks, T.M.; Pilgrim, J.D.; Konstant, W.R.; Da Fonseca, G.A.B.; Kormos, C. Wilderness and biodiversity conservation. Proc. Natl. Acad. Sci. USA 2003, 100, 10309–10313. [Google Scholar] [CrossRef] [PubMed]
- Godlee, J.L.; Gonçalves, F.M.; Tchamba, J.J.; Chisingui, A.V.; Muledi, J.I.; Shutcha, M.N.; Ryan, C.M.; Brade, T.K.; Dexter, K.G. Diversity and Structure of an Arid Woodland in Southwest Angola, with Comparison to the Wider Miombo Ecoregion. Diversity 2020, 12, 140. [Google Scholar] [CrossRef]
- Useni, S.Y.; Malaisse, F.; Cabala, K.S.; Munyemba, K.F.; Bogaert, J. Le rayon de déforestation autour de la ville de Lubumbashi (Haut-Katanga, RD Congo): Synthèse. Tropicultura 2017, 35, 215–221. [Google Scholar]
- Schneibel, A.; Stellmes, M.; Röder, A.; Finckh, M.; Revermann, R.; Frantz, D.; Hill, J. Evaluating the trade-off between food and timber resulting from the conversion of Miombo forests to agricultural land in Angola using multi-temporal Landsat data. Sci. Total Environ. 2016, 548–549, 390–401. [Google Scholar] [CrossRef]
- Gonçalves, F.M.P.; Revermann, R.; Cachissapa, M.J.; Gomes, A.L.; Aidar, M.P.M. Species diversity, population structure and regeneration of woody species in fallows and mature stands of tropical woodlands of southeast Angola. J. For. Res. 2018, 29, 1569–1579. [Google Scholar] [CrossRef]
- Khoji, M.H.; N’tambwe, N.D.; Mwamba, K.F.; Harold, S.; Munyemba, K.F.; Malaisse, F.; Bastin, J.-F.; Useni, S.Y.; Bogaert, J. Mapping and Quantification of Miombo Deforestation in the Lubumbashi Charcoal Production Basin (DR Congo): Spatial Extent and Changes between 1990 and 2022. Land 2023, 12, 1852. [Google Scholar] [CrossRef]
- Nansikombi, H.; Fischer, R.; Ferrer Velasco, R.; Lippe, M.; Kalaba, F.K.; Kabwe, G.; Günter, S. Can de facto governance influence deforestation drivers in the Zambian Miombo? For. Policy Econ. 2020, 120, 102309. [Google Scholar] [CrossRef]
- Kyale, K.J.; Wardell, D.A.; Mikwa, J.-F.; Kabuanga, J.M.; Monga Ngonga, A.M.; Oszwald, J.; Doumenge, C. Dynamique de la déforestation dans la Réserve de biosphère de Yangambi (République démocratique du Congo): Variabilité spatiale et temporelle au cours des 30 dernières années. Bois For. Trop. 2019, 341, 15–28. [Google Scholar] [CrossRef]
- Cabala, K.S.; Useni, S.Y.; Amisi, M.Y.A.; Munyemba, K.F.; Bogaert, J. Activités anthropiques et dynamique des écosystèmes forestiers dans les zones territoriales de l’Arc Cuprifère Katangais (RD Congo). Tropicultura 2022, 40, 2100. [Google Scholar] [CrossRef]
- Reyniers, C. Agroforesterie et déforestation en République démocratique du Congo. Miracle ou mirage environnemental? Mondes Dév. 2019, 187, 113–132. [Google Scholar] [CrossRef]
- Abdourhamane, H.; Morou, B.; Rabiou, H.; Amhamane, A. Caractéristiques floristiques, diversité et structure de la végétation ligneuse dans le Centre-Sud du Niger: Cas du complexe des forêts classées de Dan kada Dodo-Dan Gado. Int. J. Biol. Chem. Sci. 2013, 7, 1048. [Google Scholar] [CrossRef]
- Syampungani, S.; Geldenhuys, C.J.; Chirwa, P.W. Regeneration dynamics of miombo woodland in response to different anthropogenic disturbances: Forest characterisation for sustainable management. Agrofor. Syst. 2016, 90, 563–576. [Google Scholar] [CrossRef]
- Eba’a Atyi, R.; Hiol Hiol, F.; Lescuyer, G.; Mayaux, P.; Defourny, P.; Bayol, N.; Saracco, F.; Pokem, D.; Sufo Kankeu, R.; Nasi, R. Les Forêts du Bassin du Congo: État des Forêts 2021; CIFOR: Bogor, Indonesia, 2022; 474p. [Google Scholar] [CrossRef]
- Malaisse, F.; Bogaert, J.; Boisson, S.; Sikuzani, Y.U. La végétation naturelle d’Élisabethville (actuellement Lubumbashi) au début et au milieu du XXième siècle. Géo-Eco-Trop 2021, 45, 41–51. [Google Scholar]
- Potapov, P.V.; Turubanova, S.A.; Hansen, M.C.; Adusei, B.; Broich, M.; Altstatt, A.; Mane, L.; Justice, C.O. Quantifying forest cover loss in Democratic Republic of the Congo, 2000–2010, with Landsat ETM+ data. Remote Sens. Environ. 2012, 122, 106–116. [Google Scholar] [CrossRef]
- Holl, K.D.; Aide, T.M. When and where to actively restore ecosystems? For. Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- Rinaudo, T.; Muller, A.; Morris, M. Manuel La Régénération Naturelle Assistée (RNA). Une Ressource pour les Gestionnaires de Projets, les Utilisateurs et Tous Ceux qui ont un Intérêt à Mieux Comprendre et Soutenir le Mouvement Pour la RNA; FMNR Hub, World Vision Australia: Melbourne, Australia, 2020; 241p. [Google Scholar]
- Awono, A.; Assembe-Mvondo, S.; Tsanga, R.; Guizol, P.; Peroches, A. Restauration des Paysages Forestiers et Régimes Fonciers au Cameroun: Acquis et Handicaps; Document Occasionnel 10; Centre de Recherche Forestière Internationale (CIFOR) : Bogor, Indonesia; Centre International de Recherche en Agroforesterie (ICRAF): Nairobi, Kenya, 2023; 43p. [Google Scholar] [CrossRef]
- Ramade, F. Eléments d’écologie. In Ecologie Appliquée: Action de L’homme sur la Biosphère, 7th ed.; Dunod: Paris, France, 2012; 791p. [Google Scholar]
- Useni, S.Y.; Mpibwe, K.A.; Yona, M.J.; N’tambwe, N.D.; Malaisse, F.; Bogaert, J. Assessment of Street Tree Diversity, Structure and Protection in Planned and Unplanned Neighborhoods of Lubumbashi City (DR Congo). Sustainability 2022, 14, 3830. [Google Scholar] [CrossRef]
- Larson, J.E.; Funk, J.L. Regeneration: An overlooked aspect of trait-based plant community assembly models. J. Ecol. 2016, 104, 1284–1298. [Google Scholar] [CrossRef]
- Rondeux, J. La Mesure des Arbres et des Peuplements Forestiers, 3rd ed.; Les Presses Universitaires de Liège–Agronomie–Gembloux: Gembloux, Belgium, 2021; 738p, Available online: http://hdl.handle.net/2268/262622 (accessed on 6 February 2024).
- Syampungani, S.; Tigabu, M.; Matakala, N.; Handavu, F.; Oden, P.C. Coppicing ability of dry miombo woodland species harvested for traditional charcoal production in Zambia: A win–win strategy for sustaining rural livelihoods and recovering a woodland ecosystem. J. For. Res. 2017, 28, 549–556. [Google Scholar] [CrossRef]
- Sangeda, A.Z.; Maleko, D.D. Regeneration Effectiveness Post Tree Harvesting in Natural Miombo Woodlands, Tanzania. J. Plant Sci. Agric. Res. 2018, 2, 10. [Google Scholar]
- Muvengwi, J.; Chisango, T.; Mpakairi, K.; Mbiba, M.; Witkowski, E.T.F. Structure, composition and regeneration of miombo woodlands within harvested and unharvested areas. For. Ecol. Manag. 2020, 458, 117792. [Google Scholar] [CrossRef]
- Montfort, F.; Nourtier, M.; Grinand, C.; Maneau, S.; Mercier, C.; Roelens, J.-B.; Blanc, L. Regeneration capacities of woody species biodiversity and soil properties in Miombo woodland after slash-and-burn agriculture in Mozambique. For. Ecol. Manag. 2021, 488, 119039. [Google Scholar] [CrossRef]
- Picard, N.; Gourlet-Fleury, S. Manuel de Référence pour L’installation de Dispositifs Permanents en Forêt de Production dans le Bassin du Congo; COMIFAC: Yaoundé, Cameroon, 2008; 265p, Available online: http://hal.cirad.fr/cirad-00339816 (accessed on 7 February 2024).
- Mutondo, G.T.; Kamutanda, D.K.; Numbi, A.M. Evaluation du bilan hydrique dans les milieux anthropisés de la forêt claire (région de Lubumbashi, Province du Haut-Katanga, R.D. Congo). Méthodologie adoptée pour l’estimation de l’évapotranspiration potentielle. Geo-Eco-Trop 2018, 42, 159–172. [Google Scholar]
- Kalombo, K.D. Évolution des Éléments du Climat en RDC: Stratégies D’adaptation des Communautés de Base, Face aux Événements Climatiques de Plus en Plus Fréquents; Éditions Universitaires Européennes: Sarrebruck, Germany, 2016; 220p. [Google Scholar]
- Ngongo, M.L.; Van Ranst, E.; Baert, G.; Kasongo, E.L.; Verdoodt, A.; Mujinya, B.B.; Mukalay, J.M. Guide des sols en République Démocratique du Congo, tome I: Étude et gestion; Ed. Salama: Lubumbashi, Democratic Republic of the Congo, 2009; 260p. [Google Scholar]
- N’tambwe, D.N.; Khoji, M.H.; Kasongo, K.B.; Kouagou, S.R.; Malaisse, F.; Useni, S.Y.; Masengo, K.W.; Bogaert, J. Towards an Inclusive Approach to Forest Management: Highlight of the Perception and Participation of Local Communities in the Management of miombo Woodlands around Lubumbashi (Haut-Katanga, D.R. Congo). Forests 2023, 14, 687. [Google Scholar] [CrossRef]
- Cadre Intégré de Classification de la sécurité Alimentaire (IPC). Aperçu de l’Insécurité Alimentaire Aiguë de l’IPC; IPC: Kinshasa, Democratic Republic of the Congo, 2023. [Google Scholar]
- Khoji, M.H.; N’tambwe, N.D.; Malaisse, F.; Waselin, S.; Kouagou, R.S.; Cabala, K.S.; Munyemba, F.M.; Bastin, J.-F.; Bogaert, J.; Useni, S.Y. Quantification and Simulation of Landscape Anthropization around the Mining Agglomerations of Southeastern Katanga (DR Congo) between 1979 and 2090. Land 2022, 11, 850. [Google Scholar] [CrossRef]
- Ribeiro, N.S.; Katerere, Y.; Chirwa, P.W.; Grundy, I.M. Miombo Woodlands in a Changing Environment: Securing the Resilience and Sustainability of People and Woodlands; Springer International Publishing: Cham, Switzerland, 2020; 269p. [Google Scholar] [CrossRef]
- Buramuge, V.A.; Ribeiro, N.S.; Olsson, L.; Bandeira, R.R. Exploring Spatial Distributions of Land Use and Land Cover Change in Fire-Affected Areas of Miombo Woodlands of the Beira Corridor, Central Mozambique. Fire 2023, 6, 77. [Google Scholar] [CrossRef]
- Bolakonga, I.A.B.; Nkulu, M.F.J.; Mushakulwa, W. Filières en République Démocratique du Congo: Maïs, riz, bananes plantains et pêche; Konrad Adenauer Stiftung: Kinshasa, Democratic Republic of the Congo, 2017; 321p. [Google Scholar]
- Kalawu, S.M.; Ngoy, M.K.; Ombeni, I.; Mane, L.; Claude, P. Mapping the stratification of vegetation classes in the Miombo forests and assessing the accuracy of their classification in Katanga province in the Democratic Republic of the Congo. Int. J. Sci. Eng. Res. 2022, 13, 770–785. [Google Scholar]
- Thiombiano, A.; Glele kakaï, R.; Bayen, P.; Boussim, J.I.; Mahamane, A. Méthodes et Dispositifs d’inventaires Forestiers en Afrique de l’Ouest: État des Lieux et Propositions Pour une Harmonisation. Ann. Sci. Agron. 2016, 20, 15–31. [Google Scholar]
- Ding, Y.; Zang, R.; Lu, X.; Huang, J. The impacts of selective logging and clear-cutting on woody plant diversity after 40 years of natural recovery in a tropical montane rain forest, south China. Sci. Total Environ. 2017, 579, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Meerts, P.J.; Hasson, M. Arbres et Arbustes du Haut-Katanga; Editions Jardin Botanique de Meise: Brussels, Belgium, 2016; 386p. [Google Scholar]
- Vollesen, K.; Merrett, L. A Photo Rich Field Guide to the (Wetter) Zambian Miombo Woodland: Based on Plants from the Mutinondo Wilderness Area, Northern Zambia; Ed. Oxford: Lusaka, Zambia, 2020; 1200p. [Google Scholar]
- Zébazé, D.; Gorel, A.; Gillet, J.-F.; Houngbégnon, F.; Barbier, N.; Ligot, G.; Lhoest, S.; Kamdem, G.; Libalah, M.; Droissart, V.; et al. Natural regeneration in tropical forests along a disturbance gradient in South-East Cameroon. For. Ecol. Manag. 2023, 547, 121402. [Google Scholar] [CrossRef]
- Gonçalves, F.M.P.; Revermann, R.; Gomes, A.L.; Aidar, M.P.M.; Finckh, M.; Juergens, N. Tree Species Diversity and Composition of Miombo Woodlands in South-Central Angola: A Chronosequence of Forest Recovery after Shifting Cultivation. Int. J. For. Res. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Hakizimana, P.; Bangirinama, F.; Havyarimana, F.; Habonimana, B.; Bogaert, J. Analyse de l’effet de la structure spatiale des arbres sur la régénération naturelle de la forêt claire de Rumonge au Burundi. Bull. Sci. Inst. Natl. Environ. Conserv. Nat. 2011, 9, 46–52. [Google Scholar]
- Melingui, J.B.N.; Angoni, H.; Claude, P.A.; Kono, L. Potentiel De Régénération Naturelle De Quelques Produits Forestiers Non Ligneux Prioritaires Dans Le Bassin De Production D’akom II (Sud Cameroun). World Wide J. Multidiscip. Res. Dev. 2017, 4, 214–224. [Google Scholar]
- Colwell, R.K.; Elsensohn, J.E. EstimateS turns 20: Statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Gupta, B.; Mishra, T.K. Analysis of tree diversity and factors affecting natural regeneration in fragmented dry deciduous forests of lateritic West Bengal. Trop. Ecol. 2019, 60, 405–414. [Google Scholar] [CrossRef]
- Heinken, T.; Diekmann, M.; Liira, J.; Orczewska, A.; Schmidt, M.; Brunet, J.; Chytrý, M.; Chabrerie, O.; Decocq, G.; De Frenne, P.; et al. The European Forest Plant Species List (EuForPlant): Concept and applications. J. Veg. Sci. 2022, 33, e13132. [Google Scholar] [CrossRef]
- Albatineh, A.N.; Niewiadomska-Bugaj, M. Correcting Jaccard and other similarity indices for chance agreement in cluster analysis. Adv. Data Anal. Classi. 2011, 5, 179–200. [Google Scholar] [CrossRef]
- Kiruki, H.M.; Van Der Zanden, E.H.; Gikuma-Njuru, P.; Verburg, P.H. The effect of charcoal production and other land uses on diversity, structure and regeneration of woodlands in a semi-arid area in Kenya. For. Ecol. Manag. 2017, 391, 282–295. [Google Scholar] [CrossRef]
- Useni, S.Y.; Boisson, S.; Cabala, K.S.; Khonde, C.N.; Malaisse, F.; Halleux, J.-M.; Bogaert, J.; Kankumbi, F.M. Dynamique de l’occupation du sol autour des sites miniers le long du gradient urbain-rural de la ville de Lubumbashi, RD Congo. Biotechnol. Agron. Soc. Environ. 2020, 24, 1–14. [Google Scholar]
- Peñuelas, J.; Sardans, J. Global Change and Forest Disturbances in the Mediterranean Basin: Breakthroughs, Knowledge Gaps, and Recommendations. Forests 2021, 12, 603. [Google Scholar] [CrossRef]
- Kissanga, R.; Catarino, L.; Máguas, C.; Cabral, A.I.R.; Chozas, S. Assessing the Impact of Charcoal Production on Southern Angolan Miombo and Mopane Woodlands. Forests 2023, 15, 78. [Google Scholar] [CrossRef]
- Chinder, G.B.; Hattas, D.; Massad, T.J. Growth and functional traits of Julbernardia globiflora (Benth) resprouts and seedlings in response to fire frequency and herbivory in miombo woodlands. S. Afr. J. Bot. 2020, 135, 476–483. [Google Scholar] [CrossRef]
- Useni, S.Y.; Khoji, M.H.; Langunu, S.; Gerardy, A.; Bogaert, J. Amplification of Anthropogenic Pressure Heavily Hampers Natural Ecosystems Regeneration within the Savanization Halo Around Lubumbashi City (Democratic Republic of Congo). Int. J. Environ. Sci. Nat. Resour. 2019, 17, 555958. [Google Scholar] [CrossRef]
- Finger, C.A.G.; Costa, E.A.; Hess, A.F.; Liesenberg, V.; Bispo, P.D.C. Simulating Sustainable Forest Management Practices Using Crown Attributes: Insights for Araucaria angustifolia Trees in Southern Brazil. Forests 2023, 14, 1285. [Google Scholar] [CrossRef]
- Pretzsch, H.; Del Río, M.; Arcangeli, C.; Bielak, K.; Dudzinska, M.; Ian Forrester, D.; Kohnle, U.; Ledermann, T.; Matthews, R.; Nagel, R.; et al. Competition-based mortality and tree losses. An essential component of net primary productivity. For. Ecol. Manag. 2023, 544, 121204. [Google Scholar] [CrossRef]
- Chidumayo, E.N. Forest degradation and recovery in a miombo woodland landscape in Zambia: 22 years of observations on permanent sample plots. For. Ecol. Manag. 2013, 291, 154–161. [Google Scholar] [CrossRef]
- Lu, H.; Mohren, G.; Del Río, M.; Schelhaas, M.-J.; Bouwman, M.; Sterck, F. Species Mixing Effects on Forest Productivity: A Case Study at Stand-, Species- and Tree-Level in the Netherlands. Forests 2018, 9, 713. [Google Scholar] [CrossRef]
- Chirwa, P.W.; Larwanou, M.; Syampungani, S.; Babalola, F.D. Management and restoration practices in degraded landscapes of Southern Africa and requirements for up-scaling. Int. For. Rev. 2015, 17, 31–42. [Google Scholar] [CrossRef]
- Puig, H. Diversité spécifique et déforestation: L’exemple des forêts tropicales humides du Mexique. Bois For. Trop. 2001, 268, 41–55. [Google Scholar]
- Montfort, F. Dynamiques des Paysages Forestiers au Mozambique: Étude de L’écologie du Miombo pour Contribuer aux Stratégies de Restauration des Terres Dégradées. Thèse de Doctorat, AgroParisTech, Paris, France, 2021; 189p. Available online: https://hal.science/tel-03524870 (accessed on 12 March 2024).
- Morel, L.; Chollet, S. Naturalité et biodiversité: Des relations à préciser pour penser la valeur de conservation des écosystèmes en libre évolution. Rev. For. Fr. 2022, 73, 293–311. [Google Scholar] [CrossRef]
- N’tambwe, N.D.; Biloso, M.A.; Malaisse, F.; Useni, S.Y.; Masengo, K.W.; Bogaert, J. Socio-Economic Value and Availability of Plant-Based Non-Timber Forest Products (NTFPs) within the Charcoal Production Basin of the City of Lubumbashi (DR Congo). Sustainability 2023, 15, 14943. [Google Scholar] [CrossRef]
- Ryan, C.M.; Williams, M. How does fire intensity and frequency affect miombo woodland tree populations and biomass? Ecol. Appl. 2011, 21, 48–60. [Google Scholar] [CrossRef]
- Tarimo, B.; Dick, Ø.B.; Gobakken, T.; Totland, Ø. Spatial distribution of temporal dynamics in anthropogenic fires in miombo savanna woodlands of Tanzania. Carbon Balance Manag. 2015, 10, 18. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; De Klerk, H.M.; Stellmes, M.; Archibald, S. An analysis of the recent fire regimes in the Angolan catchment of the Okavango Delta, Central Africa. Fire Ecol. 2022, 18, 13. [Google Scholar] [CrossRef]
- Buramuge, V.A.; Ribeiro, N.S.; Olsson, L.; Bandeira, R.R.; Lisboa, S.N. Tree Species Composition and Diversity in Fire-Affected Areas of Miombo Woodlands, Central Mozambique. Fire 2023, 6, 26. [Google Scholar] [CrossRef]
- Useni, S.Y.; Mpanda, M.M.; Khoji, M.H.; Cirezi, C.N.; Malaisse, F.; Bogaert, J. Vegetation Fires in the Lubumbashi Charcoal Production Basin (The Democratic Republic of the Congo): Drivers, Extent and Spatiotemporal Dynamics. Land 2023, 12, 2171. [Google Scholar] [CrossRef]
- Sola, P.; Schure, J.; Eba’a Atyi, R.; Gumbo, D.; Okeyo, I. Politiques et pratiques en matière de bois-énergie dans certains pays d’Afrique subsaharienne–un examen critique. Bois For. Trop. 2019, 340, 27–41. [Google Scholar] [CrossRef]
- Mama, A.; Bamba, I.; Sinsin, B.; Bogaert, J.; De Cannière, C. Déforestation, savanisation et développement agricole des paysages de savanes-forêts dans la zone soudano-guinéenne du Bénin. Bois For. Trop. 2014, 322, 65–76. [Google Scholar] [CrossRef]
- Meniko, T.H.J.-P.P.; Tshibamba, M.J.; Sabongo, Y.P.; Nshimba, S.W.M.H.; Dudu, A.B.; Mate, M.J.-P.; Bogaert, J. Caractérisation floristique de quatre habitats forestiers d’un gradient d’anthropisation à Masako. In Les Forêts de la Tshopo: Écologie, Histoire et Composition; Bogaert, J., Beeckman, H., De cannière, C., Defourny, P., et Ponette, Q., Eds.; Les Presses Universitaires de Liège: Liège, Belgique, 2020; pp. 75–90. [Google Scholar]
- Guizol, P.; Guizol, P.; Diakhite, M.; Seka, J.; Mbonayem, L.; Awono, A.; Oyono, P.R.; Ndikumagenge, C.; Sonwa, D.; Ndabirorere, S.; et al. La restauration des paysages forestiers (RPF) en Afrique centrale. In Les forêts du bassin du Congo: État des Forêts 2021; Eba’a Atyi, R., Hiol Hiol, F., Lescuyer, G., Mayaux, P., Defourny, P., Bayol, N., Saracco, F., Pokem, D., Sufo Kankeu, R., Nasi, R., Eds.; CIFOR: Bogor, Indonesia, 2022; pp. 338–359. [Google Scholar]
- Talukdar, N.R.; Choudhury, P.; Barbhuiya, R.A.; Singh, B. Importance of Non-Timber Forest Products (NTFPs) in rural livelihood: A study in Patharia Hills Reserve Forest, northeast India. Trees For. People 2021, 3, 100042. [Google Scholar] [CrossRef]
- Kaumbu, J.M.K.; Mpundu, M.M.M.; Kasongo, E.L.M.; Ngoy Shutcha, M.; Tekeu, H.; Kalambulwa, A.N.; Khasa, D. Early Selection of Tree Species for Regeneration in Degraded Woodland of Southeastern Congo Basin. Forests 2021, 12, 117. [Google Scholar] [CrossRef]
- Giliba, R.A.; Mafuru, C.S.; Paul, M.; Kayombo, C.J.; Kashindye, A.M.; Chirenje, L.I.; Musamba, E.B. Human Activities Influencing Deforestation on Meru Catchment Forest Reserve, Tanzania. J. Hum. Ecol. 2011, 33, 17–20. [Google Scholar] [CrossRef]

| Habitat | Description |
|---|---|
| Unexploited forests | |
 | These forests are not exploited for charcoal production or cultivated at a human scale [6]. These refer to the land characterized by vegetation dominated by a sparse herbaceous layer under a 10–20 m forest stratum. The canopy covers 0.05 and covers at least 10–30% of the area, spanning between 0.05 and 1 hectare [15]. |
| Degraded forests | |
 | These forests have been exploited for charcoal production [43] and correspond to forests where the capacity to provide ecosystem services has been significantly reduced due to decreased woody plant density and biodiversity. |
| Post-cultivation follows | |
 | Fallows are habitats that are abandoned after subsistence farming. This refers to habitats that have been severely damaged by excessive land use, degrading soil and vegetation, and delaying woody plant diversity recovery. Vegetation is primarily dominated by grasses [44]. |
| Families | UFO (%) | DFO (%) | PCF (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genera n = 27 | Species n = 36 | Individuals n = 500 | Genera n = 33 | Species n = 48 | Individuals n = 456 | Genera n = 25 | Species n = 32 | Individuals n = 143 | |
| Anacardiaceae | 3.70 | 2.78 | 0.80 | 3.03 | 2.08 | 2.63 | - | - | - |
| Anisophylleaceae | 3.70 | 2.78 | 1.00 | 3.03 | 2.08 | 0.88 | 4.00 | 3.13 | 1.40 |
| Annonaceae | - | - | - | - | - | - | 4.00 | 3.13 | 1.40 |
| Apocynaceae | 3.70 | 2.78 | 2.00 | 3.03 | 2.08 | 6.58 | 4.00 | 3.13 | 4.20 |
| Bignoniaceae | 3.70 | 2.78 | 0.60 | 3.03 | 2.08 | 0.22 | - | - | - |
| Celastraceae | 3.70 | 2.78 | 0.20 | 3.03 | 2.08 | 0.22 | - | - | - |
| Chrysobalanaceae | 3.70 | 2.78 | 1.20 | 3.03 | 2.08 | 3.51 | 4.00 | 3.13 | 2.80 |
| Clusiaceae | 3.70 | 2.78 | 0.20 | 9.09 | 6.25 | 1.10 | 4.00 | 3.13 | 0.70 |
| Combretaceae | 3.70 | 5.56 | 0.60 | 3.03 | 6.25 | 2.41 | 4.00 | 6.25 | 2.10 |
| Dipterocarpaceae | 7.41 | 8.33 | 7.20 | 6.06 | 4.17 | 5.04 | - | - | - |
| Fabaceae | 33.33 | 33.33 | 76.00 | 27.27 | 33.33 | 59.87 | 28.00 | 34.38 | 67.83 |
| Ixonanthaceae | 3.70 | 2.78 | 1.00 | 3.03 | 2.08 | 0.88 | 4.00 | 3.13 | 0.70 |
| Lamiaceae | 3.70 | 2.78 | 0.20 | 3.03 | 6.25 | 2.85 | 4.00 | 3.13 | 2.10 |
| Loganiaceae | 3.70 | 8.33 | 1.60 | 3.03 | 4.17 | 0.44 | 4.00 | 6.25 | 1.40 |
| Malvaceae | - | - | - | - | - | - | 8.00 | 6.25 | 3.50 |
| Meliaceae | 3.70 | 2.78 | 0.20 | 3.03 | 2.08 | 0.22 | - | - | - |
| Moraceae | - | - | - | 3.03 | 4.17 | 0.66 | - | - | - |
| Myrtaceae | - | - | - | 3.03 | 2.08 | 0.66 | 4.00 | 3.13 | 3.50 |
| Ochnaceae | 3.70 | 2.78 | 1.20 | 3.03 | 2.08 | 0.22 | 4.00 | 3.13 | 1.40 |
| Olacaceae | 3.70 | 2.78 | 0.20 | - | - | - | - | - | - |
| Oleaceae | - | - | - | 3.03 | 2.08 | 0.22 | 4.00 | 3.13 | 0.70 |
| Phyllanthaceae | 7.41 | 11.11 | 5.80 | 9.09 | 10.42 | 10.96 | 12.00 | 12.50 | 5.59 |
| Proteaceae | - | - | - | 3.03 | 2.08 | 0.44 | - | - | - |
| Rubiaceae | - | - | - | - | - | - | 4.00 | 3.13 | 0.70 |
| Total of frequencies | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Parameters | UFO | DFO | PCF |
|---|---|---|---|
| Dendrometric parameters | |||
| Density (individuals/ha) | 312.50 ± 126.36 a | 285.00 ± 126.97 a | 89.38 ± 96.02 b |
| Quadratic mean diameter (cm) | 40.75 ± 15.83 a | 32.57 ± 6.78 b | 28.84 ± 11.18 b |
| Basal area (m2/ha) | 16.78 ± 7.25 a | 9.98 ± 7.14 a | 1.92 ± 2.07 b |
| Floristic parameters | |||
| Taxa_S/plot | 10.25 ± 2.86 a | 12.44 ± 4.46 a | 4.81 ± 4.28 b |
| Genera/plot | 8.44 ± 2.16 a | 9.88 ± 3.36 a | 3.94 ± 3.17 b |
| Families/plot | 4.67 ± 1.96 a | 5.88 ± 1.86 a | 2.69 ± 2.06 b |
| Species | Family | dbh < 1 cm | 1 cm ≤ dbh < 10 cm | ||||||||||
| UFO (n = 6628) | DFO (n = 6660) | PCF (n = 4676) | UFO (n = 948) | DFO (n = 2384) | PCF (n = 1756) | ||||||||
| RF | RD | RF | RD | RF | RD | RF | RD | RF | RD | RF | RD | ||
| Albizia adianthifolia (Schumach.) W. Wight | Fabaceae | 4.17 | 6.76 | 4.62 | 9.01 | 5.50 | 5.39 | 4.86 | 3.38 | 5.17 | 6.71 | 5.54 | 5.47 |
| Albizia antunesiana Harms | Fabaceae | 4.17 | 4.16 | 3.08 | 2.52 | 5.11 | 3.25 | 2.78 | 1.69 | 4.13 | 1.85 | 4.54 | 6.15 |
| Anisophyllea boehmii Engl. | Anisophylleaceae | 3.13 | 1.39 | 2.77 | 2.88 | 1.97 | 1.71 | 3.47 | 2.95 | 2.07 | 0.67 | 7.06 | 3.19 |
| Baphia bequaertii De Wild. | Fabaceae | 5.56 | 7.91 | 4.31 | 5.29 | 5.11 | 6.50 | 3.47 | 3.38 | 5.68 | 5.70 | 3.53 | 7.06 |
| Brachystegia spiciformis Benth. | Fabaceae | 5.56 | 12.61 | 4.93 | 13.33 | 6.29 | 10.61 | 10.42 | 15.19 | 6.71 | 5.54 | 6.05 | 7.97 |
| Brachystegia wangermeeana De Wild. | Fabaceae | 4.51 | 10.80 | 4.93 | 20.48 | 5.90 | 27.89 | 6.94 | 17.30 | 6.20 | 29.53 | 7.56 | 23.46 |
| Diplorhynchus condylocarpon (Müll. Arg.) Pichon | Apocynaceae | 2.78 | 2.96 | 4.31 | 2.46 | 1.18 | 0.86 | 3.47 | 5.91 | 5.68 | 8.39 | 2.02 | 1.82 |
| Garcinia huillensis Oliv. | Clusiaceae | 3.13 | 1.15 | 2.16 | 0.48 | 1.57 | 0.34 | - | - | - | - | 0.50 | 0.23 |
| Hymenocardia acida Tul. | Phyllanthaceae | - | - | 1.23 | 1.26 | 1.18 | 5.99 | 0.69 | 0.42 | 1.55 | 1.34 | 1.01 | 0.46 |
| Isoberlinia angolensis (Benth.) Hoyle & Brenan | Fabaceae | 3.82 | 11.04 | 4.62 | 5.23 | 3.54 | 4.53 | 4.86 | 2.95 | 2.07 | 4.53 | 3.53 | 6.61 |
| Isoberlinia tomentosa (Harms) Craib & Stapf | Fabaceae | 0.35 | 0.18 | - | - | - | - | - | - | 4.13 | 4.53 | 2.02 | 2.28 |
| Ochna schweinfurthiana F. Hoffm. | Ochnaceae | 4.17 | 3.02 | 3.69 | 3.78 | 3.93 | 2.65 | 4.17 | 2.53 | 3.10 | 2.18 | 1.51 | 0.91 |
| Parinari curatellifolia Planch. ex Benth. | Chrysobalanaceae | 1.74 | 0.48 | 1.85 | 0.84 | 1.57 | 0.86 | 2.08 | 1.27 | 4.13 | 3.69 | 3.53 | 2.96 |
| Pseudolachnostylis maprouneifolia Pax | Phyllanthaceae | 2.78 | 2.35 | 3.39 | 1.80 | 3.14 | 1.80 | 2.08 | 2.53 | 2.07 | 1.01 | 5.04 | 3.19 |
| Psorospermum febrifugum Spach | Clusiaceae | 4.51 | 4.47 | 2.77 | 2.04 | 4.32 | 1.88 | - | - | 1.03 | 0.34 | 3.02 | 1.37 |
| Pterocarpus angolensis DC. | Fabaceae | 0.35 | 0.06 | - | - | 0.39 | 0.17 | 5.56 | 5.49 | 3.10 | 1.17 | 3.02 | 1.59 |
| Rothmannia engleriana (K. Schum.) Keay | Rubiaceae | 3.82 | 2.53 | 2.16 | 1.50 | 4.72 | 3.25 | 1.39 | 0.84 | - | - | 1.01 | 0.46 |
| Uapaca kirkiana Müll. Arg. | Phyllanthaceae | 4.51 | 3.14 | 2.46 | 2.70 | 0.79 | 0.34 | 4.17 | 3.80 | 3.62 | 2.35 | 2.02 | 1.37 |
| dbh < 1 cm | 1 cm ≤ dbh < 10 cm | |||||
|---|---|---|---|---|---|---|
| UFO | DFO | PCF | UFO | DFO | PCF | |
| Dendrometric parameters | ||||||
| Density (individuals/ha) | 4142.50 ± 2176.33 ab | 4185.00 ± 1544.84 a | 2935.00 ± 1567.39 b | 592.50 ± 341.36 a | 1490.00 ± 1133.21 a | 1110.00 ± 954.82 a |
| Quadratic mean diameter (cm) | - | - | - | 7.52 ± 0.66 a | 7.16 ± 0.48 a | 7.06 ± 0.49 a |
| Basal area (m2/ha) | - | - | - | 2.49 ± 1.26 a | 5.76 ± 4.36 a | 4.25 ± 3.71 a |
| Floristic parameters | ||||||
| Individuals | 103.56 ± 54.41 ab | 104.63 ± 38.62 a | 73.38 ± 39.18 b | 14.81 ± 8.53 a | 37.25 ± 28.33 a | 27.75 ± 23.87 a |
| Taxa_S | 18.00 ± 3.97 ab | 20.50 ± 3.41 a | 16.06 ± 3.99 b | 16.88 ± 6.91 a | 19.13 ± 10.83 a | 19.19 ± 14.62 a |
| Genera | 15.25 ± 4.09 ab | 17.75 ± 3.49 a | 13.44 ± 3.76 b | 7.81 ± 3.62 a | 10.63 ± 5.15 a | 10.13 ± 6.39 a |
| Families | 9.56 ± 2.78 a | 10.88 ± 2.83 a | 9.31 ± 2.89 a | 5.19 ± 2.83 a | 6.50 ± 3.18 a | 6.63 ± 3.91 a |
| Chao-1 | 21.14 ± 5.85 a | 25.57 ± 4.78 a | 21.30 ± 8.29 a | 18.87 ± 11.80 a | 20.80 ± 11.22 a | 23.16 ± 17.04 a |
| Taxa_S/Chao-1 | 0.85 | 0.80 | 0.75 | 0.90 | 0.92 | 0.83 |
| Fisher_alpha | 6.78 ± 1.66 a | 8.57 ± 3.13 a | 6.99 ± 2.11 a | 10.74 ± 6.17 a | 9.96 ± 11.91 a | 8.71 ± 5.79 a |
| Species | dbh < 1 cm | 1 cm ≤ dbh < 10 cm | ||||
|---|---|---|---|---|---|---|
| UFO | DFO | PCF | UFO | DFO | PCF | |
| Albizia antunesiana Harms | 23.00 | 12.92 | 50.67 | 1.33 | 3.38 | 36.00 |
| Anisophyllea boehmii Engl. | 18.40 | 48.00 | 40.00 | 5.60 | 4.00 | 28.00 |
| Baphia bequaertii De Wild. | 23.82 | 16.76 | 50.67 | 1.45 | 6.48 | 20.67 |
| Combretum molle R.Br ex G. Don | 40.00 | 9.33 | 72.00 | 4.00 | 4.00 | 12.00 |
| Combretum zeyheri Sond. | 0.00 | 6.00 | 0.00 | 34.00 | 16.00 | |
| Diplorhynchus condylocarpon (Müll. Arg.) Pichon | 19.60 | 5.47 | 6.67 | 5.60 | 6.67 | 5.33 |
| Ekebergia benguelensis Welw. ex C.DC. | 36.00 | 24.00 | 0.00 | 12.00 | ||
| Harungana madagascariensis Lam. ex Poir. | 136.00 | 0.00 | 0.00 | |||
| Hymenocardia acida Tul. | 0.00 | 42.00 | 16.00 | |||
| Isoberlinia angolensis (Benth.) Hoyle & Brenan | 45.75 | 38.67 | 42.40 | 1.75 | 12.00 | 23.20 |
| Isoberlinia tomentosa (Harms) Craib & Stapf | 0.00 | 0.00 | 0.00 | 21.60 | 8.00 | |
| Julbernardia paniculata (Benth.) Troupin | 1.60 | 24.00 | 0.00 | 1.60 | 4.00 | 20.00 |
| Markhamia obtusifolia (Boulanger) Sprague | 2.67 | 44.00 | 2.67 | 24.00 | ||
| Mystroxylon aethiopicum (Thunb.) Lœs. | 16.00 | 60.00 | 0.00 | 12.00 | 0.00 | |
| Ochna schweinfurthiana F. Hoffm. | 33.33 | 252.00 | 62.00 | 4.00 | 52.00 | 8.00 |
| Olax obtusifolia De Wild. | 16.00 | 0.00 | 24.00 | 0.00 | 0.00 | |
| Phyllocosmus lemaireanus (De Wild. & T. Durand) T. Durand & H. Durand | 32.00 | 43.00 | 92.00 | 4.80 | 6.00 | 4.00 |
| Pseudolachnostylis maprouneifolia Pax | 78.00 | 17.14 | 42.00 | 12.00 | 3.43 | 28.00 |
| Psorospermum febrifugum Spach | 296.00 | 68.00 | 0.00 | 4.00 | ||
| Rothmannia engleriana (K. Schum.) Keay | 152.00 | 0.00 | 8.00 | |||
| Schrebera trichoclada Welw. | 0.00 | 28.00 | 0.00 | 0.00 | 28.00 | |
| Strychnos cocculoides Boulanger | 1.33 | 28.00 | 64.00 | 4.00 | 16.00 | 12.00 |
| Strychnos spinosa Lam. | 0.00 | 0.00 | 4.00 | |||
| Vitex doniana Sweet | 4.00 | 2.40 | 5.33 | 4.00 | 1.60 | 6.67 |
| Vitex mombassae Vatke | 8.00 | 0.00 | 16.00 | 0.00 | ||
| UFO < 1 | DFO < 1 | PCF < 1 | UFO ≥ 1 | DFO ≥ 1 | PCF ≥ 1 | UFO ≥ 10 | DFO ≥ 10 | |
|---|---|---|---|---|---|---|---|---|
| DFO < 1 | 0.65 | |||||||
| PCF < 1 | 0.68 | 0.65 | ||||||
| UFO ≥ 1 | 0.65 | 0.55 | 0.50 | |||||
| DFO ≥ 1 | 0.71 | 0.59 | 0.55 | 0.92 | ||||
| PCF ≥ 1 | 0.67 | 0.64 | 0.74 | 0.63 | 0.68 | |||
| UFO ≥ 10 | 0.65 | 0.48 | 0.57 | 0.71 | 0.79 | 0.63 | ||
| DFO ≥ 10 | 0.67 | 0.57 | 0.59 | 0.73 | 0.80 | 0.74 | 0.86 | |
| PCF ≥ 10 | 0.56 | 0.42 | 0.50 | 0.71 | 0.67 | 0.63 | 0.60 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nghonda, D.-d.N.; Muteya, H.K.; Salomon, W.; Mushagalusa, F.C.; Malaisse, F.; Ponette, Q.; Sikuzani, Y.U.; Kalenga, W.M.; Bogaert, J. Floristic Diversity and Natural Regeneration of Miombo Woodlands in the Rural Area of Lubumbashi, D.R. Congo. Diversity 2024, 16, 405. https://doi.org/10.3390/d16070405
Nghonda D-dN, Muteya HK, Salomon W, Mushagalusa FC, Malaisse F, Ponette Q, Sikuzani YU, Kalenga WM, Bogaert J. Floristic Diversity and Natural Regeneration of Miombo Woodlands in the Rural Area of Lubumbashi, D.R. Congo. Diversity. 2024; 16(7):405. https://doi.org/10.3390/d16070405
Chicago/Turabian StyleNghonda, Dieu-donné N’tambwe, Héritier Khoji Muteya, Waselin Salomon, Fidèle Cuma Mushagalusa, François Malaisse, Quentin Ponette, Yannick Useni Sikuzani, Wilfried Masengo Kalenga, and Jan Bogaert. 2024. "Floristic Diversity and Natural Regeneration of Miombo Woodlands in the Rural Area of Lubumbashi, D.R. Congo" Diversity 16, no. 7: 405. https://doi.org/10.3390/d16070405
APA StyleNghonda, D.-d. N., Muteya, H. K., Salomon, W., Mushagalusa, F. C., Malaisse, F., Ponette, Q., Sikuzani, Y. U., Kalenga, W. M., & Bogaert, J. (2024). Floristic Diversity and Natural Regeneration of Miombo Woodlands in the Rural Area of Lubumbashi, D.R. Congo. Diversity, 16(7), 405. https://doi.org/10.3390/d16070405






