Genetic Diversity and Profile of Red Algae Pterocladiella capillacea (Gelidiales, Rhodophyta) along the Coast of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Materials
2.2. Sequencing Molecular Markers
2.3. Data Analysis
3. Results
3.1. Molecular Data
3.2. Genetic Diversity
3.3. Genetic Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, B.M. Tomus II Rhodophyta. Part III. Gelidiales Cryptonemiales Hildenbrandiales. In Flora Algarum Marinarum Sinicarum; Science Press: Beijing, China, 2004; pp. 59–81. (In Chinese) [Google Scholar]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication. 2024. Available online: https://www.algaebase.org/ (accessed on 20 March 2024).
- Patarra, R.F. Short term effects of irradiance on the growth of Pterocladiella capillacea (Gelidiales, Rhodophyta). Arquipelago. Life Mar. Sci. 2019, 1, 85–94. [Google Scholar] [CrossRef]
- Patarra, R.F.; Iha, C.; Pereira, L.; Neto, A.I. Concise review of the species Pterocladiella capillacea (SG Gmelin) Santelices & Hommersand. J. Appl. Phycol. 2020, 32, 787–808. [Google Scholar]
- Yang, R.; Liu, B.Q.; Luo, Q.J.; Wang, Y.J.; Zhou, X.C.; Dai, J.X. Genetic variation of Porphyra haitanensis by applying AFLP. Chin. High Technol. Lett. 2002, 1, 83–86, (In Chinese with English Abstract). [Google Scholar]
- Xie, C.T.; Ji, D.H.; Chen, C.S.; Xu, Y.; Zhang, Y. Application of ISSR markers in germplasm identification of different color’s Porphyra haitanensis filament strains. J. Fish. China 2007, 1, 105–111, (In Chinese with English Abstract). [Google Scholar]
- Xie, C.T.; Ji, D.H.; Chen, C.S.; Xu, Y.; Liu, B. Sequence analysis and application of 5.8S rDNA and ITS regions of Porphyra haitanensis. Chin. High Technol. Lett. 2007, 17, 540–545, (In Chinese with English Abstract). [Google Scholar]
- Lim, P.E.; Yang, L.E.; Tan, J.; Maggs, C.A.; Brodie, J. Advancing the taxonomy of economically important red seaweeds (Rhodophyta). Eur. J. Phycol. 2017, 52, 438–451. [Google Scholar] [CrossRef]
- Boo, G.H.; Cai, Y.; Boo, S.M. Molecular identification of Gelidioid algae (Gelidiales, Rhodophyta) from Singapore with a description of Gelidium sentosaense sp. nov. Phycologia 2006, 55, 247–256. [Google Scholar] [CrossRef]
- Wang, X.L. The Preliminary Analysis of Molecular Phylogeny of Marinerhodophyta and the Taxonomic Study of Gelidiales (Rhodophyta) from China; Chinese Academy of Sciences (Institute of Oceanology): Qingdao, China, 2016. (In Chinese) [Google Scholar]
- Wu, X.W.; Wang, T.G.; Liu, Y.; Zhang, P. Genetic diversity of Pyropia haitanensis alongthe southeast coast of China. J. Mar. Sci. 2020, 4, 58–64, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, X.L.; Yan, S.H.; Wang, Y.Q.; Sun, Z.M.; Xia, B.M.; Wang, G.C. Study of the phylogeny and distribution of Pterocladiella (Pterocladiaceae, Rhodophyta) from China. Phycologia 2020, 59, 165–176. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Rueness, J. Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia 1994, 33, 187–194. [Google Scholar] [CrossRef]
- Shimada, S.; Horiguchi, T.; Masuda, M. Two new species of Gelidium (Rhodophyta, Gelidiales), Gelidium tenuifolium and Gelidium koshikianum, from Japan. Phycol. Res. 2000, 48, 37–46. [Google Scholar] [CrossRef]
- Millar, A.J.; Freshwater, D.W. Morphology and molecular phylogeny of the marine algal order Gelidiales (Rhodophyta) from New South Wales, including Lord Howe and Norfolk Islands. Aust. Syst. Bot. 2005, 18, 215–263. [Google Scholar] [CrossRef]
- Shimada, S.; Horiguchi, T.; Masuda, M. Phylogenetic affinities of genera Acanthopeltis and Yatabella (Gelidiales, Rhodophyta) inferred from molecular analyses. Phycologia 1999, 38, 528–540. [Google Scholar] [CrossRef]
- Tronchin, E.M.; Freshwater, D.W. Four Gelidiales (Rhodophyta) new to southern Africa, Aphanta pachyrrhiza gen. et sp. nov., Gelidium profundum sp. nov., Pterocladiella caerulescens and P. psammophila sp. nov. Phycologia 2007, 46, 325–348. [Google Scholar] [CrossRef]
- Thomas, D.T.; Freshwater, D.W. Studies of Costa Rican Gelidiales (Rhodophyta): Four Caribbean taxa including Pterocladiella beachii sp. nov. Phycologia 2001, 40, 340–350. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algol. 2010, 31, 435. [Google Scholar]
- Iha, C.; Milstein, D.; Guimaraes, S.M.; Freshwater, D.W.; Oliveria, M.C. DNA barcoding reveals high diversity in the Gelidiales of the Brazilian southeast coast. Bot. Mar. 2015, 58, 295–305. [Google Scholar] [CrossRef]
- Iha, C.; Jamas, M.; Guimaraes, S.M.; Fuji, M.T.; Freshwater, D.W.; Oliveria, M.C. Pterocladiella (Gelidiales, Rhodophyta) species of Brazil including morphological studies of Pterocladiella media and a reassessment of Pterocladiella taylorii. Phycologia 2017, 56, 624–637. [Google Scholar] [CrossRef]
- Lin, S.M.; Fredericq, S.; Hommersand, M.H. Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov. J. Phycol. 2001, 37, 881–899. [Google Scholar] [CrossRef]
- Geraldino, P.J.L.; Yang, E.C.; Boo, S.M. Morphology and molecular phylogeny of Hypnea flexicaulis (Gigartinales, Rhodophyta) from Korea. Algae 2006, 21, 417–423. [Google Scholar] [CrossRef]
- Boo, G.H.; Park, J.K.; Boo, S.M. Gelidiophycus (Gelidiales, Rhodophyta): A new genus of marine algae from East Asia. Taxon 2013, 62, 1105–1116. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T.A. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Ful-feature software for haplotype network construction. Methods Ecol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Yang, E.C.; Kim, M.S.; Geraldino, P.J.L.; Sahoo, D.; Shin, J.A.; Boo, S.M. Mitochondrial cox1 and plastid rbcL genes of Gracilaria vermiculophylla (Gracilariaceae, Rhodophyta). J. Appl. Phycol. 2008, 20, 161–168. [Google Scholar] [CrossRef]
- Kong, F.; Mao, Y.; Gao, Z.; Wang, L.; Sun, P.; Shi, X. Genetic Polymorphic Analysis from the Shilaoren Sea Area of Qingdao. Period. Ocean Univ. China 2010, 40, 75–78. [Google Scholar]
- Shi, L.M. Genetic diversity and its preservation. Bio. Sci. Inf. 1993, 2, 159–164. (In Chinese) [Google Scholar]
- Zhang, C.X.; Zhou, W.N.; Sun, S.L.; Song, Z.G. Seasonal succession of macroalgae community in Naozhou Island. J. Trop. Oceanogr. 2020, 1, 74–84, (In Chinese with English Abstract). [Google Scholar]
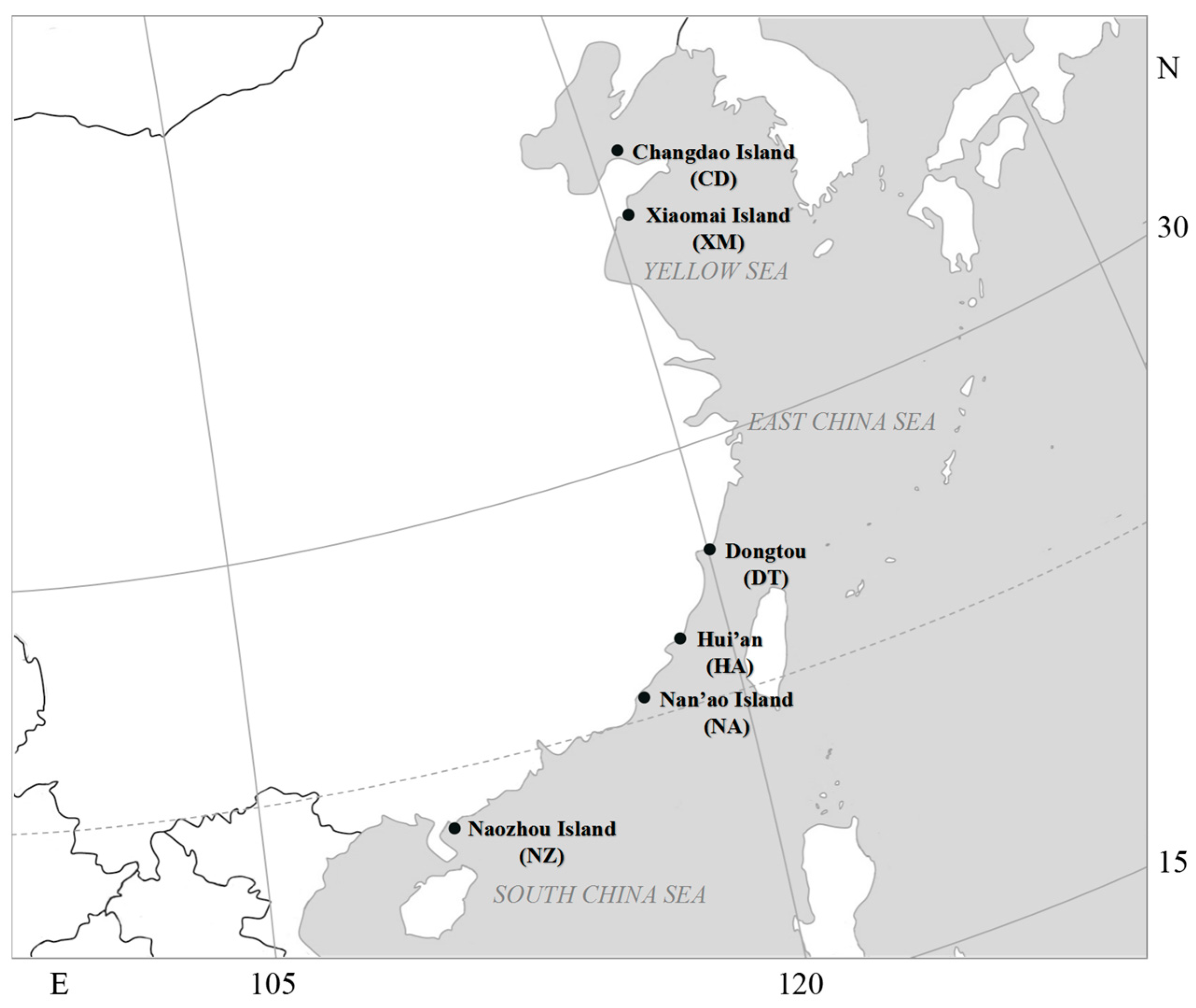
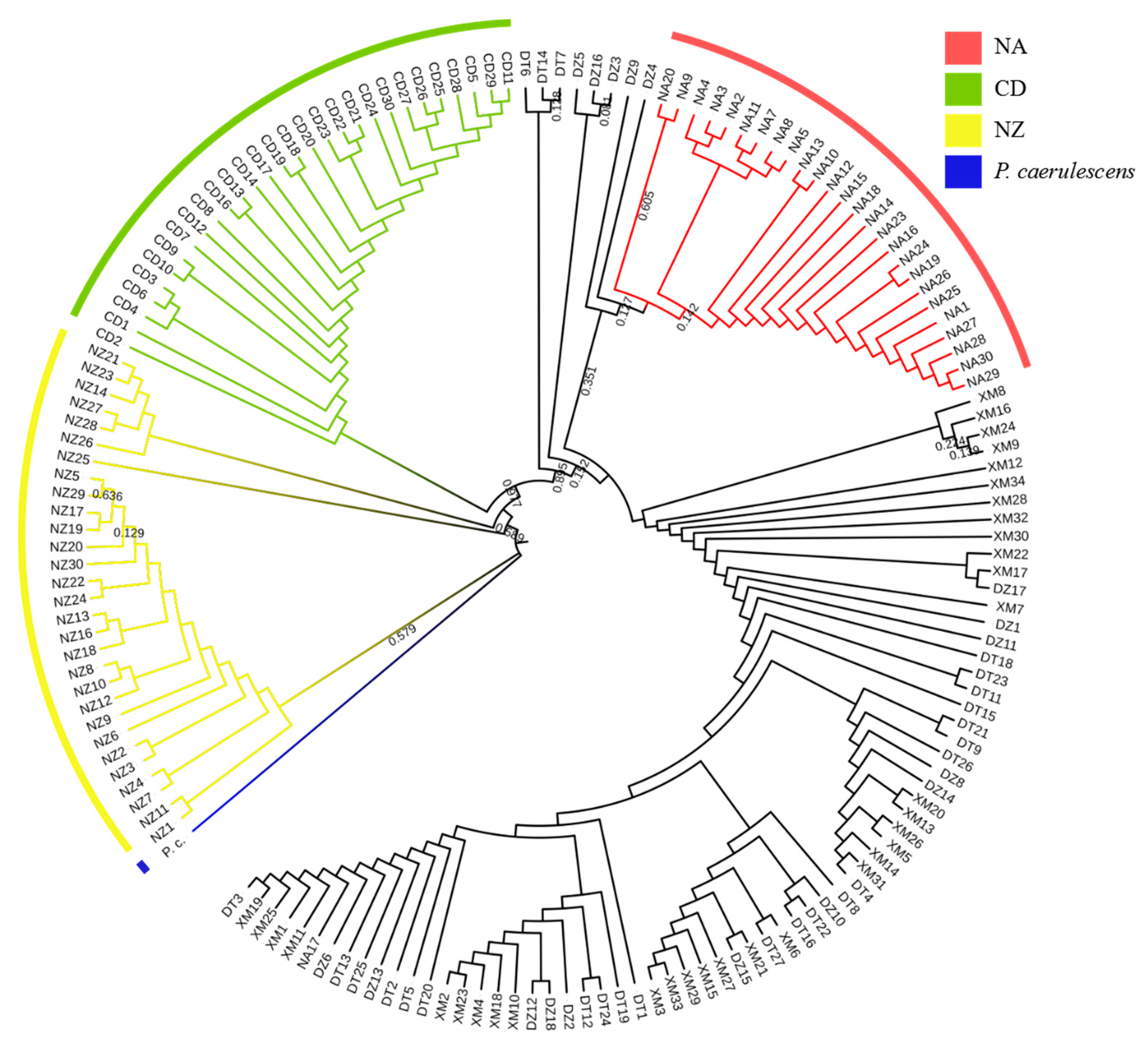

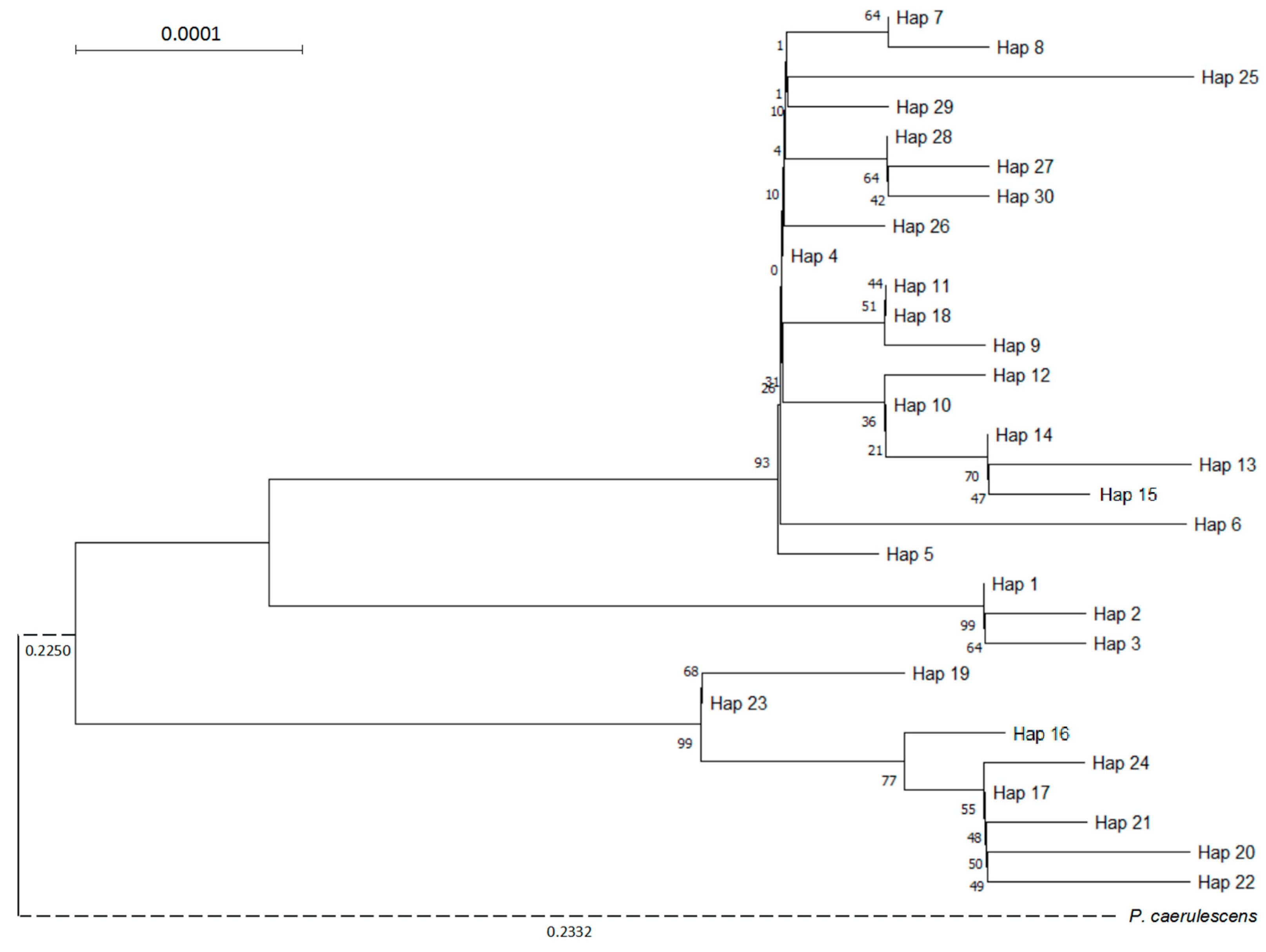
| Sampling Location | Code | Longitude (E) | Latitude (N) | Quantity | |
|---|---|---|---|---|---|
| Shandong Province | Changdao Island | CD | 120°44′41″ | 37°57′3″ | 29 |
| Xiaomai Island | XM | 120°25′8″ | 36°3′54″ | 34 | |
| Zhejiang Province | Dongtou | DT | 121°9′2″ | 27°41′57″ | 25 |
| Fujian Province | Hui’an | HA | 118°59′30″ | 24°52′53″ | 17 |
| Guangdong Province | Nan’ao Island | NA | 117°6′37″ | 23°28′54″ | 28 |
| Naozhou Island | NZ | 110°34′6″ | 20°53′31″ | 28 | |
| Total | 161 | ||||
| Population | Quantity | Nh | Hd | Nsv | Npi | Pi | k | Tajima’s D |
|---|---|---|---|---|---|---|---|---|
| CD | 29 | 3 | 0.135 ± 0.085 | 2 | 0 | 0.00006 ± 0.00004 | 0.138 | −1.50906, p > 0.10 |
| XM | 34 | 7 | 0.373 ± 0.105 | 9 | 1 | 0.00031 ± 0.00012 | 0.684 | −2.08287, p < 0.05 |
| DT | 25 | 5 | 0.363 ± 0.120 | 6 | 1 | 0.00031 ± 0.00023 | 0.700 | −1.92457, p < 0.05 |
| HA | 17 | 5 | 0.507 ± 0.140 | 3 | 2 | 0.00033 ± 0.00011 | 0.735 | −0.48541, p > 0.10 |
| NA | 28 | 5 | 0.328 ± 0.112 | 16 | 3 | 0.00070 ± 0.00046 | 1.556 | −2.39364, p < 0.01 |
| NZ | 28 | 8 | 0.643 ± 0.088 | 9 | 3 | 0.00070 ± 0.00014 | 1.558 | −1.63850, 0.10 > p > 0.05 |
| Total | 161 | 30 | 0.805 ± 0.023 | 26 | 28 | 0.00368 ± 0.00023 | 8.22042 | −0.51893, p > 0.1 |
| CD | XM | DT | HA | NA | NZ | |
|---|---|---|---|---|---|---|
| CD | ||||||
| XM | 0.005591 | |||||
| DT | 0.005594 | 0.000319 | ||||
| HA | 0.005618 | 0.000343 | 0.000343 | |||
| NA | 0.005593 | 0.001280 | 0.001283 | 0.001159 | ||
| NZ | 0.007647 | 0.006868 | 0.006870 | 0.006895 | 0.007407 |
| CD | XM | DT | HA | NA | NZ | |
|---|---|---|---|---|---|---|
| CD | ||||||
| XM | 0.96690 | |||||
| DT | 0.96629 | 0.02903 | ||||
| HA | 0.96502 | 0.07168 | 0.06728 | |||
| NA | 0.93186 | 0.60741 | 0.60567 | 0.55673 | ||
| NZ | 0.95000 | 0.92645 | 0.92596 | 0.92507 | 0.90523 |
| Source of Variance | df | Sum of Squares | Variance Components | Percentage Variation | p |
|---|---|---|---|---|---|
| Among populations | 5 | 588.090 | 4.39891 | 90.74 | <0.0010 |
| Within populations | 155 | 69.543 | 0.44867 | 9.26 | <0.0010 |
| Total | 160 | 657.634 | 4.84757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, Z.; Sun, Z.; Chen, W. Genetic Diversity and Profile of Red Algae Pterocladiella capillacea (Gelidiales, Rhodophyta) along the Coast of China. Diversity 2024, 16, 389. https://doi.org/10.3390/d16070389
Li J, Chen Z, Sun Z, Chen W. Genetic Diversity and Profile of Red Algae Pterocladiella capillacea (Gelidiales, Rhodophyta) along the Coast of China. Diversity. 2024; 16(7):389. https://doi.org/10.3390/d16070389
Chicago/Turabian StyleLi, Jianning, Zepan Chen, Zhongmin Sun, and Weizhou Chen. 2024. "Genetic Diversity and Profile of Red Algae Pterocladiella capillacea (Gelidiales, Rhodophyta) along the Coast of China" Diversity 16, no. 7: 389. https://doi.org/10.3390/d16070389
APA StyleLi, J., Chen, Z., Sun, Z., & Chen, W. (2024). Genetic Diversity and Profile of Red Algae Pterocladiella capillacea (Gelidiales, Rhodophyta) along the Coast of China. Diversity, 16(7), 389. https://doi.org/10.3390/d16070389






