Intertidal Species of Gelidium from the Temperate Coast of Argentina
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Molecular Identification of the Morphotypes
3.2. Distribution and Phylogeny of G. crinale and G. carolinianum
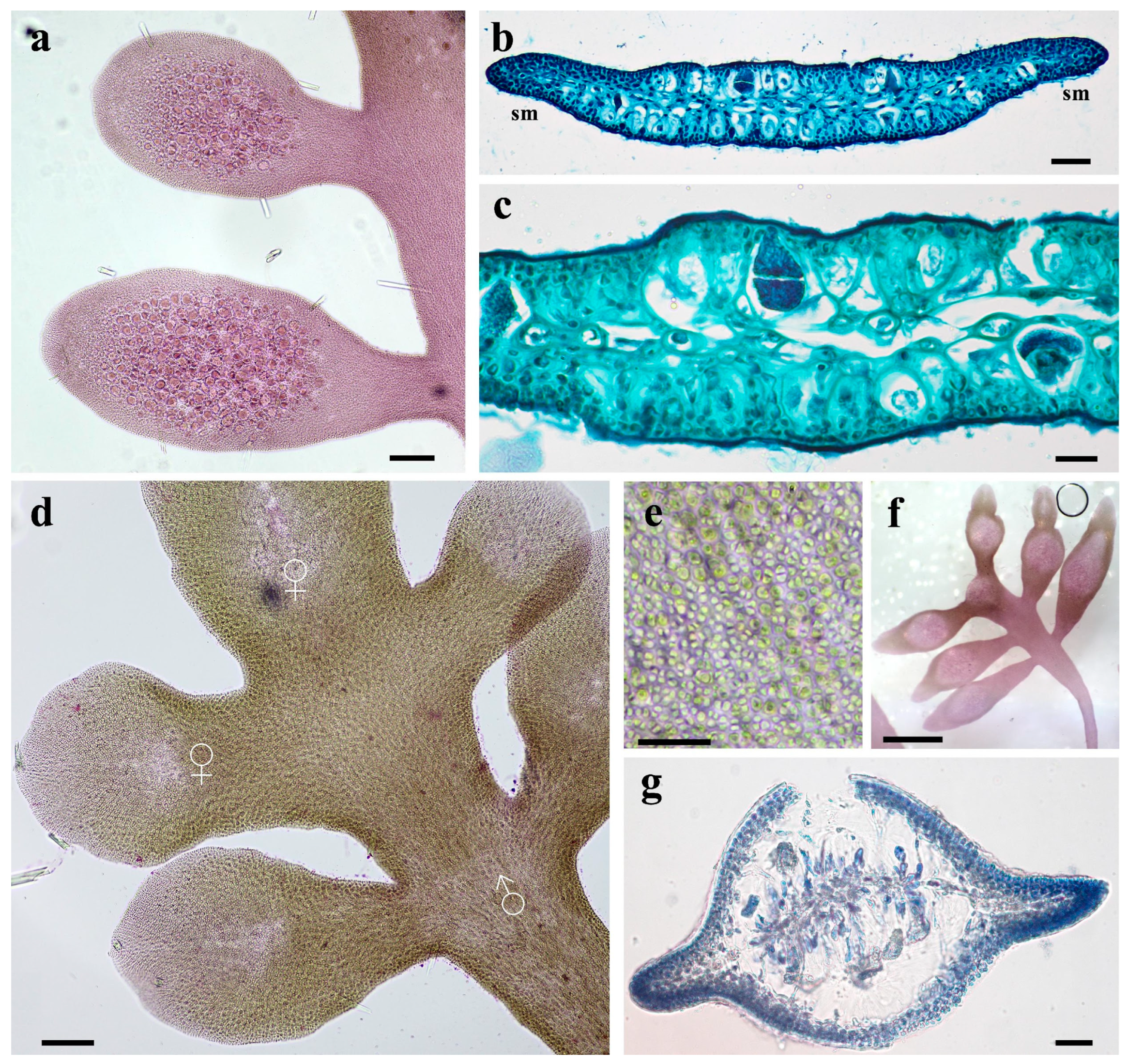
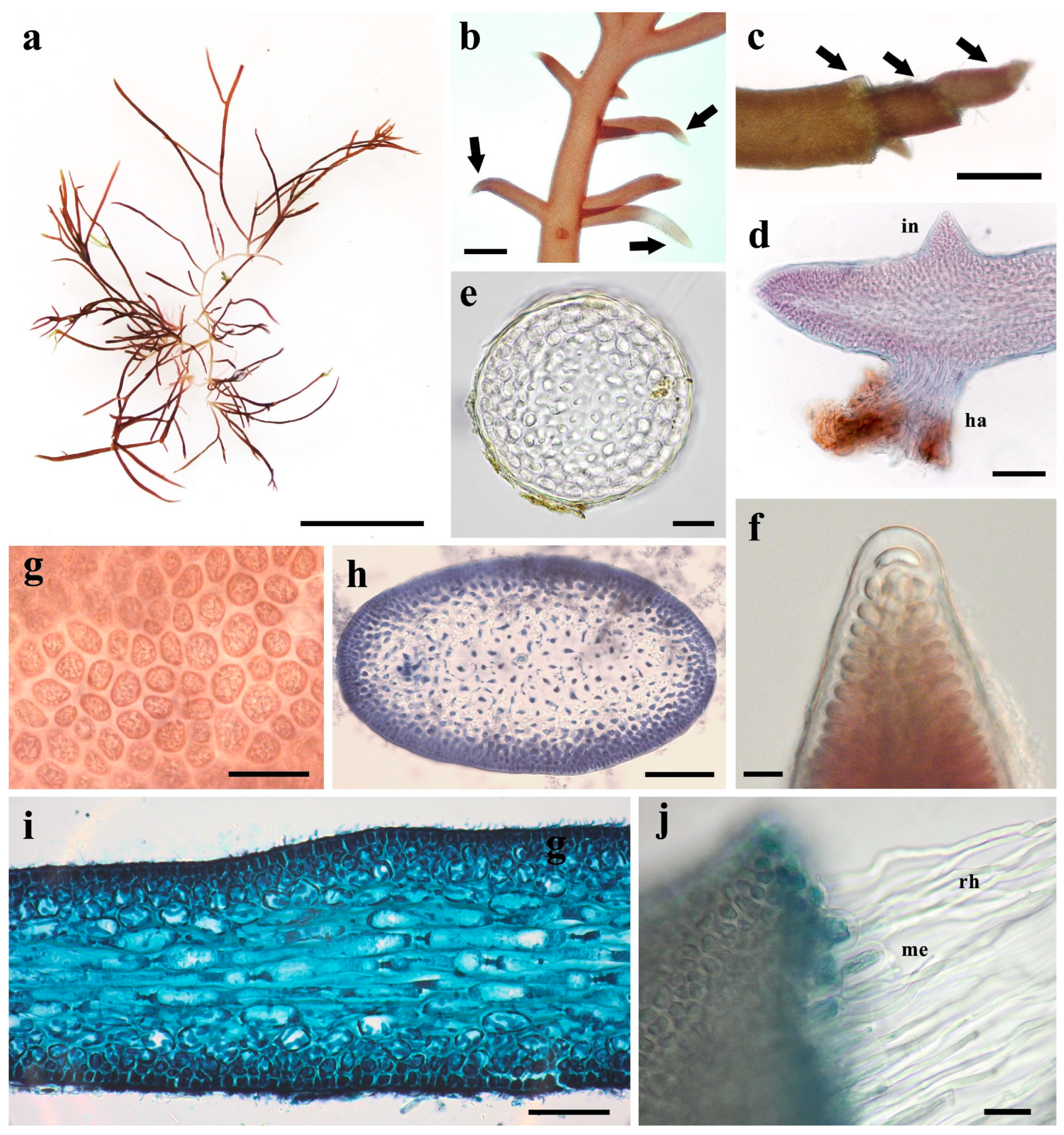
3.3. Morphological Characteristics of Gelidium carolinianum
3.4. Morphological Characteristics of Gelidium crinale
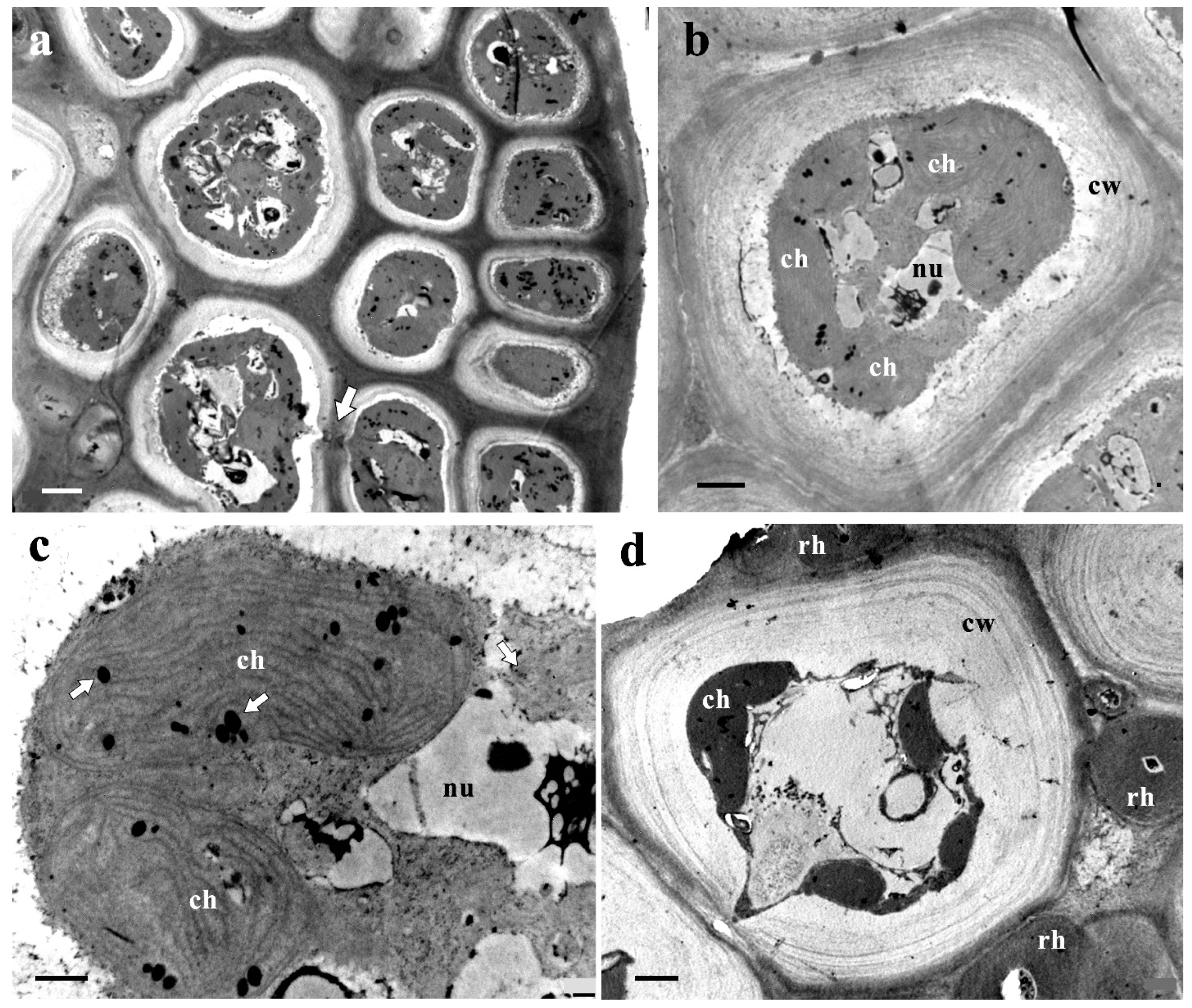
3.5. Ultrastructure of Vegetative Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Womersley, H.B.S.; Guiry, M.D. The Marine Benthic Flora of Southern Australia. Part IIIA; Australian Biological Resources Study and State Herbarium of South Australia: Canberra, Australia, 1994; pp. 118–142. [Google Scholar]
- Kim, K.M.; Boo, S.M. Phylogenetic relationships and distribution of Gelidium crinale and G. pusillum (Gelidiales, Rhodophyta) using cox1 and rbcL sequences. Algae 2012, 27, 83–94. [Google Scholar] [CrossRef][Green Version]
- Boo, G.H.; Kim, K.M.; Nelson, W.A.; Riosmena-Rodríguez, R.; Yoon, K.J.; Boo, S.M. Taxonomy and distribution of selected species of the agarophyte genus Gelidium (Gelidiales, Rhodophyta). J. Appl. Phycol. 2014, 26, 1243–1251. [Google Scholar] [CrossRef]
- Fujita, D.; Ishikawa, T.; Kodama, S.; Kato, Y.; Notoya, M. Distribution and recent reduction of Gelidium beds in Toyama Bay, Japan. J. Appl. Phycol. 2006, 18, 591–598. [Google Scholar] [CrossRef]
- Boo, G.H.; Mansilla, A.; Nelson, W.A.; Bellgrove, A.; Boo, S.M. Genetic connectivity between trans-oceanic populations of Capreolia implexa (Gelidiales, Rhodophyta) in cool temperate waters of Australasia and Chile. Aquat. Bot. 2014, 119, 73–79. [Google Scholar] [CrossRef]
- López, B.A.; Tellier, F.; Retamal-Alarcón, J.C.; Pérez-Araneda, K.; Fierro, A.O.; Macaya, E.C.; Tala, F.; Thiel, M. Phylogeography of two intertidal seaweeds, Gelidium lingulatum and G. rex (Rhodophyta: Gelidiales), along the South East Pacific: Patterns explained by rafting dispersal? Mar. Biol. 2017, 164, 1–19. [Google Scholar] [CrossRef]
- Boo, G.H.; Hughey, J.R. Phylogenomics and multigene phylogenies decipher two new cryptic marine algae from California, Gelidium gabrielsonii and G. kathyanniae (Gelidiales, Rhodophyta). J. Phycol. 2019, 55, 160–172. [Google Scholar] [CrossRef]
- Callaway, E. Lab staple agar runs low: Dwindling seaweed harvest imperils reagent essential for culturing microbes. Nature 2015, 528, 171–172. [Google Scholar] [CrossRef]
- Boo, G.H.; Le Gall, L.; Miller, K.A.; Freshwater, D.W.; Wernberg, T.; Terada, R.; Yoon, K.J.; Boo, S.M. A novel phylogeny of the Gelidiales (Rhodophyta) based on five genes including the nuclear CesA, with descriptions of Orthogonacladia gen. nov. and Orthogonacladiaceae fam. nov. Mol. Phylogenetics Evol. 2016, 101, 359–372. [Google Scholar] [CrossRef]
- Perrone, C.; Felicini, G.P.; Bottalico, A. The prostrate system of the Gelidiales: Diagnostic and taxonomic importance. Bot. Mar. 2006, 49, 23–33. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 14 June 2024).
- Meinita, M.D.N.; Harwanto, D.; Amron, M.T.; Hannan, M.A.; Jeong, G.T.; Moon, I.S.; Choi, J.S. A concise review of the potential utilization based on bioactivity and pharmacological properties of the genus Gelidium (Gelidiales, Rhodophyta). J. Appl. Phycol. 2023, 35, 1499–1523. [Google Scholar] [CrossRef]
- Airoldi, L.; Fabiano, M.; Cinelli, F. Sediment deposition and movement over a turf assemblage in a shallow rocky coastal area of the Ligurian Sea. Mar. Ecol. Prog. Ser. 1996, 133, 241–251. [Google Scholar] [CrossRef]
- Iha, C.; Milstein, D.; Guimarães, S.M.P.B.; Freshwater, D.W.; Oliveira, M.C. DNA barcoding reveals high diversity in the Gelidiales of the Brazilian southeast coast. Bot. Mar. 2015, 58, 295–305. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Rueness, J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’Shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the ‘barcoding gap’. Cryptogam. Algol. 2010, 31, 435–449. [Google Scholar]
- Kim, M.K.; Hwang, L.K.; Yoon, H.S.; Boo, S.M. Four novel Gelidium species (Gelidiales, Rhodophyta) discovered in Korea: G. coreanum, G. jejuensis, G. minimum and G. prostratum. Phycologia 2012, 51, 461–474. [Google Scholar] [CrossRef]
- Bottalico, A.; Boo, G.H.; Russo, C.; Boo, S.M.; Perrone, C. Parviphycus albertanoae sp. nov. (Gelidiales, Rhodophyta) from the Mediterranean Sea. Phycologia 2014, 53, 243–251. [Google Scholar] [CrossRef]
- Boo, G.H.; Park, J.K.; Han, K.S.; Yoon, H.S. Gelidium rosulatum (Gelidiales, Rhodophyta), a new species of subtidal marine algae from Korea. Phycologia 2022, 61, 332–340. [Google Scholar] [CrossRef]
- Santelices, B.; Montalva, S. Taxonomic studies on Gelidiaceae (Rhodophyta) from central Chile. Phycologia 1983, 22, 185–196. [Google Scholar] [CrossRef]
- Santelices, B.; Stewart, J.G. Pacific species of Gelidium Lamouroux and other Gelidiales (Rhodophyta), with keys and descriptions to the common or economically important species. In Taxonomy of Economic Seaweeds with Reference to Some Pacific and Caribbean Species; Abbott, I.A., Norris, J.N., Eds.; California Sea Grant College Program: San Diego, CA, USA, 1985; pp. 17–31. [Google Scholar]
- Hoffmann, A.; Santelices, B. Flora Marina de Chile Central; Ediciones Universidad Católica de Chile: Santiago, Chile, 1997; p. 434. [Google Scholar]
- Boo, G.H.; Hughey, J.R.; Miller, K.A.; Boo, S.M. Mitogenomes from type specimens, a genotyping tool for morphologically simple species: Ten genomes of agar-producing red algae. Sci. Rep. 2016, 6, 35337. [Google Scholar] [CrossRef]
- Boo, G.H.; Calderon, M.S.; Boo, S.M. A new marine alga, Pterocladiella andresii sp. nov.(Gelidiales, Rhodophyta) and its relationship to P. caloglossoides from Pacific South America. Phytotaxa 2017, 319, 139–148. [Google Scholar] [CrossRef]
- Iha, C.; O’Shaughnessy, K.A.; Guimarães, S.M.P.B.; Oliveira, M.C.; Freshwater, D.W. Taxonomic reappraisal of Gelidium coarctacum (Gelidiales, Rhodophyta) and Gelidium lineare sp. nov. from the tropical western Atlantic. Phycologia 2016, 55, 555–563. [Google Scholar] [CrossRef]
- Iha, C.; Jamas, M.; Guimarães, S.M.P.B.; Fujii, M.T.; Freshwater, D.W.; Oliveira, M.C. Pterocladiella (Gelidiales, Rhodophyta) species of Brazil including morphological studies of Pterocladiella media and a reassessment of Pterocladiella taylorii. Phycologia 2017, 56, 624–637. [Google Scholar] [CrossRef]
- Iha, C.; Freshwater, D.W.; Guimarães, S.M.P.B.; Oliveira, M.C. Gelidiorariphycus gen. nov. (Gelidiales, Rhodophyta): A rare genus found in the Americas. Phycologia 2022, 61, 473–483. [Google Scholar] [CrossRef]
- Jamas, M.; Iha, C.; Oliveira, M.C.; Guimarães, S.M.P.B.; Fujii, M.T. Morphological and molecular studies on Gelidiaceae and Gelidiellaceae (Gelidiales, Rhodophyta) from Brazil with description of the new species Gelidium calidum. Phytotaxa 2017, 314, 195–218. [Google Scholar] [CrossRef]
- Brunelli, B.; Milstein, D.; Boo, S.M.; Fujii, M.T. Gelidium guimaraesiae sp. nov. (Gelidiaceae, Rhodophyta) from the Western Atlantic segregated from G. floridanum by morphological and molecular evidence. Phytotaxa 2019, 388, 275–286. [Google Scholar] [CrossRef]
- Brunelli, B.; Jamas, M.; Milstein, D.; Boo, S.M.; Fujii, M.T. Gelidium brasiliense sp. nov. (Gelidiales, Rhodophyta): A diminutive agarophyte from Brazil. J. Appl. Phycol. 2019, 31, 951–958. [Google Scholar] [CrossRef]
- Steigleder, K.M.; Copertino, M.S.; Lanari, M.; Camargo, M.; Fujii, M.T. Latitudinal gradient in intertidal seaweed composition off the coast of southern Brazil and Uruguay. Aquat. Bot. 2019, 156, 47–56. [Google Scholar] [CrossRef]
- Croce, M.E.; Gauna, M.C.; Fernández, C.; Parodi, E.R. Intertidal seaweeds from North Atlantic Patagonian coasts, Argentina. Checklist 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Croce, M.E.; Parodi, E.R. Seasonal dynamic of macroalgae in intertidal pools formed by beds of Crassostrea gigas (Mollusca, Bivalvia) on the north Patagonian Atlantic coast. Bot. Mar. 2012, 55, 49–58. [Google Scholar] [CrossRef]
- Pujals, C. Catálogo de Rhodophyta citadas para la Argentina; Museo Argentino de Ciencias Naturales e Instituto Nacional de Investigación de las Ciencias Naturales: Buenos Aires, Argentina, 1963; p. 139. [Google Scholar]
- Sar, E.; Pascual, M.; Parma, A. Consideraciones ecológicas sobre las algas del litoral rocoso bonaerense. Rev. Mus. Plata 1984, 13, 143–147. [Google Scholar]
- Parma, A.; Pascual, M.; Sar, E. Clave Para el Reconocimiento de los Géneros de Algas Macrofitas del Intermareal Rocoso Bonaerense; Universidad Nacional de La Plata, Serie Técnica y Didáctica: La Plata, Argentina, 1987; p. 29. [Google Scholar]
- Croce, M.E.; Parodi, E.R. The turf-forming alga Gelidium crinale (Florideophyceae, Rhodophyta) on Atlantic Patagonian coasts. Bot. Mar. 2013, 56, 131–141. [Google Scholar] [CrossRef]
- Boraso, A.L. Elementos Para el Estudio de las Macroalgas de Argentina; Editorial Universitaria de la Patagonia: Comodoro Rivadavia, Argentina, 2013; p. 204. [Google Scholar]
- López Gappa, J.; Tablado, A.; Magaldi, N.H. Seasonal changes in an intertidal community affected by sewage pollution. Environ. Pollut. 1993, 82, 157–165. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 20 June 2024).
- Taylor, R.L.; Bailey, J.C.; Freshwater, D.W. Systematics of Cladophora spp. (Chlorophyta) from North Carolina, USA, based upon morphology and DNA sequence data with a description of Cladophora subtilissima sp. nov. J. Phycol. 2017, 53, 541–556. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Scott, S.; Tronchin, E.M.; Saunders, G.W. Reassessment of Tristan da Cunha Gelidium (Gelidiales, Rhodophyta) species. Bot. Mar. 2020, 63, 455–462. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 30, 754–775. [Google Scholar] [CrossRef]
- Armiñana, J.R.; Breijo, F.G. Técnicas de Histología Vegetal; Jardín Botánico de Valencia: Valencia, Spain, 2006; p. 40. [Google Scholar]
- Cáceres, E.J. Métodos de preparación de algas para su observación con microscopía electrónica de transmisión convencional (METC). In Manual de Métodos Ficológicos; Alveal, K., Ferrario, M.E., Oliveira, E.C., Sar, E., Eds.; Universidad de Concepción: Concepción, Chile, 1995; pp. 147–168. [Google Scholar]
- Raffo, M.P.; Dellatorre, F.; Ciancia, M. Seaweed resources of Argentina (S W Atlantic): Production, bio-ecological, applied research and challenges for sustainable development. Appl. Phycol. 2022, 3, 383–421. [Google Scholar] [CrossRef]
- Perrone, C.; Bottalico, A.; Boo, G.H.; Boo, S.M.; Miller, K.A.; Freshwater, D.W. Gelidium adriaticum sp. nov. and Gelidium carolinianum sp. nov. (Gelidiales, Rhodophyta) from the Mediterranean Sea. Phycologia 2019, 58, 359–373. [Google Scholar] [CrossRef]
- Patarra, R.F.; Iha, C.; Pereira, L.; Neto, A.I. Concise review of the species Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand. J. Appl. Phycol. 2020, 32, 787–808. [Google Scholar] [CrossRef]
- Boo, G.H.; Bottalico, A.; Le Gall, L.; Yoon, H.S. Genetic diversity and phylogeography of a turf-forming cosmopolitan marine alga, Gelidium crinale (Gelidiales, Rhodophyta). Int. J. Mol. Sci. 2023, 24, 5263. [Google Scholar] [CrossRef]
- Hariot, P. Algues. Algues: Mission Scientifique du Cap Horn (1882–83); Botanique: Paris, France, 1888; pp. 1–109. [Google Scholar]
- Cotton, A.D. Cryptogams from the Falkland Islands collected by Mrs. Vallentin. J. Linn. Soc. Bot. 1915, 43, 137–231. [Google Scholar] [CrossRef]
- Skottsberg, C. Marine Algae. 2. Rhodophyceae. In Botan. Ergebn. der Schwed. Expedition Nach Patagonien und dem Feuerlande 1907–1909, IX; Svenska, K., Ed.; Vetenkapasakad Handlingar: Stockholm, Sweden, 1923; 70p. [Google Scholar]
- Murray, J. On the deep and shallow-water marine fauna of the Kerguelen Region of the Great Southern Ocean. Trans. R. Soc. Edinb. 1897, 38, 343–500. [Google Scholar] [CrossRef][Green Version]
- Okolodkov, Y.B. Biogeografía Marina; Universidad Autónoma de Campeche: Campeche, México, 2010; p. 217. [Google Scholar]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Collin, R.; Ramos-Esplá, A.A.; Izquierdo, A. Identification of the South Atlantic spiny slipper limpet Bostrycapulus odites Collin, 2005 (Caenogastropoda: Calyptraeidae) on the Spanish Mediterranean coast. Aquat. Invasions 2010, 5, 197–200. [Google Scholar] [CrossRef]
- Gammill, S.P.; Hosier, P.E. Coastal saltmarsh development at Southern Topsail sound, North Carolina. Estuaries 1992, 15, 122–129. [Google Scholar] [CrossRef]
- Gauna, M.C.; Croce, M.E. Evaluación del crecimiento de Gelidium en cultivos con bioestimulantes a base de algas. In Boletín de la Sociedad Argentina de Botánica, Proceedings of the XXXIX Jornadas Argentinas de Botánica, Catamarca, Argentina, 21 September 2023; Sociedad Argentina de Botánica: La Plata, Argentina, 2023. [Google Scholar]
- Echegaray Taborga, J.; Seaone Camba, J.A. Estudio comparativo sobre la variación morfológica y fisiológica de Gelidium crinale y Gelidium spathulatum colectados en el mediterráneo catalán. Collect. Botánica 1982, 13, 803–816. [Google Scholar]
- Prathep, A.; Lewmanomontb, K.; Buapeta, P. Effects of wave exposure on population and reproductive phenology of an algal turf, Gelidium pusillum (Gelidales, Rhodophyta), Songkhla, Thailand. Aquat. Bot. 2009, 90, 179–183. [Google Scholar] [CrossRef]
- Renfrew, D.E.; Gabrielson, P.W.; Scagel, R.F. The marine algae of British Columbia, northern Washington, and southeast Alaska: Division Rhodophyta (red algae), class Rhodophyceae, order Gelidiales. Can. J. Bot. 1989, 67, 3297–3314. [Google Scholar] [CrossRef]
- Santelices, B.; Flores, V. Spermatangial sori on cystocarpic branchlets of species of Gelidium and Pterocladia (Gelidiales, Rhodophyta). Phycologia 1995, 34, 337–341. [Google Scholar] [CrossRef]
- Thomas, D.T.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Rhodophyta): Four Caribbean taxa including Pterocladiella beachii sp. nov. Phycologia 2001, 40, 340–350. [Google Scholar] [CrossRef]
- Tronchin, E.M.; Freshwater, D.W. Four Gelidiales (Rhodophyta) new to southern Africa, Aphanta pachyrrhiza gen. et sp. nov., Gelidium profundum sp. nov., Pterocladiella caerulescens and P. psammophila sp. nov. Phycologia 2007, 46, 325–348. [Google Scholar] [CrossRef]
- Santelices, B. Synopsis of Biological Data on the Seaweed Genera Gelidium and Pterocladia (Rhodophyta); Food & Agriculture Organization of the United Nations: Rome, Italy, 1988; Volume 145, pp. 1–55. [Google Scholar]
- Whorff, J.S.; Whorff, L.L.; Sweet, M.H. Spatial variation in an algal turf community with respect to substratum slope and wave height. J. Mar. Biol. Assoc. UK 1995, 75, 429–444. [Google Scholar] [CrossRef]
- Pell, M.V.; Carcedo, M.C.; Fernández, C.; Croce, M.E. Evaluación de algas Gelidiales cespitosas como formadoras de hábitat del estuario de Bahía Blanca. In Boletín de la Sociedad Argentina de Botánica, Proceedings of the XXXIX Jornadas Argentinas de Botánica, Catamarca, Argentina, 21 September 2023; Sociedad Argentina de Botánica: La Plata, Argentina, 2023. [Google Scholar]
- Hommersand, M.H. Global biogeography and relationships of the Australasian marine macroalgae. In Algae of Australia: Introduction; McCarthy, P.M., Ed.; Australian Biological Resources Study and CSIRO Publishing: Canberra, Australia, 2007; pp. 511–542. [Google Scholar]
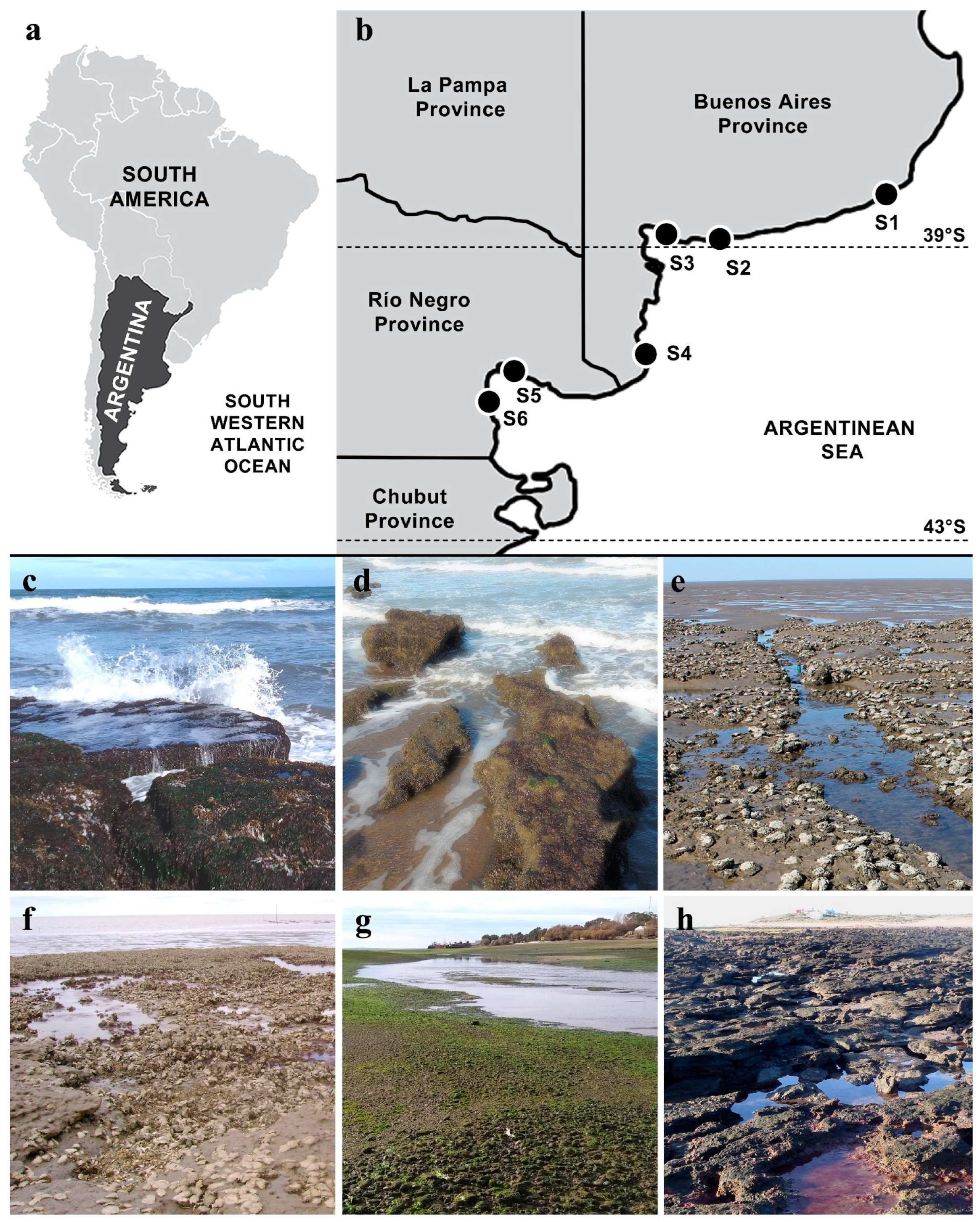

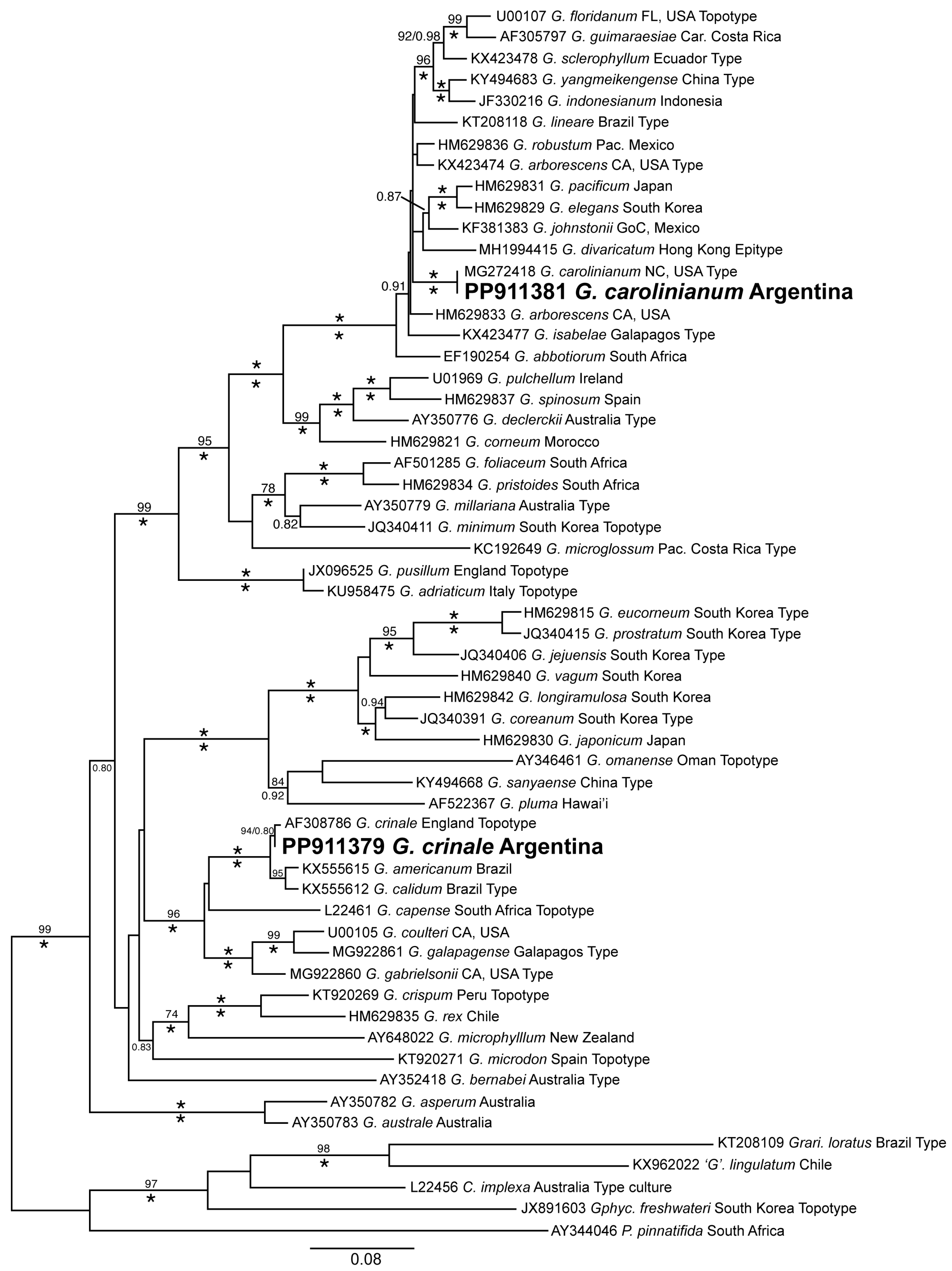

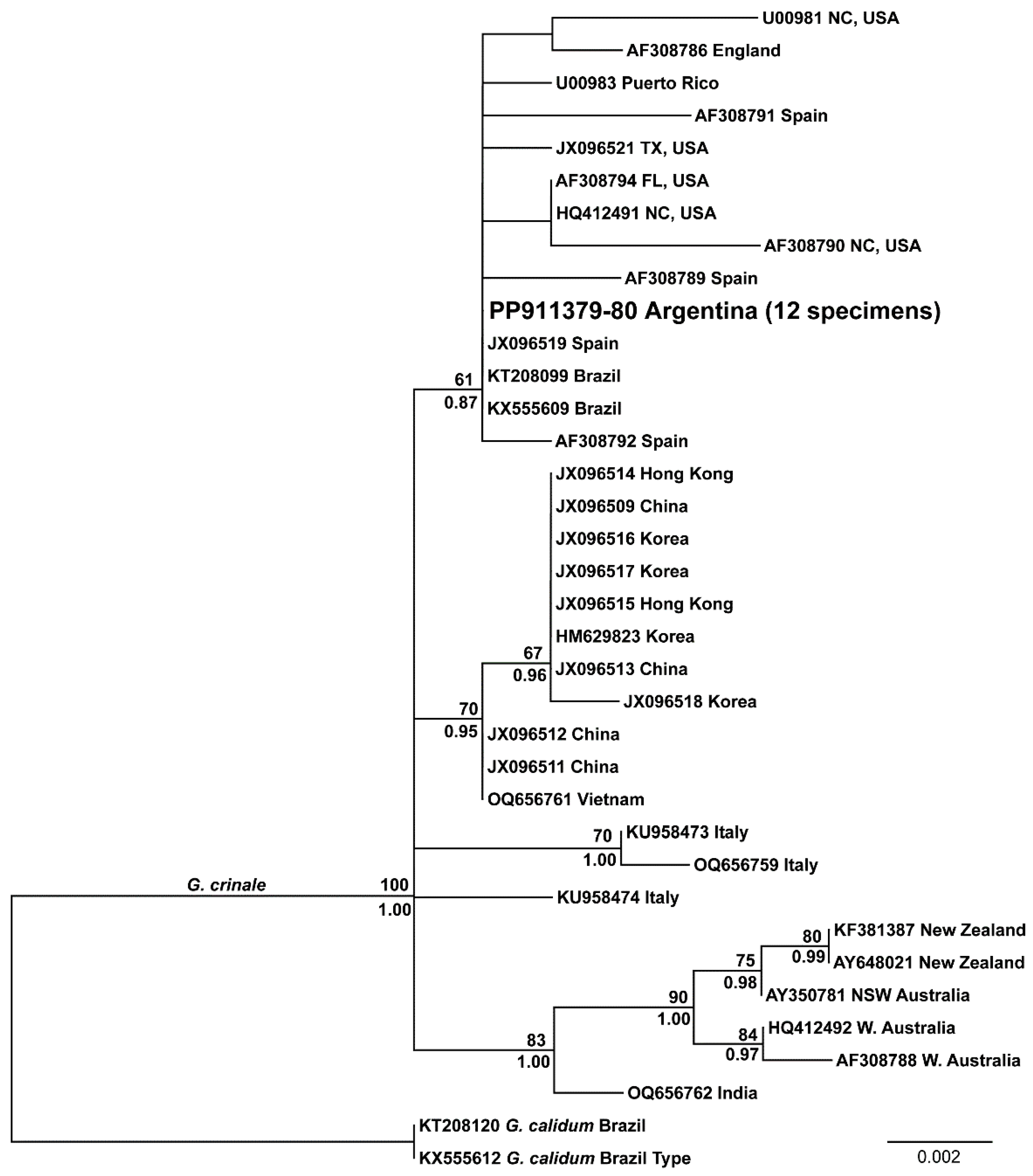
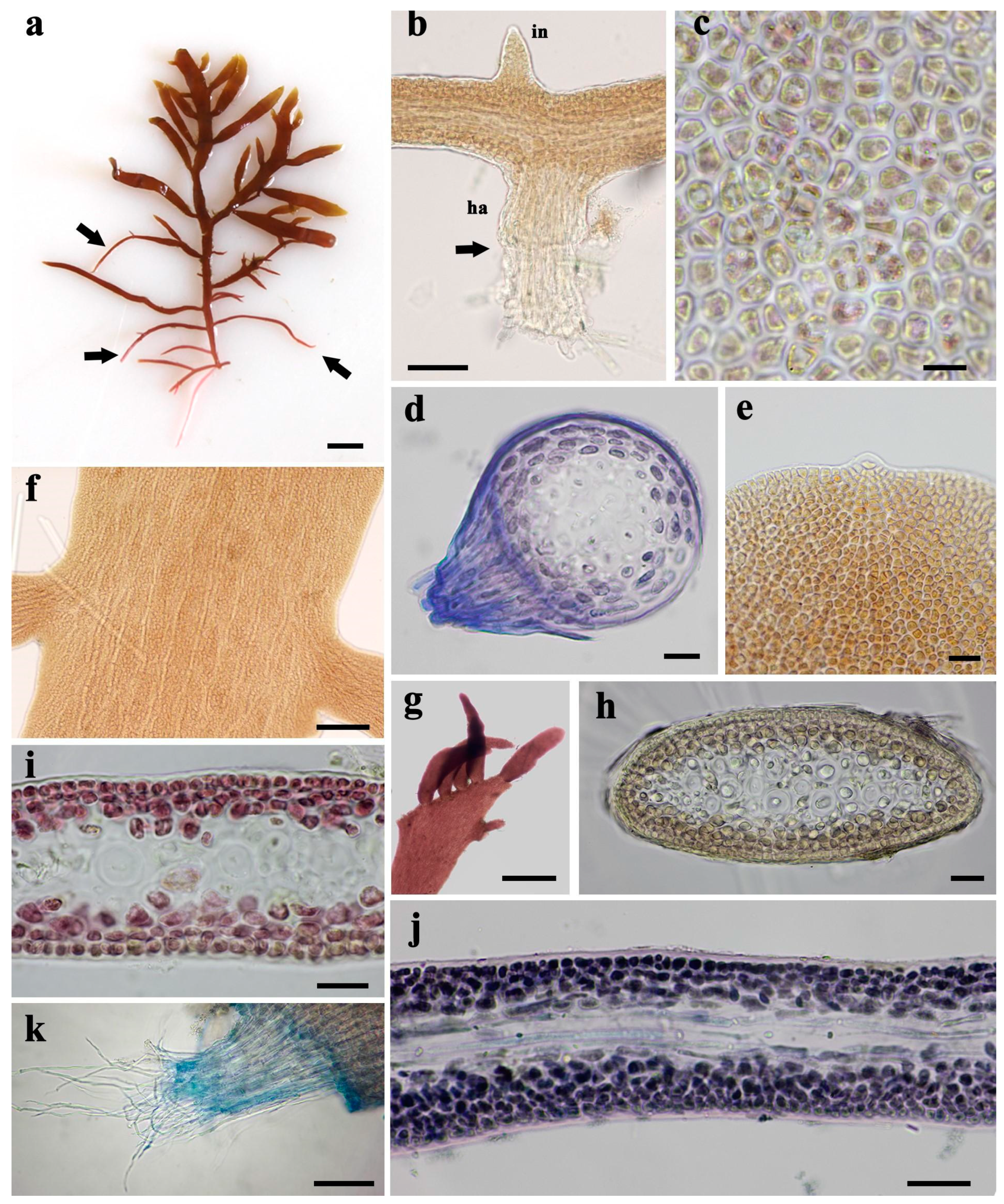


| Site | Locality, Province | Coordinates | Collection Dates | Type of Coast | Tidal Amplitude | Type of Hard Substrate | Dominant Sediment | Surf Exposure | Water Turbidity |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Mar del Plata, Buenos Aires | 37°59′48.5″ S 57°32′27.7″ W | April 2015; March 2023 | Exposed sandy beach | Micro-tidal | Orthoquartzite rocks | Sand | High | Low/Medium |
| S2 | Pehuen Co, Buenos Aires | 39°00′13.0″ S 61°36′55.5″ W | June 2015 | Exposed sandy beach | Meso-tidal | Sandstone outcrops | Sand | High/Medium | Medium |
| S3 | Villa del Mar, Buenos Aires | 38°51′24.6″ S 62°07′04.5″ W | May 2015; Jan–Dec 2022 | Protected estuarial bay with tidal flats and salt marshes | Meso-tidal | Sandstone outcrops | Lime–clay | Low | High |
| S4 | Los Pocitos, Buenos Aires | 40°26′30.0″ S 62°25′15.1″ W | Jan–Dec 2009 | Protected bay with salt marshes | Meso-tidal | Sandstone outcrops | Lime–clay | Low | High |
| S5 | San Antonio Oeste, Río Negro | 40°43′37.0″ S 64°57′05.3″ W | April 2017 | Protected tidal channels | Macro-tidal | Pebbles/cobbles | Sand-gravel | Low | Medium |
| S6 | Las Grutas, Río Negro | 40°53′02.3″ S 65°07′29.1″ W | March 2016 | Sandy beach with meadows | Macro-tidal | Sandstone outcrops | Sand | Medium | Low |
| Argentina (n = 11) | North Carolina (n = 2) | Italy (n = 1) | Bermuda (n = 2) | |
|---|---|---|---|---|
| Argentina (n = 11) | 0 bp 0.00% | |||
| North Carolina (n = 2) | 0 bp 0.00% | 0 bp 0.00% | ||
| Italy (n = 1) | 1 bp 0.08% | 1 bp 0.08% | 0 bp 0.00% | |
| Bermuda (n = 2) | 1 bp 0.13% | 1 bp 0.13% | 1 bp 0.13% | 0 bp 0.00% |
| Australia, New Zealand, and India (n = 6) | Eastern Asia (n = 11) | Atlantic Ocean and Mediterranean (n = 17) | |
|---|---|---|---|
| Australia, New Zealand, and India (n = 6) | 0–6 bp 0.00–0.48% | ||
| Eastern Asia (n = 11) | 4–10 bp 0.32–0.81% | 0–2 bp 0.00–0.16% | |
| Atlantic Ocean and Mediterranean (n = 17) | 4–15 bp 0.32–1.21% | 2–11 bp 0.16–0.89% | 0–14 bp 0.00–1.13% |
| Pehuen Co | Villa del Mar | San Antonio Oeste | Las Grutas | ||
|---|---|---|---|---|---|
| Character | S2 | S3 | S5 | S6 | F |
| Height (cm) | 0.74 (±0.33) a | 1.22 (±0.36) b | 0.67 (±0.25) a | 0.63 (±0.11) a | 24.01 |
| Basal width of erect axes (mm) | 0.16 (±0.03) c | 0.15 (±0.03) bc | 0.13 (±0.04) ab | 0.12 (±0.02) a | 6.54 |
| Maximum width of erect axes (mm) | 0.78 (±0.28) b | 0.65 (±0.20) b | 0.39 (±0.12) c | 0.22 (±0.04) a | 43.03 |
| Diameter of stolon (mm) | 0.15 (±0.02) b | 0.10 (±0.02) a | 0.11 (±0.03) a | 0.12 (±0.02) a | 13.56 |
| Length of 1st-order branches (mm) | 1.78 (±0.90) a | 3.31 (±1.61) b | 1.97 (±1.17) a | 2.02 (±1.08) a | 10.46 |
| Width of apical cell (µm) | 8.19 (±1.40) a | 8.93 (±1.69) a | 10.21 (±1.72) b | 9.25 (±1.88) ab | 9.68 |
| Width of cortical cell (µm) | 8.47 (±2.77) a | 7.53 (±1.46) ab | 6.86 (±1.79) b | 8.17 (±1.63) a | 5.58 |
| Distance between haptera (mm) | 0.62 (±0.35) b | 0.78 (±0.45) ab | 0.64 (±0.28) b | 1.02 (±0.41) a | 4.68 |
| Distance between 1st-order branches (mm) | 0.98 (±0.45) bc | 1.21 (±0.70) c | 0.55 (±0.26) a | 0.70 (±0.47) ab | 14.90 |
| Width of sterile margin (µm) | 187.5 (±72.17) a | 89.10 (±36.43) c | 32.88 (±20.36) b | - | 45.01 |
| Diameter of cortical cells (µm) | 9.73 (±2.08) b | 9.43 (±2.11) b | 8.63 (±2.49) b | 5.92 (±1.45) a | 12.33 |
| Diameter of medullary cells (µm) | 17.43 (±3.02) b | 15.55 (±3.27) b | 16.43 (±3.50) b | 11.93 (±1.71) a | 7.70 |
| Mar del Plata | Pehuen Co | Villa del Mar | Los Pocitos | Las Grutas | ||
|---|---|---|---|---|---|---|
| Character | S1 | S2 | S3 | S4 | S6 | F |
| Height (cm) | 1.12 (±0.33) a | 1.64 (±0.66) ab | 3.16 (±1.27) c | 2.07 (±0.76) b | 1.38 (±0.31) a | 26.93 ** |
| Basal width of erect axes (mm) | 0.17 (±0.04) a | 0.16 (±0.03) a | 0.16 (±0.03) a | 0.17 (±0.02) a | 0.15 (±0.02) a | 1.57 |
| Maximum width of erect axes (mm) | 0.29 (±0.07) a | 0.38 (±0.09) b | 0.29 (±0.05) a | 0.28 (±0.05) a | 0.19 (±0.04) c | 39.9 ** |
| Diameter of stolon (mm) | 0.18 (±0.04) a | 0.17 (±0.04) a | 0.15 (±0.03) a | 0.18 (±0.03) a | 0.18 (±0.03) a | 2.5 |
| Length of 1st-order branches (mm) | 1.72 (±1.24) a | 6.22 (±3.44) a | 19.96 (±10.04) b | 5.91 (±3.06) a | 3.27 (±2.64) a | 45.7 ** |
| Width of apical cell (µm) | 9.06 (±1.53) a | 10.97 (±1.49) b | 10.26 (±2.52) ab | 9.26 (±1.66) a | 9.42 (±1.25) a | 5.39 ** |
| Width of cortical cell (µm) | 6.76 (±1.26) a | 8.48 (±1.51) b | 8.20 (±1.57) b | 9.03 (±2.58) b | 9.17 (±1.97) b | 8.16 ** |
| Distance between haptera (mm) | 0.87 (±0.39) a | 0.65 (±0.29) a | 0.66 (±0.35) a | 0.81 (±0.54) a | 0.70 (±0.38) a | 1.49 |
| Distance between 1st-order branches (mm) | 0.66 (±0.34) a | 0.68 (±0.75) a | 2.01 (±1.61) b | 1.01 (±0.59) a | 1.18 (±0.33) ab | 5.34 ** |
| Diameter of cortical cells (µm) | 10.18 (±2.86) a | 14.80 (±2.52) c | 11.70 (±2.98) ab | 9.74 (±1.77) a | 13.00 (±3.56) bc | 15.54 ** |
| Diameter of medullary cells (µm) | 17.12 (±3.60) bc | 14.29 (±1.96) a | 19.22 (±2.85) c | 19.61 (±3.94) c | 14.65 (±3.47) ab | 11.33 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, M.E.; Freshwater, D.W. Intertidal Species of Gelidium from the Temperate Coast of Argentina. Diversity 2024, 16, 399. https://doi.org/10.3390/d16070399
Croce ME, Freshwater DW. Intertidal Species of Gelidium from the Temperate Coast of Argentina. Diversity. 2024; 16(7):399. https://doi.org/10.3390/d16070399
Chicago/Turabian StyleCroce, María Emilia, and D. Wilson Freshwater. 2024. "Intertidal Species of Gelidium from the Temperate Coast of Argentina" Diversity 16, no. 7: 399. https://doi.org/10.3390/d16070399
APA StyleCroce, M. E., & Freshwater, D. W. (2024). Intertidal Species of Gelidium from the Temperate Coast of Argentina. Diversity, 16(7), 399. https://doi.org/10.3390/d16070399






